Prostate cancer (PCa) is one of the most significant
causes of morbidity and mortality in the global male population,
presenting as the second most commonly diagnosed malignancy and the
fifth most predominant cause of mortality in men worldwide
(1). A number of risk factors
relating to PCa incidence have been identified, with the most
prominent being age; 75% of all patients diagnosed with PCa are
over the age of 65. Additionally to this, other risk factors which
predispose an individual to developing PCa have been identified,
including being of African-American heritage, living in a more
economically-developed country, and certain genetic factors
(1–3).
Patients with suspected PCa are primarily diagnosed
through the use of an initial digital rectal examination (DRE) and
the current gold-standard prostate-specific antigen (PSA) blood
test, followed by confirmation of diagnosis through magnetic
resonance imaging (MRI) or transrectal ultrasound (TRUS) guided
biopsy (4,5). The growth and development of PCa is
largely driven through androgen receptor (AR) signalling.
Therefore, following diagnosis, the most widely utilised approach
to treating patients is androgen deprivation therapy (ADT).
However, despite the initial disease regression commonly observed
with ADT, 2–3 years after commencing treatment a significant
majority of patients will go on to develop ADT-resistant tumours,
which are termed castrate-resistant PCa (CRPC) (6). The treatment options available for
treating CRPC patients has significantly improved in recent years,
most predominantly having been revolutionised through the
development of androgen receptor-axis targeted (ARAT) agents, such
as abiraterone and enzalutamide (7,8).
Despite these advances, CRPC remains to be a predominantly fatal
disease, with the median survival for these patients being
relatively dire at 9–36 months (9,10).
Bone metastases is a commonly observed clinical
occurrence for PCa patients, with secondary skeletal tumours being
apparent in around 10% of patients diagnosed with primary disease
and up to 80% of patients with later-stage PCa (11,12).
The presence or absence of bone metastases in PCa patients has
significant prognostic implications. This can be highlighted by the
findings of one study in which it was reported that the 3- and
5-year survival rate for PCa patients with bone metastases was
47.70 and 32.42%, respectively, in comparison to 98.43 and 97.28%
in patients without bone metastases (12). In addition to the prognostic impact
of bone metastases, it is also associated with significant
debilitating co-morbidities, including severe bone pain and
fractures (13).
One of the most extensively described features of
PCa cells which possess the capability to form secondary tumours at
the bone is the ability to interact with and modulate the activity
of the resident bone cells, osteoblasts and osteoclasts, in order
to generate a preferential microenvironment to support tumour
establishment. Osteoblasts and osteoclasts are involved in the bone
remodelling process, which is a naturally occurring process in
which bone tissue is progressively broken down and built back up
again in order to maintain healthy skeletal structure and function,
as well as playing an integral role in calcium homeostasis
(14,15). However, a number of studies have
documented that cancer cells which colonise the bone have the
ability to interact with these resident bone cells in order to
disrupt the carefully regulated balance of bone resorption in order
to promote the formation of secondary tumours (16–18).
Despite PCa having a well-known predilection for
forming secondary tumours at the bone, the molecular mechanisms
which underpin this tropism remain to be fully elucidated. Gaining
a better understanding of these mechanisms may provide the
opportunity to identify more clinically relevant biomarkers to
enable the earlier detection of metastatic disease to the bone, in
addition to potentially providing novel therapeutic targets.
In order for a secondary skeletal tumour to be
established, PCa cells must first undergo a step-wise process in
which successive molecular expression changes must occur to support
detachment from the primary tumour site, survival in the blood
stream and settlement at the distal site (19). According to the ‘seed and soil’
hypothesis first stipulated by Paget in 1889, in order for a
circulating tumour cell to colonise a secondary site the
microenvironment of the distal loci must be hospitable to support
the settlement and establishment of a secondary tumour (20). A key feature underpinning the
capacity of a circulating PCa cell in establishing a secondary
tumour at the site of the bone is the ability to interact with the
resident osteoblasts and osteoclasts in order to generate a
microenvironment that can support the growth of a tumour (18).
Bone metastases can be defined as either
osteoblastic, osteolytic or mixed, depending on the mechanistic
involvement of osteoblasts and osteoclasts in the formation of
secondary tumours (21). In the
instance of PCa, the significant majority of cases present with
osteoblastic metastases (22–24).
Osteoblastic (also called sclerotic) bone lesions
are characterised by an imbalance in resorption activity in which
osteoblasts are preferentially activated whilst the activity of
osteoclasts are downregulated (25). Excessive osteoblast activity results
in a net gain in bone tissue that presents in patients as irregular
woven bone that is highly under-mineralised. This results in the
generation of highly frail bone tissue that is liable to fracture
(26). Areas of osteoblastic bone
metastases formation are commonly associated with parallel areas of
heightened osteoclast activity, which gives rise to both osteopenic
and osteodense areas arising within the same lesion (27,28).
In comparison, osteolytic bone lesions arise as a
result of hyper-activity of osteoclasts; the cells responsible for
bone resorption (29). Osteolytic
bone metastases are characterised by excessive bone degradation due
to a heightened bone resorption activity of osteoclasts. The
formation of osteolytic bone metastases has been described as a
‘vicious cycle’ in which PCa cells secrete factors which enhance
the differentiation and activity of osteoclasts, and in-turn,
osteoclasts secrete factors which further promote the survival and
growth of localised PCa cells (18).
Despite the marked difference in the activity of
osteoblasts and osteoclasts in sclerotic and osteolytic bone
lesions, the subtype of bone lesion is not currently utilised to
inform treatment approach in the clinic. This may be a result of
the fact that the subtype of bone lesion has been shown to result
in no difference in patient survival (30). However, personalised treatment
approaches and patient stratification based on bone metastases
presentation may provide the opportunity to improve the current
survival statistics and quality of life for patients that have PCa
which has metastasised to the bone.
The vast majority of PCa patients that present with
bone metastases have osteoblastic lesions. For example, one study
of 55 PCa patients found that 70.9% had osteoblastic metastases,
compared to 16.4% of patients having osteolytic metastases and the
remaining 12.7% having mixed osteolytic and osteoblastic lesions
(30). Despite the high occurrence
of osteoblastic bone lesions in PCa and a number of other cancer
types the mechanisms through which cancer cells may promote the
formation of osteoblastic lesions are not well established.
However, studies have demonstrated an irrefutable relationship
between PCa cells and osteoblasts in the formation of sclerotic
lesions, and some of the potential signalling pathways involved
have been highlighted in the literature. For example, one study
reported that culturing the CRPC cell line, C4-2B, in osteoblast
conditioned media, resulted in increased PCa cell proliferation
through an androgen-independent signalling mechanism (31). Furthermore, it has been demonstrated
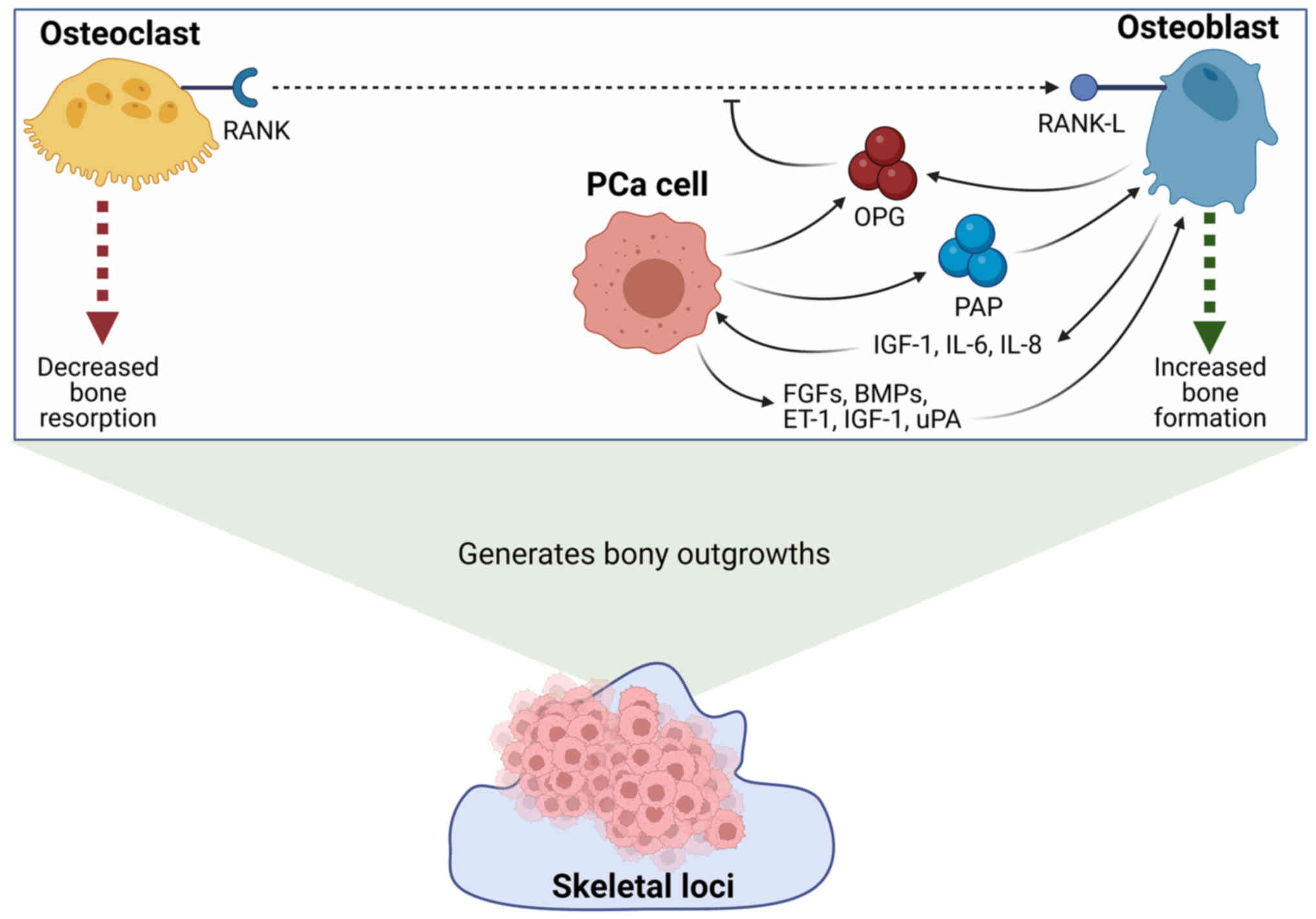
that PCa cells secrete a number of factors which promote the
differentiation and activity of osteoblasts. One example of this is
prostatic acid phosphatase (PAP) which has been shown to be
secreted by PCa cells and plays an important role in
osteoblastogenesis. The importance of PAP in sclerotic bone
metastases can be highlighted by the findings of Kirschenbaum et
al (32) who reported that 100%
of PCa bone metastases tissue samples had high expression of PAP.
Furthermore, it was found that co-culture of mouse pre-osteoblast
cells with the PAP-secretory human PCa cell line, VCaP, induced
osteoblast maturation. The secretion of PAP has been shown to play
a role in modulating the key nuclear factor-κB
(RANK)/RANK-L/osteoprotegerin (OPG) signalling axis involved in
controlling osteoclast and osteoblast differentiation. For example,
it has been demonstrated that PAP overexpression in a metastatic
bone-derived PCa cell line results in a concurrent increase in OPG
and decrease in RANK/RANK-L expression. This change in expression
profile was demonstrated to result in elevated osteoblast
maturation and the generation of osteoblastic lesions in an in
vivo model (33).
Another factor which may be utilised by PCa cells in
order to promote the formation of osteoblastic bone lesions is OPG
(34). OPG is a soluble decoy
receptor which acts to prevent the interaction between RANK and
RANK-L, and thereby inhibits osteoclast maturation (35,36).
It has been previously shown that OPG expression is significantly
higher in PCa cell lines compared to normal cell lines (34,37,38).
Furthermore, in one study it was reported that 73% of patient
samples taken from metastatic PCa (mPCa) were positive for OPG
expression, in comparison to 19% of primary carcinoma samples, with
a marked increase in OPG expression being most apparent for bone
lesions (34). Additionally to
this, C4-2 PCa cells which were stably transfected to overexpress
OPG were found to inhibit osteoclast maturation and resulted in
heightened bone mineral density and bone volume in vivo
(36). In addition to PAP and OPG,
a number of additional PCa-derived secretory factors, such as
fibroblast growth factors (FGFs), endothelin-1 (ET-1), insulin-like
growth factor-1 (IGF-1) and urokinase-type plasminogen activator
(uPA) have all been demonstrated to induce dysfunctional
osteoblastic activity giving rise to sclerotic lesions (39–43).
The enhancement of osteoblast differentiation and
activity then in turn further promotes the proliferation of
localised PCa cells through the release of growth factors, such as
IGF-1, and cytokines, such as interleukin-6 and −8 (IL-6 and −8)
(40,43). A summary of the mechanisms through
which osteoblasts and PCa cells may interact in order to facilitate
the formation of sclerotic lesions are summarised below in Fig. 1.
Osteolytic bone metastases are not a common
occurrence in PCa and when they do occur, they primarily present as
a solitary metastasis (44,45). However, there are some limited
documented cases of diffuse osteolytic lesions occurring in PCa
patients (45–53).
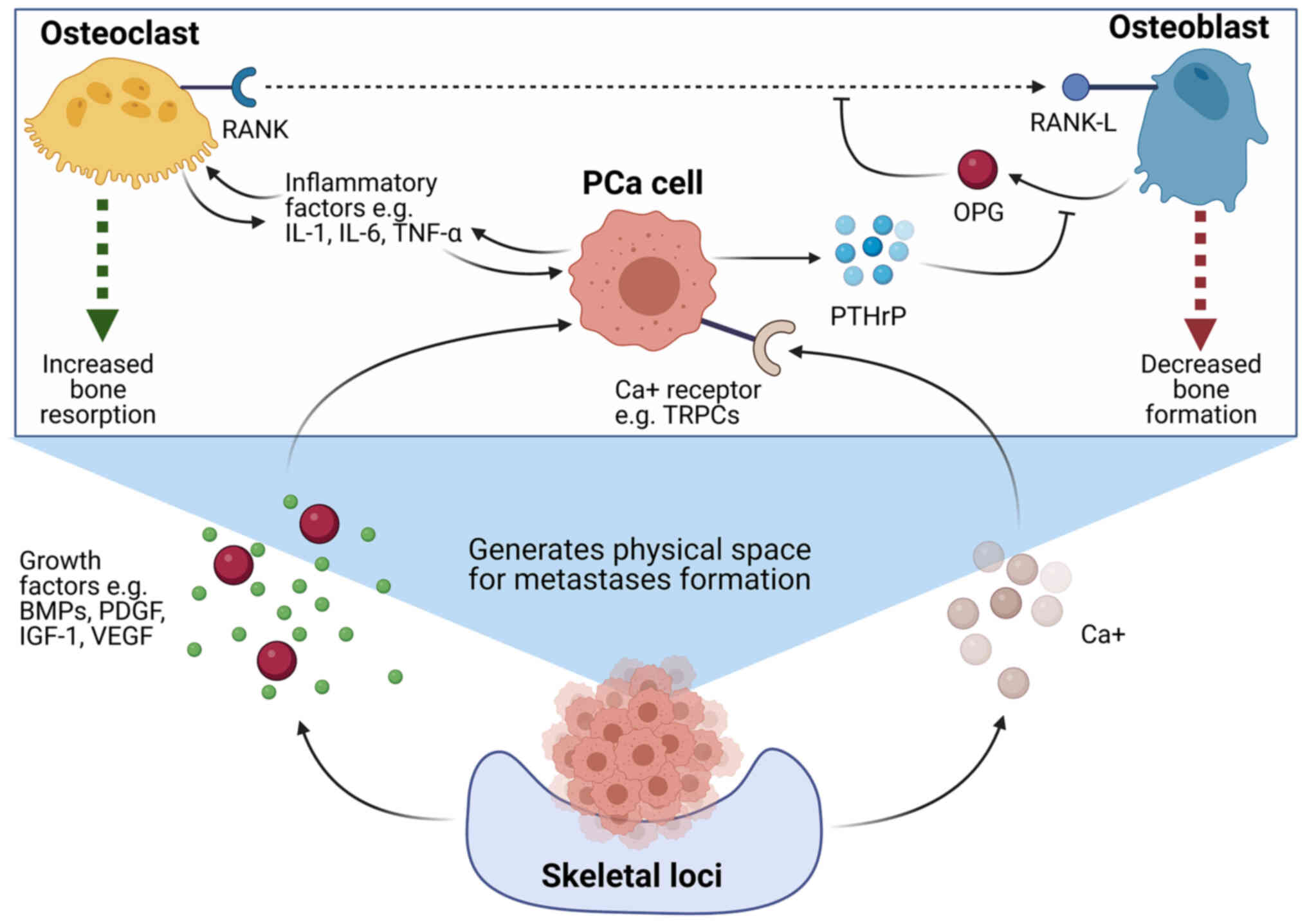
The generation of osteolytic bone metastases arises
as a result of specific signalling factors expressed by localised
PCa cells that promote the maturation and activity of osteoclasts.
For example, it has been demonstrated that PCa cells with the
ability to form osteolytic bone lesions express elevated levels of
parathyroid hormone-related peptide (PTHrP), which binds to
receptors on osteoblasts and facilitates a downregulation in
expression of OPG and an upregulation in receptor activator of
RANK-L expression (18,54–56).
The expression of RANK-L on the surface of osteoblasts facilitates
binding to the RANK receptor which is expressed on the surface of
pre-osteoclasts, resulting in enhanced osteoclast maturation and
activity (57,58). In addition to these factors, PCa
cells may also upregulate osteoclast activity through the release
of a number of pro-inflammatory factors, such as tumour necrosis
factor α (TNF-α), and cytokines, such as interleukin-1 (IL-1) and
IL-6, via downstream signalling cascades (59).
Ultimately, the excessive resorptive activity of
osteoclasts and inhibition of osteoblasts results in a bone
resorption imbalance that favours uncontrolled bone degradation.
This generates a ‘vicious cycle’, as the degradation of bone tissue
leads to the release of a number of growth factors, including
IGF-1, vascular endothelial growth factor (VEGF), bone morphogenic
proteins (BMPs), platelet-derived growth factor (PDGF) and
excessive calcium, which then further promotes the growth and
proliferation of PCa cells (18,60–62).
Not only do these changes in the microenvironment help to further
aid and support the growth of PCa cells, but it also leads to the
formation of the physical space to enable the formation of a
metastatic tumour (63). The
interplay between PCa cells and osteoclasts in order to promote the
establishment of secondary tumours is a highly complex and dynamic
process which remains to be fully investigated. However, a summary
of some of the key interactions outlined in the literature to date
can be summarised in Fig. 2.
In addition to interacting with the resident bone
cells in order to promote secondary bone metastases formation, PCa
cells with the ability to metastasise must undergo sequential
changes to protein expression which will facilitate dissociation
from the primary tumour site, survival in the circulatory system,
extravasation at a distal site, and establishment of a secondary
tumour. Due to the highly complex, multi-step nature of this
process vast numbers of molecular changes must occur to enable a
metastatic event to take place. Although the principle molecular
changes which underpin the metastatic process are already
well-documented in literature, novel aberrations to both protein
expression and post-translational modifications specific to bone
cancer metastases are becoming apparent in the literature (19).
One example of an important molecular change which
underpins the ability of a circulating cancer cell to establish
secondary tumours at the site of the bone is alternative integrin
expression. Integrins are a family of transmembrane receptors that
mediate cellular adhesion to the extracellular matrix (ECM) and to
other neighbouring cells (64,65). A
number of changes to integrin expression have been reported to play
a role in cancer metastases, including integrin αvβ3. Abnormal
expression of αvβ3 plays a role in facilitating bone metastases in
PCa through a multitude of mechanisms. For example, it has been
shown that αvβ3 on PCa cells may enable tumour cell adhesion to the
localised bone ECM through interacting with the RDG motif on
fibronectin. This facilitates a downstream signalling cascade that
ultimately results in cytoskeletal protein rearrangement, leading
to protein fibre contraction and enabling invasion of the PCa cell
into the bone ECM (66,67). In addition to the direct
interactions of αvβ3 in facilitating ECM invasion, it has also been
shown that αvβ3 modulates the activity of matrix metalloproteinases
via aberrations to the phosphoinositide 3-kinase (PI3K) signalling
pathway in order to promote the degradation of localised ECM
(68,69). Similarly, integrin αvβ3 interactions
promotes vascularisation via PI3K/Akt pathway activation leading to
VEGF expression, further supporting the establishment of a
secondary tumour (70–72). Furthermore, αvβ3 has been shown to
recruit and activate additional downstream proteins which help to
prevent immune-recognition, such as TGFβ, which further promotes
tumour cell survival in the circulation (73).
The therapeutic potential of targeting integrins to
prevent bone metastases in PCa patients has been investigated. An
example of this is in one phase II study which investigated the use
of a pan-αv integrin inhibitor, abituzumab, in a cohort of patients
with asymptomatic or mildly symptomatic mCRPC. Despite finding no
significant difference in progression-free survival (PFS) between
the abituzumab-treatment group and the placebo group, significantly
fewer patients in the abituzumab-treatment group experienced bone
lesion progression relative to the patients on placebo (74,75).
In addition to integrins, a number of other protein
expression changes have been shown to play a role in modulating the
ability of PCa cells to colonise the bone. For example, alterations
to the expression profile of cadherins are a well-characterised
aberration which play a key role in cancer metastases through
promoting epithelial-to-mesenchymal transition (EMT). EMT is
generally characterised by a loss of E-cadherin expression and an
increase in N-cadherin expression, which promotes cellular
dissociation from the primary site and enables migration and
invasion of cancer cells at distal sites (65,76).
For example, in one study looking at paired prostate bone
metastases and primary tumour samples, it was found that both
E-cadherin and β-catenin were uniformly downregulated in expression
in all of the metastatic tissues assessed compared to the matched
primary samples (77). Furthermore,
a number of studies have reported an upregulation of N-cadherin in
patients and in vivo studies in the instance of metastases
(78–80). The clinical utility of targeting
N-cadherin using antibodies have been shown to block metastases and
result in disease regression in in vivo studies (81).
In addition to the interactions between PCa cells
and bone cells in the establishment of secondary bone tumours, a
number of other biological processes have been implicated as
playing a key role in bone metastases formation. One example of
this is glycosylation. Glycosylation is a ubiquitous feature of
cellular proteins in which sugar groups are enzymatically added
post-translationally to proteins and lipids in order to generate
glycan chains (82). Alterations to
glycosylation in cancer has been identified as a potential driver
of metastasis, and moreover, may play a role in premeditating the
site-preference exhibited by cancer cells when forming secondary
metastatic tumours (83,84). Although not widely investigated, a
cohort of studies have published findings indicating that changes
to glycans and their associated glycosylation enzymes may play a
role in predisposing PCa cells to establishing secondary tumours at
the bone.
One example of how abnormal glycosylation may impact
metastasis and osteotropism in PCa is the glycosphingolipid, GM1.
The bone-mPCa cell line, C4-2B, has been shown to express an
abnormally glycosylated form of GM1, which has the capacity to
interact the integrin, α2β1, to facilitate invasive cell behaviour.
The role of the α2β1 integrin in bone metastasis has been
previously outlined in literature, with PCa patients that have bone
metastases being found to have significantly greater expression of
α2β1 relative to patients with soft tissue metastases (85). In addition to this, elevated levels
of α2,3-sialylation and expression of the responsible
sialyltransferase enzyme, ST3GAL3, were found to be more highly
expressed in the bone-invasive PCa cell lines relative to
non-invasive counterparts (86).
This may provide a potential insight into one of the mechanisms
through which PCa exhibits a preference for settlement at the bone,
through altering the cellular glycosylation profile to promote
attachment and invasiveness at secondary skeletal sites.
Furthermore, one of the most extensively documented
interactions involved in bone metastasis is the interaction between
E-selectin and its preferential ligand, sialyl-LewisX (sLeX)
(87). It has been previously
demonstrated that extravasation of PCa cells at the site of the
bone is mediated by interactions between E-selectin ligands, such
as sLeX, and E-selectin, which is expressed on endothelial bone
cells. Moreover, it has been demonstrated that PCa cell lines with
the capacity to invade bone tissue express the fucosyltransferase
enzymes, 3 and 6 (FUT3 and FUT6), at a 31- and 10-fold higher
expression, respectively, in comparison to normal, non-invasive
prostate tissue. These FUT3 and FUT6 enzymes play an integral role
in the catalytic creation of sLeX ligand (88).
Another example which highlights a potential role
for aberrant glycosylation in PCa metastasis is cleaved galectin-3
(Gal-3). Cleaved Gal-3 expression is positively associated with
metastasis in PCa and is closely related to PSA levels (89). Furthermore, secreted Gal-3 has been
found to be elevated specifically in PCa patients with bone
metastases. The secreted, cleaved version of Gal-3 interacts with
myosin-2A which attenuates osteoclast differentiation, and thereby
generates a bone niche which is supportive of sclerotic bone
metastases formation (90).
Although glycosylation appears to play a role in
determining the ability of circulating PCa cells to colonise
secondary skeletal sites, the metastatic process is highly complex
and involves a multitude of interactions and alterations to
cellular expression profiles to facilitate each stage of metastasis
(91). Extensive and dynamic
sequential changes to the expression of a great number of proteins,
including cadherins, integrins and GTP-binding proteins underpins
the ability of PCa to establish a secondary tumour at the bone.
However, as alterations to glycosylation poses as a plausible
contributing factor to the ability of PCa to metastasise to the
bone it may provide a potentially efficacious therapeutic target
for the discovery of novel treatments and biomarkers for patient
disease identification and treatment stratification.
Due to the clinical relevance of bone metastases in
cancer, the ability to study this process in vitro is of
high importance. However, due to the extensive complexity of the
molecular pathways involved in the metastatic cascade, in addition
to difficulties in recapitulating patient physiology, models are
often limited and lack translational efficacy (92).
However, significant recent advances have led the
development of promising models for studying metastases both in
vitro and in vivo. For example, improved knowledge of
the molecular pathways which underpin a metastatic event have led
to studies which have demonstrated that knocking down CD44 and
CD147 in PCa cells results in reduced invasiveness. Furthermore,
xenografts of these knock-down cell lines in in vivo models
have demonstrated reduced tumour growth and metastatic capacity
(93). This knowledge can then be
applied to generate models in which key drivers of metastases, such
as CD44 and CD147, can be modulated to somewhat recapitulate the
molecular profile of metastatic tumour cells (94,95).
Despite advances in the understanding of the
molecular pathways which underpin metastasis, human cell line-based
models remain limited in their translational efficacy. This is due
to a lack of clinical relevance in recapitulating the complex
interplay between the multiple cell types that are involved in
tumour formation. For example, increasing evidence has indicated
that the stromal microenvironment plays an integral role in the
metastatic cascade (96,97). Therefore, 2-dimensional (2D) models
which aim to allow the investigation of tumour cell interactions
with the surrounding ECM have been generated, including scratch or
wound healing assays and transwell cell migration assays (92). Although these assays provide
improved translational efficacy in comparison to human cell
line-based models, they fail to fully recapitulate the
architectural and cellular complexity of metastatic tumour
formation in vivo. Therefore, an alternative approach to
modelling metastasis experimentally is through the use of 3-D
models. One example of a 3-D model of metastasis in vitro is
the use of perfusion bioreactors which aim to experimentally
recreate the mechanical tension on the ECM caused by constant
tissue perfusion. Studies have indicated that the inclusion of
perfusion bioreactors in modelling metastasis enables a better
representation of the cellular functionality of human mesenchymal
stem cells (hMSCs) and initiates the expression of EMT markers in
bone-metastasised PCa models (98).
In addition to this, the capacity to model metastasis in
vitro has further been improved through the ability to grow 3-D
models of prostate tumours. For example, establishing human
prostate organoids from induced pluripotent stem cells (iPSCs) and
the growth of patient-derived xenograft models. These models then
have the capacity to be co-cultured alongside other cell types
commonly observed in the tumour microenvironment to better model
tumour-stromal interactions (99,100).
Furthermore, the translational capacity of these
models has been significantly improved due to recent advances in
the development of novel nanocomposite materials and scaffolds.
These materials can provide biophysical and biochemical signals to
cells to promote tissue formation, whilst also enabling nutrient
supply and waste removal to occur due the porous nature of the
structures. Therefore, these materials enable better recreation of
the metastatic microenvironment in which a secondary tumour forms
in vivo (101,102). These novel biomaterials can be
utilised in models which allow the study of tumour cells which
metastasise to the bone. For example, the use of
calcium-phosphate-based biomaterials, such as hydroxyapatite (HAP),
have been used to model bone metastases due to their similarity
with the mineral composition observed in human bone. These HAP
nanoparticles in combination with collagen have been shown to
support osteoblast colonisation and promote bone-ingrowths.
Additionally to this, it has been shown that PCa cell lines undergo
morphogenic changes and have the ability to colonise these
scaffolds (102,103).
These recent developments in the generation of
novel, efficacious models of metastasis will enable more in-depth
study of the molecular mechanisms which underpin the formation of
secondary tumours, and hopefully inform the development of future
targets for treating and preventing bone metastasis occurrence.
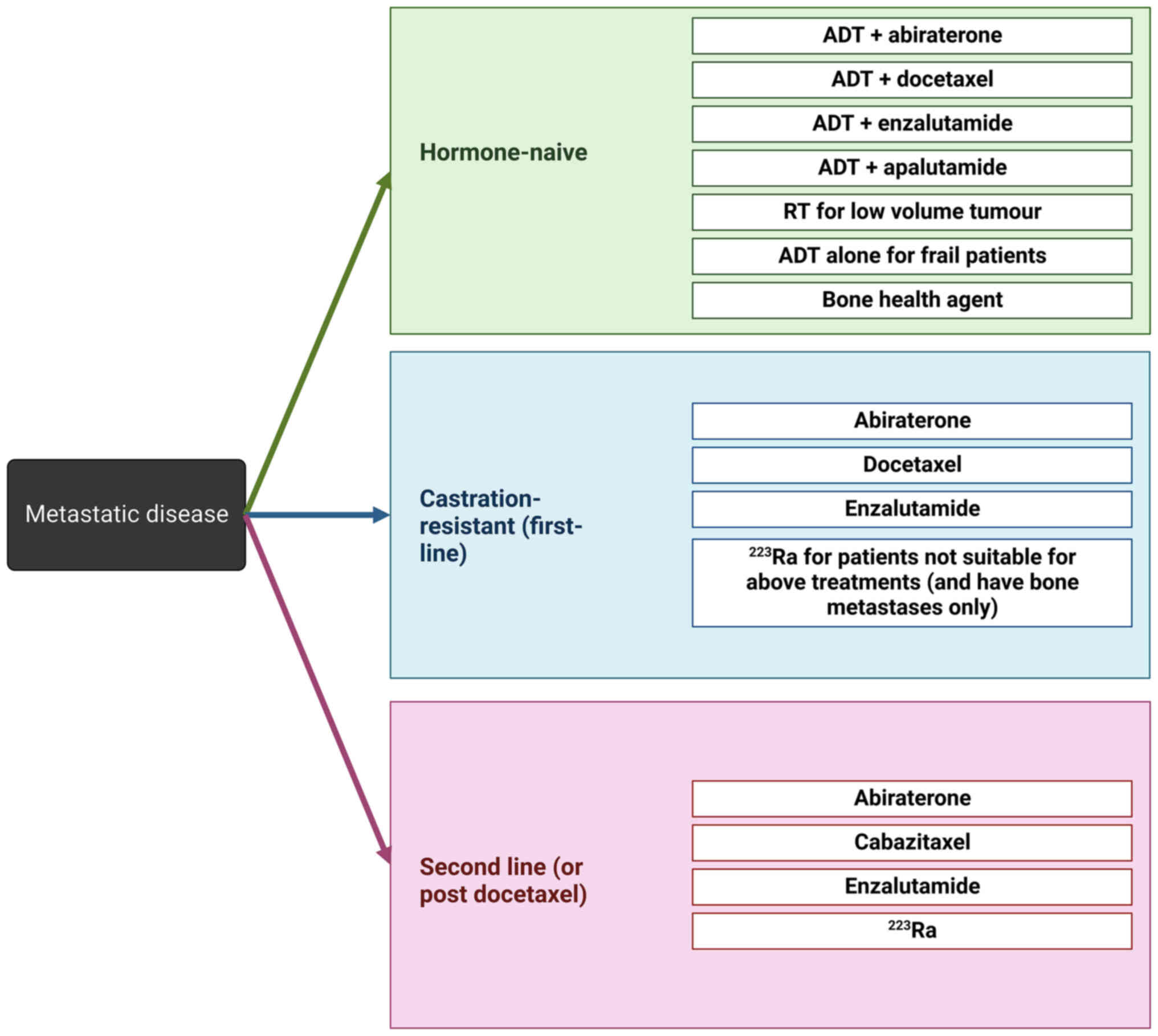
The treatment options for patients diagnosed with
metastatic disease has improved significantly over recent years,
however the survival statistics for this subset of patients still
remains relatively poor, with the medium overall survival for
patients with mPCa being around 21 months (10). For patients diagnosed with mPCa, the
treatment options available are dependent on the hormone-status of
the disease and prior treatment regimes. Patients diagnosed with
hormone-naïve metastatic disease may be treated with ADT in
combination with abiraterone, docetaxel, enzalutamide or
apalutamide, or for patients that are deemed to not have the
capacity to tolerate combination therapy may receive ADT alone.
Patients who have a low tumour volume may alternatively be treated
with radiotherapy. In addition to this, patients may be prescribed
bone health agents, such as zoledronic acid, which are utilised to
help manage bone pain, initiate disease regression and prevent
further skeletal related events (SREs) (104,105). For patients diagnosed with
castrate-resistant metastatic disease, the first-line treatment
approaches are abiraterone, docetaxel or enzalutamide
monotherapeutically. For those patients who are found to be not
suitable for these treatment approaches and have the presence of
bone metastases, patients may be prescribed the bone-targeting
agent, radium 223 dichloride (radium-223). Patients needing
treatment as a second-line approach or following disease
progression that is refractory to docetaxel are treated with
abiraterone, cabazitaxel, enzalutamide, or radium-223 (104). The currently utilised approach to
clinical management of PCa patients with metastatic disease is
summarised in Fig. 3.
The high prevalence of PCa bone metastases and
resultant life-limiting nature of the associated symptoms
necessitates the ability to successfully prevent and treat bone
metastases formation and progression. A number of treatment
approaches with the aim of limiting the progression of bone
metastases and managing the associated symptoms have been
developed, including bisphosphonates and other bone-targeting
therapies, corticosteroids, radiation therapies and surgical
intervention.
Despite the fact that the vast majority of
metastatic events in PCa are osteoblastic, osteoclast-inhibiting
therapeutics have, until recently, been the only treatment
approaches approved for managing PCa bone metastases.
Osteolytic-based interventions are thought to demonstrate a degree
of success as previous studies have indicated that a significant
proportion of patients with bone metastases have mixed osteolytic
and osteoblastic lesions. Furthermore, it is a widely accepted
dogma that simultaneous activation of osteoclasts may be concurrent
with osteoblastic lesions. This is thought to be due to the natural
feedback system designed to maintain the homeostasis of bone
resorption, in which activated osteoblasts may promote the
maturation of pre-osteoclasts in order to achieve an equilibrated
rate of bone turnover (27,28).
Bisphosphonates act to inhibit osteoclast maturation
and ultimately result in osteoclast apoptosis (106,107). Bisphosphonates were originally
developed with the view to treat osteolytic bone lesions. However,
due to the concurrent upregulation of osteolytic activity in areas
of sclerotic metastases, these drugs have been applied to the
clinical management of mPCa disease, with variable success
(108–110). For example, pamidronate disodium
has been investigated for its utility in treating skeletal
metastases progression and the associated bone pain experienced by
patients. However, results have been largely inconclusive and show
no notable significant improvement to disease or symptom management
(111,112). This may be a result of pamidronate
disodium not being as potent as some of the more recently developed
bisphosphate treatments available in the clinic, such as zoledronic
acid (113). Zoledronic acid is
the most widely utilised bisphosphonate for the management of bone
metastasis in PCa patients due to its reported ability to delay the
time to SRE and a reduction in bone pain experienced by patients
(114). Despite reported efficacy
for disease management with zoledronic acid, results are variable
and some studies report inconclusive findings. For example, in one
study assessing the clinical utility of zoledronic acid in 645
patients with early stage PCa, it was found there was no
significant improvement to the time to first SRE (31.9 months with
zoledronic acid vs. 29.8 months with placebo) or overall survival
(111). However, a number of
studies have reported findings in support of a clinical benefit for
the use of zoledronic in treating mPCa disease. For example, Kamba
et al (115) reported
finding a significant improvement to the time to the first SRE of
18.8 months in patients treated with zoledronic acid and ADT
compared to patients that received ADT alone. As a result of the
findings of this study and others, NICE guidelines currently
recommend the use of bisphosphonates for pain management in PCa
patients with bone metastases, and specifically zoledronic acid for
preventing or reducing the occurrence of SREs (116,117).
A number of studies have reported the results of
treating bone mPCa patients with bisphosphonates, as summarised
below Table I.
As an alternative to bisphosphonates, a human
monoclonal antibody which targets RANK-L, called denosumab, has
been developed. In one phase 3 study, 1,904 patients with CRPC with
no previous exposure to bisphosphonates were allocated to either
denosumab, or the current gold-standard therapy, zoledronic acid.
It was found that there was a significant improvement to the median
duration to the first detected SRE in the denosumab group (20.7
months), compared to zoledronic acid (17.1 months). Both drugs were
found to be similarly well-tolerated between the treatment groups,
however a greater occurrence of adverse events such as
hypocalcaemia (13% vs. 6%) and osteonecrosis of the jaw (2% vs. 1%)
was observed with the denosumab treatment group (118). Despite the relatively improved
efficacy of treating mPCa patients with denosumab relative to
bisphosphonate intervention, the cost associated with denosumab
treatment is considerably higher. For example, one cost-benefit
study carried out indicated that 1 year treatment of a PCa patient
with denosumab would cost an estimated $35,341 in comparison to
$27,528 for zoledronic acid treatment, and this increase in cost
could not be justified by the predicted improvement to the
occurrence of SREs (119).
Despite denosumab being approved for the treatment
of other solid tumours which have metastasised to the bone, such as
breast cancers, denosumab is not currently recommended for PCa bone
metastases treatment or management according to NICE guidelines
(120).
An alternative, more recently introduced approach to
managing bone metastases in patients with PCa is the use of
radiopharmaceuticals. A number of radiopharmaceutical agents have
received approval by the FDA to manage skeletal secondary tumours
in patients with advanced PCa [Phosphorus-32 (32P), Strontium-89
(89Sr), Samarium-153 (153Sm) in combination with
ethylenediaminetetramethylenephosphonic acid (Sm-EDTMP or 153Sm
lexidronam)]. Despite improvements to patient-reported pain, these
interventions have been shown to provide no survival benefit
(121–126). However, a promising alternative is
radium-223. Radium-223 is an FDA-approved alpha-emitting
radionucleotide that is selectively taken up in areas of high bone
turnover (due it being a calcium-mimetic) and subsequently
initiates apoptosis in resident PCa cells, thereby facilitating
tumour regression (127). Despite
other bone-specific radiopharmaceuticals not having demonstrated a
significant impact on patient survival to date, a phase 3 trial
reported finding treatment with radium-223 achieved a significant
improvement to patient quality of life, and an improvement to
overall survival of 3.6 months (128,129). As a result of this trial,
radium-223 is now approved for the treatment of patients with mCRPC
with symptomatic bone metastases and no known visceral metastases
(104,127).
Despite the recent improvements to the currently
available therapeutic interventions for managing bone metastases in
patients with PCa, there remains only a marginal improvement to
patient survival and pain management. The current limitations to
the efficacy of therapeutic interventions necessitates the
development of novel treatment targets for the clinical management
of patients with mPCa to the bone.
The mainstay of intervention for PCa patients with
bone metastases is currently predominantly limited to
osteoclast-targeting therapies despite the significant majority of
PCa patients having sclerotic (osteoblastic) bone lesions (30). Therefore, alternative therapeutic
targets based on inhibiting the aberrant activity of osteoblasts
may be a more efficacious approach to treating PCa bone metastases.
One target that may have future clinical utility for preventing
osteoblast-mediated metastatic tumour formation is PAP. PAP is a
secretory glycoprotein produced by PCa cells that was the first
described human tumour marker (130). Circulatory levels of PAP have been
found to be higher in PCa patients with more advanced disease,
particularly in those with the presence of bone metastases
(131,132). PAP has been shown to promote
osteoblast maturation and activity through modulating the
RANK/RANK-L/OPG system to promote OPG levels, leading to a
concurrent inhibition of osteoclasts and promotion of osteoblast
activity (32,33). The clinical efficacy of inhibiting
PAP for the treatment of PCa patients has already been demonstrated
through the development of the first FDA-approved anti-tumour
vaccine, sipuleucel-T, which targets PAP for generating a targeted
host immune response (133,134).
Sipuleucel-T had been shown to reduce the risk of death by 33% in
patients with advanced PCa in two phase III randomised,
double-blind, placebo-controlled trials, leading to its FDA
approval (135). Furthermore,
early phase trials are currently being conducted to investigate the
potential clinical utility of combining sipuleucel-T treatment with
radium-223 and have shown improvements to overall survival in
patients with mCRPC (136).
However, no treatments directly targeting PAP enzymatic activity
for the treatment of sclerotic bone metastasis in PCa are currently
in clinical trials.
Another alternative proposed target for the
development of therapies for treating PCa bone metastases is ET-1.
ET-1 is secreted by PCa cells and promotes osteoblastogenesis
(41,137,138). This knowledge has led to the
development of an inhibitor targeting the ET-1 receptor, ETA,
called atrasentan (ABT627). Despite pre-clinical indications
yielding positive results, the reported findings of clinical trials
to date have been variable and inconclusive for treatment efficacy
in PCa patients with skeletal secondary tumours (139–141). Atrasentan has also been
investigated for clinical efficacy in combination with docetaxel
for the treatment of patients with mCRPC in a phase III trial,
however the trial was terminated prematurely due to no indication
of patient benefit (142). The
potential to develop novel, more potent inhibitors of ET-1 may have
the capacity to provide clinical benefit for PCa patients with
sclerotic bone metastases.
A number of alternative therapeutic approaches to
treating patients with mPCa are currently being investigated,
however, further future elucidation of the molecular interplay
between metastasising PCa cells and resident bone cells will
hopefully enable the identification of novel, more efficacious
therapeutic targets for the clinical management of PCa bone
metastasis (125).
The presence of bone metastases for PCa patients has
significant prognostic implications, and the treatments available
to manage patients at this stage of disease remain limited and have
little effect on prolonging patient survival (12,143).
Current diagnostic approaches utilised for patients which present
with suspected bone metastases in the clinic encompass a blood test
for a variety of factors (serum calcium, phosphorous,
25-hydroxyvitamin D, alkaline phosphatase, creatine, parathyroid
hormone, thyroid-stimulating hormone and specific globulins
detected by serum protein electrophoresis) in combination with
imaging approaches (144).
Although this method of diagnosis has a relatively high success
rate for identifying bone metastases in patients, secondary tumours
are already well-established at the point of being detectable
through imaging and blood-based assessments. Therefore, it would be
beneficial to have the capacity to identify patients with a high
risk of developing secondary bone metastases prior to the
metastatic event taking place, or in lieu of this, at the earliest
stage of development possible. This would enable better
stratification of the clinical management and treatment of PCa
patients. Currently, there is no singular reliable biomarker widely
utilised in the clinic to identify or stratify PCa patients in
regards to the presence or absence of bone metastases, however a
number of approaches have been suggested in the literature to
date.
Identifying specific biomarkers with the ability to
distinguish between osteolytic and osteoblastic metastatic lesions
would be desirable to achieve better patient stratification and
enable personalised treatment approaches. However, areas of
osteolytic bone metastases are commonly associated with a
concurrent, parallel upregulation of osteoblastic activity, which
is thought to be as a result of a localised bone-formation response
in an attempt to repair an osteolytic lesion (145). This phenomenon gives rise to a
difficulty in establishing biomarkers that are specific to the
individual sub-class of bone cell involvement in metastases
formation.
One of the most widely utilised biomarkers in
identifying bone metastases currently in the clinic is serum
alkaline phosphatase. Alkaline phosphatase is a marker of
osteoblast differentiation and activity, and studies have indicated
a link between the progression of bone metastases and serum
phosphatase levels, although the clinical utility of serum
phosphatase as a biomarker of PCa disease progression remains
inconclusive (21,146). Despite this, alkaline phosphatase
is currently implicated as part of a panel of factors assessed to
monitor disease progression and treatment response in PCa (144). Previous studies have indicated
that serum alkaline phosphatase may prove to be an efficacious
marker for the identification of patients with bone metastases. For
example, Karhade et al (147) found that following investigation
of serum alkaline phosphatase levels in 732 patients with
metastatic disease that levels were significantly higher in
patients with both bone and visceral metastases. Furthermore, it
was found that elevated serum alkaline phosphatase levels acted as
a prognostic factor for the survival of patients with bone
metastases (147). However, it was
reported that elevated serum alkaline phosphatase was associated
with both skeletal and non-skeletal metastases, and therefore this
marker may not be exclusive to the presence of bone metastases.
Another potential marker of the presence of bone
metastases is serum levels of calcium. Hypercalcaemia has been
demonstrated to be associated with a higher incidence of osteolytic
bone lesions, greater bone pain and a significantly poorer
prognosis in PCa patients (148).
In addition to this, elevated calcium levels may act as a predictor
for the risk of patients developing fatal PCa. One study of 2,814
men found that those with the highest serum calcium levels were
three times more likely to die from PCa in later life (149). In addition to the potential
prognostic use of calcium for the identification of lytic bone
lesions, it may similarly be utilised in identifying sclerotic bone
lesions. Heightened osteoblast activity and subsequent bone
formation could result in a reduction in circulatory calcium levels
as calcium is utilised in the bone formation process. One study
investigating the clinical utility of urine-based calcium for
predicting the success of treating osteoblastic bone lesions found
that PCa patients with sclerotic metastasis that demonstrated
regression after treatment had increased urine calcium levels
following chemotherapy, and a concurrent decrease to urine calcium
levels in those patients which had disease progression in spite of
chemotherapy (150). This
indicates that monitoring serum calcium levels may enable the
identification of sclerotic bone lesions in PCa patients, in
addition to having the potential utility to monitor treatment
success following chemotherapeutic intervention.
Relying on the discovery of a singular biomarker for
accurate identification of bone metastases in PCa patients may be
limiting due to the highly dynamic nature of the bone resorption
process, individual patient variability and the impact of
biological variance on the expression of these factors. Therefore,
a more efficacious approach may be to identify a panel of
biomarkers of bone turnover to identify aberrations to this process
in metastases formation. In one study, the diagnostic accuracy of a
panel of serum biomarkers for bone formation and bone
osteoclastogenesis markers were assessed in 117 PCa patients in
comparison to 35 patients with benign prostatic hyperplasia (BPH)
and a further 35 normal samples. It was found that the bone
formation markers, total and bone-specific alkaline phosphatase
(tALP and bALP) and amino terminal procollagen pro-peptides of type
1 collagen (P1NP), and the bone resorption markers, bone
sialoprotein (BSP), tartrate-resistant acid phosphatase (TRAP),
cross-linked N-terminal (NTX) telopeptide of type 1 collagen and
OPG, were all found at significantly greater levels in the serum of
patients with metastases compared to those without metastases.
Furthermore, it was found that elevated levels of these factors was
associated with significantly reduced survival compared to patients
with lower detected serum levels (151). Despite the efficacy of these
biomarkers collaboratively in identifying the presence of bone
metastases, the serum levels of these factors individually do not
reliably distinguish between lytic and sclerotic bone
metastases.
Attempts to identify efficacious biomarkers that are
specific to the predominant bone cell activity in metastases
formation have been carried out. For example, markers of osteoblast
activity, such as osteocalcin, hydroxyproline, pyridinolene and
markers of collagen degradation have all been investigated for
their efficacy as biomarkers for sclerotic bone metastases
formation, with variable success (152–155).
Despite some biomarkers, such as serum alkaline
phosphatase and calcium, already being utilised as part of a panel
of factors in some clinics to identify and diagnose bone
metastases, these factors remain to have limited specificity and
sensitivity in their diagnostic capacity. Therefore, future
identification of novel biomarkers specific for osteolytic or
sclerotic bone tumours may provide earlier and more efficacious
diagnostic potential for the identification and treatment of
patients with bone metastases.
Circulating tumour cells (CTCs) are cancer cells
that have originated from a primary or metastatic tumour that can
be isolated from patient blood. CTCs have been investigated in a
number of cancer subtypes for their prognostic efficacy with
variable success reported to date (156). It has been demonstrated that the
use of CTCs in order to identify PCa patients with metastatic
disease may have some clinical utility. For example, in one study
in which 76 mCRPC patient blood samples were compared to 180
patient samples with localised disease and 19 healthy samples, the
expression levels of KLK3, KLK2 and PSCA in isolated CTCs was
assessed to determine variability in expression between metastatic
and non-metastatic disease. It was reported that patients with
metastatic disease were found to be significantly more likely to
test positive for KLK2 and KLK3 expression in comparison to
patients with localised disease (49% vs. 8%), and normal samples
were negative for the expression of both genes. Furthermore, the
expression of KLK2 and KLK3 was found to be correlated with
survival and acted as a better prognostic indicator in this cohort
in comparison to serum PSA (157).
Furthermore, circulating tumour cells have been shown to
recapitulate the expression profile of skeletal metastasis, which
could be utilised to identify the presence of secondary bone
metastases and also inform the implementation of individualised
treatment approaches for patients with skeletal disease (158).
Another potential avenue for the discovery of an
efficacious biomarker for the diagnosis of bone metastatic disease
in PCa patients is the use of microRNAs (miRNAs). miRNAs are short,
non-coding RNAs which are typically 19–26 nucleotides in length.
They are thought to function by suppressing gene expression
post-transcriptionally by binding to complementary messenger RNA
(159). miRNAs can be found free
in the serum or contained in extracellular vesicles in the serum,
urine and semen. Once isolated, they can then be reliably profiled
through easily accessible techniques such as quantitative
polymerase chain reaction (qPCR) (160–163). A number of miRNAs have been
identified as potentially playing a role in PCa development and
progression (160,162,164–166). One miRNA which has gained
particular interest in the respect of being a potential biomarker
for the identification of bone metastases in PCa patients is
miR-141. For example, in one study comparing the serum miR-141
levels in 6 patients with BPH, 20 with localised PCa and 30 with
bone-mPCa it was found that miR-141 levels were significantly
higher in those patients with bone metastases and were also
correlated with the number of bone metastatic lesions.
Interestingly, no correlation was found between serum miR-141 and
PSA (the currently utilised gold-standard biomarker for the
diagnosis of PCa), indicating an additive diagnostic potential to
what is currently available in the clinic (167).
Another potential miRNA that could be used to
identify bone metastases in PCa patients is miR-205. miR-205 is
predominantly expressed by basal cells in the prostate tissue and
has been shown to inhibit tumour proliferation, apoptosis and
cancer cell aggressiveness (165,168). Despite miR-205 expression levels
being lower in the serum of individuals with PCa relative to
healthy controls, it has been shown that miR-205 expression is
significantly elevated in patients with bone metastases compared to
PCa patients without bone metastases (164). Furthermore, a number of additional
miRNAs have been identified as showing promising indications for
the ability to distinguish patients with bone metastases, including
miR-96, miR-135b, miR-15, miR-16, miR-21 and miR505-3p (169–173).
In addition to the potential application of miRNAs
as clinical biomarkers, they have also been investigated for their
utility as potential therapeutic interventions (174,175).
Despite promising emerging evidence supporting the
potential of using miRNAs as biomarkers in PCa, inconsistencies in
the findings of different studies are a common occurrence making it
difficult to draw definitive conclusions on the efficacy of
individual miRNA-based biomarkers. This may largely be a result of
variability in experimental design in the way that miRNAs are
isolated from PCa patients and subsequently processed. To
circumvent these issues, specific guidelines outlining
recommendations for sample acquisition, handling and analytical
processing have been suggested in the literature in order to
improve reliability and reproducibility in this field of research
(176). An example of one method
which may enable more accurate quantification of miRNAs in patient
samples is the use of spike-ins in order to allow for sample
normalisation (176,177). Another approach to improving the
certainty in identifying which miRNAs may act as the most
efficacious biomarkers for diagnosing and monitoring PCa is through
the use of meta-analysis approaches (178,179). For example, Pashaei et al
(179) carried out a meta-analysis
on six miRNA datasets looking at PCa recurrence following radical
prostatectomy and identified a common upregulation in 15 miRNAs,
and a common downregulation in 22 miRNA genes across the datasets
analysed.
As a result of the seemingly high efficacy in
identifying bone metastases, coupled with being easily and
non-invasively isolated from patients and quantitatively assessed
through widely accessible laboratory techniques, miRNAs may pose an
attractive alternative diagnostic tool for the identification of
bone-metastatic disease in PCa patients in the future.
Despite recent advances to the treatment options
available for PCa patients with bone metastases, significant
limitations still remain which is reflected in the insubstantial
options for patient symptom management and poor overall survival
for this subset of patients. Improvements to our knowledge of the
molecular signalling changes which occur during the formation of
bone metastases, and the molecular interplay between metastasising
PCa cells and localised osteoclasts and osteoblasts, will in the
future potentially enable the development of improved novel
targeted therapeutics and biomarkers for disease diagnosis and
management. An example of how this knowledge can be utilised to
improve the morbidity and mortality of patients with mPCa is the
implementation of Radium-223 in the clinical pathway, manifesting
in significant improvements to both patient survival and pain
experienced as a result of bone metastases. An alternative future
directive for diagnosing and treating PCa patients with metastatic
disease may come from further elucidation of the roles of
post-translational modification processes, such as glycosylation,
which have been shown to play an integral role in bone metastases
formation and development.
However, despite promising advances in the field of
developing novel targeted therapeutics and identification of bone
metastases specific biomarkers, significant further work remains to
be carried out in order to improve the treatment landscape for
patients with incurable mPCa.
Not applicable.
This work was supported by the MRC Discovery Medicine North
Doctoral Training Partnership and Prostate Cancer Research (grant
reference 6961).
Not applicable.
EAG carried out a literature search, wrote and
formatted the review, and produced the figures. JM and NW
contributed to the review content and reviewed the original draft.
Data authentication is not applicable. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare they have no competing
interests.
|
1
|
Rawla P: Epidemiology of prostate cancer.
World J Oncol. 10:63–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pernar CH, Ebot EM, Wilson KM and Mucci
LA: The epidemiology of prostate cancer. Cold Spring Harb Perspect
Med. 8:a0303612018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vietri MT, D'Elia G, Caliendo G, Resse M,
Casamassimi A, Passariello L, Albanese L, Cioffi M and Molinari AM:
Hereditary prostate cancer: Genes related, target therapy and
prevention. Int J Mol Sci. 22:37532021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed HU, El-Shater Bosaily A, Brown LC,
Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG,
Freeman A, et al: Diagnostic accuracy of multi-parametric MRI and
TRUS biopsy in prostate cancer (PROMIS): A paired validating
confirmatory study. Lancet. 389:815–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Descotes JL: Diagnosis of prostate cancer.
Asian J Urol. 6:129–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wadosky KM and Koochekpour S: Molecular
mechanisms underlying resistance to androgen deprivation therapy in
prostate cancer. Oncotarget. 7:64447–64470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chi K, Hotte SJ, Joshua AM, North S, Wyatt
AW, Collins LL and Saad F: Treatment of mCRPC in the
AR-axis-targeted therapy-resistant state. Ann Oncol. 26:2044–2056.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah H and Vaishampayan U: Therapy of
advanced prostate cancer: Targeting the androgen receptor axis in
earlier lines of treatment. Target Oncol. 13:679–689. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing, and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antonov P, Raycheva G and Popov V:
Unexpected long-term survival in an adult patient with metastatic
prostate cancer. Urol Case Rep. 37:1016342021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bubendorf L, Schöpfer A, Wagner U, Sauter
G, Moch H, Willi N, Gasser TC and Mihatsch MJ: Metastatic patterns
of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol.
31:578–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu D, Kuai Y, Zhu R, Zhou C, Tao Y, Han W
and Chen Q: Prognosis of prostate cancer and bone metastasis
pattern of patients: A SEER-based study and a local hospital based
study from China. Sci Rep. 10:91042020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nieder C, Haukland E, Pawinski A and
Dalhaug A: Pathologic fracture and metastatic spinal cord
compression in patients with prostate cancer and bone metastases.
BMC Urol. 10:232010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Veldurthy V, Wei R, Oz L, Dhawan P, Jeon
YH and Christakos S: Vitamin D, calcium homeostasis and aging. Bone
Res. 4:160412016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM
and Xie C: Osteoblast-osteoclast interactions. Connect Tissue Res.
59:99–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suva LJ, Washam C, Nicholas RW and Griffin
RJ: Bone metastasis: Mechanisms and therapeutic opportunities. Nat
Rev Endocrinol. 7:208–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nordstrand A, Bovinder Ylitalo E, Thysell
E, Jernberg E, Crnalic S, Widmark A, Bergh A, Lerner UH and
Wikström P: Bone cell activity in clinical prostate cancer bone
metastasis and its inverse relation to tumor cell androgen receptor
activity. Int J Mol Sci. 19:12232018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong SK, Mohamad NV, Giaze TR, Chin KY,
Mohamed N and Ima-Nirwana S: Prostate cancer and bone metastases:
The underlying mechanisms. Int J Mol Sci. 20:25872019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther. 5:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 133:571–573. 1889.
View Article : Google Scholar
|
|
21
|
Macedo F, Ladeira K, Pinho F, Saraiva N,
Bonito N, Pinto L and Goncalves F: Bone metastases: An overview.
Oncol Rev. 11:3212017.PubMed/NCBI
|
|
22
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conti G, La Torre G, Cicalese V,
Micheletti G, Ludovico MG, Vestita GD, Cottonaro G, Introini C and
Cecchi M: Prostate cancer metastases to bone: Observational study
for the evaluation of clinical presentation, course and treatment
patterns. Presentation of the METAURO protocol and of patient
baseline features. Arch Ital Urol Androl. 80:59–64. 2008.PubMed/NCBI
|
|
24
|
Kitajima K, Yamamoto S, Kawanaka Y, Komoto
H, Shimatani K, Hanasaki T, Taguchi M, Nagasawa S, Yamada Y,
Kanematsu A and Yamakado K: Assessment of the viability and
treatment response of bone metastases in patients with metastatic
castration-resistant prostate cancer using choline PET/CT. Medicine
(Baltimore). 100:e262062021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin SC, Yu-Lee LY and Lin SH: Osteoblastic
factors in prostate cancer bone metastasis. Curr Osteoporos Rep.
16:642–647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roudier MP, Morrissey C, True LD, Higano
CS, Vessella RL and Ott SM: Histopathological assessment of
prostate cancer bone osteoblastic metastases. J Urol.
180:1154–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garnero P, Buchs N, Zekri J, Rizzoli R,
Coleman RE and Delmas PD: Markers of bone turnover for the
management of patients with bone metastases from prostate cancer.
Br J Cancer. 82:858–864. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khan MA and Partin AW: Bisphosphonates in
metastatic prostate cancer. Rev Urol. 5:204–206. 2003.PubMed/NCBI
|
|
29
|
Kim JM, Lin C, Stavre Z, Greenblatt MB and
Shim JH: Osteoblast-osteoclast communication and bone homeostasis.
Cells. 9:20732020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheville JC, Tindall D, Boelter C, Jenkins
R, Lohse CM, Pankratz VS, Sebo TJ, Davis B and Blute ML: Metastatic
prostate carcinoma to bone: Clinical and pathologic features
associated with cancer-specific survival. Cancer. 95:1028–1036.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ribelli G, Simonetti S, Iuliani M, Rossi
E, Vincenzi B, Tonini G, Pantano F and Santini D: Osteoblasts
promote prostate cancer cell proliferation through androgen
receptor independent mechanisms. Front Oncol. 11:7898852021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kirschenbaum A, Liu XH, Yao S, Leiter A
and Levine AC: Prostatic acid phosphatase is expressed in human
prostate cancer bone metastases and promotes osteoblast
differentiation. Ann N Y Acad Sci. 1237:64–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kirschenbaum A, Izadmehr S, Yao S,
O'Connor-Chapman KL, Huang A, Gregoriades EM, Yakar S and Levine
AC: Prostatic acid phosphatase alters the RANKL/OPG system and
induces osteoblastic prostate cancer bone metastases.
Endocrinology. 157:4526–4533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen G, Sircar K, Aprikian A, Potti A,
Goltzman D and Rabbani SA: Expression of RANKL/RANK/OPG in primary
and metastatic human prostate cancer as markers of disease stage
and functional regulation. Cancer. 107:289–298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Udagawa N, Takahashi N, Yasuda H, Mizuno
A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K
and Suda T: Osteoprotegerin produced by osteoblasts is an important
regulator in osteoclast development and function. Endocrinology.
141:3478–3484. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Corey E, Brown LG, Kiefer JA, Quinn JE,
Pitts TE, Blair JM and Vessella RL: Osteoprotegerin in prostate
cancer bone metastasis. Cancer Res. 65:1710–1718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brown JM, Corey E, Lee ZD, True LD, Yun
TJ, Tondravi M and Vessella RL: Osteoprotegerin and rank ligand
expression in prostate cancer. Urology. 57:611–616. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Holen I, Croucher PI, Hamdy FC and Eaton
CL: Osteoprotegerin (OPG) is a survival factor for human prostate
cancer cells. Cancer Res. 62:1619–1623. 2002.PubMed/NCBI
|
|
39
|
Chiao JW, Moonga BS, Yang YM, Kancherla R,
Mittelman A, Wu-Wong JR and Ahmed T: Endothelin-1 from prostate
cancer cells is enhanced by bone contact which blocks osteoclastic
bone resorption. Br J Cancer. 83:360–365. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fizazi K, Yang J, Peleg S, Sikes CR,
Kreimann EL, Daliani D, Olive M, Raymond KA, Janus TJ, Logothetis
CJ, et al: Prostate cancer cells-osteoblast interaction shifts
expression of growth/survival-related genes in prostate cancer and
reduces expression of osteoprotegerin in osteoblasts. Clin Cancer
Res. 9:2587–2597. 2003.PubMed/NCBI
|
|
41
|
Yin JJ, Mohammad KS, Käkönen SM, Harris S,
Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM and
Guise TA: A causal role for endothelin-1 in the pathogenesis of
osteoblastic bone metastases. Proc Natl Acad Sci USA.
100:10954–10959. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Valta MP, Tuomela J, Bjartell A, Valve E,
Väänänen HK and Härkönen P: FGF-8 is involved in bone metastasis of
prostate cancer. Int J Cancer. 123:22–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Quiroz-Munoz M, Izadmehr S, Arumugam D,
Wong B, Kirschenbaum A and Levine AC: Mechanisms of osteoblastic
bone metastasis in prostate cancer: Role of prostatic acid
phosphatase. J Endocr Soc. 3:655–664. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ikuerowo SO, Omisanjo OA, Bioku MJ, Ajala
MO, Mordi VP and Esho JO: Prevalence and characteristics of
prostate cancer among participants of a community-based screening
in Nigeria using serum prostate specific antigen and digital rectal
examination. Pan Afr Med J. 15:1292013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Idowu BM: Prostate carcinoma presenting
with diffuse osteolytic metastases and supraclavicular
lymphadenopathy mimicking multiple myeloma. Clin Case Rep.
6:253–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maharaj B, Kalideen JM, Leary WP and
Pudifin DJ: Carcinoma of the prostate with multiple osteolytic
metastases simulating multiple myeloma. A case report. S Afr Med J.
70:227–228. 1986.PubMed/NCBI
|
|
47
|
Fukuoka H, Ishibashi Y, Masuda M, Gotoh A,
Murai T and Kitamura H: A case of prostatic carcinoma with
osteolytic bone metastases. Hinyokika Kiyo. 34:1805–1809. 1988.(In
Japanese). PubMed/NCBI
|
|
48
|
Migita T, Maeda K and Ogata N: A case of
prostate cancer associated with osteolytic bone metastases.
Hinyokika Kiyo. 45:371–374. 1999.(In Japanese). PubMed/NCBI
|
|
49
|
Rajendiran G, Green L and Chhabra G: A
rare presentation of prostate cancer with diffuse osteolytic
metastases and PSA of 7242 ng/ml. Int J Case Rep Image. 2:16–20.
2011. View Article : Google Scholar
|
|
50
|
Segamwenge IL, Mgori NK, Abdallahyussuf S,
Mukulu CN, Nakangombe P, Ngalyuka PK and Kidaaga F: Cancer of the
prostate presenting with diffuse osteolytic metastatic bone
lesions: A case report. J Med Case Rep. 6:4252012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sharma P, Karunanithi S, Singh Dhull V,
Jain S, Bal C and Kumar R: Prostate cancer with lytic bone
metastases: 18F-fluorodeoxyglucose positron emission
tomography-computed tomography for diagnosis and monitoring
response to medical castration therapy. Indian J Nucl Med.
28:178–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bird VY, Domino PM, Sutkowski R, Stillings
SA and Trejo-Lopez JA: Prostate cancer with metastatic lytic bone
lesions: Positive bone scan post docetaxel chemotherapy in the
setting of clinically successful treatment. Urol Case Rep. 6:12–14.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rummel K, Benson J and Roller L: Prostate
adenocarcinoma with osteolytic metastases: Case report and review
of the literature. Radiol Case Rep. 16:3565–3568. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bryden AAG, Hoyland JA, Freemont AJ,
Clarke NW and George NJR: Parathyroid hormone related peptide and
receptor expression in paired primary prostate cancer and bone
metastases. Br J Cancer. 86:322–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang JC, Sakata T, Pfleger LL, Bencsik M,
Halloran BP, Bikle DD and Nissenson RA: PTH differentially
regulates expression of RANKL and OPG. J Bone Miner Res.
19:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ongkeko WM, Burton D, Kiang A, Abhold E,
Kuo SZ, Rahimy E, Yang M, Hoffman RM, Wang-Rodriguez J and Deftos
LJ: Parathyroid hormone related-protein promotes
epithelial-to-mesenchymal transition in prostate cancer. PLoS One.
9:e858032014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen X, Zhi X, Wang J and Su J: RANKL
signaling in bone marrow mesenchymal stem cells negatively
regulates osteoblastic bone formation. Bone Res. 6:342018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ikebuchi Y, Aoki S, Honma M, Hayashi M,
Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, et
al: Coupling of bone resorption and formation by RANKL reverse
signalling. Nature. 561:195–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang M, Xia F, Wei Y and Wei X: Molecular
mechanisms and clinical management of cancer bone metastasis. Bone
Res. 8:302020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Feng J, Xu X, Li B, Brown E, Farris AB,
Sun SY and Yang JJ: Prostate cancer metastatic to bone has higher
expression of the calcium-sensing receptor (CaSR) than primary
prostate cancer. Receptors Clin Investig. 1:e2702014.PubMed/NCBI
|
|
61
|
Kuchimaru T, Hoshino T, Aikawa T, Yasuda
H, Kobayashi T, Kadonosono T and Kizaka-Kondoh S: Bone resorption
facilitates osteoblastic bone metastatic colonization by
cooperation of insulin-like growth factor and hypoxia. Cancer Sci.
105:553–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Russo S, Scotto di Carlo F and
Gianfrancesco F: The osteoclast traces the route to bone tumors and
metastases. Front Cell Dev Biol. 10:8863052022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hadjidakis DJ and Androulakis II: Bone
remodeling. Ann N Y Acad Sci. 1092:385–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Schwartz MA, Schaller MD and Ginsberg MH:
Integrins: Emerging paradigms of signal transduction. Annu Rev Cell
Dev Biol. 11:549–599. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Schneider JG, Amend SR and Weilbaecher KN:
Integrins and bone metastasis: Integrating tumor cell and stromal
cell interactions. Bone. 48:54–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Singh R, Kapur N, Mir H, Singh N, Lillard
JW Jr and Singh S: CXCR6-CXCL16 axis promotes prostate cancer by
mediating cytoskeleton rearrangement via Ezrin activation and αvβ3
integrin clustering. Oncotarget. 7:7343–7353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lu J, Doyle AD, Shinsato Y, Wang S,
Bodendorfer MA, Zheng M and Yamada KM: Basement membrane regulates
fibronectin organization using sliding focal adhesions driven by a
contractile winch. Dev Cell. 52:631–646.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
He Y, Liu XD, Chen ZY, Zhu J, Xiong Y, Li
K, Dong JH and Li X: Interaction between cancer cells and stromal
fibroblasts is required for activation of the uPAR-uPA-MMP-2
cascade in pancreatic cancer metastasis. Clin Cancer Res.
13:3115–3124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sun LC, Luo J, Mackey LV, Fuselier JA and
Coy DH: A conjugate of camptothecin and a somatostatin analog
against prostate cancer cell invasion via a possible signaling
pathway involving PI3K/Akt, alphaVbeta3/alphaVbeta5 and MMP-2/-9.
Cancer Lett. 246:157–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Somanath PR, Malinin NL and Byzova TV:
Cooperation between integrin alphavbeta3 and VEGFR2 in
angiogenesis. Angiogenesis. 12:177–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dong Y, Xie X, Wang Z, Hu C, Zheng Q, Wang
Y, Chen R, Xue T, Chen J, Gao D, et al: Increasing matrix stiffness
upregulates vascular endothelial growth factor expression in
hepatocellular carcinoma cells mediated by integrin β1. Biochem
Biophys Res Commun. 444:427–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tang L, Xu M, Zhang L, Qu L and Liu X:
Role of αVβ3 in prostate cancer: Metastasis initiator and important
therapeutic target. Onco Targets Ther. 13:7411–7422. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Brown NF and Marshall JF:
Integrin-mediated TGFβ activation modulates the tumour
microenvironment. Cancers (Basel). 11:12212019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hussain M, Le Moulec S, Gimmi C, Bruns R,
Straub J and Miller K; PERSEUS Study Group, : Differential effect
on bone lesions of targeting integrins: Randomized phase II trial
of abituzumab in patients with metastatic castration-resistant
prostate cancer. Clin Cancer Res. 22:3192–3200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bryden AAG, Hoyland JA, Freemont AJ,
Clarke NW, Schembri Wismayer D and George NJR: E-cadherin and
beta-catenin are down-regulated in prostatic bone metastases. BJU
Int. 89:400–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jennbacken K, Tesan T, Wang W, Gustavsson
H, Damber JE and Welén K: N-cadherin increases after androgen
deprivation and is associated with metastasis in prostate cancer.
Endocr Relat Cancer. 17:469–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cui Y and Yamada S: N-cadherin dependent
collective cell invasion of prostate cancer cells is regulated by
the N-terminus of α-catenin. PLoS One. 8:e550692013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sun Y, Jing J, Xu H, Xu L, Hu H, Tang C,
Liu S, Wei Q, Duan R, Guo J and Yang L: N-cadherin inhibitor
creates a microenvironment that protect TILs from immune
checkpoints and Treg cells. J Immunother Cancer. 9:e0021382021.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tanaka H, Kono E, Tran CP, Miyazaki H,
Yamashiro J, Shimomura T, Fazli L, Wada R, Huang J, Vessella RL, et
al: Monoclonal antibody targeting of N-cadherin inhibits prostate
cancer growth, metastasis and castration resistance. Nat Med.
16:1414–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Reily C, Stewart TJ, Renfrow MB and Novak
J: Glycosylation in health and disease. Nat Rev Nephrol.
15:346–366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Garnham R, Scott E, Livermore KE and
Munkley J: ST6GAL1: A key player in cancer. Oncol Lett. 18:983–989.
2019.PubMed/NCBI
|
|
84
|
Bindeman WE and Fingleton B: Glycosylation
as a regulator of site-specific metastasis. Cancer Metastasis Rev.
41:107–129. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sottnik JL, Daignault-Newton S, Zhang X,
Morrissey C, Hussain MH, Keller ET and Hall CL: Integrin alpha2beta
1 (α2β1) promotes prostate cancer skeletal metastasis. Clin Exp
Metastasis. 30:569–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Van Slambrouck S, Groux-Degroote S,
Krzewinski-Recchi MA, Cazet A, Delannoy P and Steelant WF:
Carbohydrate-to-carbohydrate interactions between α2,3-linked
sialic acids on α2 integrin subunits and asialo-GM1 underlie the
bone metastatic behaviour of LNCAP-derivative C4-2B prostate cancer
cells. Biosci Rep. 34:e001382014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Julien S, Ivetic A, Grigoriadis A, QiZe D,
Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, et
al: Selectin ligand sialyl-Lewis × antigen drives metastasis of
hormone-dependent breast cancers. Cancer Res. 71:7683–7693. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Barthel SR, Gavino JD, Wiese GK, Jaynes
JM, Siddiqui J and Dimitroff CJ: Analysis of glycosyltransferase
expression in metastatic prostate cancer cells capable of rolling
activity on microvascular endothelial (E)-selectin. Glycobiology.
18:806–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gao J, Li T, Mo Z, Hu Y, Yi Q, He R, Zhu
X, Zhou X, She S and Chen Y: Overexpression of the galectin-3
during tumor progression in prostate cancer and its clinical
implications. Int J Clin Exp Pathol. 11:839–846. 2018.PubMed/NCBI
|
|
90
|
Nakajima K, Kho DH, Yanagawa T, Harazono
Y, Hogan V, Chen W, Ali-Fehmi R, Mehra R and Raz A: Galectin-3
cleavage alters bone remodeling: different outcomes in breast and
prostate cancer skeletal metastasis. Cancer Res. 76:1391–1402.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: Cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pouliot N, Pearson HB and Burrows A:
Investigating metastasis using in vitro platforms. Madame Curie
Bioscience Database [Internet] Austin (TX): Landes Bioscience;
2013
|
|
93
|
Hao J, Madigan MC, Khatri A, Power CA,
Hung TT, Beretov J, Chang L, Xiao W, Cozzi PJ, Graham PH, et al: In
vitro and in vivo prostate cancer metastasis and chemoresistance
can be modulated by expression of either CD44 or CD147. PLoS One.
7:e407162012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li W, Qian L, Lin J, Huang G, Hao N, Wei
X, Wang W and Liang J: CD44 regulates prostate cancer
proliferation, invasion and migration via PDK1 and PFKFB4.
Oncotarget. 8:65143–65151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fang F, Li Q, Wu M, Nie C, Xu H and Wang
L: CD147 promotes epithelial-mesenchymal transition of prostate
cancer cells via the Wnt/β-catenin pathway. Exp Ther Med.
20:3154–3160. 2020.PubMed/NCBI
|
|
96
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Guo S and Deng CX: Effect of stromal cells
in tumor microenvironment on metastasis initiation. Int J Biol Sci.
14:2083–2093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jasuja H, Kar S, Katti DR and Katti KS:
Perfusion bioreactor enabled fluid-derived shear stress conditions
for novel bone metastatic prostate cancer testbed. Biofabrication.
13:2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Fong ELS, Wan X, Yang J, Morgado M, Mikos
AG, Harrington DA, Navone NM and Farach-Carson MC: A 3D in vitro
model of patient-derived prostate cancer xenograft for controlled
interrogation of in vivo tumor-stromal interactions. Biomaterials.
77:164–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hepburn AC, Curry EL, Moad M, Steele RE,
Franco OE, Wilson L, Singh P, Buskin A, Crawford SE, Gaughan L, et
al: Propagation of human prostate tissue from induced pluripotent
stem cells. Stem Cells Transl Med. 9:734–745. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fitzgerald KA, Guo J, Tierney EG, Curtin
CM, Malhotra M, Darcy R, O'Brien FJ and O'Driscoll CM: The use of
collagen-based scaffolds to simulate prostate cancer bone
metastases with potential for evaluating delivery of
nanoparticulate gene therapeutics. Biomaterials. 66:53–66. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Katti KS, Jasuja H, Kar S and Katti DR:
Nanostructured biomaterials for in vitro models of bone metastasis
cancer. Curr Opin Biomed Eng. 17:1002542021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cruz-Neves S, Ribeiro N, Graça I, Jerónimo
C, Sousa SR and Monteiro FJ: Behavior of prostate cancer cells in a
nanohydroxyapatite/collagen bone scaffold. J Biomed Mater Res A.
105:2035–2046. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Parker C, Castro E, Fizazi K, Heidenreich
A, Ost P, Procopio G, Tombal B and Gillessen S; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Prostate cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 31:1119–1134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kuppen MCP, Westgeest HM, van den Eertwegh
AJM, van Moorselaar RJA, van Oort IM, Tascilar M, Mehra N, Lavalaye
J, Somford DM, Aben KKH, et al: Symptomatic skeletal events and the
use of bone health agents in a real-world treated metastatic
castration resistant prostate cancer population: Results from the
CAPRI-study in the netherlands. Clin Genitourin Cancer. 20:43–52.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Benford HL, McGowan NW, Helfrich MH,
Nuttall ME and Rogers MJ: Visualization of bisphosphonate-induced
caspase-3 activity in apoptotic osteoclasts in vitro. Bone.
28:465–473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gralow J and Tripathy D: Managing
metastatic bone pain: The role of bisphosphonates. J Pain Symptom
Manage. 33:462–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Percival RC, Urwin GH, Harris S, Yates AJ,
Williams JL, Beneton M and Kanis JA: Biochemical and histological
evidence that carcinoma of the prostate is associated with
increased bone resorption. Eur J Surg Oncol. 13:41–49.
1987.PubMed/NCBI
|
|
109
|
Clarke NW, McClure J and George NJ:
Morphometric evidence for bone resorption and replacement in
prostate cancer. Br J Urol. 68:74–80. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Berruti A, Dogliotti L, Tucci M, Tarabuzzi
R, Fontana D and Angeli A: Metabolic bone disease induced by
prostate cancer: Rationale for the use of bisphosphonates. J Urol.
166:2023–2031. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Small EJ, Smith MR, Seaman JJ, Petrone S
and Kowalski MO: Combined analysis of two multicenter, randomized,
placebo-controlled studies of pamidronate disodium for the
palliation of bone pain in men with metastatic prostate cancer. J
Clin Oncol. 21:4277–4284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu J, Zhao C, Liu B, Liu H and Wang L:
Analgesia and curative effect of pamidronate disodium combined with
chemotherapy on elderly patients with advanced metastatic bone
cancer. Oncol Lett. 18:771–775. 2019.PubMed/NCBI
|
|
113
|
Widler L, Jaeggi KA, Glatt M, Müller K,
Bachmann R, Bisping M, Born AR, Cortesi R, Guiglia G, Jeker H, et
al: Highly potent geminal bisphosphonates. From pamidronate
disodium (Aredia) to zoledronic acid (Zometa). J Med Chem.
45:3721–3738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Finianos A and Aragon-Ching JB: Zoledronic
acid for the treatment of prostate cancer. Expert Opin
Pharmacother. 20:657–666. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kamba T, Kamoto T, Maruo S, Kikuchi T,
Shimizu Y, Namiki S, Fujimoto K, Kawanishi H, Sato F, Narita S, et
al: A phase III multicenter, randomized, controlled study of
combined androgen blockade with versus without zoledronic acid in
prostate cancer patients with metastatic bone disease: Results of
the ZAPCA trial. Int J Clin Oncol. 22:166–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Weinfurt KP, Anstrom KJ, Castel LD,
Schulman KA and Saad F: Effect of zoledronic acid on pain
associated with bone metastasis in patients with prostate cancer.
Ann Oncol. 17:986–989. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
NICE. Prostate cancer, . Diagnosis and
management. 2019.15/12/2021 [cited 2022 21/11/2022]; Available
from:. https://www.nice.org.uk/guidance/ng131/chapter/Recommendations
|
|
118
|
Fizazi K, Carducci M, Smith M, Damião R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Xie J, Namjoshi M, Wu EQ, Parikh K, Diener
M, Yu AP, Guo A and Culver KW: Economic evaluation of denosumab
compared with zoledronic acid in hormone-refractory prostate cancer
patients with bone metastases. J Manag Care Pharm. 17:621–643.
2011.PubMed/NCBI
|
|
120
|
NICE, . Denosumab is not recommended for
preventing skeletal-related events in adults with bone metastases
from prostate cancer. 2012.Available from. https://www.nice.org.uk/donotdo/denosumab-is-not-recommended-for-preventing-skeletalrelated-events-inadults-with-bone-metastases-from-prostate-cancer
|
|
121
|
Smart JG: The use of P32 in the treatment
of severe pain from bone metastases of carcinoma of the prostate1.
Br J Urol. 37:139–147. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Porter AT, McEwan AJ, Powe JE, Reid R,
McGowan DG, Lukka H, Sathyanarayana JR, Yakemchuk VN, Thomas GM,
Erlich LE, et al: Results of a randomized phase-III trial to
evaluate the efficacy of strontium-89 adjuvant to local field
external beam irradiation in the management of endocrine resistant
metastatic prostate cancer. Int J Radiat Oncol Biol Phys.
25:805–813. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Serafini AN, Houston SJ, Resche I, Quick
DP, Grund FM, Ell PJ, Bertrand A, Ahmann FR, Orihuela E, Reid RH,
et al: Palliation of pain associated with metastatic bone cancer
using samarium-153 lexidronam: A double-blind placebo-controlled
clinical trial. J Clin Oncol. 16:1574–1581. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Sartor O, Reid RH, Hoskin PJ, Quick DP,
Ell PJ, Coleman RE, Kotler JA, Freeman LM and Olivier P; Quadramet
424Sm10/11 Study Group, : Samarium-153-lexidronam complex for
treatment of painful bone metastases in hormone-refractory prostate
cancer. Urology. 63:940–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Powers E, Karachaliou GS, Kao C, Harrison
MR, Hoimes CJ, George DJ, Armstrong AJ and Zhang T: Novel therapies
are changing treatment paradigms in metastatic prostate cancer. J
Hematol Oncol. 13:1442020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Terrisse S, Karamouza E, Parker CC, Sartor
AO, James ND, Pirrie S, Collette L, Tombal BF, Chahoud J, Smeland
S, et al: Overall survival in men with bone metastases from
castration-resistant prostate cancer treated with bone-targeting
radioisotopes: A meta-analysis of individual patient data from
randomized clinical trials. JAMA Oncol. 6:206–216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Den RB, George D, Pieczonka C and McNamara
M: Ra-223 treatment for bone metastases in castrate-resistant
prostate cancer: Practical management issues for patient selection.
Am J Clin Oncol. 42:399–406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Nilsson S, Cislo P, Sartor O, Vogelzang
NJ, Coleman RE, O'Sullivan JM, Reuning-Scherer J, Shan M, Zhan L
and Parker C: Patient-reported quality-of-life analysis of
radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol.
27:868–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Gutman EB, Sproul EE and Gutman AB:
Significance of increased phosphatase activity of bone at the site
of osteoplastic metastases secondary to carcinoma of the prostate
gland. Am J Cancer. 28:485–495. 1936. View Article : Google Scholar
|
|
131
|
Ozu C, Nakashima J, Horiguchi Y, Oya M,
Ohigashi T and Murai M: Prediction of bone metastases by
combination of tartrate-resistant acid phosphatase, alkaline
phosphatase and prostate specific antigen in patients with prostate
cancer. Int J Urol. 15:419–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Larson SR, Chin J, Zhang X, Brown LG,
Coleman IM, Lakely B, Tenniswood M, Corey E, Nelson PS, Vessella RL
and Morrissey C: Prostate cancer derived prostatic acid phosphatase
promotes an osteoblastic response in the bone microenvironment.
Clin Exp Metastasis. 31:247–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cheever MA and Higano CS: PROVENGE
(Sipuleucel-T) in prostate cancer: The first FDA-approved
therapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wargowski E, Johnson LE, Eickhoff JC,
Delmastro L, Staab MJ, Liu G and McNeel DG: Prime-boost vaccination
targeting prostatic acid phosphatase (PAP) in patients with
metastatic castration-resistant prostate cancer (mCRPC) using
sipuleucel-T and a DNA vaccine. J Immunother Cancer. 6:212018.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Higano CS, Schellhammer PF, Small EJ,
Burch PA, Nemunaitis J, Yuh L, Provost N and Frohlich MW:
Integrated data from 2 randomized, double-blind,
placebo-controlled, phase 3 trials of active cellular immunotherapy
with sipuleucel-T in advanced prostate cancer. Cancer.
115:3670–3679. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Marshall CH, Fu W, Wang H, Park JC,
DeWeese TL, Tran PT, Song DY, King S, Afful M, Hurrelbrink J, et
al: Randomized phase II trial of sipuleucel-T with or without
radium-223 in men with bone-metastatic castration-resistant
prostate cancer. Clin Cancer Res. 27:1623–1630. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kasperk CH, Börcsök I, Schairer HU,
Schneider U, Nawroth PP, Niethard FU and Ziegler R: Endothelin-1 is
a potent regulator of human bone cell metabolism in vitro. Calcif
Tissue Int. 60:368–374. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Montironi R, Mazzucchelli R, Barbisan F,
Stramazzotti D, Santinelli A, Lòpez Beltran A, Cheng L, Montorsi F
and Scarpelli M: Immunohistochemical expression of endothelin-1 and
endothelin-A and endothelin-B receptors in high-grade prostatic
intraepithelial neoplasia and prostate cancer. Eur Urol.
52:1682–1690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Vogelzang NJ, Nelson JB, Schulman CC,
Dearnaley DP, Saad F, Sleep DJ, Isaacson D and Carducci MA:
Meta-analysis of clinical trials of atrasentan 10 mg in metastatic
hormone-refractory prostate cancer. J Clin Oncol. 23 (Suppl
16):S45632005. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Carducci MA, Saad F, Abrahamsson PA,
Dearnaley DP, Schulman CC, North SA, Sleep DJ, Isaacson JD and
Nelson JB; Atrasentan Phase III Study Group Institutions, : A phase
3 randomized controlled trial of the efficacy and safety of
atrasentan in men with metastatic hormone-refractory prostate
cancer. Cancer. 110:1959–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Drake JM, Danke JR and Henry MD:
Bone-specific growth inhibition of prostate cancer metastasis by
atrasentan. Cancer Biol Ther. 9:607–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Quinn DI, Tangen CM, Hussain M, Lara PN
Jr, Goldkorn A, Moinpour CM, Garzotto MG, Mack PC, Carducci MA,
Monk JP, et al: Docetaxel and atrasentan versus docetaxel and
placebo for men with advanced castration-resistant prostate cancer
(SWOG S0421): A randomised phase 3 trial. Lancet Oncol. 14:893–900.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Teo MY, Rathkopf DE and Kantoff P:
Treatment of advanced prostate cancer. Annu Rev Med. 70:479–499.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Selvaggi G and Scagliotti GV: Management
of bone metastases in cancer: A review. Crit Rev Oncol Hematol.
56:365–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Keller ET and Brown J: Prostate cancer
bone metastases promote both osteolytic and osteoblastic activity.
J Cell Biochem. 91:718–729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Li D, Lv H, Hao X, Hu B and Song Y:
Prognostic value of serum alkaline phosphatase in the survival of
prostate cancer: Evidence from a meta-analysis. Cancer Manag Res.
10:3125–3139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Karhade AV, Thio QCBS, Kuverji M, Ogink
PT, Ferrone ML and Schwab JH: Prognostic value of serum alkaline
phosphatase in spinal metastatic disease. Br J Cancer. 120:640–646.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Tucci M, Mosca A, Lamanna G, Porpiglia F,
Terzolo M, Vana F, Cracco C, Russo L, Gorzegno G, Tampellini M, et
al: Prognostic significance of disordered calcium metabolism in
hormone-refractory prostate cancer patients with metastatic bone
disease. Prostate Cancer Prostatic Dis. 12:94–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Skinner HG and Schwartz GG: Serum calcium
and incident and fatal prostate cancer in the national health and
nutrition examination survey. Cancer Epidemiol Biomarkers Prev.
17:2302–3205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Francini G, Petrioli R, Gonnelli S,
Correale P, Pozzessere D, Marsili S, Montagnani A, Lucani B, Rossi
S, Monaco R, et al: Urinary calcium excretion in the monitoring of
bone metastases from prostatic carcinoma. Cancer. 92:1468–1474.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Jung K, Lein M, Stephan C, Von Hösslin K,
Semjonow A, Sinha P, Loening SA and Schnorr D: Comparison of 10
serum bone turnover markers in prostate carcinoma patients with
bone metastatic spread: Diagnostic and prognostic implications. Int
J Cancer. 111:783–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Cooper EH, Whelan P and Purves D: Bone
alkaline phosphatase and prostate-specific antigen in the
monitoring of prostate cancer. Prostate. 25:236–242. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kylmälä T, Tammela TL, Risteli L, Risteli
J, Kontturi M and Elomaa I: Type I collagen degradation product
(ICTP) gives information about the nature of bone metastases and
has prognostic value in prostate cancer. Br J Cancer. 71:1061–1064.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Maeda H, Koizumi M, Yoshimura K, Yamauchi
T, Kawai T and Ogata E: Correlation between bone metabolic markers
and bone scan in prostatic cancer. J Urol. 157:539–543. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Klepzig M, Jonas D and Oremek GM:
Procollagen type 1 amino-terminal propeptide: A marker for bone
metastases in prostate carcinoma. Anticancer Res. 29:671–673.
2009.PubMed/NCBI
|
|
156
|
Sundling KE and Lowe AC: Circulating tumor
cells: Overview and opportunities in cytology. Adv Anat Pathol.
26:56–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Helo P, Cronin AM, Danila DC, Wenske S,
Gonzalez-Espinoza R, Anand A, Koscuiszka M, Väänänen RM, Pettersson
K, Chun FK, et al: Circulating prostate tumor cells detected by
reverse transcription-PCR in men with localized or
castration-refractory prostate cancer: Concordance with CellSearch
assay and association with bone metastases and with survival. Clin
Chem. 55:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Josefsson A, Larsson K, Månsson M,
Björkman J, Rohlova E, Åhs D, Brisby H, Damber JE and Welén K:
Circulating tumor cells mirror bone metastatic phenotype in
prostate cancer. Oncotarget. 9:29403–29413. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Saxby H, Mikropoulos C and Boussios S: An
update on the prognostic and predictive serum biomarkers in
metastatic prostate cancer. Diagnostics (Basel). 10:5492020.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhang HL, Yang LF, Zhu Y, Yao XD, Zhang
SL, Dai B, Zhu YP, Shen YJ, Shi GH and Ye DW: Serum miRNA-21:
Elevated levels in patients with metastatic hormone-refractory
prostate cancer and potential predictive factor for the efficacy of
docetaxel-based chemotherapy. Prostate. 71:326–331. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Bhagirath D, Yang TL, Bucay N, Sekhon K,
Majid S, Shahryari V, Dahiya R, Tanaka Y and Saini S: microRNA-1246
is an exosomal biomarker for aggressive prostate cancer. Cancer
Res. 78:1833–1844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Tinay I, Tan M, Gui B, Werner L, Kibel AS
and Jia L: Functional roles and potential clinical application of
miRNA-345-5p in prostate cancer. Prostate. 78:927–937. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Roest HP, IJzermans JNM and van der Laan
LJW: Evaluation of RNA isolation methods for microRNA
quantification in a range of clinical biofluids. BMC Biotechnol.
21:482021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Guo X, Han T, Hu P, Guo X, Zhu C, Wang Y
and Chang S: Five microRNAs in serum as potential biomarkers for
prostate cancer risk assessment and therapeutic intervention. Int
Urol Nephrol. 50:2193–2200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Yamada Y, Nishikawa R, Kato M, Okato A,
Arai T, Kojima S, Yamazaki K, Naya Y, Ichikawa T and Seki N:
Regulation of HMGB3 by antitumor miR-205-5p inhibits cancer cell
aggressiveness and is involved in prostate cancer pathogenesis. J
Hum Genet. 63:195–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Casanova-Salas I, Rubio-Briones J,
Fernández-Serra A and López-Guerrero JA: miRNAs as biomarkers in
prostate cancer. Clin Transl Oncol. 14:803–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhang HL, Qin XJ, Cao DL, Zhu Y, Yao XD,
Zhang SL, Dai B and Ye DW: An elevated serum miR-141 level in
patients with bone-metastatic prostate cancer is correlated with
more bone lesions. Asian J Androl. 15:231–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Nordby Y, Richardsen E, Ness N, Donnem T,
Patel HRH, Busund LT, Bremnes RM and Andersen S: High miR-205
expression in normal epithelium is associated with biochemical
failure-an argument for epithelial crosstalk in prostate cancer?
Sci Rep. 7:163082017. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Haflidadóttir BS, Larne O, Martin M,
Persson M, Edsjö A, Bjartell A and Ceder Y: Upregulation of miR-96
enhances cellular proliferation of prostate cancer cells through
FOXO1. PLoS One. 8:e724002013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ma Y, Yang HZ, Dong BJ, Zou HB, Zhou Y,
Kong XM and Huang YR: Biphasic regulation of autophagy by miR-96 in
prostate cancer cells under hypoxia. Oncotarget. 5:9169–9182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Bonci D, Coppola V, Patrizii M, Addario A,
Cannistraci A, Francescangeli F, Pecci R, Muto G, Collura D, Bedini
R, et al: A microRNA code for prostate cancer metastasis. Oncogene.
35:1180–1192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Tang Y, Wu B, Huang S, Peng X, Li X, Huang
X, Zhou W, Xie P and He P: Downregulation of miR-505-3p predicts
poor bone metastasis-free survival in prostate cancer. Oncol Rep.
41:57–66. 2019.PubMed/NCBI
|
|
173
|
Olivan M, Garcia M, Suárez L, Guiu M, Gros
L, Méndez O, Rigau M, Reventós J, Segura MF, de Torres I, et al:
Loss of microRNA-135b enhances bone metastasis in prostate cancer
and predicts aggressiveness in human prostate samples. Cancers
(Basel). 13:62022021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Aigner A and Fischer D:
Nanoparticle-mediated delivery of small RNA molecules in tumor
therapy. Pharmazie. 71:27–34. 2016.PubMed/NCBI
|
|
175
|
Oh-Hohenhorst SJ and Lange T: Role of
metastasis-related microRNAs in prostate cancer progression and
treatment. Cancers (Basel). 13:44922021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Abramovic I, Ulamec M, Katusic Bojanac A,
Bulic-Jakus F, Jezek D and Sincic N: miRNA in prostate cancer:
Challenges toward translation. Epigenomics. 12:543–558. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Locati MD, Terpstra I, de Leeuw WC, Kuzak
M, Rauwerda H, Ensink WA, van Leeuwen S, Nehrdich U, Spaink HP,
Jonker MJ, et al: Improving small RNA-seq by using a synthetic
spike-in set for size-range quality control together with a set for
data normalization. Nucleic Acids Res. 43:e892015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Zhang W, Zang J, Jing X, Sun Z, Yan W,
Yang D, Shen B and Guo F: Identification of candidate miRNA
biomarkers from miRNA regulatory network with application to
prostate cancer. J Transl Med. 12:662014. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Pashaei E, Pashaei E, Ahmady M, Ozen M and
Aydin N: Meta-analysis of miRNA expression profiles for prostate
cancer recurrence following radical prostatectomy. PLoS One.
12:e01795432017. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Sottnik JL and Keller ET: Understanding
and targeting osteoclastic activity in prostate cancer bone
metastases. Curr Mol Med. 13:626–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Macherey S, Monsef I, Jahn F, Jordan K,
Yuen KK, Heidenreich A and Skoetz N: Bisphosphonates for advanced
prostate cancer. Cochrane Database Syst Rev.
12:Cd0062502017.PubMed/NCBI
|
|
182
|
Elomaa I, Kylmälä T, Tammela T, Viitanen
J, Ottelin J, Ruutu M, Jauhiainen K, Ala-Opas M, Roos L, Seppänen
J, et al: Effect of oral clodronate on bone pain. A controlled
study in patients with metastic prostatic cancer. Int Urol Nephrol.
24:159–166. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Vorreuther R, Klotz T and Engelking R:
Clodronate in the palliative therapy of bone-metastasized prostatic
carcinoma. Urologe A. 31:63–66. 1992.(In German). PubMed/NCBI
|
|
184
|
Adami S and Mian M: Clodronate therapy of
metastatic bone disease in patients with prostatic carcinoma.
Recent Results Cancer Res. 116:67–72. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Strang P, Nilsson S, Brändstedt S, Sehlin
J, Borghede G, Varenhorst E, Bandman U, Borck L, Englund G and
Selin L: The analgesic efficacy of clodronate compared with placebo
in patients with painful bone metastases from prostatic cancer.
Anticancer Res. 17:4717–4721. 1997.PubMed/NCBI
|
|
186
|
Kylmälä T, Taube T, Tammela TL, Risteli L,
Risteli J and Elomaa I: Concomitant i.v. and oral clodronate in the
relief of bone pain-a double-blind placebo-controlled study in
patients with prostate cancer. Br J Cancer. 76:939–942. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Ernst DS, Tannock IF, Winquist EW, Venner
PM, Reyno L, Moore MJ, Chi K, Ding K, Elliott C and Parulekar W:
Randomized, double-blind, controlled trial of
mitoxantrone/prednisone and clodronate versus
mitoxantrone/prednisone and placebo in patients with
hormone-refractory prostate cancer and pain. J Clin Oncol.
21:3335–3342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Mason MD, Sydes MR, Glaholm J, Langley RE,
Huddart RA, Sokal M, Stott M, Robinson AC, James ND, Parmar MK, et
al: Oral sodium clodronate for nonmetastatic prostate
cancer-results of a randomized double-blind placebo-controlled
trial: Medical research council PR04 (ISRCTN61384873). J Natl
Cancer Inst. 99:765–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Dearnaley DP, Sydes MR, Mason MD, Stott M,
Powell CS, Robinson AC, Thompson PM, Moffat LE, Naylor SL and
Parmar MK; Mrc Pr05 Collaborators, : A double-blind,
placebo-controlled, randomized trial of oral sodium clodronate for
metastatic prostate cancer (MRC PR05 trial). J Natl Cancer Inst.
95:1300–1311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Dearnaley DP, Mason MD, Parmar MK, Sanders
K and Sydes MR: Adjuvant therapy with oral sodium clodronate in
locally advanced and metastatic prostate cancer: Long-term overall
survival results from the MRC PR04 and PR05 randomised controlled
trials. Lancet Oncol. 10:872–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Lipton A, Glover D, Harvey H, Grabelsky S,
Zelenakas K, Macerata R and Seaman J: Pamidronate in the treatment
of bone metastases: Results of 2 dose-ranging trials in patients
with breast or prostate cancer. Ann Oncol. 5 (Suppl 7):S31–S35.
1994.PubMed/NCBI
|
|
192
|
Figg WD, Liu Y, Arlen P, Gulley J,
Steinberg SM, Liewehr DJ, Cox MC, Zhai S, Cremers S, Parr A, et al:
A randomized, phase II trial of ketoconazole plus alendronate
versus ketoconazole alone in patients with androgen independent
prostate cancer and bone metastases. J Urol. 173:790–796. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Sweeney C, Dugan WM II, Dreicer R, Chu F,
Parks G, Baker K, Reed D, Jansz K, Zadra J and Yiannoutsos CT: A
randomized placebo-controlled trial of daily high-dose oral
risedronate in men with metastatic prostate cancer commencing
androgen deprivation therapy (ADT). J Clin Oncol. 28 (15
Suppl):e150002010. View Article : Google Scholar
|
|
194
|
Meulenbeld HJ, van Werkhoven ED, Coenen
JL, Creemers GJ, Loosveld OJ, de Jong PC, Ten Tije AJ, Fosså SD,
Polee M, Gerritsen W, et al: Randomised phase II/III study of
docetaxel with or without risedronate in patients with metastatic
castration resistant prostate cancer (CRPC), the Netherlands
prostate study (NePro). Eur J Cancer. 48:2993–3000. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Hahn NM, Yiannoutsos CT, Kirkpatrick K,
Sharma J and Sweeney CJ: Failure to suppress markers of bone
turnover on first-line hormone therapy for metastatic prostate
cancer is associated with shorter time to skeletal-related event.
Clin Genitourin Cancer. 12:33–40.e4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Hoskin P, Sundar S, Reczko K, Forsyth S,
Mithal N, Sizer B, Bloomfield D, Upadhyay S, Wilson P, Kirkwood A,
et al: A multicenter randomized trial of ibandronate compared with
single-dose radiotherapy for localized metastatic bone pain in
prostate cancer. J Natl Cancer Inst. 107:djv1972015. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Saad F, Gleason DM, Murray R, Tchekmedyian
S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA and Chen B;
Zoledronic Acid Prostate Cancer Study Group, : A randomized,
placebo-controlled trial of zoledronic acid in patients with
hormone-refractory metastatic prostate carcinoma. J Natl Cancer
Inst. 94:1458–1468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Abetz L, Barghout V, Arbuckle R, Bosch V,
Shirina N and Saad F: Impact of zoledronic acid (Z) on pain in
prostate cancer patients with bone metastases in a randomised
placebo-control trial. J Clin Oncol. 24 (Suppl 18):S46382006.
View Article : Google Scholar
|
|
199
|
Leto G, Badalamenti G, Arcara C,
Crescimanno M, Flandina C, Tumminello FM, Incorvaia L, Gebbia N and
Fulfaro F: Effects of zoledronic acid on proteinase plasma levels
in patients with bone metastases. Anticancer Res. 26:23–26.
2006.PubMed/NCBI
|
|
200
|
Cózar Olmo JM, Carballido Rodriguez J,
Luque Galvez P, Tabernero Gómez AG, Barreiro Mouro A, Sánchez
Sánchez E, González Enguita C, Alcover García J, Garcia-Galisteo E,
Abascal García JM, et al: Effectiveness and tolerability of
zoledronic acid in the treatment of metastatic prostate cancer.
Actas Urol Esp. 32:492–501. 2008.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Saad F, Gleason DM, Murray R, Tchekmedyian
S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA and Zheng M;
Zoledronic Acid Prostate Cancer Study Group, : Long-term efficacy
of zoledronic acid for the prevention of skeletal complications in
patients with metastatic hormone-refractory prostate cancer. J Natl
Cancer Inst. 96:879–882. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Fulfaro F, Leto G, Badalamenti G, Arcara
C, Cicero G, Valerio MR, Di Fede G, Russo A, Vitale A, Rini GB, et
al: The use of zoledronic acid in patients with bone metastases
from prostate carcinoma: Effect on analgesic response and bone
metabolism biomarkers. J Chemother. 17:555–559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Saad F: Clinical benefit of zoledronic
acid for the prevention of skeletal complications in advanced
prostate cancer. Clin Prostate Cancer. 4:31–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Saad F, Chen YM, Gleason DM and Chin J:
Continuing benefit of zoledronic acid in preventing skeletal
complications in patients with bone metastases. Clin Genitourin
Cancer. 5:390–396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Saad F and Eastham J: Zoledronic acid
improves clinical outcomes when administered before onset of bone
pain in patients with prostate cancer. Urology. 76:1175–1181. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Paparella S, Finkelberg E, Varisco D,
Tondelli E and Rocco F: Use of zoledronic acid in patients with
prostate cancer bone metastases: Control of pain and
musculoskeletal complications. Urologia. 78:300–304. 2011.(In
Italian). View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Uemura H, Yanagisawa M, Ikeda I, Fujinami
K, Iwasaki A, Noguchi S, Noguchi K and Kubota Y; Yokohama Bone
Metastasis Study Group, : Possible anti-tumor activity of initial
treatment with zoledronic acid with hormonal therapy for
bone-metastatic prostate cancer in multicenter clinical trial. Int
J Clin Oncol. 18:472–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Wang F, Chen W, Chen H, Mo L, Jin H, Yu Z,
Li C, Liu Q, Duan F and Weng Z: Comparison between zoledronic acid
and clodronate in the treatment of prostate cancer patients with
bone metastases. Med Oncol. 30:6572013. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Ueno S, Mizokami A, Fukagai T, Fujimoto N,
Oh-Oka H, Kondo Y, Arai G, Ide H, Horie S, Ueki O, et al: Efficacy
of combined androgen blockade with zoledronic acid treatment in
prostate cancer with bone metastasis: The ZABTON-PC (zoledronic
acid/androgen blockade trial on prostate cancer) study. Anticancer
Res. 33:3837–3844. 2013.PubMed/NCBI
|
|
210
|
Chiang PH, Wang HC, Lai YL, Chen SC,
Yen-Hwa W, Kok CK, Ou YC, Huang JS, Huang TC and Chao TY:
Zoledronic acid treatment for cancerous bone metastases: A phase IV
study in Taiwan. J Cancer Res Ther. 9:653–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Pan Y, Jin H, Chen W, Yu Z, Ye T, Zheng Y,
Weng Z and Wang F: Docetaxel with or without zoledronic acid for
castration-resistant prostate cancer. Int Urol Nephrol.
46:2319–2326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Smith MR, Halabi S, Ryan CJ, Hussain A,
Vogelzang N, Stadler W, Hauke RJ, Monk JP, Saylor P, Bhoopalam N,
et al: Randomized controlled trial of early zoledronic acid in men
with castration-sensitive prostate cancer and bone metastases:
Results of CALGB 90202 (alliance). J Clin Oncol. 32:1143–1150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Wirth M, Tammela T, Cicalese V, Gomez
Veiga F, Delaere K, Miller K, Tubaro A, Schulze M, Debruyne F,
Huland H, et al: Prevention of bone metastases in patients with
high-risk nonmetastatic prostate cancer treated with zoledronic
acid: Efficacy and safety results of the Zometa European Study
(ZEUS). Eur Urol. 67:482–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
James ND, Pirrie SJ, Pope AM, Barton D,
Andronis L, Goranitis I, Collins S, Daunton A, McLaren D,
O'Sullivan J, et al: Clinical outcomes and survival following
treatment of metastatic castrate-refractory prostate cancer with
docetaxel alone or with strontium-89, zoledronic acid, or both: The
TRAPEZE randomized clinical trial. JAMA Oncol. 2:493–499. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Denham JW, Wilcox C, Joseph D, Spry NA,
Lamb DS, Tai KH, Matthews J, Atkinson C, Turner S, Christie D, et
al: Quality of life in men with locally advanced prostate cancer
treated with leuprorelin and radiotherapy with or without
zoledronic acid (TROG 03.04 RADAR): Secondary endpoints from a
randomised phase 3 factorial trial. Lancet Oncol. 13:1260–1270.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Denham JW, Joseph D, Lamb DS, Spry NA,
Duchesne G, Matthews J, Atkinson C, Tai KH, Christie D, Kenny L, et
al: Short-term androgen suppression and radiotherapy versus
intermediate-term androgen suppression and radiotherapy, with or
without zoledronic acid, in men with locally advanced prostate
cancer (TROG 03.04 RADAR): An open-label, randomised, phase 3
factorial trial. Lancet Oncol. 15:1076–1089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Denham JW, Joseph D, Lamb DS, Spry NA,
Duchesne G, Matthews J, Atkinson C, Tai KH, Christie D, Kenny L, et
al: Short-term androgen suppression and radiotherapy versus
intermediate-term androgen suppression and radiotherapy, with or
without zoledronic acid, in men with locally advanced prostate
cancer (TROG 03.04 RADAR): 10-Year results from a randomised, phase
3, factorial trial. Lancet Oncol. 20:267–281. 2019. View Article : Google Scholar : PubMed/NCBI
|