Introduction
Renal cell carcinoma (RCC) is a heterogeneous group
of types of kidney cancer that arise from renal tubular epithelial
cells. These types of cancer display divergent epigenetic and
genetic abnormalities and are amongst the 10 most common types of
cancer worldwide (1–4). Genetic factors such as the Von
Hippel-Lindau and protein polybromo-1 genes have been associated
with the pathogenesis of RCC (5).
Over the last two decades, the classification of RCCs have
undergone major changes based on histological presentation and
molecular pathology and, in 2004, the World Health Organisation
(WHO) classification recognized numerous histological RCC subtypes
(6,7) with distinct genetic, biological and
clinical behaviors (3,8). The most frequent subtypes of all cases
of RCC are clear cell RCC (ccRCC, ~75%) (3,5),
papillary RCC (pRCC, ~15%) (9,10) and
chromophobe RCC (chRCC, ~5%) (11).
The growing understanding of the morphology,
immunohistochemistry (IHC), genomics and epidemiology of RCC has
allowed for an improved insight into the tumor biology and
characterization of this disease (4,12–15).
Therefore, in 2013, the International Society of Urological
Pathology (ISUP) Vancouver consensus proposed a new classification
of renal neoplasia including newly characterized RCC subtypes and
other additional emerging/provisional entities (14–17).
The new classification of RCC was revised by the WHO Renal Tumor
Panel in 2015, with the results published in the 4th (14) and 5th (14,15)
editions of the WHO Classification of Tumors of the Urinary System
and Male Genital Organs Bluebook in 2016 and 2022 respectively.
Among the newly recognized epithelial renal tumors
were fumarate hydratase (FH)-deficient RCC, succinate dehydrogenase
(SDH)-deficient RCC, tubulocystic RCC, acquired cystic
disease-associated RCC and clear cell papillary RCC (12,15,18,19).
The aforementioned entities are now considered separate entities as
their morphologies, immune-profiles and molecular characteristics
have been adequately recognized (4,12,19,20).
Previously, some of these entities were identified as unclassified
types of RCC with aggressive features in younger adults with a mean
age of ≤35 years (21–23). Other tumor types have been proposed
as emerging/provisional entities; however, they have not yet been
recognized by the WHO as separate entities due to a lack of
sufficient evidence (14,19,24).
Furthermore, certain oncocytic renal tumors have been described
(19), such as eosinophilic
vacuolated tumor and low-grade oncocytic renal tumor. High-grade
oncocytic tumor has also been proposed to represent a potentially
new renal entity (19,25). These new entities show divergent
prognoses, varying from indolent to aggressive renal tumors
(26) with correlated treatment
implications. Moreover, correct diagnosis of the hereditary forms
of these tumors has implications for affected family members
(27). Therefore, accurate
classification is important for prognosis, therapeutic treatment
and genetic counselling (28).
In addition to the newly proposed and recognized
subtypes, the Vancouver consensus proposed a new ISUP grading
(16,29). The ISUP grading is similar to the
well-established Fuhrman grading (30), i.e., it is also a four grade system,
but relies on nucleolus identification to determine WHO/ISUP grade
1–3 and the presence of polymorphic giant tumor cells, sarcomatoid
or rhabdoid differentiation (RD) features for assigning grade 4
(15,18). This new grading system was
recommended to replace the Fuhrman grading as it is a more
reproducible system (31,32).
Tumor-Node-Metastasis (TNM) staging has been
considered the gold standard when predicting the prognosis of
patients with RCC and for the guidance of patient management,
surveillance and treatment (33).
Moreover, it is continuously revised to improve its prognostic
accuracy and predictive ability, and the 8th edition (12,34) is
presently used by clinicians and pathologists (12,33,34).
In addition to TNM staging (35) and Fuhrman grading (36), tumor necrosis (TN) has been
considered to be a prognostic factor (37) and evidence for this has been
published for ccRCC and chRCC, independent of tumor stage and grade
(14,37–39).
Moreover, lymphovascular (microvascular) invasion (LVI), excluding
that within the perinephric or renal fat which is already described
in the pT3a part of TNM staging, has been reported to correlate
with survival, independent of tumor size, grade or type (40,41).
Considering these developments, the aim of the
present study was to review pathological slides of kidney tumors
from the Netherlands Cohort Study on Diet and Cancer (NLCS)
(42–44) in order to reclassify them by
morphotype, TNM stage, ISUP grade, LVI and necrosis, according to
the ISUP and the 2022 WHO classification (5th edition). A further
aim was to assess whether newly accepted entities could be
identified in this dataset of elderly patients. To the best of our
knowledge, this is the first study to report such a re-evaluation
on a large, unselected population-based series of RCCs with
extensive clinical data.
Materials and methods
Study population
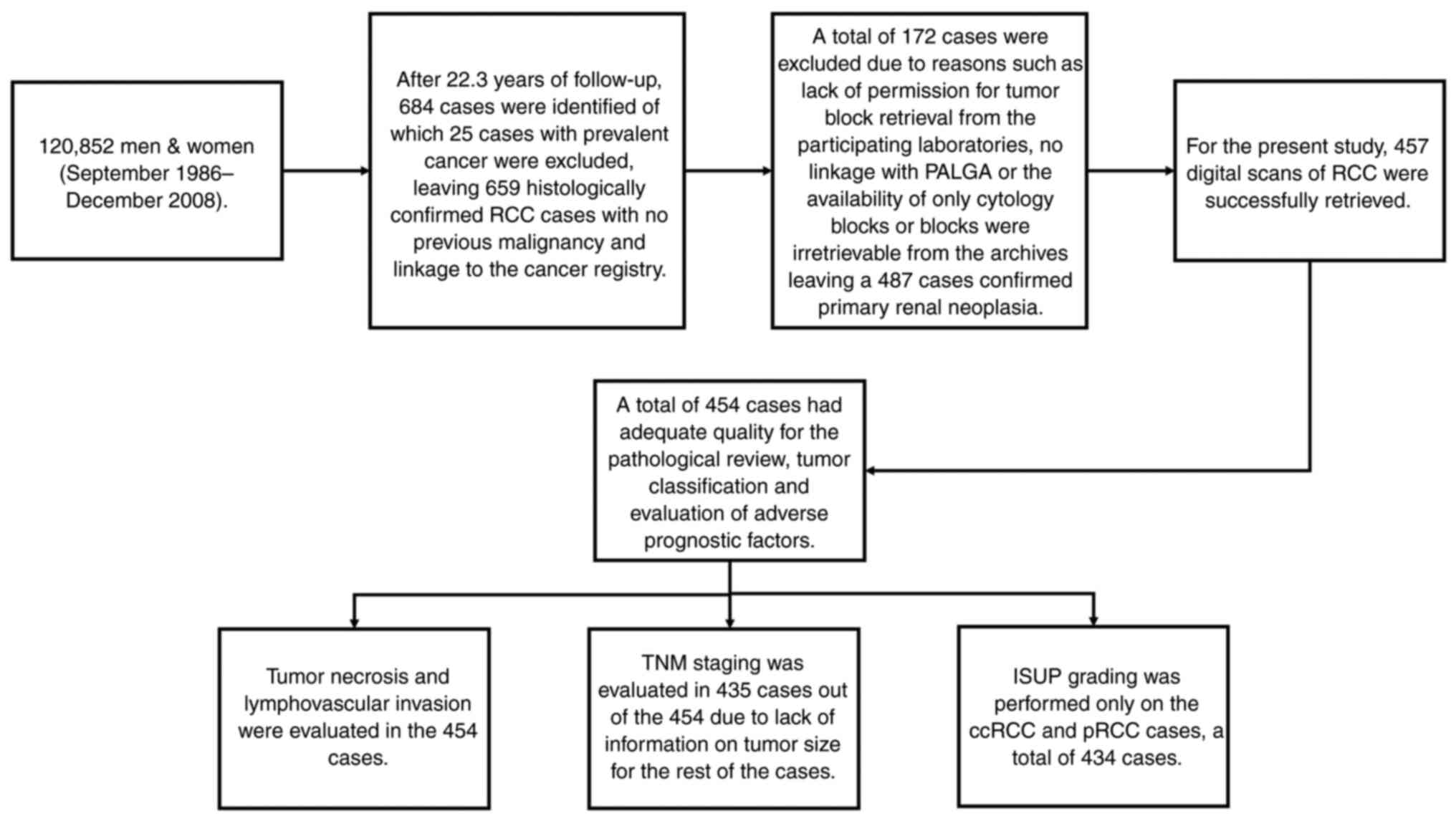
A total of 457 cases of RCC from the NLCS were
reviewed. The NLCS is a prospective cohort study that has
previously been described in detail (44–46).
In summary, this series was initiated in September 1986 and
included 120,852 men and women, aged 55–69 years at diagnosis. The
collection of the samples was limited to cases diagnosed before the
31st of December 2008. Follow-up for cancer occurrence was
available through computerized record linkage with the Netherlands
Cancer Registry (NCR) and Pathologisch Anatomisch Landelijk
Geautomatiseerd Archief (PALGA), a national database of pathology
reports (47) as described
previously (46). After 22.3 years
of follow-up, 659 histologically confirmed RCC cases were eligible
for collection of formalin-fixed-paraffin-embedded (FFPE) tumor
tissue from 51 pathology laboratories throughout The Netherlands.
Data on tumor characteristics, such as laterality, date of
diagnosis, TNM stage, initial treatment and other
clinicopathological characteristics were obtained through record
linkage with the NCR (46).
Pathology reports were used to record the tumor size and to verify
the staging information from the cancer registry.
Tissue collection
FFPE tumor tissues were collected after ethical
approval by the Medical Ethical Committees of Maastricht University
(Maastricht, The Netherlands), PALGA and the NCR. Tissue collection
was performed at the time of diagnosis for the cases diagnosed
between 1986–1996 and was later extended to include cases diagnosed
between 1997–2008 (45,47). Urothelial cell carcinomas were
excluded and only histologically confirmed epithelial cancers were
included. This resulted in the collection of a total of 487 cases
of confirmed primary renal neoplasia.
Original pathology review
The original tumor blocks were retrieved from all
participating laboratories and hematoxylin and eosin (H&E)
slides were made at the laboratories of Maastricht University
Medical Center (Maastricht, The Netherlands) and Radboud university
(Nijmegen, The Netherlands). H&E-stained slides of all
collected FFPE tumor tissues were assessed by two experienced
urogenital pathologists, to confirm tumor histological subtype
based on the 2004 WHO classification (33). Nuclear grading was performed
according to the Fuhrman grading system. After revision, cases
showing <10% malignant cells or cases that were reclassified as
urothelial cell carcinomas were excluded, leaving a total of 487
confirmed RCC cases.
Second pathology review according to
the new classifications of renal tumors, using the new ISUP grading
and the 8th TNM edition
For the present study, 457 digital scans of RCC
cases were retrieved for inclusion in the re-evaluation which
applied the new 2022 ISUP/WHO classification. Fig. 1 shows the NLCS cases from the
collection to the second pathology review. These scans were
originally made from the tumor slides that were selected by the
pathologists as those being the most representative of the tumor
subtype. The scans were made using a Ventana iScan HT scanner
(series number, BI12N7070; Roche Tissue Diagnostics), in the
diagnostics facility at the Department of Pathology at the
Maastricht University Medical Center, which met an internal
standard quality assurance check procedure (48). If the quality of the digital slides
were inadequate for the evaluation of the nucleus details, the
original H&E slides were re-scanned and evaluated for a second
time. Two experienced urogenital pathologists both confirmed that
all digital slides included in the present study were of the
quality required for the re-evaluation process.
All tumors were re-evaluated and reassigned
according to histological subtype, nuclear grade, TN, sarcomatoid
differentiation, RD, novel nuclear ISUP grade and LVI, using the
latest TNM version and, ISUP and WHO diagnostic criteria (4,15,16,20),
independently by a urogenital pathologist at Maastricht University
Medical Center and an expert urogenital pathologist from the Johns
Hopkins University School of Medicine (Baltimore, USA). A total of
two autopsy cases were included in the pathological revision but
were excluded from the TNM reclassification. Furthermore, data on
tumor size were used to assign the pathological T stage according
to the TNM 8th edition. Both pathologists were blinded for the
outcomes of previous pathological reviews.
IHC
IHC staining for carbonic anhydrase IX (CAIX) was
performed on ccRCC cases that warranted further subtyping
confirmation. The staining was performed on RCC tissue sections
(3–4 µm) from the FFPE tissue blocks. Firstly, the slides were
deparaffinized at room temperature in xylene, rinsed in a
decreasing alcohol concentration series and then rinsed in water.
Samples were thereafter treated at room temperature with 0.3%
hydrogen peroxide in methanol for 20 min. Afterwards, the slides
were washed 3× at room temperature in 1X PBS. Subsequently, the
antigen retrieval pretreatment was performed using 1X citrate
antigen retrieval buffer (pH 6.0, 10X diluted in water; Dako;
Agilent Technologies, Inc.; cat. no. S2369) in the microwave for 20
min at 600 watt. Staining was performed on an Autostainer Plus Link
48 System (Agilent Technologies, Inc.). All steps were performed at
room temperature. Samples were covered with Endogenous Peroxidase
Blocking Reagent (Agilent Technologies, Inc.) for 5 min and then
washed with 1X PBS. The slides were then incubated for 20 min with
rabbit anti-CAIX primary antibody (1:1,000; cat. no. NB100-417;
Novus Biologicals, LCC) diluted in Agilent antibody diluent (cat.
no. K8006; Agilent Technologies, Inc.). Secondary detection and
visualization were performed using the EnVision FLEX+ detection
system (cat. no. K8002; Agilent Technologies, Inc.). In brief,
slides were incubated for 20 min with labelled polymer (EnVision
FLEX-HRP; Agilent Technologies, Inc.) and then the slides were
incubated for 10 min with substrate buffer and DAB chromogen
solution. Counterstaining with hematoxylin for 90 sec at room
temperature, subsequent dehydration in an increasing alcohol series
and cover slips were added using a Leica Histocore (Leica
Microsystems, Inc.). Slides were evaluated by a urogenital
pathologist (IS) using a light microscope.
Statistical analysis
In the present study, the χ2 test was
performed using SPSS 28 (IBM Corp.).
Results
Patient characteristics. A total of 457 patients
were included in the present study. There was a predominance of
male patients (62.6%). The age at diagnosis of the included
patients ranged from 56–88 years with a mean age of 71.4±6.3 years.
The mean tumor size was 67.2±31.7 mm (Table I).
 | Table I.Clinical characteristics of patients
included in the present study. |
Table I.
Clinical characteristics of patients
included in the present study.
| Clinical
characteristic | Number |
|---|
| Number of patients,
n | 457 |
| Mean age at
diagnosis (range), years | 71.4 (56–88) |
| Sex, n (%) |
|
|
Male | 286 (62.6) |
|
Female | 171 (37.4) |
| Mean tumor size ±
SD, mm | 67.2±31.7 |
| Original
histological review, n (%) |
|
| Clear
cell | 375 (82.1) |
|
Papillary | 62 (13.6) |
|
Chromophobe | 13 (2.8) |
|
Collecting duct carcinoma | 3 (0.7) |
|
Oncocytoma | 4 (0.9) |
| Pathological T
stagea, n, (%) | 435 (95.2) |
| 1 | 22 (5.1) |
| 1a | 1 (0.2) |
| 1b | 3 (0.7) |
| 2 | 262 (60.2) |
| 3 | 1 (0.2) |
| 3a | 73 (16.8) |
| 3b | 69 (15.9) |
| 4 | 4 (0.9) |
| Fuhrman
gradeb, n (%) | 434 (95.0) |
| 1 | 54 (12.4) |
| 2 | 196 (45.2) |
| 3 | 143 (32.9) |
| 4 | 41 (9.4) |
Pathological review and classification of the
tumors. A total of three scans from the 457 cases were excluded due
to poor quality and digital scans of the representative sections
for 454 cases were available for revision. These scans were
independently reviewed and classified based on morphological
criteria by two pathologists based on the 2022 WHO classification,
the ISUP recommendations and the Genitourinary Pathology Society
(GUPS) update on renal neoplasia. The subtyping showed a 100%
overlap with the previous diagnoses. The renal cell neoplasia
included 373 ccRCC cases (82.1%), 61 pRCC cases (13.4%), 13 chRCC
cases (2.9%), 3 cases of collecting duct carcinoma (0.7%) and 4
cases of oncocytoma (0.9%) (Table
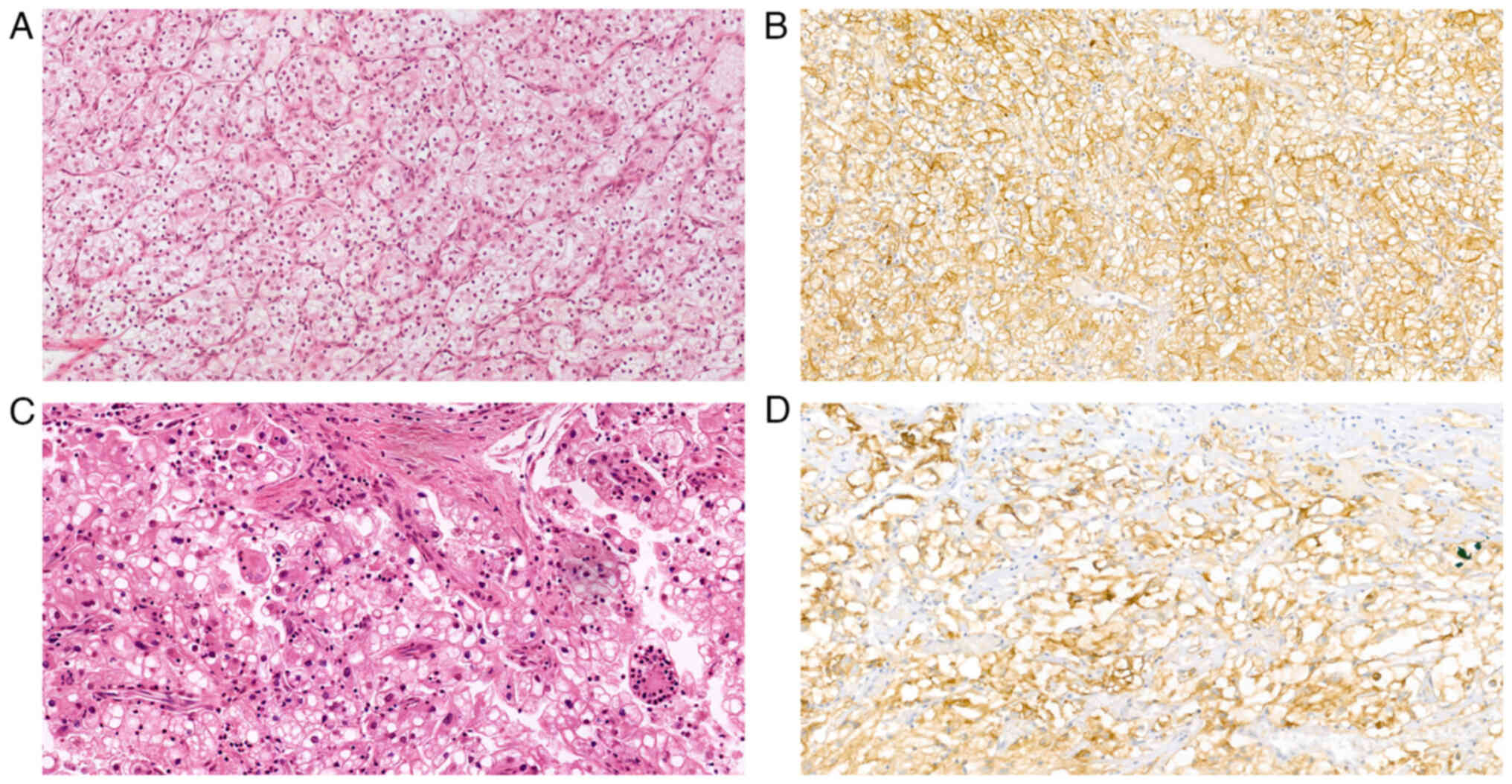
II). In 30 ccRCC cases, the diagnosis was confirmed by IHC
staining for CAIX, which showed a box-like staining pattern
(Fig. 2). Furthermore, none of the
tumors demonstrated features compatible with FH-deficient RCC,
SDH-deficient RCC, eosinophilic solid and cystic RCC or other
recently described entities (12).
 | Table II.Clinical characteristic results of
the re-evaluation of the histology of a population-based series of
RCC cases from the NLCS 1986-2008, according to the 2022 ISUP
grading systems and WHO classification. |
Table II.
Clinical characteristic results of
the re-evaluation of the histology of a population-based series of
RCC cases from the NLCS 1986-2008, according to the 2022 ISUP
grading systems and WHO classification.
| Clinical
characteristic | Number (%) |
|---|
| Histological
reviewa, n (%) | 454 (99.3) |
| Clear
cell | 373 (82.2) |
|
Papillary | 61 (13.4) |
|
Chromophobe | 13 (2.9) |
|
Collecting duct carcinoma | 3 (0.7) |
|
Oncocytoma | 4 (0.9) |
| Pathologic T
stageb, n (%) | 435 (95.8) |
| 1a | 94 (21.6) |
| 1b | 115 (26.4) |
| 2 | 4 (0.9) |
| 2a | 50 (11.5) |
| 2b | 25 (5.7) |
| 3 | 1 (0.2) |
| 3a | 73 (16.8) |
| 3b | 69 (15.9) |
| 4 | 4 (0.9) |
| Sarcomatoid
differentiation, n/total (%) | 23/454 (5.1) |
| Rhabdoid
differentiation, n/total (%) | 19/454 (4.2) |
| Lymphovascular
invasion, n/total (%) | 64/454 (14.1) |
| Necrosis present, n
(%) | 152/454 (33.5) |
| Necrosis present
per morphotype, n necrosis present/n total cases of the morphotype
(%) |
|
| ccRCC,
n (%) | 111/373 (29.8) |
| pRCC, n
(%) | 38/61 (62.3) |
| chRCC,
n (%) | 2/13 (15.4) |
| CDC, n
(%) | 1/3 (33.3) |
|
Oncocytoma, n (%) | 0/4 (0.0) |
| ISUP grade for
ccRCC and PRCCc n
(%), | 434 (95.6) |
| 1 | 93 (21.4) |
| 2 | 191 (44.0) |
| 3 | 108 (24.9) |
| 4 | 42 (9.7) |
Tumor grade according to the new ISUP grading system
and reporting on sarcomatoid and rhabdoid features. Initially, all
434 ccRCC and pRCC cases were graded according to the Fuhrman
grading system by two pathologists (Tables I and III). Tumors were assigned grade 1 in 54
cases (12.4%), grade 2 in 196 cases (45.2%), grade 3 in 143 cases
(32.9%) and grade 4 in 41 cases (9.4%). All ccRCC and pRCC cases
were regraded using the new WHO/ISUP grade by two pathologists
blinded to the original Fuhrman grading (Tables II and III). This resulted in the assignment of
ISUP grade 1 in 93 cases (21.4%), grade 2 in 191 cases (44.0%),
grade 3 in 108 cases (24.9%) and grade 4 in 42 cases (9.7%).
Comparison of the two grading systems showed the same tumor grade
in 245 cases (56.5%), whereas a different grade was reported in 189
cases (43.5%) (Table III). Of the
454 cases histologically reviewed, sarcomatoid differentiation was
identified in 23 patients (5.1%) and RD in 19 (4.2%).
 | Table III.Comparison of nuclear grading
classification according to the Fuhrman and ISUP grading systems on
the Netherlands Cohort Study on Diet and Cancer, 1986-2008. |
Table III.
Comparison of nuclear grading
classification according to the Fuhrman and ISUP grading systems on
the Netherlands Cohort Study on Diet and Cancer, 1986-2008.
|
| ISUP, n |
|
|---|
|
|
|
|
|---|
| Grading system
Fuhrman, n | 1 | 2 | 3 | 4 | Total, n |
|---|
| 1 | 34 | 17 | 3 | 0 | 54 |
| 2 | 58 | 111 | 26 | 1 | 196 |
| 3 | 1 | 61 | 70 | 11 | 143 |
| 4 | 0 | 2 | 9 | 30 | 41 |
| Total, n | 93 | 191 | 108 | 42 | 434 |
Adverse prognostic factors
TN and LVI were also assessed and evaluated in the
454 cases. TN was identified in 152 cases (33.5%) and, tumor
necrosis (TN) was evaluated as the percentage of tumor necrosis in
relation to the total tumor volume as previously described
(49,50). A total of 35 tumors (23.0% of the
152 cases) exhibited a TN of ≤5%, 84 tumors (55.3% of the 152
cases) showed 6–49% TN, 29 tumors (19.1% of the 152 cases) showed
50–89% TN, and 4 tumors (2.6% of the 152 cases) showed ≥90% TN. TN
was present more often in pRCC (38/61, 62.3%) compared with ccRCC
(111/372, 29.8%) (χ2 24.46, P<0.05).
LVI was identified in 64 renal neoplasms (14.1%).
Invasion in the renal vein or its segmental branches was classified
as a pT3a tumor (Table I). LVI was
seen more often in ISUP grade 3 tumors (27/64 cases, 42.2%),
followed by ISUP grade 2 (19/64 cases, 29.7%) and ISUP grade 4
(13/64 cases, 20.3%). Notably, LVI was seen in 5 cases of ISUP
grade 1 ccRCC.
TNM staging according to the 8th edition. A total of
435 RCC cases were restaged according to the 8th edition of the TNM
version as shown in Table II as
information on tumor size was only available for 435 cases. This
restaging resulted in the assignment of pT1a in 94 cases (21.6%),
pT1b in 115 cases (26.4%), pT2 in 4 cases (0.9%), pT2a in 50 cases
(11.5%), pT2b in 25 cases (5.7%), pT3 in 1 case (0.2%), pT3a in 73
cases (16.8%), pT3b in 69 cases (15.9%) and pT4 in 4 cases (0.9%).
Comparison of the 8th edition of the TNM staging with the 3rd
edition of the TNM staging that was originally applied to the NLCS
cases showed a restaging in 65.5% of the cases. Table IV presented the comparison of the
3rd and the 8th edition of the TNM classification of the NLCS
cases. The restaging in the present study showed that more cases
were categorized in a lower TNM stage compared to the original
classification, as 60.2% of the cases were originally assigned as
pT2.
 | Table IV.Comparison of the 3rd and 8th edition
of the TNM classification on the Netherlands Cohort Study on Diet
and Cancer, 1986-2008. |
Table IV.
Comparison of the 3rd and 8th edition
of the TNM classification on the Netherlands Cohort Study on Diet
and Cancer, 1986-2008.
|
| TNM 8th, n |
|
|---|
|
|
|
|
|---|
| TNM edition TNM
3rd, n | 1a | 1b | 2 | 2a | 2b | 3 | 3a | 3b | 4 | Total, n |
|---|
| 1 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 |
| 1a | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 1b | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| 2 | 70 | 114 | 4 | 50 | 24 | 0 | 0 | 0 | 0 | 262 |
| 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 3a | 0 | 0 | 0 | 0 | 0 | 0 | 73 | 0 | 0 | 73 |
| 3b | 0 | 0 | 0 | 0 | 0 | 0 |
| 69 | 0 | 69 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 |
| Total, n | 94 | 115 | 4 | 50 | 25 | 1 | 73 | 69 | 4 | 435 |
Discussion
In the present study, the aim was to review RCC
cases from the large, prospective NLCS cohort according to the
latest 2022 WHO classification and the latest updates of GUPS and
ISUP on renal tumors, to evaluate the presence of newly recognized
or emerging/provisional entities. Furthermore, an evaluation of
whether recently accepted renal tumor subtypes can be identified in
an existing cohort of patients with RCC was performed. Moreover,
the present study also aimed to classify these cases according to
the new ISUP grading, to assess TN, LVI and the presence of
sarcomatoid differentiation and RD features, and to apply the
latest TNM edition. None of the reviewed cases showed any of the
newly described entities and all the cases showed the formerly
well-recognized and most common RCC subtypes. The re-evaluation of
the subtyping was completely in accordance with the previous
diagnoses of these RCC cases.
Comparison of the ISUP and Fuhrman gradings on the
ccRCC and pRCC cases showed a different grading in 43.5% of the
cases. Evaluation of the presence of sarcomatoid differentiation
and RD features revealed their presence in the NLCS cases.
Furthermore, assessment of the presence of adverse prognostic
features showed that TN was also present in 33.5% of the cases and
that it was present more often in pRCC cases (62.3%) compared with
other subtypes. Hemorrhage and necrosis, however, are known to be
related to the pRCC subtype but not to prognosis, and therefore are
not used as adverse prognostic indicators in pRCC. Furthermore, LVI
was similarly identified in few cases and was mostly detected in
tumors with ISUP grade 3. Comparison of the TNM 8th edition and the
previously applied TNM 3rd edition revealed a restaging in the
majority of cases. However, this difference, as well as the
difference seen between the Fuhrman and the ISUP grading, were to
be expected as in both situations different grading criteria were
applied.
In the present study, none of the newly described or
emerging entities were identified. This could be explained by the
fact that the NLCS cohort included patients with a mean age of 71.4
years and some of the newly described entities, such as the
SDH-deficient RCC subtype, have been reported to be particularly
seen in younger adults (12). Thus,
Kwon et al (51) reported a
reclassification of a proportion (13%) of adults with unclassified
RCC in a patient cohort with a mean age at presentation of 58 years
old. Clemmensen et al (52)
reclassified a subset of early onset RCC in patients aged <46
years. Li et al (23)
re-evaluated oncocytic renal tumors in patients aged ≤35 years.
These findings suggested a low chance of finding a newly described
entity in the research databases and diagnostic archives consisting
of an elderly patient cohort.
However, in the present study there were 30 cases
with a differential diagnosis between ccRCC and the
translocation-related RCC (such as Xp11 translocation RCC), based
on the morphological images. Therefore, an additional test was
necessary to confirm the diagnoses of these cases. According to the
literature (53) and the widely
used WHO 2022 diagnostic criteria, IHC CAIX staining is a specific
and sensitive marker of ccRCC, since ccRCC has a ‘box-shape’
staining pattern and translocation-related RCC has a negative
staining result. All tested cases showed a strong membranous
‘box-shaped’ expression of CAIX. Therefore, CAIX was used in the
present study to confirm the diagnosis of ccRCC. It can therefore
be postulated that using a limited IHC panel can also be sufficient
to confirm the diagnosis, especially when reviewing a large cohort
database that is used for multiple research purposes and where
limited tissue availability can be a limitation.
The differences in grading that were seen for the
originally applied Fuhrman grading on the NLCS cases and the
recently applied ISUP can be explained by the differences between
these two grading systems. Despite the fact that Fuhrman grading
was accepted worldwide and has been employed for many years,
several studies have reported its pitfalls, including questionable
prognostic value and suboptimal interobserver reproducibility
(24,31,54).
This is due to the fact that Fuhrman grading relies on the
simultaneous assessment of nuclear size, nuclear shape and
nucleolar prominence and there is no further direction on how this
should be handled when these three parameters provide conflicting
information (29). In contrast, the
ISUP grading system relies only on the size of the nucleolus for
grading tumors 1–3 and on the presence of giant cells or, the
presence of sarcomatoid differentiation or RD features for
assigning grade 4 (18,29). Previously, several studies compared
Furman grading with WHO/ISUP grading (55,56).
In these studies, the WHO/ISUP grading was shown to provide
superior prognostic information compared to Fuhrman grading
(55,56). Therefore, the regrading of the
kidney tumors in the research databases could be a reasonable
procedure.
Additionally, reporting on adverse prognostic
factors such as TN and LVI was also proposed in the ISUP consensus.
TN has been reported to have prognostic significance for ccRCC and
chRCC, independent of tumor stage and grade (50,57).
However, pRCC tumors often contain areas of necrosis, the presence
of which in this tumor type lacks the same significance (12). TN may also influence treatment
efficacy as, for example, the response to VEGF/tyrosine kinase
inhibitor-targeted therapy has been shown to be poor in patients
with metastatic disease where there was ≥10% necrosis in the
primary ccRCC tumor (58).
Therefore, assessment of the extent of tumor necrosis is
recommended for reporting of kidney specimens by the International
Collaboration on Cancer Reporting (59). LVI, either intratumoral, peritumoral
or perirenal, has been reported to relate to metastasis rate and
patient disease free survival, independent of tumor size, primary
tumor category and grade (59).
However, despite the fact that macroscopic tumor invasion into the
renal and caval vein has been incorporated into the well-known
American Joint Committee on Cancer and University of California,
Los Angeles integrated staging system (UISS) (14), the predictive ability of LVI is
debatable (40). This is due to the
fact that certain studies have reported LVI as having been
correlated with prognosis whereas others reported no association
with prognosis (60–64).
The NLCS cases in the present study were originally
evaluated using the TNM version that was applicable at the time of
diagnosis, but all the cases were later converted to the 1987 3rd
TNM version (65). However, there
are significant differences between the 1987 TNM version and the
currently used 8th edition, as the new TNM version has proven to be
more concise and reproducible than all the previously published
versions. The restaging performed in the present study showed that
more cases were categorized to a lower TNM stage compared to the
original classification. For example, 60.2% of cases were
previously assigned pT2 while with restaging, only 11.6% of cases
were assigned to pT2a and 5.8% to pT2b. Furthermore, a higher
percentage in the pT1a and pT1b stages was seen, which were 21.6
and 26.4%, respectively, compared to the previously assigned
staging (6% according to the TNM 3rd version). This could be
explained by the major differences between the TNM classifications,
namely the boundary values for assigning pT1a, pT1b, pT2a and pT2b.
In the TNM 8th edition, T1a is assigned to tumors that are confined
to the kidney and are <4 cm, and pT1b is assigned to tumors that
are also confined to the kidney but are 4–7 cm. Furthermore, T2a is
assigned to tumors that are limited to the kidney and are 7–10 cm
and T2b is assigned to the tumors that are >10 cm but confined
to the kidney; however, in the TNM 3rd edition, pT1 tumor was
defined as <2 cm.
In the present study, despite the unique
characteristics of the large population-based series with extensive
clinical characteristics, there were certain limitations.
Specifically, the age of the patients included in the analysis,
including patients aged ≥55 years at baseline (46), hindered the possibility of reporting
on some of the new entities that are mostly identified in younger
patients. Another limitation was the lack of tumor size information
for 5 cases, which impeded the conversion to the latest TNM
version. This was hampered by the lack of access to the original
clinical files and a reliance on the information obtained from the
NCR and pathology reports. Furthermore, assessment was performed on
the TN of representative digital slides and these slides were
originally chosen by the pathologists as the ones being most
representative of the tumor subtype and not necrosis. However,
these scans should have been a reliable representation of tumor
necrosis since a range of necrosis was observed in the cohort. If
only viable tumor sections had been selected as representative
slides, there would be fewer cases with tumor necrosis. Only one
IHC marker, CAIX, was used to confirm the diagnosis of ccRCC in 30
cases, which was considered sufficient due to its specificity and
sensitivity (53). Furthermore, in
large research cohorts, the application of extensive tests must be
carefully considered when it comes to the use of tissues with
limited availability and costs. Molecular testing was not performed
on the revised slides as no recently described entities were
identified based on the re-evaluation by two urogenital
pathologists. Molecular studies are also mostly indicated when IHC
is not conclusive and molecular studies are not routinely used.
In conclusion, to the best of our knowledge, the
present study is the first to re-evaluate renal neoplasms from a
large population-based prospective cohort that is extensively used
for research purposes. The findings emphasize that the newly
described entities are a minor component of the cases when
analyzing a cohort of patients with a high average age. Moreover,
the evaluation of additional prognostic factors in this existing
cohort, such as ISUP, TNM 8th edition and rhabdoid/sarcomatoid
features, allows for the updating of previously published
prognostic models and comparison of these to other current
prognostic models, such as the UISS and the Stage, Size, Grade and
Necrosis system.
Acknowledgements
Not applicable.
Funding
This work was supported by the PPP Allowance made available by
Health Holland, Top Sector Life Sciences and Health, to stimulate
public-private partnerships [grant no. LSHM17059 (Prognosis Renal
Cancer and Detection, PRECEDE)].
Availability of data and materials
The datasets used and/or analyzed during the current
are available from the corresponding author on reasonable
request.
Authors' contributions
SO, IVS, LJS and KMS were responsible for the
conception and design of the study and the acquisition of data. SO,
IVS, AM, LJS and KMS were responsible for the analysis and
interpretation of data. SO, IVS, LJS and KMS were responsible for
drafting the manuscript and revising it critically for important
intellectual content. JAAVDP, JVDM, GR, EG, MVE and AZH were
responsible for the acquisition, analysis and interpretation of
data, and drafting and critically revising the manuscript. MMLLB
and CAHVDK were responsible for the conception and design of the
study, drafting the manuscript and revising it critically for
important intellectual content. All authors read and approved the
final version of the manuscript. SO, IVS, LJS and KMS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Medical Ethical
Committee of the Maastricht University (Maastricht, The
Netherlands; approval nos. MEC 00-086.2 and MEC 85-012-8).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RCC
|
renal cell carcinoma
|
|
ISUP
|
International Society of Urological
Pathologists
|
|
WHO
|
World Health Organization
|
|
TNM
|
Tumor-Node-Metastasis
|
|
TN
|
tumor necrosis
|
|
LVI
|
lymphovascular (microvascular)
invasion
|
|
NLCS
|
Netherlands Cohort Study on Diet and
Cancer
|
|
GUPS
|
Genitourinary Pathology Society
|
|
NCR
|
Netherlands Cancer Registry
|
|
PALGA
|
Pathologisch-Anatomisch Landelijk
Geautomatiseerd Archief
|
|
ccRCC
|
clear cell RCC
|
|
pRCC
|
papillary RCC
|
|
chRCC
|
chromophobe RCC
|
|
FH
|
fumarate hydratase
|
|
FFPE
|
formalin-fixed-paraffin-embedded
|
|
RD
|
rhabdoid differentiation
|
|
CAIX
|
carbonic anhydrase IX
|
|
SDH
|
succinate dehydrogenase
|
|
UISS
|
University of California, Los Angeles
integrated staging system
|
References
|
1
|
John N, Eble GS, Jonathan I and Epstein
Isabell A; Sesterhenn World health organization classification of
tumors, . Pathology and genetics of tumors of the urinary system
and male genital organs. IARC Press; Lyon: 2004
|
|
2
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cairns P: Renal cell carcinoma. Cancer
Biomark. 9:461–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inamura K: Renal cell tumors:
Understanding their molecular pathological epidemiology and the
2016 WHO classification. Int J Mol Sci. 18:21952017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nabi S, Kessler ER, Bernard B, Flaig TW
and Lam ET: Renal cell carcinoma: A review of biology and
pathophysiology. F1000Res. 7:3072018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prasad SR, Humphrey PA, Catena JR, Narra
VR, Srigley JR, Cortez AD, Dalrymple NC and Chintapalli KN: Common
and uncommon histologic subtypes of renal cell carcinoma: Imaging
spectrum with pathologic correlation. Radiographics. 26:1795–1810.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yap NY, Rajandram R, Ng KL, Pailoor J,
Fadzli A and Gobe GC: Genetic and chromosomal aberrations and their
clinical significance in renal neoplasms. Biomed Res Int.
2015:4765082015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cancer Genome Atlas Research Network, .
Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis
C, Wheeler DA, Murray BA, Schmidt L, et al: Comprehensive molecular
characterization of papillary renal-cell carcinoma. N Engl J Med.
374:135–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rhoades Smith KE and Bilen MA: A review of
papillary renal cell carcinoma and MET inhibitors. Kidney Cancer.
3:151–161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vera-Badillo FE, Conde E and Duran I:
Chromophobe renal cell carcinoma: A review of an uncommon entity.
Int J Urol. 19:894–900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warren AY and Harrison D: WHO/ISUP
classification, grading and pathological staging of renal cell
carcinoma: Standards and controversies. World J Urol. 36:1913–1926.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frew IJ and Moch H: A clearer view of the
molecular complexity of clear cell renal cell carcinoma. Annu Rev
Pathol. 10:263–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
WHO Classification of Tumours Editorial
Board, Urinary and male genital tumours and WHO Classification of
Tumours, . 5th edition. 8. World Health Organization; 2022
|
|
16
|
Delahunt B, Srigley JR, Montironi R and
Egevad L: Advances in renal neoplasia: Recommendations from the
2012 international society of urological pathology consensus
conference. Urology. 83:969–974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Srigley JR, Delahunt B, Eble JN, Egevad L,
Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, et
al: The international society of urological pathology (ISUP)
vancouver classification of renal neoplasia. Am J Surg Pathol.
37:1469–1489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moch H: The WHO/ISUP grading system for
renal carcinoma. Pathologe. 37:355–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trpkov K and Hes O: New and emerging renal
entities: A perspective post-WHO 2016 classification.
Histopathology. 74:31–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delahunt B, Cheville JC, Martignoni G,
Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch
H, Grignon DJ, et al: The international society of urological
pathology (ISUP) grading system for renal cell carcinoma and other
prognostic parameters. Am J Surg Pathol. 37:1490–1504. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YB, Xu J, Skanderup AJ, Dong Y,
Brannon AR, Wang L, Won HH, Wang PI, Nanjangud GJ, Jungbluth AA, et
al: Molecular analysis of aggressive renal cell carcinoma with
unclassified histology reveals distinct subsets. Nat Commun.
7:131312016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trpkov K, Williamson SR, Gill AJ, Adeniran
AJ, Agaimy A, Alaghehbandan R, Amin MB, Argani P, Chen YB, Cheng L,
et al: Novel, emerging and provisional renal entities: The
genitourinary pathology society (GUPS) update on renal neoplasia.
Mod Pathol. 34:1167–1184. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Reuter VE, Matoso A, Netto GJ,
Epstein JI and Argani P: Re-evaluation of 33 ‘unclassified’
eosinophilic renal cell carcinomas in young patients.
Histopathology. 72:588–600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eble JN and Delahunt B: Emerging entities
in renal cell neoplasia: Thyroid-like follicular renal cell
carcinoma and multifocal oncocytoma-like tumours associated with
oncocytosis. Pathology. 50:24–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williamson SR, Kum JB, Goheen MP, Cheng L,
Grignon DJ and Idrees MT: Clear cell renal cell carcinoma with a
syncytial-type multinucleated giant tumor cell component:
Implications for differential diagnosis. Hum Pathol. 45:735–744.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robila V, Kraft AO and Smith SC: New
entities, new technologies, new findings: A review of the cytologic
features of recently established subtypes of renal cell carcinoma.
Cancer Cytopathol. 127:79–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stratton KL, Alanee S, Glogowski EA,
Schrader KA, Rau-Murthy R, Klein R, Russo P, Coleman J and Offit K:
Outcome of genetic evaluation of patients with kidney cancer
referred for suspected hereditary cancer syndromes. Urol Oncol.
34:238.e1–e7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yunker A, Holder L and Nething J: Newly
described eosinophilic, solid and cystic renal cell carcinoma: A
case report and review of the literature. Arch Nephrol Urol.
3:38–45. 2020. View Article : Google Scholar
|
|
29
|
Samaratunga H, Gianduzzo T and Delahunt B:
The ISUP system of staging, grading and classification of renal
cell neoplasia. J Kidney Cancer VHL. 1:26–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Delahunt B: Advances and controversies in
grading and staging of renal cell carcinoma. Mod Pathol. 22 (Suppl
2):S24–S36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Delahunt B, Eble JN, Egevad L and
Samaratunga H: Grading of renal cell carcinoma. Histopathology.
74:4–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Swami U, Nussenzveig RH, Haaland B and
Agarwal N: Revisiting AJCC TNM staging for renal cell carcinoma:
Quest for improvement. Ann Transl Med. 7 (Suppl 1):S182019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paner GP, Stadler WM, Hansel DE, Montironi
R, Lin DW and Amin MB: Updates in the eighth edition of the
tumor-node-metastasis staging classification for urologic cancers.
Eur Urol. 73:560–569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qayyum T, McArdle P, Orange C, Seywright
M, Horgan P, Oades G, Aitchison M and Edwards J: Reclassification
of the Fuhrman grading system in renal cell carcinoma-does it make
a difference? Springerplus. 2:3782013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khor LY, Dhakal HP, Jia X, Reynolds JP,
McKenney JK, Rini BI, Magi-Galluzzi C and Przybycin CG: Tumor
necrosis adds prognostically significant information to grade in
clear cell renal cell carcinoma: A study of 842 consecutive cases
from a single institution. Am J Surg Pathol. 40:1224–1231. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Avulova S, Cheville JC, Lohse CM, Gupta S,
Potretzke TA, Tsivian M, Thompson RH, Boorjian SA, Leibovich BC and
Potretzke AM: Grading chromophobe renal cell carcinoma: Evidence
for a four-tiered classification incorporating coagulative tumor
necrosis. Eur Urol. 79:225–231. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang L, Zha Z, Qu W, Zhao H, Yuan J, Feng
Y and Wu B: Tumor necrosis as a prognostic variable for the
clinical outcome in patients with renal cell carcinoma: A
systematic review and meta-analysis. BMC Cancer. 18:8702018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Belsante M, Darwish O, Youssef R, Bagrodia
A, Kapur P, Sagalowsky AI, Lotan Y and Margulis V: Lymphovascular
invasion in clear cell renal cell carcinoma-association with
disease-free and cancer-specific survival. Urol Oncol.
32:30.e23–e8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Delahunt B, Srigley JR, Judge M, Amin M,
Billis A, Camparo P, Fleming S, Griffiths D, Lopez-Beltran A,
Martignoni G, et al: Dataset for the reporting of renal biopsy for
tumour: Recommendations from the international collaboration on
cancer reporting (ICCR). J Clin Pathol. 72:573–578. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Joosten SC, Odeh SNO, Koch A, Buekers N,
Aarts MJB, Baldewijns MMLL, Van Neste L, van Kuijk S, Schouten LJ,
van den Brandt PA, et al: Development of a prognostic risk model
for clear cell renal cell carcinoma by systematic evaluation of DNA
methylation markers. Clin Epigenetics. 13:1032021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Vlodrop IJH, Joosten SC, De Meyer T,
Smits KM, Van Neste L, Melotte V, Baldewijns MMLL, Schouten LJ, van
den Brandt PA, Jeschke J, et al: A four-gene promoter methylation
marker panel consisting of GREM1, NEURL, LAD1, and NEFH predicts
survival of clear cell renal cell cancer patients. Clin Cancer Res.
23:2006–2018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Deckers IA, van Engeland M, van den Brandt
PA, Van Neste L, Soetekouw PM, Aarts MJ, Baldewijns MM, Keszei AP
and Schouten LJ: Promoter CpG island methylation in ion transport
mechanisms and associated dietary intakes jointly influence the
risk of clear-cell renal cell cancer. Int J Epidemiol. 46:622–631.
2017.PubMed/NCBI
|
|
45
|
van Houwelingen KP, van Dijk BA,
Hulsbergen-van de Kaa CA, Schouten LJ, Gorissen HJ, Schalken JA,
van den Brandt PA and Oosterwijk E: Prevalence of von Hippel-Lindau
gene mutations in sporadic renal cell carcinoma: Results from The
Netherlands cohort study. BMC Cancer. 5:572005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van den Brandt PA, Goldbohm RA, van't Veer
P, Volovics A, Hermus RJ and Sturmans F: A large-scale prospective
cohort study on diet and cancer in The Netherlands. J Clin
Epidemiol. 43:285–295. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Casparie M, Tiebosch AT, Burger G,
Blauwgeers H, van de Pol A, van Krieken JH and Meijer GA: Pathology
databanking and biobanking in The Netherlands, a central role for
PALGA, the nationwide histopathology and cytopathology data network
and archive. Cell Oncol. 29:19–24. 2007.PubMed/NCBI
|
|
48
|
Klufah F, Mobaraki G, Hausen AZ and
Samarska IV: Reactivation of BK polyomavirus in urine cytology is
not associated with urothelial cell carcinoma. Viruses.
12:14122020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Katz MD, Serrano MF, Grubb RL III,
Skolarus TA, Gao F, Humphrey PA and Kibel AS: Percent microscopic
tumor necrosis and survival after curative surgery for renal cell
carcinoma. J Urol. 183:909–914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Foria V, Surendra T and Poller DN:
Prognostic relevance of extensive necrosis in renal cell carcinoma.
J Clin Pathol. 58:39–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kwon R, Argani P, Epstein JI, Lombardo KA,
Wang X, Pierorazio PM, Mehra R and Matoso A: Contemporary
characterization and recategorization of adult unclassified renal
cell carcinoma. Am J Surg Pathol. 45:450–462. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Clemmensen T, Matoso A, Graham T, Lai WS,
Rais-Bahrami S and Gordetsky J: Pathologic and clinical
characteristics of early onset renal cell carcinoma. Hum Pathol.
74:25–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ross H, Martignoni G and Argani P: Renal
cell carcinoma with clear cell and papillary features. Arch Pathol
Lab Med. 136:391–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sika-Paotonu D, Bethwaite PB, McCredie MR,
William Jordan T and Delahunt B: Nucleolar grade but not Fuhrman
grade is applicable to papillary renal cell carcinoma. Am J Surg
Pathol. 30:1091–1096. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rabjerg M, Gerke O, Engvad B and Marcussen
N: Comparing World Health Organization/international society of
urological pathology grading and Fuhrman grading with the
prognostic value of nuclear area in patients with renal cell
carcinoma. Uro. 1:2–13. 2021. View Article : Google Scholar
|
|
56
|
Xiao Q, Yi X, Guan X, Yin H, Wang C, Zhang
L, Pang Y, Li M, Gong G, Chen D and Liu L: Validation of the World
Health Organization/international society of urological pathology
grading for Chinese patients with clear cell renal cell carcinoma.
Transl Androl Urol. 9:2665–2674. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shuch B, Bratslavsky G, Linehan WM and
Srinivasan R: Sarcomatoid renal cell carcinoma: A comprehensive
review of the biology and current treatment strategies. Oncologist.
17:46–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Park JY, Lee JL, Baek S, Eo SH, Go H, Ro
JY and Cho YM: Sarcomatoid features, necrosis, and grade are
prognostic factors in metastatic clear cell renal cell carcinoma
with vascular endothelial growth factor-targeted therapy. Hum
Pathol. 45:1437–1444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Delahunt B, Srigley JR, Amin MB, Billis A,
Camparo P, Evans AJ, Fleming S, Griffiths D, Lopez-Beltran A,
Martignoni G, et al: Invasive carcinoma of renal tubular origin,
histopathology reporting guide. 1st edition. International
Collaboration on Cancer Reporting; Sydney; Australia: 2017
|
|
60
|
Bengió RG, Arribillaga LC, Epelde J,
Orellana S, Montedoro A, Bengió V, Cordero E and Guevara M:
Evaluation of microvascular invasion as a prognostic factor in the
progression of non-metastatic renal cancer. Cent European J Urol.
71:386–390. 2018.PubMed/NCBI
|
|
61
|
Lang H, Lindner V, Letourneux H, Martin M,
Saussine C and Jacqmin D: Prognostic value of microscopic venous
invasion in renal cell carcinoma: Long-term follow-up. Eur Urol.
46:331–335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Roos FC, Weirich J, Victor A, Elsässer A,
Brenner W, Biesterfeld S, Hampel C and Thüroff JW: Impact of
several histopathological prognosticators and local tumour
extension on oncological outcome in pT3b/c N0M0 renal cell
carcinoma. BJU Int. 104:461–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sevinç M, Kirkali Z, Yörükoğlu K, Mungan U
and Sade M: Prognostic significance of microvascular invasion in
localized renal cell carcinoma. Eur Urol. 38:728–733. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Van Poppel H, Vandendriessche H, Boel K,
Mertens V, Goethuys H, Haustermans K, Van Damme B and Baert L:
Microscopic vascular invasion is the most relevant prognosticator
after radical nephrectomy for clinically nonmetastatic renal cell
carcinoma. J Urol. 158:45–49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hermanek P, Scheibe O, Spiessl B and
Wagner G: TNM classification of malignant tumors: The new 1987
edition. Rontgenblatter. 40:2001987.(In German). PubMed/NCBI
|