Introduction
Ovarian cancer is the eighth most common cancer
affecting women world-wide (1) and
has a UK incidence of over 7,400 cases per year (2). Approximately 70% of women present at
an advanced stage with evidence of metastatic disease (3,4). More
than 80% of women with stage 3 and 4 disease respond to surgical
debulking and chemotherapy (5),
however recurrences tend to occur within 22 months. The overall 5
year survival rate is poor (27%) (4,6).
It was not until 2003 that Zhang et al
(7) demonstrated the association
between tumour-infiltrating T cells (CD3+) and prognosis in ovarian
cancer. They showed a 5-year survival of 38% in cancers with CD3+
infiltration compared to 4.3% in those where such infiltrates were
not evident. It also significantly affected progression-free
survival and correlated with delayed recurrence and delayed death.
Tumours with high T cell infiltration were more likely to achieve
complete cytoreduction (7).
The relevance of TILs has been highlighted in
several other solid cancers such as breast and liver (8,9).
Additionally, it is not simply the presence or absence of TILs that
is of importance, it is their location within the tissue that is
becoming of interest. A study by Sato et al (10) highlighted the importance of
identifying the precise in situ localisation of the TILs suggesting
that their topographic location within an ovarian tumour could be
of significance to the outcome. This has also been explored by Toss
et al (11) in breast
carcinoma. It is known from prior studies that the presence of TILs
in invasive breast cancer positively augmented the effect of
chemotherapy mainly in triple-negative cancers (12), prompting the publishing of
guidelines recommending TILs evaluation.
Many studies have demonstrated that a lack of TIL
infiltration is associated with a poorer outcome in many solid
cancers, however the population used in these amongst ovarian
cancer studies tends to be older women with a median age in the
fifth or sixth decade of life. A meta-analysis performed on TILs in
ovarian cancer identified ten suitable studies and the mean age of
patients was between 55 and 62 (13).
The significance of TILs in young women with ovarian
cancer is yet to be explored. Therefore, this study was conducted
to analyse, in detail, the expression and topographical
distribution of CD20 and CD3 tumour-infiltrating lymphocytes and
their impact on prognosis or response to therapy in ovarian cancer
women under the age of 50. Comparisons were drawn between
histologically borderline tumours and frankly invasive
carcinomas.
Materials and methods
Study cohort
This was a retrospective study of women under the
age of 50 who underwent surgery for a borderline or invasive
ovarian tumour between 1st January 2010 and 31st December 2015 at
the Royal Wolverhampton NHS Trust, Wolverhampton, United Kingdom.
Ethical approval was sought and granted by the West Midlands-Black
Country NRES Committee (07/Q2702/24). Women aged less than 50 were
specifically chosen because this age group is under-represented in
the current literature and may demonstrate a different
pathophysiological course in comparison to women of an older
age.
The patients were identified by searching the
clinical and pathology databases. Clinicopathological data
including patients' age, tumour type, disease stage, management,
recurrence and survival were retrieved from case notes and
electronic records. Data including overall survival and disease
specific survival was collected till 1st December 2019. Information
was gathered on standard treatment regimens but data on
complementary and alternative medicine was not available and has
not been included. Women received standard therapy as per local
guidance so would have received the same treatment in accordance
with their diagnosis. The influence of any additional treatment
factors within this cohort would be marginal and so are likely
representative of the general patient population.
Immunohistochemistry
Slide review and histological selection of
representative full face formalin fixed paraffin wax embedded
tumour blocks of the primary treatment naïve tumours was performed.
Representative 4 microsections were prepared and
immunohistochemically stained for CD3 and CD20 using the Dako Link
autostainer using ready-to-use CD3 and CD20 antibodies following
the manufacturer's instructions. CD3 staining was completed
utilising the Agilent anti-human CD3 polyclonal ready-to-use
antibody (product GA503, CE-IVD). CD20 staining was completed
utilising the Agilent anti-human CD20 ready to use antibody, clone
L26 (product GA604, CE-IVD). Both assays were completed on the
Agilent OMNIS platform and visualised with the EnVision FLEX DAB
detection kit. The CD3 assay is a pan T-cell marker well suited to
labelling reactive T-cells in tissue with lymphoid infiltrates, the
CD20 assay reacts with an intracytoplasmic epitope localised on the
CD20 antigen and labels cells of the B-cell lineage. Both assays
reliably label human B-cells or T-cells in histological tissue
preparations. Antibodies were used as per their published protocol.
Sections were anonymised using unique identification numbers by a
third party and assessors were blinded to the patient and clinical
data. Slides were scored manually by one investigator (DO) overseen
by a pathologist (AMS). Guidelines produced by the International
Immuno-Oncology Biomarker Working Group (8) aim to standardise the assessment of
TILs in solid tumours and this guidance was applied to counting the
TIL populations in our samples. Briefly, the number of positively
stained CD3 or CD20 cells present in ten high-power fields (HPFs)
was counted and average per HPF calculated. The percentage
infiltration of TILs representing the area occupied by the immune
cells in relation to the total stromal area present was also
estimated for each patient following the guidelines of the
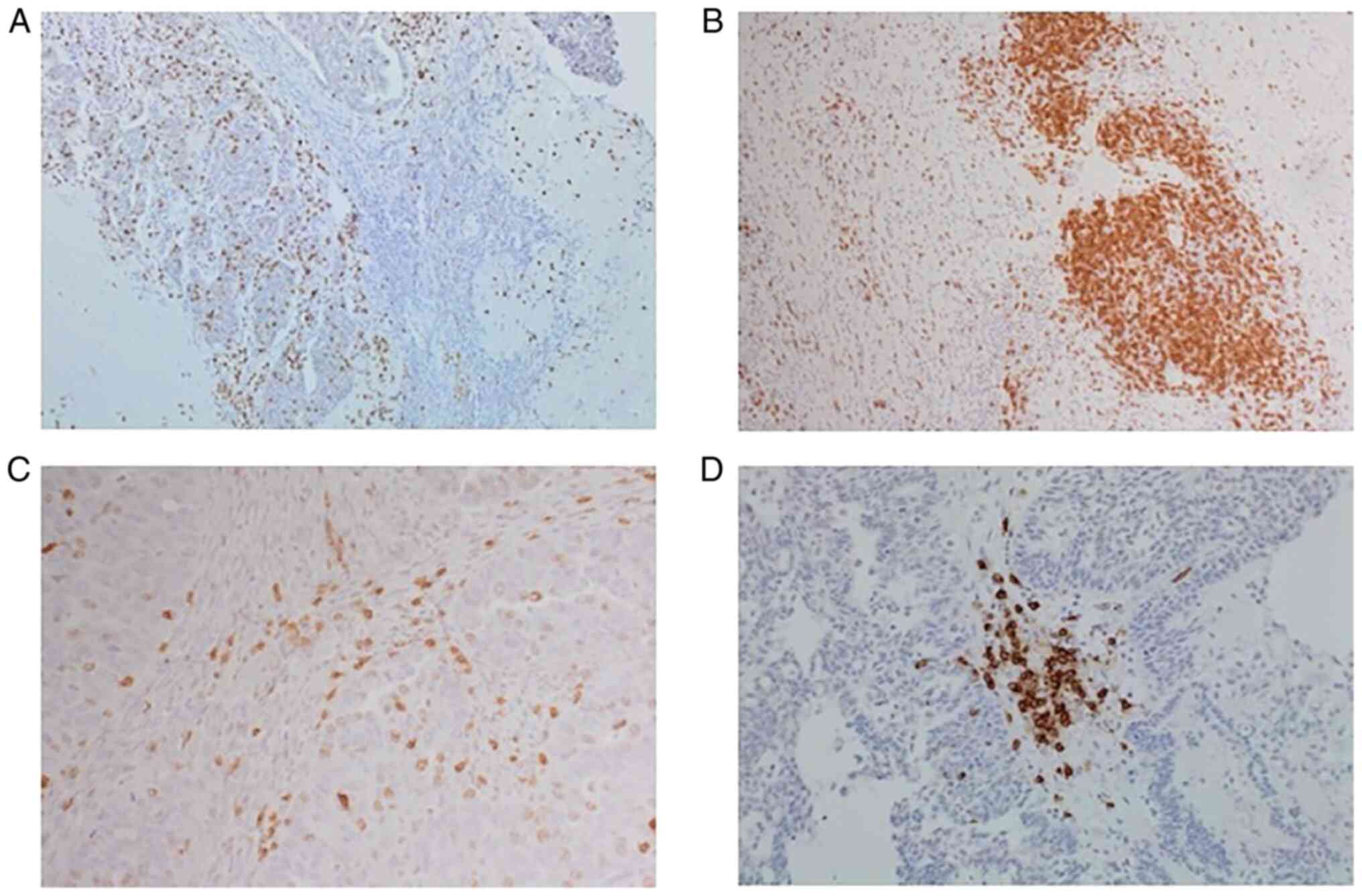
International ImmunoOncology Group (8). Representative slides of CD3 and CD20
IHC staining are shown in Fig.
1.
Scoring of TILs expression
Intratumoural lymphocytes were defined as those in
direct contact with tumour cells or within the tumour itself. The
stromal lymphocytes were defined as those lying within the tumoural
stroma. Hotspots representing regions with highest densities were
recorded if present. Touching lymphocytes were defined as the
number of lymphocytes touching the basement membrane or within one
lymphocyte cell diameter from the basement membrane as previously
described and are shown in Fig. 1C
(11). This method was shown to
have minimal intra-observer variance and good concordance (11). Lymphoid aggregates (well defined,
densely populated areas of lymphocytes within the stroma) where
present, were also scored and are shown in Fig. 1D. Any discrepancies were
subsequently reviewed by both assessors and a consensus was
reached.
Statistical analysis
Statistical analysis was performed using SPSS
statistical software version 26 (IBM Corp.). Normally distributed
data are presented as the mean ± standard deviation. For data
without normal distribution, such as age, median values (with
interquartile range) are presented. Chi-squared analysis
(χ2) was used to correlate patient outcome (dead vs.
alive) with tumour type. The nonparametric independent samples
median test [Mann-Whitney-U Wilcoxon (rank sum) test (MWW)] and
Kruskal-Wallis test (KWt) were performed to compare the means and
medians of the variables, including age, CD3, CD20, touching
lymphocytes count, aggregates and hot spot counts among different
groups. Spearman's rank correlation coefficient (rs) was
used to correlate continuous variables of biomarker expression with
clinical variables (e.g., age, CA125, RMI). All comparisons were
two sided and significance threshold was set to a p-value of less
than 0.05. Kaplan Meier analysis was used to determine overall and
disease specific survival in relation to TILs expression in the
studied cohort. In accordance with the journal's guidelines, we
will provide our data for the reproducibility of this study in
other centres if such is requested.
Results
Patient characteristics
A total of 58 patients fulfilled the initial
inclusion criteria, however one was subsequently excluded due to a
diagnosis of primary colonic malignancy, giving a total of 57 cases
in the cohort. There were 23 women in the borderline cohort and 34
in the invasive cancer cohort. The histological diagnoses and
patient characteristics are described in Table I. The median age was 41
(Interquartile range 28.5-46), with over a third of women belonging
to the over 45 category. There was no significant difference in age
at diagnosis between invasive ovarian cancer and borderline ovarian
tumour (BOT) (MWW, P=0.33). The majority of women (89.5%) were of
white ethnicity, with representation from black Caribbean,
Pakistani and Indian ethnicities included. The most common
diagnosis was serous epithelial ovarian cancer (n=21, 36.8%),
followed by mucinous BOT and serous BOT. Women largely presented in
stage 1 (59.6%) or stage 3 (26.3%) disease.
 | Table I.Patient and clinicopathological
characteristics of the studied cohort. |
Table I.
Patient and clinicopathological
characteristics of the studied cohort.
| Variable | Value |
|---|
| Age at diagnosis, n
(%) |
|
| 15–19
years | 1 (1.8) |
| 20–24
years | 6 (10.5) |
| 25–29
years | 9 (15.7) |
| 30–34
years | 3 (5.3) |
| 35–39
years | 5 (8.8) |
| 40–44
years | 11 (19.3) |
| 45–49
years | 22 (38.6) |
| Median age (IQR),
years | 41 (28.5–46.0) |
| Ethnicity, n
(%) |
|
| White
British | 51 (89.5) |
|
Indian | 2 (3.5) |
|
Pakistani | 1 (1.8) |
| Black
Caribbean | 1 (1.8) |
| Not
stated | 2 (3.5) |
| Histological
diagnosis, n (%) |
|
| Clear
cell cancer | 5 (8.8) |
|
Endometrioid cancer | 5 (8.8) |
|
Mucinous cancer | 3 (5.3) |
| Serous
cancer | 21 (36.8) |
| Serous
BOT | 11 (19.3) |
|
Mucinous BOT | 12 (21.1) |
| Stage, n (%) |
|
| 1 | 34 (59.6) |
| 2 | 3 (5.3) |
| 3 | 15 (26.3) |
| 4 | 5 (8.8) |
| RMI, n (%) |
|
|
0–250 | 22 (38.6) |
|
251–500 | 7 (12.3) |
|
501–1,000 | 3 (5.3) |
|
1,001+ | 14 (24.6) |
| Not
stated | 11 (19.3) |
| Mean RMI ± SD |
3,008.6±3,810.7 |
| Chemotherapy, n
(%) |
|
| No | 25 (43.9) |
|
Yes | 32 (56.1) |
| Recurrence, n
(%) |
|
|
Yes | 15 (26.3) |
| No | 38 (66.6) |
| Not
stated | 4 (7.0) |
| Deceased |
|
|
Yes | 15
(26.3)a |
| No | 42 (73.7) |
The median follow up was 56 months (range 1–114
months). A total of 15 women (44%) succumbed to their disease and
this was exclusively within the cancer cohort. No deaths were seen
in the BOT category. The risk of malignancy index (RMI) was
significantly greater among the women diagnosed with cancer (MWW,
P=0.04) and the CA125 serum level tended to be higher within the
cancer cohort (MWW, P=0.05).
CD3 expression
Regardless of the underlying histology, we noted
that within our cohort there was a greater infiltration of CD3
cells within the stroma compared to CD20 cells. The mean CD3
infiltration was 91.3 (SD 102.4) whilst with CD20 cells the mean
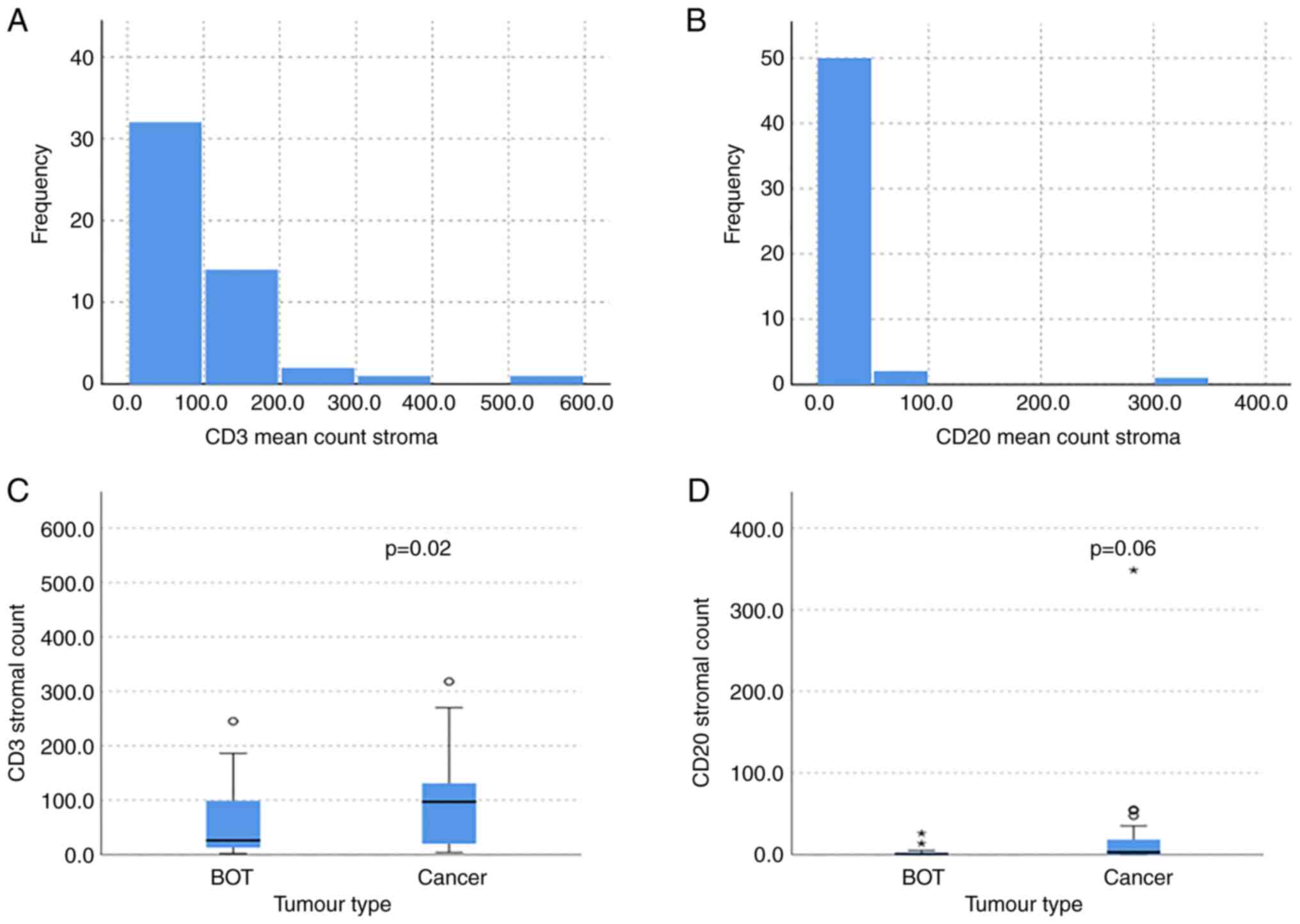
was 14.6 (SD 18.7) as shown in Fig. 2A
and B. There were significantly more CD3 and CD20 TILs residing
in the stroma compared to within the tumour (MWW, P=0.01 and
P=0.008). The infiltration of T cells (CD3 positive) was
significantly greater within the stroma of cancer cases compared to
BOT (MWW, P=0.02) as shown in Fig.
2C. The median CD3 stromal count for both clear cell and
endometrioid cancers was 139, compared to 84.5 in serous cancer per
10 high power fields. Within the BOT samples, the median CD3
stromal count was much lower, with 23 and 46.5 for serous and
mucinous BOT respectively. When considering the percentage stromal
infiltration, the clear cell cancers had the greatest abundance
with a median of 25%, compared to 15% in endometrioid, 7% in serous
cancer and 5% in mucinous cancer. The percentage stromal
infiltration was much greater in cancer compared to BOT, however,
as the mucinous BOT had a median percentage of 1.25%, whilst the
serous BOT had 0.5%. The mean CD3 stromal and intra-tumoural
lymphocyte count did not significantly differ between the different
epithelial cancer histological subtypes (KWt, stromal P=0.36 and
intra-tumoural P=0.15). The intra-tumoural infiltration of CD3
cells was not significantly greater in cancer compared to BOT (MWW,
P=0.25).
CD20 expression
Overall, the expression of CD20 in various
compartments was much less than that of CD3. There was a trend to a
greater presence of B cells (CD20 positive) within the stroma of
cancerous tumours in comparison to BOT (MWW, P=0.06) as shown in
Fig. 2D. The median CD20 stromal
infiltration was greatest in endometrioid cancer with a score of
13.8, whilst in serous cancer it was 0 (absent). Within the
borderline cancers, mucinous BOT had a median CD20 infiltration of
1 and serous BOT had a median of 0. Similar to our findings with
CD3 aggregates, there was no significant difference in the presence
of CD20 aggregates within the samples (MWW, P=0.22). The stromal
CD20 infiltration was not significantly greater in the different
histological subtypes, nor was it significant for intra-tumoural
infiltration. The intra-tumoural infiltration of CD20 positive
lymphocytes attained significance, with more CD20 TILs present in
the cancer samples compared to BOT (MWW, P=0.048).
Touching lymphocyte
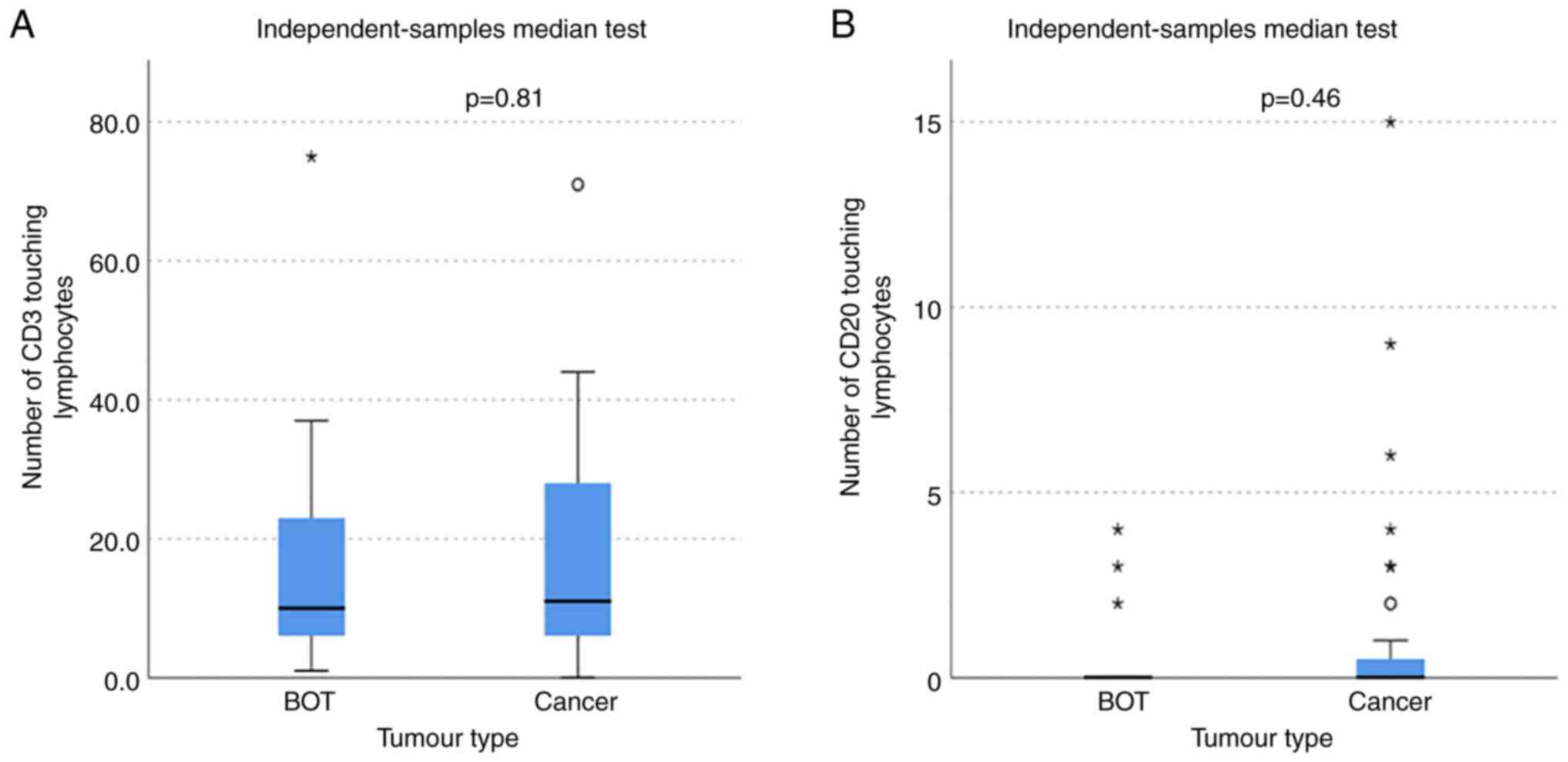
The number of T and B lymphocytes within the stroma
touching the tumour (touching lymphocytes) was not significantly
different between ovarian cancer and BOT (MWW, CD3 P=0.81 and CD20
P=0.46), demonstrated in Fig. 3. In
addition, there was no significant difference between the number of
touching lymphocytes between the different ovarian cancer subtypes
with CD3 cells (KWt, P=0.67) or CD20 cells (KWt, P=0.72).
TILs aggregates and hotspots
The presence of CD3 aggregates were not
significantly different in either the cancer or BOT samples (MWW,
P=0.09), nor were there more CD3 hotspots in one cohort over the
other (MWW, P=0.81).
Correlation with patients'
outcome
We evaluated the relationship between the presence
of TILs and prognostic indicators. Primarily, all of the deaths
occurred within the cancer cohort [n=15/34 (44%) χ2,
P=0.001] with no mortality within the BOT group. The median
survival was 34.8 months in the cancer cohort and mortality did not
depend on the underlying histological cancer subtype, with a
significance value of P=0.10 (KWt). The infiltration of CD3 or CD20
TILs in the stroma or tumour and the number of aggregates or
hotspots were not predictive of mortality. Additionally, the number
of touching lymphocytes of CD3 or CD20 cells was not associated
with mortality (MWW, P=0.64 and P=0.33 respectively). The
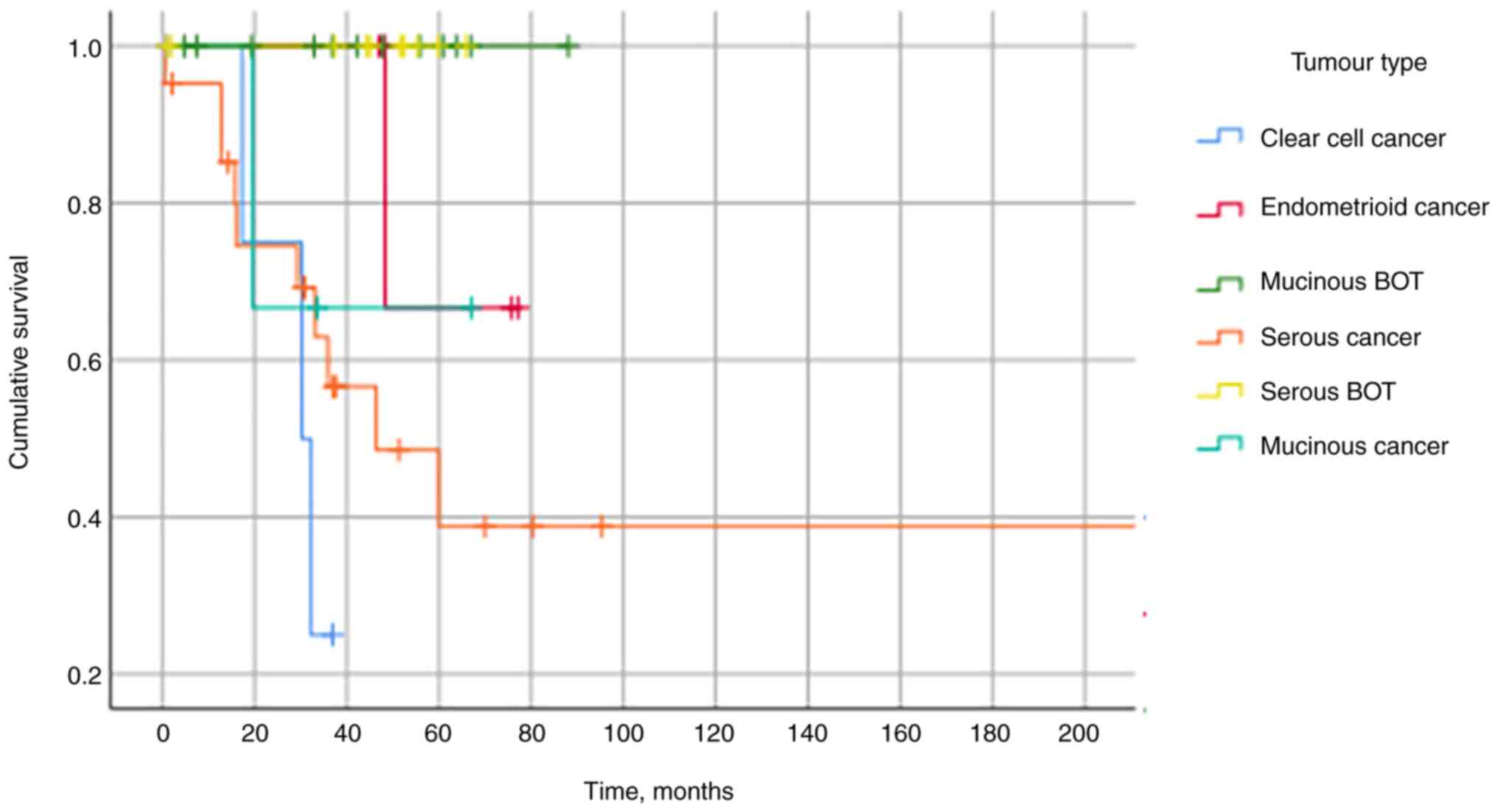
Kaplan-Meier curve in Fig. 4
demonstrates no mortality within the BOT cohort and that clear cell
carcinoma was associated with the poorest prognosis.
CD3 and CD20 stromal infiltration was significantly
correlated with the serum CA125 level (rs, P=0.005 and
P<0.001 respectively). No statistically significant correlation
was found between, intra-tumoural CD3 or CD20 TILs, the presence of
aggregates or hotspots and serum CA125.
Discussion
Our results have primarily demonstrated that there
were significantly more CD3 than CD20 TILs in the stroma of our
specimens. There were significantly more CD3 TILs within the stroma
of ovarian cancers in comparison to BOT and significantly more CD20
within the tumour of cancerous neoplasms in comparison to BOT.
Collectively, however, these results failed to impact on prognosis
or mortality or response to therapy.
In the context of published literature, this is the
first study to analyse the role of TILs in ovarian neoplasms of
young women. Our results show a predominant infiltration by CD3
TILs and their greater infiltration within the stroma of ovarian
cancer cases compared to those with BOT. This finding is consistent
with other literature in ovarian cancer of older women, but also in
other solid cancers. Stromal TILs have been found to be prognostic
of outcome in triple negative breast cancer and as such, their
routine reporting has been proposed (14,15).
Within ovarian cancer, this has been previously demonstrated
comparing benign and malignant ovarian tumours whereby those which
were malignant had a greater stromal infiltration (16). A recent systematic review on TILs in
ovarian cancer highlighted a lack of reliable studies investigating
stromal CD3 TILs therefore a conclusion was not able to be formed,
however intratumoural CD3 TILs were associated with increased
progression-free survival and overall survival (17). Our results did not identify a
significant relationship between TIL infiltration and prognosis, a
finding that has been corroborated in other published work
(18). In addition, a comparison
between malignant and BOT, to our knowledge, has not been performed
previously.
CD20 positive B cells have been shown to be
associated with an improved clinical outcome in high grade serous
(HGS) cancer (19–21) as well as other cancers including
lung, melanoma and breast (22–26).
We demonstrated that CD20 infiltration was considerably lower than
that of CD3 infiltration in both the stroma and intra-tumoural
compartments.
In our cohort, CD20 prevalence was reduced in HGS
ovarian cancer in comparison to endometrioid and mucinous cancers.
This is in contrast to the findings by Milne et al (27) who demonstrated a greater
infiltration of CD20 positive cells in HGS cancer. They also found
that disease-specific survival was greater in those with greater
CD20 infiltration (27). CD20 TILs
are thought to have antigen-presenting cell functionality as well
as a direct cytolytic response (19) so contribute greatly to the immune
microenvironment. The cohort used within that study, however, was
considerably older than our cohort. Whether age affects the tumour
biology and subsequent immune infiltration is unknown but work is
currently underway to investigate this. There are no published
studies identifying the differences in stromal infiltration of B
cells between the different ovarian cancer subtypes.
Aside from primary tumours, CD20 positive B cells
have also been found to be present in omental metastases of HGS
ovarian cancer, localising mainly in lymphoid aggregates within the
stroma. Their presence was unaffected by the exposure to
chemotherapy, however those who had been exposed to neo-adjuvant
chemotherapy had a greater proportion of class-switched memory B
cells, potentially contributing to an improved response to therapy
(28). The presence of stromal CD20
cells has also been associated with the absence of lymph node
metastases and therefore may play a role in preventing metastatic
spread.
It is known that there is significant heterogeneity
of immune cells within the tumour microenvironment between patients
and also between different sites in the same patient (29–31).
Additionally, the degree of infiltration of CD3+ cells prior to
chemotherapy has, however, been shown to vary wildly within HGS
cancer patients (21). As such, the
interrelationship of both T and B cells should be considered. A
meta-analysis demonstrated that the presence of intraepithelial
CD3+ and CD8+ lymphocytes improved survival despite significant
heterogeneity between the published studies (13).
Despite multiple publications demonstrating
correlation of TILs infiltration and prognosis in HGS ovarian
cancer and other solid tumours, our results did not corroborate
with this in this young age group. This may be due to the
population we sampled as we chose a specific population of women
presenting with ovarian tumours under the age of 50. As such these
women may present atypically and demonstrate a different
biology/immune infiltrate when compared to the more common
presentation of women in their 5th or 6th decade of life. There has
been one study identified which split their cohort into 2; those
aged less than 52 and those greater than 52. This did not show any
difference in CD3 infiltration between the cohorts, suggesting that
age may not be an important factor. They also found that tumour
size or its location, presence of ascites, and lymph node
metastasis had no significant association with the densities of CD3
cells (32).
In addition to this, our population was rather
unique as the vast majority of women presented in stage 1 of their
disease. Usually, women tend to present at stage 3 or 4 due to the
vague presenting symptoms and late diagnosis. Whether this also has
contributed to our findings is unclear; perhaps women presenting in
later stage disease have greater stromal and intra-tumoural
infiltration compared to those women in stage 1, potentially
dictated by their histological sub-type and host genetic and immune
factors. Our population was also largely consisting of white women
with very few women representing ethnic minority groups. This needs
to be considered and further work is required to detect any
interracial differences within the population.
We showed that touching lymphocytes were not
predictive of histology or prognosis. We assessed this parameter
because dense touching lymphocytes in other cancers such as breast
cancers have been associated with more aggressive tumours, a
shorter recurrence-free interval and ductal carcinoma in situ
recurrence. When more traditional methods of scoring were used,
such as counting stromal or intra-epithelial TILs, there was
considerable variability in association with the outcome. This
method of counting was also shown to be superior with regards to
reproducibility between assessors (11). Whether touching lymphocytes become
more reliable and predictive in the older population with ovarian
cancer is unknown.
Our findings suggested that CD3 and CD20
infiltration was greatest in endometrioid cancers but there was no
statistically significant difference demonstrated between the
different ovarian cancer subtypes or the prognosis. The density of
infiltration can vary considerably between patients and even within
the same patient, as mentioned above, so it may be necessary to
expand our cohort to see if any of results attain significance. It
has been found that TIL infiltration was greatest in HGS and
endometrioid ovarian cancer in both intra-tumoural and stromal
samples, however it was unclear as to the cell types included
within the ‘TIL’ definition. Interestingly, our results echo their
findings that there was no statistically significant difference in
prognosis between those with a greater TIL infiltration and those
with few TILs (33). No existing
publications were identified that investigated T and B cell
infiltration specifically in the various sub-types of ovarian
cancer; most focus solely on the most common, HGS cancer. As such
this research would be a welcome contribution to existing
knowledge.
In accordance with the findings of Toss et al
(11) in breast carcinoma, we found
no significant association between the presence of TILs hotspots or
aggregates with prognosis.
We found that the risk of malignancy index (RMI) and
CA125 were greater in women with cancer compared to women with a
diagnosis of BOT. It has been shown that CA125 has a linear
relationship with CD3 infiltration (32), which corresponds to our findings.
RMI is used to differentiate cancer from benign lesions (34), however it has also been shown to
differentiate benign from borderline tumours (35). There is limited published data
concerning the use of RMI to differentiate BOT and ovarian cancer
as usually these diagnoses are grouped together and compared to
benign tumours.
While this is a retrospective cohort, there are
several strengths to this study. The patient cohort is unique
(including young women with ovarian cancer and separating
borderline and ovarian malignancies; two cohorts that are often
grouped together) with a long follow up. There was rigorous
histological assessment with slide review, selection of
representative tumour blocks, optimal staining following diagnostic
standards in a UKAS accredited laboratory and detailed TILs
assessment following international guidance and overseen by
experienced pathologists. Potential weaknesses with this study lie
in its small size of cohort and consequently poor ethnic
diversity.
This research has shown an increase in TILs in
cancer specimens but that this does not impact on mortality or
prognosis, therefore TILs assessment in routine histological
assessment is not duly warranted. In addition, the assessment of
touching lymphocytes was not predictive of prognosis and as such
does not require particular assessment, unlike that advised in the
histological assessment of breast carcinoma.
To further this work, it would be prudent to
investigate the relationship of both CD3 and CD20 positive cells to
other important immunoregulatory cells, such as CD8 T cells and
macrophages. It has been extensively demonstrated that CD8 cells
are found in tertiary lymphoid structures with CD20 B cells,
alongside dendritic cells (20).
Tumour-associated macrophages (TAMs) have been found to exhibit an
immunomodulatory effect on the tumour microenvironment with M2
subset enabling tumour growth in both the primary and metastatic
sites. It has been shown that TAMs have been identified in greater
numbers at sites of metastasis in comparison to primary sites,
demonstrating their potential role in creating an environment
conducive to metastatic deposit and development (31,36).
Additionally, further work is required in an older population to
determine if our findings remain true in women presenting over the
age of 50.
Acknowledgements
Not applicable.
Funding
AMS is funded by Birmingham Cancer Research UK Centre (grant no.
C17422/A25154).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AMS and AEG conceived the original idea for the
research, designed the study, performed the statistical analysis
and contributed to writing. KR and AB reviewed tumour slides and
selected representative blocks. DK performed the
immunohistochemical staining. DO analysed the immunohistochemical
staining under the supervision of AS, collected the outcome data
and wrote the first draft. FB sought ethical approval, contributed
to the study design and edited the manuscript. DO, AEG and AMS
reviewed and confirmed the authenticity of the raw data. All
authors contributed to editing the manuscript. The authors agreed
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was sought and granted by the West
Midlands-Black Country (Wolverhampton, Birmingham, UK) NRES
Committee (07/Q2702/24). Tissue was obtained via the University of
Birmingham Human Biomaterials Resource Centre (HBRC) and therefore
individual patient consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BOT
|
borderline ovarian tumour
|
|
RMI
|
Risk of Malignancy Index
|
|
TILs
|
tumour-infiltrating lymphocytes
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Research UK, . Available from.
https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/vulval-cancer#heading-ZeroSeptember
14–2018
|
|
3
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giornelli GH: Management of relapsed
ovarian cancer: A review. Springerplus. 5:11972016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmed N and Stenvers KL: Getting to know
ovarian cancer ascites: Opportunities for targeted therapy-based
translational research. Front Oncol. 3:2562013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kipps E, Tan DSP and Kaye SB: Meeting the
challenge of ascites in ovarian cancer: New avenues for therapy and
research. Nat Rev Cancer. 13:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hendry S, Salgado R, Gevaert T, Russell
PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV,
Gonzalez-Ericsson PI, et al: Assessing tumor-infiltrating
lymphocytes in solid tumors: A practical review for pathologists
and proposal for a standardized method from the international
immunooncology biomarkers working group: Part 1: Assessing the host
immune response, TILs in invasive breast carcinoma and ductal
carcinoma in situ, metastatic tumor deposits and areas for further
research. Adv Anat Pathol. 24:235–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garnelo M, Tan A, Her Z, Yeong J, Lim CJ,
Chen J, Lim KH, Weber A, Chow P, Chung A, et al: Interaction
between tumour-infiltrating B cells and T cells controls the
progression of hepatocellular carcinoma. Gut. 66:342–351. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sato E, Olson SH, Ahn J, Bundy B,
Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone
C, et al: Intraepithelial CD8+ tumor-infiltrating lymphocytes and a
high CD8+/regulatory T cell ratio are associated with favorable
prognosis in ovarian cancer. Proc Natl Acad Sci USA.
102:18538–18543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toss MS, Miligy I, Al-Kawaz A, Alsleem M,
Khout H, Rida PC, Aneja R, Green AR, Ellis IO and Rakha EA:
Prognostic significance of tumor-infiltrating lymphocytes in ductal
carcinoma in situ of the breast. Mod Pathol. 31:1226–1236. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loi S, Sirtaine N, Piette F, Salgado R,
Viale G, Van Eenoo F, Rouas G, Francis P, Crown JPA, Hitre E, et
al: Prognostic and predictive value of tumor-infiltrating
lymphocytes in a phase III randomized adjuvant breast cancer trial
in node-positive breast cancer comparing the addition of docetaxel
to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J
Clin Oncol. 31:860–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang WT, Adams SF, Tahirovic E, Hagemann
IS and Coukos G: Prognostic significance of tumor-infiltrating T
cells in ovarian cancer: A meta-analysis. Gynecol Oncol.
124:192–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burstein HJ, Curigliano G, Loibl S, Dubsky
P, Gnant M, Poortmans P, Colleoni M, Denkert C, Piccart-Gebhart M,
Regan M, et al: Estimating the benefits of therapy for early stage
breast cancer. The St gallen international consensus guidelines for
the primary therapy of early breast cancer 2019. Ann Oncol.
30:1541–1557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kos Z, Roblin E, Kim RS, Michiels S,
Gallas BD, Chen W, van de Vijver KK, Goel S, Adams S, Demaria S, et
al: Pitfalls in assessing stromal tumor infiltrating lymphocytes
(sTILs) in breast cancer. NPJ Breast Cancer. 6:172020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Lima CA, Jammal MP, Etchebehere RM,
Murta EFC and Nomelini RS: Lymphocytes in peritumoral stroma:
Evaluation in epithelial ovarian neoplasms. Immunol Invest.
49:397–405. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao J, Yu H, Zhang T, An R and Xue Y:
Prognostic impact of tumor-infiltrating lymphocytes in high grade
serous ovarian cancer: A systematic review and meta-analysis. Ther
Adv Med Oncol. 31:17588359209672412020.PubMed/NCBI
|
|
18
|
Darb-Esfahani S, Kolaschinski I, Trillsch
F, Mahner S, Concin N, Vergote I, Nieuwenhuysen EL, Achimas-Cadariu
P, Glajzer J, Woopen H, et al: Morphology and tumour-infiltrating
lymphocytes in high-stage, high-grade serous ovarian carcinoma
correlated with long-term survival. Histopathology. 73:1002–1012.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nielsen JS, Sahota RA, Milne K, Kost SE,
Nesslinger NJ, Watson PH and Nelson BH: CD20+ tumor-infiltrating
lymphocytes have an atypical CD27-memory phenotype and together
with CD8+ T cells promote favorable prognosis in ovarian cancer.
Clin Cancer Res. 18:3281–3292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Truxova I, Kasikova L, Hensler M, Skapa P,
Laco J, Pecen L, Belicova L, Praznovec I, Halaska MJ, Brtnicky T,
et al: Mature dendritic cells correlate with favorable immune
infiltrate and improved prognosis in ovarian carcinoma patients. J
Immunother Cancer. 4:1392018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lo CS, Sanii S, Kroeger DR, Milne K,
Talhouk A, Chiu DS, Rahimi K, Shaw PA, Clarke BA and Nelson BH:
Neoadjuvant chemotherapy of ovarian cancer results in three
patterns of tumor-infiltrating lymphocyte response with distinct
implications for immunotherapy. Clin Cancer Res. 23:925–934. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Germain C, Gnjatic S, Tamzalit F,
Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S,
Bizouard G, et al: Presence of B cells in tertiary lymphoid
structures is associated with a protective immunity in patients
with lung cancer. Am J Respir Crit Care Med. 189:832–844. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ladányi A, Kiss J, Mohos A, Somlai B,
Liszkay G, Gilde K, Fejös Z, Gaudi I, Dobos J and Tímár J:
Prognostic impact of B-cell density in cutaneous melanoma. Cancer
Immunol Immunother. 60:1729–1738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iglesia MD, Vincent BG, Parker JS, Hoadley
KA, Carey LA, Perou CM and Serody JS: Prognostic B-cell signatures
using mRNA-seq in patients with subtype-specific breast and ovarian
cancer. Clin Cancer Res. 20:3818–3829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nedergaard BS, Ladekarl M, Nyengaard JR
and Nielsen K: A comparative study of the cellular immune response
in patients with stage IB cervical squamous cell carcinoma. Low
numbers of several immune cell subtypes are strongly associated
with relapse of disease within 5 years. Gynecol Oncol. 108:106–111.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arias-Pulido H, Cimino-Mathews A, Chaher
N, Qualls C, Joste N, Colpaert C, Marotti JD, Foisey M, Prossnitz
ER, Emens LA and Fiering S: The combined presence of CD20 + B cells
and PD-L1 + tumor-infiltrating lymphocytes in inflammatory breast
cancer is prognostic of improved patient outcome. Breast Cancer Res
Treat. 171:273–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Milne K, Köbel M, Kalloger SE, Barnes RO,
Gao D, Gilks CB, Watson PH and Nelson BH: Systematic analysis of
immune infiltrates in high-grade serous ovarian cancer reveals
CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One.
4:e64122009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montfort A, Pearce O, Maniati E, Vincent
BG, Bixby L, Böhm S, Dowe T, Wilkes EH, Chakravarty P, Thompson R,
et al: A strong B-cell response is part of the immune landscape in
human high-grade serous ovarian metastases. Clin Cancer Res.
23:250–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maley CC, Aktipis A, Graham TA, Sottoriva
A, Boddy AM, Janiszewska M, Silva AS, Gerlinger M, Yuan Y, Pienta
KJ, et al: Classifying the evolutionary and ecological features of
neoplasms. Nat Rev Cancer. 17:605–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hensler M, Kasikova L, Fiser K, Rakova J,
Skapa P, Laco J, Lanickova T, Pecen L, Truxova I, Vosahlikova S, et
al: M2-like macrophages dictate clinically relevant
immunosuppression in metastatic ovarian cancer. J Immunother
Cancer. 8:e0009792020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai D, Liu L, Huang H, Chen S, Chen B, Cao
J, Luo X, Wang F, Luo R and Liu J: Nomograms to predict the density
of tumor-infiltrating lymphocytes in patients with high-grade
serous ovarian cancer. Front Oncol. 25:5904142021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
James FR, Jiminez-Linan M, Alsop J, Mack
M, Song H, Brenton JD, Pharoah PDP and Ali HR: Association between
tumour infiltrating lymphocytes, histotype and clinical outcome in
epithelial ovarian cancer. BMC Cancer. 20:6572017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geomini P, Kruitwagen R, Bremer GL,
Cnossen J and Mol BWJ: The accuracy of risk scores in predicting
ovarian malignancy: A systematic review. Obstet Gynecol. 113((2 Pt
1)): 384–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Yu S, Hou W, Li X, Ning C, Wu Y,
Zhang F, Jiao YF, Lee LTO and Sun L: Diagnostic extended usefulness
of RMI: Comparison of four risk of malignancy index in preoperative
differentiation of borderline ovarian tumors and benign ovarian
tumors. J Ovarian Res. 12:872019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robinson-Smith TM, Isaacsohn I, Mercer CA,
Zhou M, Van Rooijen N, Husseinzadeh N, McFarland-Mancini MM and
Drew AF: Macrophages mediate inflammation-enhanced metastasis of
ovarian tumors in mice. Cancer Res. 67:5708–5716. 2007. View Article : Google Scholar : PubMed/NCBI
|