Introduction
Cervical cancer is a malignancy in women that can
result from persistent infection with the high-risk human
papillomavirus and has a high mortality rate, especially in low and
middle-income countries (1). In
2020, the estimated cervical cancer mortality rate across all 78
LMICs was 13.2 (range, 12.9-14.1) per 100 000 women (2). First occurrence of sexual intercourse
at younger ages, increase in the number of sexual partners,
smoking, long-term use of oral contraceptives and viral infections
can all increase the risk of cervical cancer (3). Screening, vaccination, preventive
education and physical examination programs have all decreased the
prevalence of cervical cancer. However, women with this disease do
not have access to curative treatment, since there is no adequate
and effective method to treat cervical cancer due to relapse,
metastasis and drug resistance (1,4).
Therefore, the mechanism underlying cervical carcinogenesis must be
elucidated to aid in the development of effective treatment
modalities.
Hepatitis B X-interacting protein (HBXIP) has been
previously reported to serve as a carcinogenic factor in numerous
cancer types, including hepatocellular carcinoma, breast cancer,
gastric cancer and colorectal cancer (5–9). Its
carcinogenic effects have been demonstrated to involve a variety of
complex mechanisms, including the promotion of mitosis to sustain
tumor cell proliferation, activation of transcription factors to
regulate the cancer cell phenotype and the induction of signaling
pathways to promote cancer development (10). Over the past two decades, high
expression levels of HBXIP have been reported in hepatocellular
carcinoma, breast cancer, gastric cancer and colorectal cancer,
which are in turn associated with poorer prognosis (6–9). In
particular, it has been also been previously reported that the
HBXIP expression levels are elevated in cervical cancer tissues,
which is associated with worse prognosis (11). However, the exact mechanism of
action mediated by HBXIP remains unelucidated (11). Therefore, evaluation of the specific
mechanism of action of HBXIP in cervical cancer may enrich the
target pool for cervical cancer therapy and provide novel
approaches for the cancer therapeutic interventions.
The novel LIM domain protein four and a half LIM
domain 2 (FHL2) is encoded by the human FHL2 gene (12). The reported role of FHL2 in cancer
biology remains controversial. FHL2 expression has been reported to
be downregulated in prostate cancer, rhabdomyosarcoma and
hepatocellular carcinoma (13–15),
but it has also been reported to be upregulated in epithelial
ovarian cancer, human hearts and human melanoma (16–18).
Previous studies have reported that FHL2 expression was higher in
squamous cervical tissues compared with that in non-cancerous
cervical tissues, where its high expression was associated with
poorer prognosis (19,20). In addition to its high affinity with
cancer-associated proteins, FHL2 can also regulate signaling
pathways associated with the malignant transformation of cells
(21). Furthermore, FHL2 knockdown
has been reported to suppress the activation of Wnt signaling,
which has been frequently reported to be involved in the malignant
transformation of cervical cancer (22). Based on the observations made by
these aforementioned previous studies, the present study evaluated
whether HBXIP can bind to the FHL2 protein and exert effects on the
Wnt signaling pathway in cervical cancer.
To support this evaluation, the present study
assessed the mRNA and protein expression levels of HBXIP and FHL2
in cervical cancer cells, evaluated how HBXIP influenced the
malignant development of cervical cancer cells and assessed whether
FHL2 overexpression affected these results. This investigation
might offer potential novel targets for cervical cancer
treatment.
Materials and methods
Bioinformatics tools
The BioGRID database (https://thebiogrid.org/) was used by searching the
interactors of HBXIP to predict the relationship between FHL2 and
HBXIP.
Cell culture
The human endocervical epithelial End1/E6E7 cell
line, the human cervical cancer HeLa, C33A and SiHa cell lines, and
the cervical squamous cell carcinoma Ca-Ski cell line were
purchased from BioVector NTCC, Inc. End1/E6E7 cells were cultured
in keratinocyte serum-free medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 50 µg/ml bovine pituitary extract (Absin
Bioscience, Inc.), 0.1 ng/ml recombinant epidermal growth factor
(Absin Bioscience, Inc.), 0.4 mmol/l CaCl2 and 1%
antibiotics (penicillin, 100 U/ml; streptomycin, 100 mg/ml). HeLa
and CaSki cervical cancer cell lines were cultured in RPMI-1640
(Procell Life Science & Technology Co., Ltd.) containing 10%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
1% penicillin-streptomycin. C33A and SiHa cells were cultured in
DMEM (MilliporeSigma) containing 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin. All cells were cultured under the
conditions of 37°C with 5% CO2.
Cell transfection
Small interfering RNAs (siRNA) targeting HBXIP
(siRNA-HBXIP-1, 5′-GTGATGTTTTCCAGTAAAGAACG-3′; and siRNA-HBXIP-2,
5′-CAGATAATGGGAACATTATGATC-3′) and the non-targeting control
(siRNA-NC, 5′-AAGACAUUGUGUGUCCGCCTT-5′) were supplied by Santa Cruz
Biotechnology, Inc. The pcDNA3.1 vector containing the full-length
cDNA sequence of FHL2 (Ov-FHL2) and the empty vector (Ov-NC) were
supplied by Shanghai GenePharma Co., Ltd. siRNAs (5 nM) and
overexpression plasmids (50 nM) were transfected into HeLa cells
using a Lipofectamine® 2000 kit (Invitrogen; Thermo
Fisher Scientific, Inc.) for 48 h at 37°C according to the
manufacturer's protocols. At 48 h post-transfection, the
transfected cells were harvested for use for subsequent
experiments.
Cell Counting Kit-8 (CCK-8) assay
HeLa cells were inoculated into 96-well plates
(2×103 cells/well) and were cultured at 37°C with 5%
CO2 for 24, 48 or 72 h. Following the addition of 10 µl
CCK-8 solution (Shanghai Yeasen Biotechnology Co., Ltd.), cells
were cultured at 37°C for 3 h. A microplate reader (Bio-Rad
Laboratories, Inc.) was then used to assess the absorbance in each
well at 450 nm.
5-ethynyl-2′-deoxyuridine (EDU)
staining
Cell proliferation was evaluated using a BeyoClick™
EdU Cell Proliferation Kit (cat. no. C0085S; Beyotime Institute of
Biotechnology). HeLa cells were seeded into 96-well plates at
1×104 cells/well. At 48 h post-transfection, 100 µl EdU
solution was added into each well and the cells were cultured at
37°C with 5% CO2 for 2 h. HeLa cells were then fixed
using 4% paraformaldehyde at room temperature for 10 min and
permeabilized using 0.3% Triton X-100 for 15 min at room
temperature. After washing with PBS, HeLa cells were incubated with
a Click reaction solution for 30 min in the dark at room
temperature. Finally, the nuclear DNA was stained with DAPI (5
µg/ml; Beyotime Institute of Biotechnology) for 10 min at room
temperature. Cells were imaged using a fluorescence microscope
(Olympus Corporation).
Wound healing assay
The migratory capacity of the cells was assessed
using a wound healing assay. The HeLa cells were inoculated into
six-well plates at 4×105 cells/well and cultured at
37°C. Upon reaching ~95% confluence, a sterile 200-µl pipette tip
was used to scratch the cells to create a wound in the cell
monolayer. After washing with PBS, cells were incubated with
serum-free RPMI-1640 medium for 48 h at 37°C. Images at 0 and 48 h
were captured using a light microscope (Olympus Corporation). The
relative cell migration rate of each group (scratch distance at 24
h-initial distance at 0 h) was normalized according to the average
migrated distance of the control group and analyzed using ImageJ
software 1.52r (National Institutes of Health).
Transwell assay
HeLa cells (5×104 cells/well) were
resuspended in serum-free RPMI-1640 medium and were seeded into the
upper chambers of Transwell plates (pore size, 8 mm; BD
Biosciences), which were pre-coated with 60 µl Matrigel (2 mg/ml;
BD Biosciences) for 1 h at room temperature. Complete RPMI-1640
medium (500 µl; Procell Life Science & Technology Co., Ltd.)
containing 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) was
added into the lower chamber. After 48 h incubation at 37°C with 5%
CO2, a cotton swab was used to remove the cells from the
upper chamber. Cells that penetrated the membranes were fixed using
4% paraformaldehyde for 10 min at room temperature and stained
using 0.1% crystal violet for 15 min at room temperature. The
number of invasive cells in five randomly selected fields of view
were then assessed using a light microscope (Olympus Corporation)
and quantified using ImageJ software 1.52r (National Institutes of
Health).
Flow cytometry
HeLa cells were harvested by trypsinization using
0.05% trypsin (MilliporeSigma), washed with pre-cooled PBS and then
fixed using 70% ethanol at 4°C overnight. After washing with PBS,
cells were incubated with 5 µl RNase A (10 mg/ml; Sigma-Aldrich;
Merck KGaA) and 2.5 µl propidium iodide (5 mg/ml; Beyotime
Institute of Biotechnology) for 30 min at room temperature in the
dark. Finally, the cell cycle progression was analyzed using a BD
FACSCalibur flow cytometer (BD Biosciences) before the percentage
of cells at each phase of the cell cycle
(G0/G1, S and G2/M) was assessed
using the FlowJo v.10.7.1 software (FlowJo LLC).
Western blotting
HeLa cells were lysed using RIPA buffer (Thermo
Fisher Scientific, Inc.) to extract the total protein. Following
determination of the protein concentration using a BCA assay
(Beyotime Institute of Biotechnology), the proteins (25 µg per
lane) were separated using 12% SDS-PAGE and then transferred onto
PVDF membranes. After blocking using 5% skimmed milk for 1 h at
room temperature, the membranes were probed overnight at 4°C with
primary antibodies against HBXIP (1:1,000; cat. no. ab157480;
Abcam), FHL2 (1:1,000; cat. no. ab202584; Abcam), CyclinD1 (1:200;
cat. no. ab16663; Abcam), CyclinD2 (1:1,000; cat. no. ab207604;
Abcam), MMP2 (1:1,000; cat. no. ab92536; Abcam), MMP9 (1:1,000;
cat. no. ab76003; Abcam), β-catenin (1:5,000; cat. no. ab32572;
Abcam), c-Myc (1:1,000; cat. no. ab32072; Abcam), GAPDH (1:2,500;
cat. no. ab9485; Abcam) and β-actin (1:1,000; cat. no. ab8227;
Abcam). Membranes were then probed using the HRP-conjugated goat
anti-rabbit IgG (1:2,000; cat. no. ab6721; Abcam) secondary
antibodies for 2 h at room temperature. Pierce™ ECL Plus Western
Blotting Substrate (Pierce; Thermo Fisher Scientific, Inc.) were
used to visualize blots, followed by semi-quantification using
ImageJ (version 1.46; National Institutes of Health) with GAPDH as
the internal control.
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA expression levels of HBXIP and FHL2 in HeLa
cells were assessed using RT-qPCR. In brief, an RNeasy Universal
Mini Kit (Qiagen GmbH) was used for the extraction of total RNA
according to the manufacturer's protocol, followed by cDNA
synthesis using a PrimeScript™ 1st strand cDNA Synthesis kit
(Takara Bio, Inc.). The reverse transcription was performed at 37°C
for 30 min, followed by 85°C for 5 sec and 4°C for maintenance.
Subsequently, qPCR was performed using SYBR Green PCR Master mix
(Takara Bio, Inc.) in an ABI 7500 PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling condition used
were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 45 sec, then 95°C for 15 sec, 60°C for 1 min,
95°C for 15 sec and 60°C for 15 sec. The primer sequences used were
as follows: HBXIP forward, 5′-GAGCCCAAGCCTTCGTCAG-3′ and reverse,
5′-GGCACGTCCTTCTCCACCA-3′; FHL2 forward,
5′-GTACAGACTGCTATTCCAACGAG-3′ and reverse,
5′-GCACTGCATGGCATGTTGTT-3′ and GAPDH forward,
5′-CCATGGGGAAGGTGAAGGTC-3′ and reverse, 5′-AGTGATGGCATGGACTGTGG-3′.
The 2−ΔΔCq method was used to assess the relative
expression of aforementioned genes with GADPH as the constitutive
internal control (23).
Co-immunoprecipitation (Co-IP)
assay
HeLa cells were lysed on ice in RIPA lysis buffer
(Thermo Fisher Scientific, Inc.) containing 1 µM PMSF protease
inhibitor (Thermo Fisher Scientific, Inc.) for 30 min, followed by
centrifugation at 13,000 × g for 15 min at 4°C and supernatant
collection. The lysates (500 µg) were incubated with a total of 1
µg antibodies targeting HBXIP (1:100; cat. no. sc-373980; Santa
Cruz Biotechnology, Inc.) and FHL2 (1:30; cat. no. ab202584; Abcam)
and then rotated at 4°C with a mixture of 30 µl protein A/G agarose
beads for an additional incubation at 4°C overnight. After the IP
reaction, 30 µl agarose beads were centrifuged at 1,000 × g for 3
min at 4°C to the bottom of the tube. The supernatant was then
carefully absorbed, and the agarose beads were washed three times
with 1 ml lysis buffer. A total of 15 µl 2X SDS sample buffer was
finally added for boiling at 100°C for 5 min, followed by analysis
by western blotting according to the aforementioned protocol.
Statistical analysis
All experimental data are represented by at least
three independent experimental repeats and are presented as the
mean ± standard deviation and were assessed using GraphPad Prism
8.0 (GraphPad Software, Inc.). One-way ANOVA followed by Tukey's
post hoc test was used to assess the differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
HBXIP is highly expressed in cervical
cancer cells
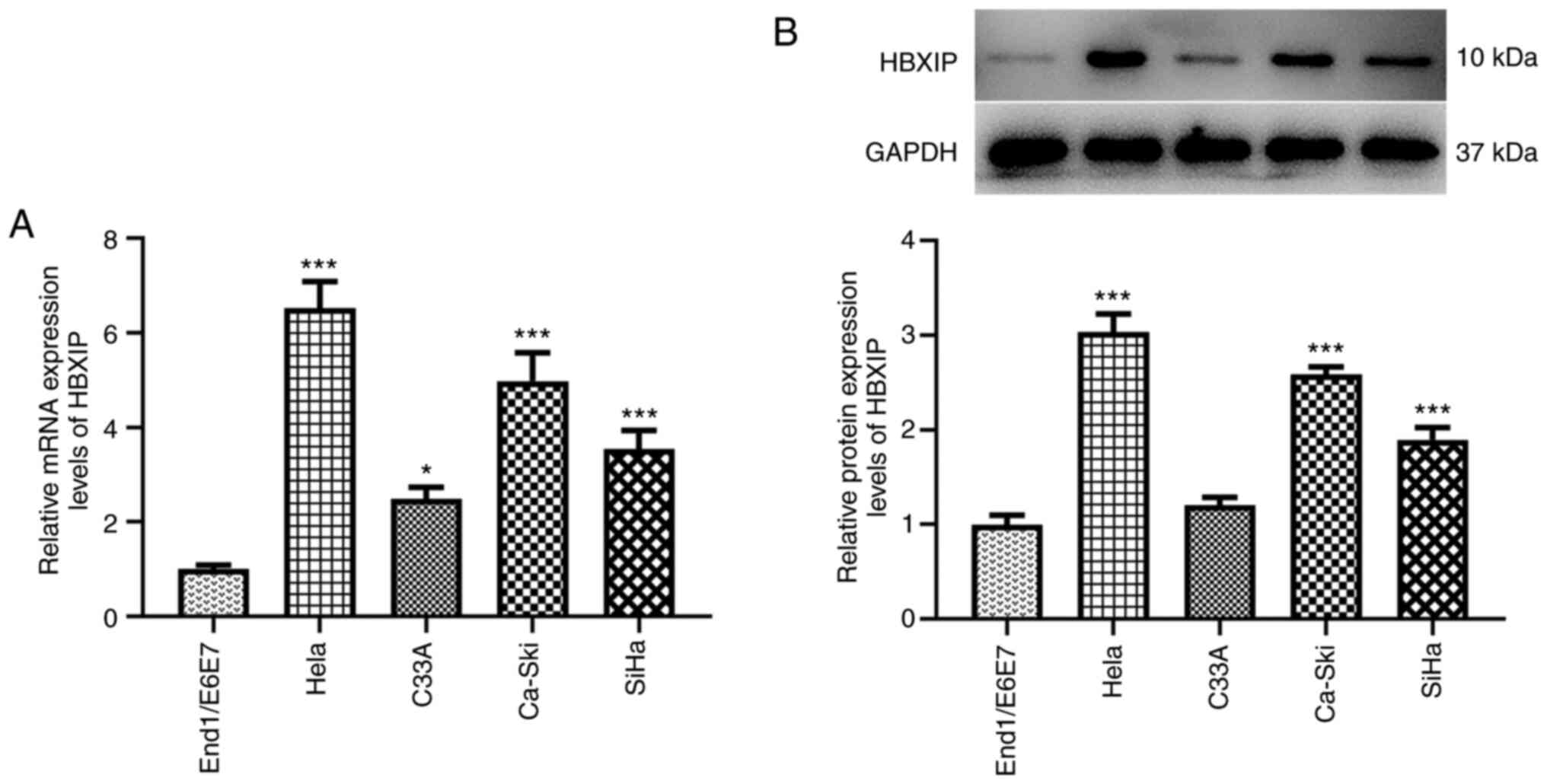
RT-qPCR and western blotting demonstrated a marked
elevation in the HBXIP mRNA and protein expression levels in HeLa,
C33A, Ca-Ski and SiHa cervical cancer cell lines compared with
those in the End1/E6E7 human cervical epithelial cell line
(Fig. 1). In particular, the HeLa
cell line exhibited the highest mRNA and protein expression levels
of HBXIP (Fig. 1). Therefore, it
was chosen for subsequent experiments.
HBXIP knockdown inhibits the
proliferation of cervical cancer cells
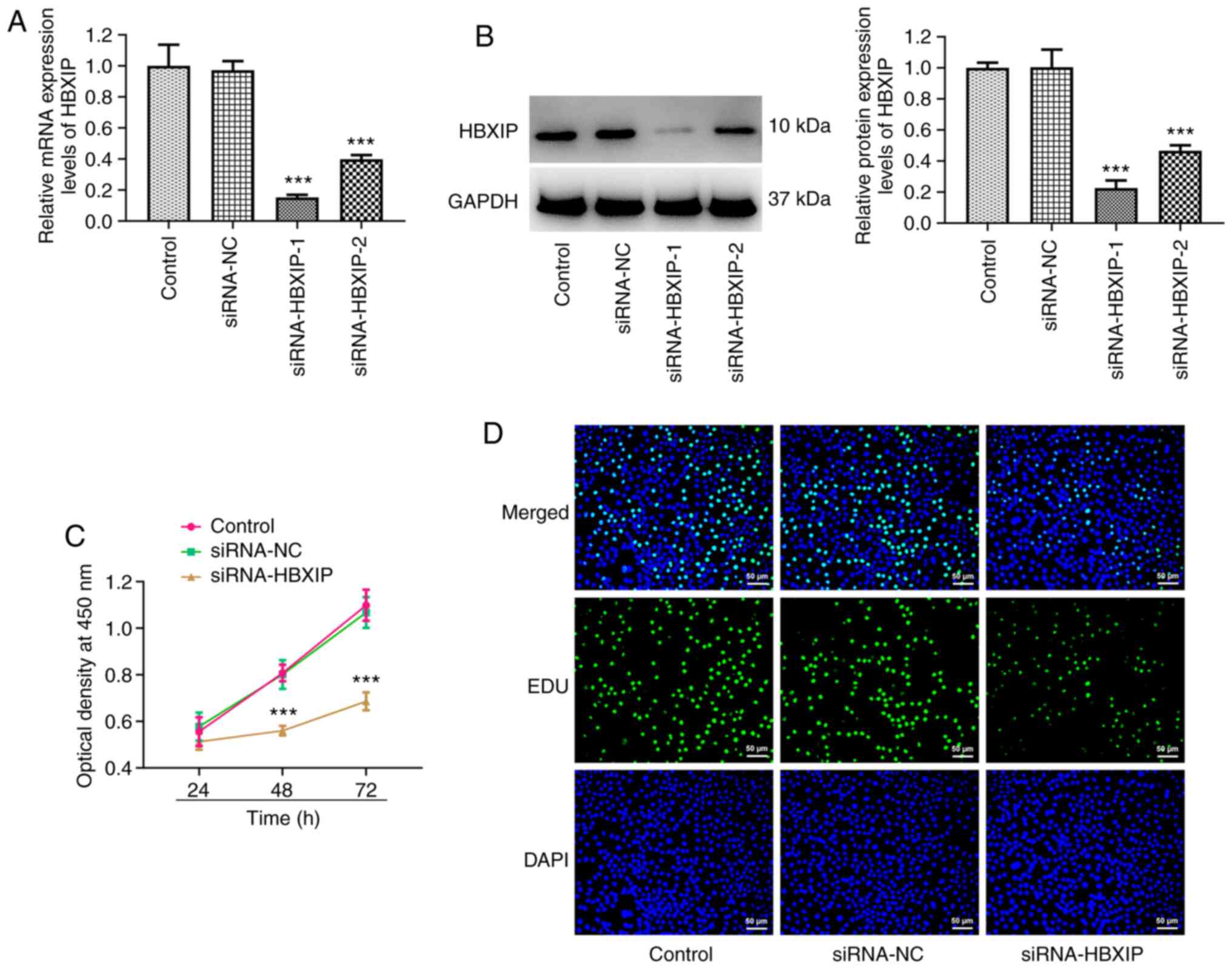
HBXIP expression in HeLa cells was subsequently
knocked down and it was demonstrated that the HBXIP mRNA and
protein expression levels were significantly reduced compared with
those in the siRNA-NC group (Fig. 2A
and B). Since siRNA-HBXIP-1 resulted in higher knockdown
efficiency, siRNA-HBXIP-1 was chosen for subsequent experiments,
which was referred to as ‘siRNA-HBXIP’ thereafter. Compared with
that in the siRNA-NC group, a significant decrease in cell
viability was observed in the siRNA-HBXIP group at both 48 and 72 h
(Fig. 2C). Furthermore, EDU
staining revealed markedly decreased levels of cell proliferation
in the siRNA-HBXIP group compared with that in the siRNA-NC group
(Fig. 2D).
HBXIP knockdown promotes
G0/G1 cycle arrest in cervical cancer
cells
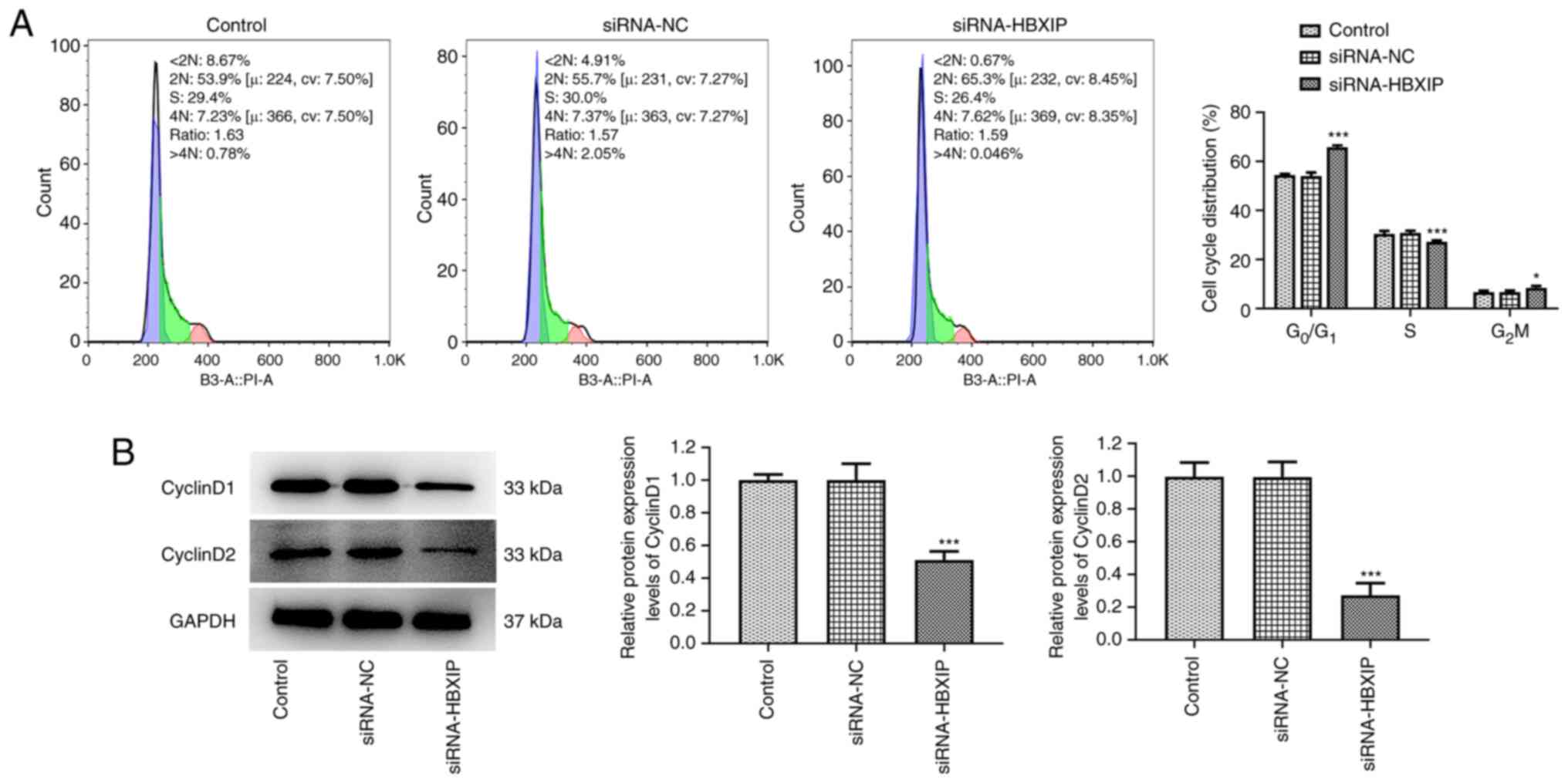
HBXIP knockdown significantly promoted the cell
cycle arrest of HeLa cells at the G0/G1
phase, slightly accelerated cell cycle arrest at the
G2/M phase and slightly obstructed cell cycle arrest at
the S phase compared with that in the siRNA-NC group (Fig. 3A). Furthermore, the protein
expression levels of CyclinD1 and CyclinD2, which are associated
with G1/S phase cell-cycle transition (24), were significantly reduced in HeLa
cells transfected with siRNA-HBXIP compared with those in the
siRNA-NC group (Fig. 3B).
HBXIP knockdown suppresses the
invasion and migration of cervical cancer cells
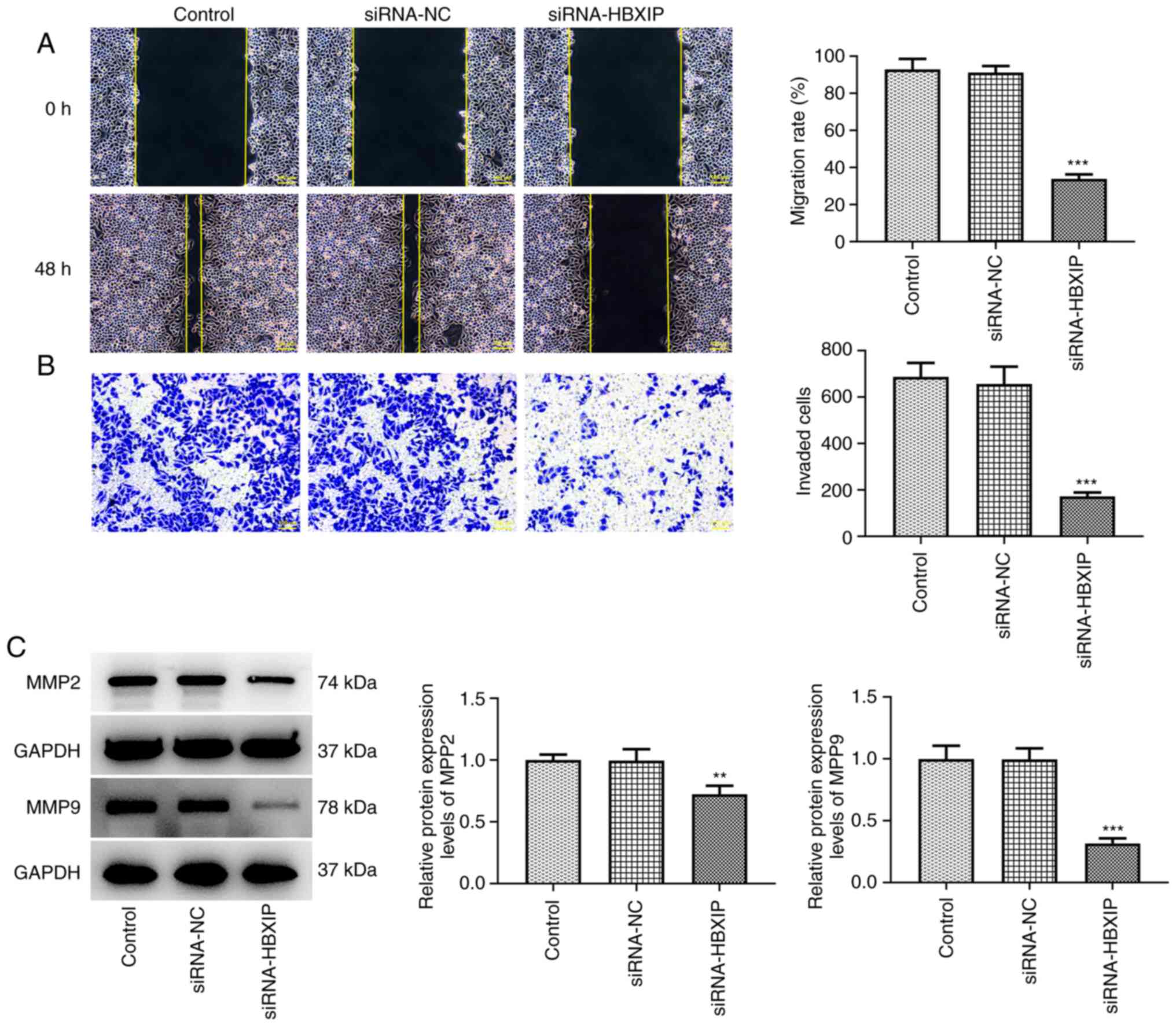
The migration rate was significantly decreased in
HeLa cells following the knockdown of HBXIP expression compared
with that in the siRNA-NC group (Fig.
4A). Similarly, the number of invasive cells was also
significantly decreased in the siRNA-HBXIP group compared with that
in the siRNA-NC group (Fig. 4B).
Furthermore, the protein expression levels of cell invasion markers
MMP2 and MMP9 were significantly decreased in HBXIP-knockdown HeLa
cells compared with those in the siRNA-NC group (Fig. 4C).
FHL2 overexpression reverses the
inhibitory effects of HBXIP knockdown on the proliferation of
cervical cancer cells
The BioGRID database predicted that FHL2 was likely
to bind to HBXIP. Similarly to HBXIP, FHL2 also demonstrated high
mRNA and protein expression levels in cervical cancer cell lines,
especially in HeLa cells, where its mRNA and protein expression
levels were significantly increased compared with those in
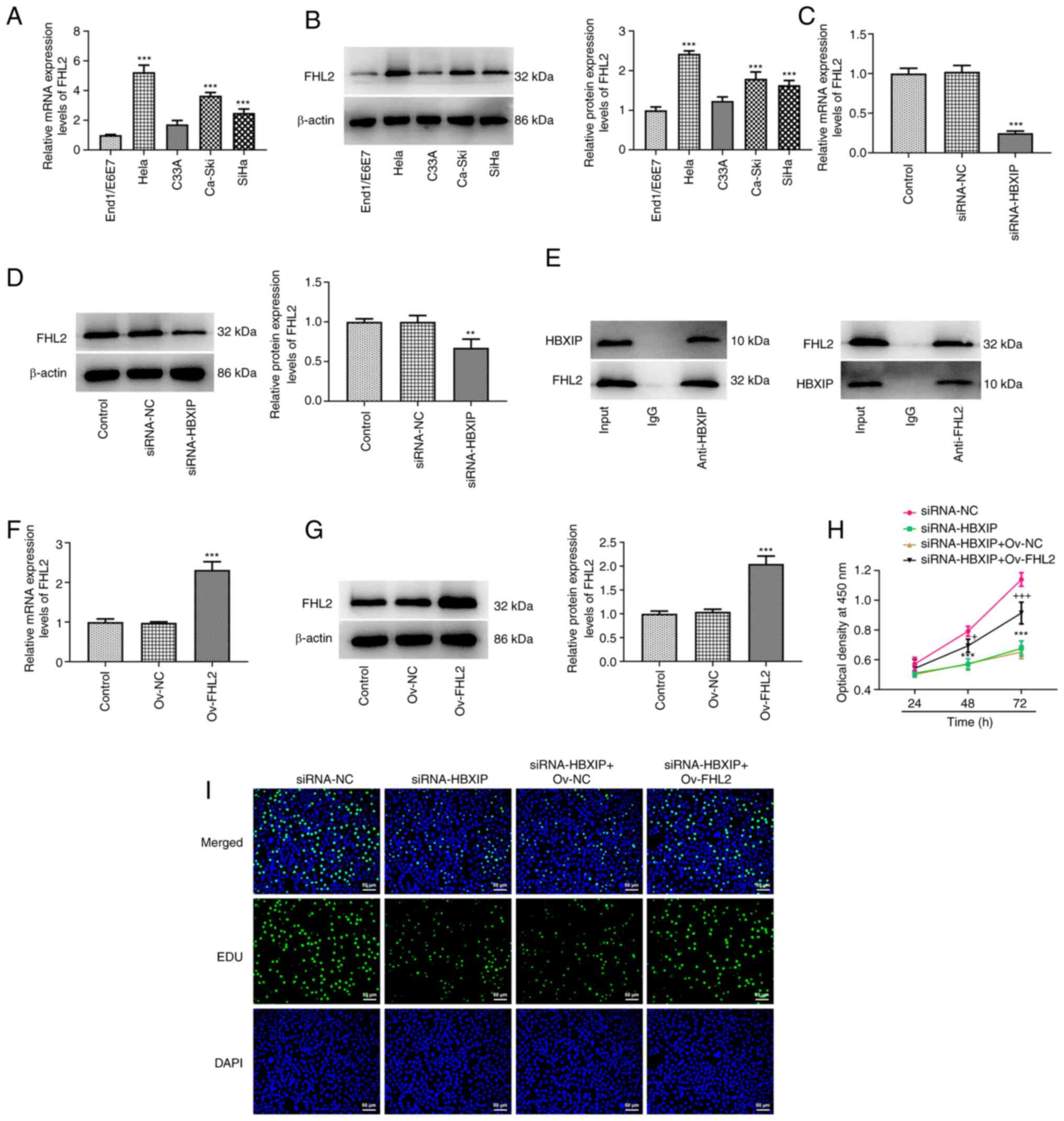
End1/E6E7 cells apart from C33A cells (Fig. 5A and B). FHL2 mRNA and protein
expression levels in HeLa cells were significantly downregulated
after HBXIP knockdown compared with those in the siRNA-NC group
(Fig. 5C and D). Furthermore, Co-IP
demonstrated the interaction between HBXIP and FHL2 (Fig. 5E). Significant elevations in FHL2
mRNA and protein expression was demonstrated after Ov-FHL2 was
transfected into HeLa cells compared with those in the Ov-NC group
(Fig. 5F and G). Subsequently, HeLa
cells co-transfected with siRNA-HBXIP and Ov-FHL2 demonstrated
significantly enhanced cell viability at 72 h compared with that in
the siRNA-HBXIP + Ov-NC group (Fig.
5H). The EDU staining results were also consistent with the
trend of changes observed in the cell viability assay (Fig. 5I).
FHL2 overexpression reverses the
promoting effects of HBXIP knockdown on cervical cancer cell cycle
arrest
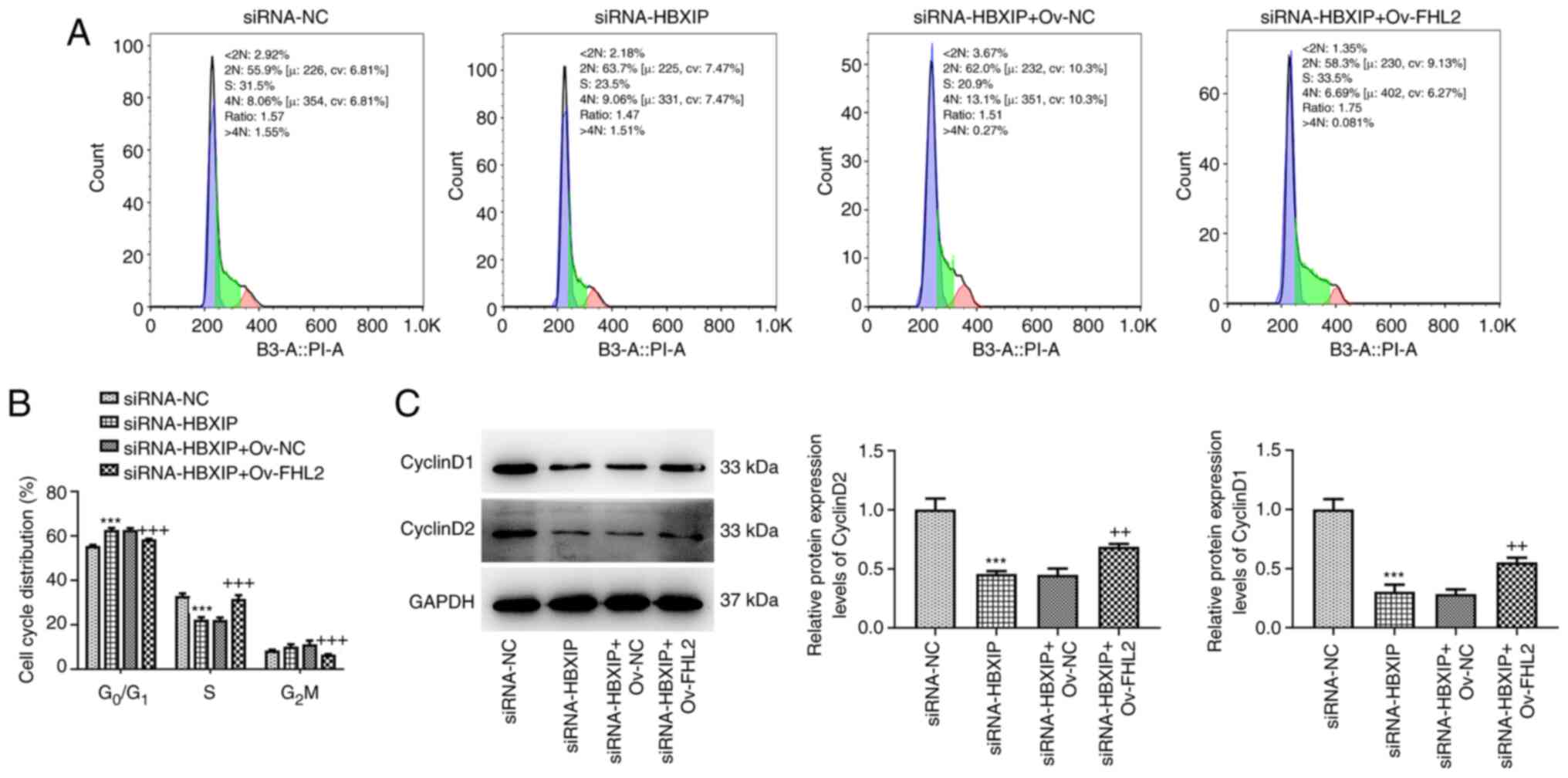
The results of the flow cytometry assay demonstrated
that overexpression of FHL2 significantly decreased the number of
cells at the G0/G1 phase and G2/M
phase, and increased the number of cells at the S phase compared
with that in the siRNA-HBXIP + Ov-NC group (Fig. 6A and B). Furthermore, western
blotting revealed significantly increased CyclinD1 and CyclinD2
protein expression levels in HeLa cells co-transfected with
siRNA-HBXIP and Ov-FHL2 compared with those in the siRNA-HBXIP +
Ov-NC group (Fig. 6C).
FHL2 overexpression counteracts the
inhibitory effects of HBXIP knockdown on cervical cancer cell
invasion and migration
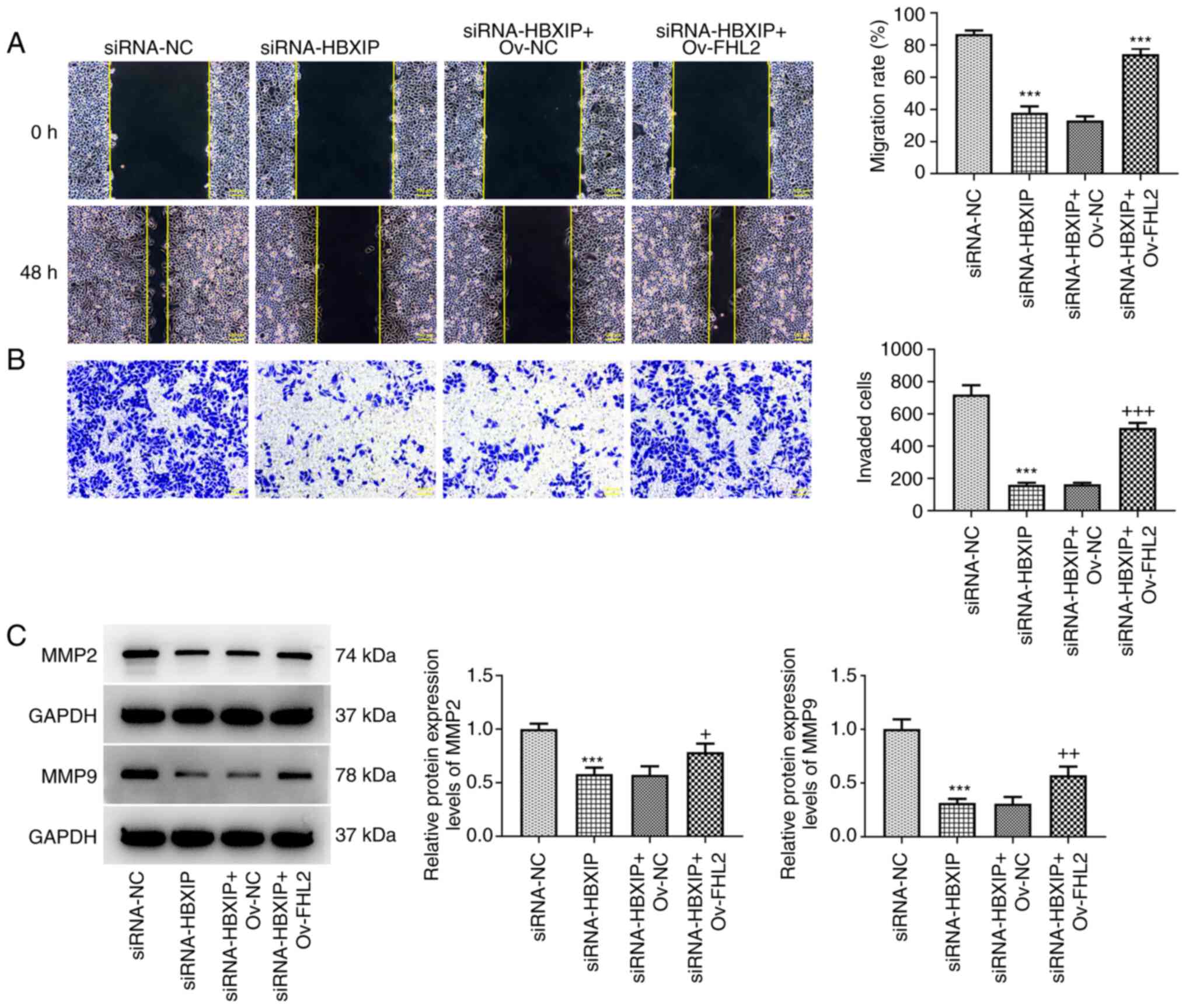
The migration and invasion of HeLa cells were
demonstrated to be significantly enhanced in the siRNA-HBXIP +
Ov-FHL2 group compared with that in the siRNA-HBXIP + Ov-NC group
(Fig. 7A and B). Furthermore,
compared with those in the siRNA-HBXIP + Ov-NC group, it was
demonstrated that the protein expression levels of MMP2 and MMP9
were significantly elevated following FHL2 overexpression in
HBXIP-knockdown HeLa cells (Fig.
7C).
HBXIP knockdown inhibits Wnt signaling
and is counteracted by FHL2 overexpression
To evaluate the potential regulatory mechanism,
markers of the Wnt/β-catenin signaling pathway in HeLa cells was
assessed after HBXIP knockdown and/or FHL2 overexpression. The
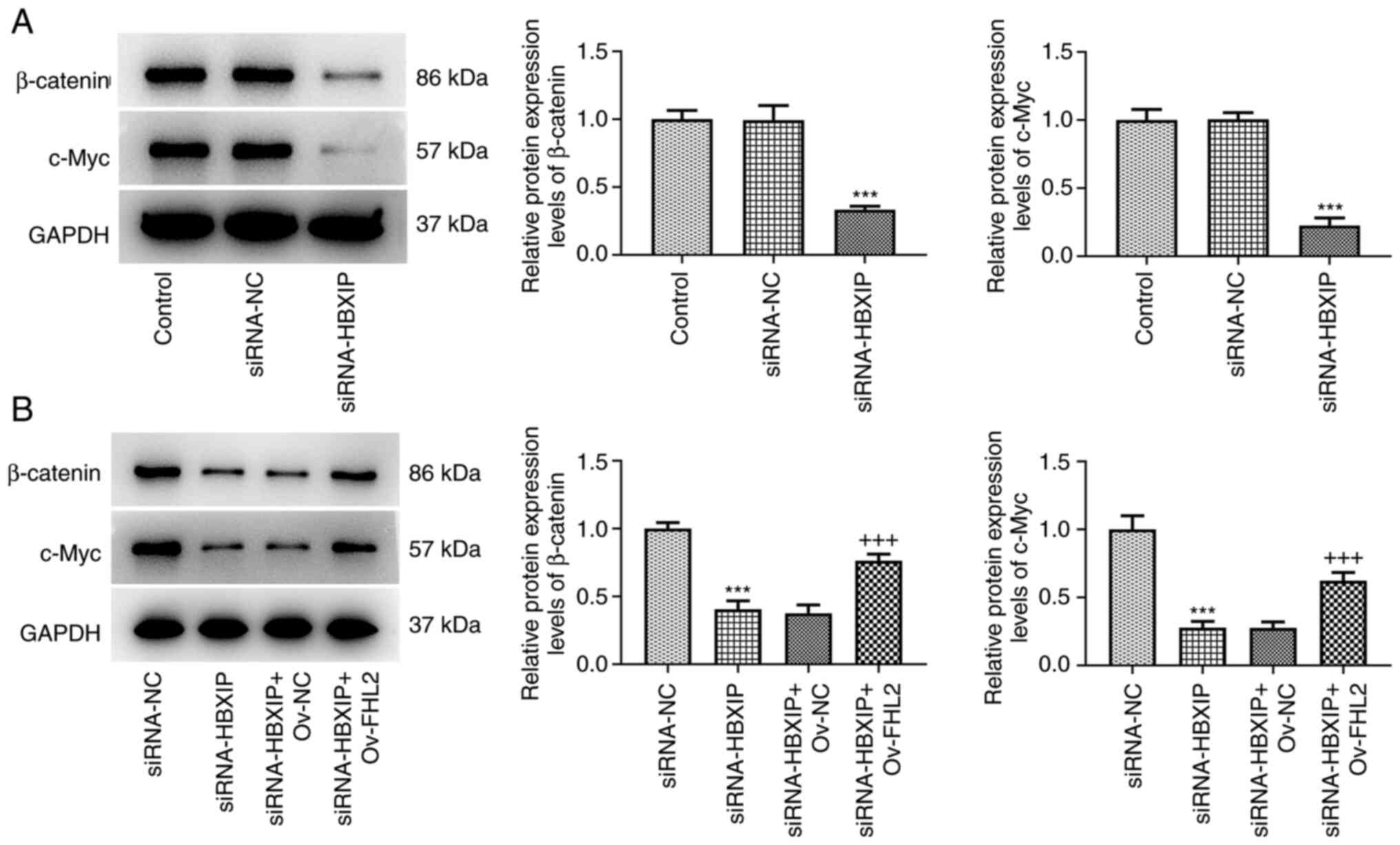
protein expression levels of the Wnt/β-catenin signaling marker
proteins β-catenin and c-Myc were demonstrated to be significantly
reduced in HeLa cells transfected with siRNA-HBXIP compared with
those in the siRNA-NC group (Fig.
8A). By contrast, this downregulated protein expression levels
of β-catenin and c-Myc caused by siRNA-HBXIP transfection were
significantly reversed by co-transfection with Ov-FHL2 compared
with those in the siRNA-HBXIP + Ov-NC group (Fig. 8B).
Discussion
Cervical cancer has high mortality rates among women
in low and middle-income countries (1). Although a previous study has reported
the oncogenic mechanisms of HBXIP in a variety of tumors (10), the effects of HBXIP on tumor
progression in cervical cancer have not been previously elucidated.
Therefore, the present study attempted to elucidate the pathogenic
mechanism of HBXIP in cervical cancer to further the understanding
of tumor progression in cervical cancer.
The present study demonstrated that HBXIP mRNA level
was significantly upregulated in cervical cancer cells (Hela, C33A
and SiHa cells) and the cervical squamous cell carcinoma Ca-Ski
cell line, while its protein level was significantly upregulated in
Hela, Ca-Ski and SiHa cells, which is consistent with the results
of a previous study (11) in which
immunohistochemical and immunofluorescence staining showed that
HBXIP expression was higher in cervical intraepithelial neoplasia
and cervical cancer cells. During the development of cancer, cells
can invade and spread to surrounding tissues and blood vessels
through proliferation and migration (25,26).
The prognosis of patients with cervical cancer is associated with
the degree of tumor cell proliferation, local infiltration and
metastasis (27). Numerous studies
have previously reported that HBXIP upregulation can promote the
tumor cell proliferation, migration and invasion capabilities by
triggering various signaling pathways, such as the PI3K/AKT, ERK1/2
and NF-κB (28–30). However, knockdown of HBXIP may
inhibit the malignant behaviors of tumor cells. HBXIP silencing has
been reported to be associated with decreased methyltransferase 3
and N6-adenosine-methyltransferase complex catalytic subunit
expression, which resulted in the repression of the proliferation,
migration and invasion of gastric cancer cells (5). To evaluate this hypothesis, HBXIP
expression was knocked down in HeLa cells in the present study,
which resulted in cell proliferation, invasion and migration being
significantly decreased. This was also accompanied by the
significantly decreased protein expression levels of cell migration
markers MMP2 and MMP9. These results suggest that HBXIP served an
important role in the physiology of cervical cancer.
Abnormalities in cell cycle progression can lead to
uncontrolled cell proliferation and conversion to tumor cells
(31). It has been reported that
HBXIP can act as a mediator of DNA damage response signals and
activate the G2/M checkpoint to maintain genomic
integrity (32). HBXIP has also
been reported to indirectly upregulate cyclin D1 expression to
promote hepatocellular carcinoma tumor growth through the PI3K/Akt
pathway (28). However, HBXIP
knockdown has been reported to abrogate ionizing radiation-induced
G2/M cell cycle checkpoints, sensitize osteosarcoma and
liver cancer cells to chemotherapy and regulate cell cycle
progression through modulating the DNA damage response (32). In the present study, HBXIP knockdown
significantly promoted cell cycle arrest in cervical cancer cells
at the G0/G1 and G2/M phases with
reduced the cell proportion in S phase. A corresponding decrease in
cyclin D1 and cyclin D2 protein expression was also found. These
results indicate that HBXIP served a role in the facilitation of
cell cycle arrest in cervical cancer.
In the present study, evaluation using the BioGRID
database predicted that FHL2 was likely to bind to HBXIP, which was
then demonstrated using Co-IP in the present study. FHL2 has been
reported to be highly expressed in cervical cancer cells (19,20),
which was supported by the results of the present study. The
effects of FHL2 on tumor progression have been reported to be
regulated by a number of transcription factors, such as P53, serum
response factor, specificity protein 1 and activator protein-1.
They have been found to be involved in various biological
functions, including cell adhesion, apoptosis, invasion,
proliferation and differentiation (21). In addition, FHL2 overexpression can
promote cell proliferation, migration and invasion in cancer types
such as epithelial ovarian cancer, ovarian granulosa cell tumor and
hepatocellular carcinoma (33–35).
In the present study, FHL2 overexpression significantly abrogated
the inhibitory effects of HBXIP knockdown on the proliferation,
migration and invasion of HeLa cells, suggesting that HBXIP
knockdown exerted suppressive effects on the malignant behavior of
HeLa cells at least partially through the downregulation of FHL2
expression.
Wnt/β-catenin signaling has been reported to
regulate several important cellular functions, including cell
proliferation, differentiation, invasion, migration and metastasis
(36). Wnt can bind the stabilizing
transcription factor β-catenin, which can enter the nucleus to
regulate the expression of target genes, such as Axin2, c-Myc and
cyclin D1, which are associated with tumorigenesis (37). FHL2 has been reported to regulate
the Wnt/β-catenin signaling pathway by increasing β-catenin
dephosphorylation at the Ser37/Thr41 site and nuclear translocation
to upregulate the β-catenin-mediated transcription of its target
genes in rat tubular epithelial cells (38). Furthermore, HBXIP has been
previously reported to induce the dysregulation of c-Myc, c-Jun and
p53 mRNA and protein levels to promote tumor progression, such as
that of breast cancer, hepatocellular carcinoma and gastric cancer
(10). In the present study, HBXIP
knockdown was demonstrated to significantly inhibit FHL2 mRNA and
protein expression. In addition, knockdown of HBXIP inhibited the
expression of β-catenin and c-Myc, which was reversed by the
overexpression of FHL2, suggesting that HBXIP knockdown suppressed
Wnt/β-catenin signaling by downregulating FHL2.
The main limitation of the present study was the
lack of in vivo verification. Future in vivo research
would be beneficial to verify the results of the present study.
Further studies are needed to further explore the precise oncogenic
function and molecular mechanism of HBXIP-induced
carcinogenesis.
Considering all the data, the present study has
provided an evaluation of the role of HBXIP in cervical cancer.
HBXIP knockdown repressed cervical cancer cell proliferation,
invasion and migration, possibly by inhibiting Wnt/β-catenin
signaling and downregulating FHL2 expression. The present study
revealed that HBXIP or FHL2 might be function as a potential
therapeutic target biomarker for cervical cancer therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG conceived and designed the study. XG performed
the functional experiments and LY performed the mechanism assays.
XG and LY collected the data. LY analyzed and interpreted the data,
and drafted the manuscript. XG revised the manuscript. XG and LY
confirm the authenticity of all the raw data. Both authors read and
approved final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lopez MS, Baker ES, Maza M, Fontes-Cintra
G, Lopez A, Carvajal JM, Nozar F, Fiol V and Schmeler KM: Cervical
cancer prevention and treatment in Latin America. J Surg Oncol.
115:615–618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Canfell K, Kim JJ, Brisson M, Keane A,
Simms KT, Caruana M, Burger EA, Martin D, Nguyen DTN, Bénard É, et
al: Mortality impact of achieving WHO cervical cancer elimination
targets: A comparative modelling analysis in 78 low-income and
lower-middle-income countries. Lancet. 395:591–603. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson CA, James D, Marzan A and Armaos
M: Cervical cancer: An overview of pathophysiology and management.
Semin Oncol Nurs. 35:166–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zimet GD, Rosberger Z, Fisher WA, Perez S
and Stupiansky NW: Beliefs, behaviors and HPV vaccine: Correcting
the myths and the misinformation. Prev Med. 57:414–418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Z and Jiang X, Li D and Jiang X:
HBXIP promotes gastric cancer via METTL3-mediated MYC mRNA m6A
modification. Aging (Albany NY). 12:24967–24982. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng S, Wu H, Wang F, Lv J, Lu J, Fang Q,
Wang F, Lu Y, Zhang S, Xu Y, et al: The oncoprotein HBXIP
facilitates metastasis of hepatocellular carcinoma cells by
activation of MMP15 expression. Cancer Manag Res. 11:4529–4540.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Li H, Zhang Y, Li L, Fang R, Li Y,
Liu Q, Zhang W, Qiu L, Liu F, et al: Oncoprotein HBXIP modulates
abnormal lipid metabolism and growth of breast cancer cells by
activating the LXRs/SREBP-1c/FAS signaling cascade. Cancer Res.
76:4696–4707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu L, Lu F, Zhang L, Wang G, Geng R and
Miao Y: HBXIP regulates gastric cancer glucose metabolism and
malignancy through PI3K/AKT and p53 signaling. Onco Targets Ther.
13:3359–3374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Feng Q, Yu H, Zhou X, Shan C,
Zhang Q and Liu S: HBXIP: A potential prognosis biomarker of
colorectal cancer which promotes invasion and migration via
epithelial-mesenchymal transition. Life Sci. 245:1173542020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiu M, Zeng X, Shan R, Wen W, Li J and Wan
R: The oncogenic role of HBXIP. Biomed Pharmacother.
133:1110452021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li N, Wang Y, Che S, Yang Y, Piao J, Liu S
and Lin Z: HBXIP over expression as an independent biomarker for
cervical cancer. Exp Mol Pathol. 102:133–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan KK, Tsui SK, Lee SM, Luk SC, Liew CC,
Fung KP, Waye MM and Lee CY: Molecular cloning and characterization
of FHL2, a novel LIM domain protein preferentially expressed in
human heart. Gene. 210:345–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinoshita M, Nakagawa T, Shimizu A and
Katsuoka Y: Differently regulated androgen receptor transcriptional
complex in prostate cancer compared with normal prostate. Int J
Urol. 12:390–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Genini M, Schwalbe P, Scholl FA, Remppis
A, Mattei MG and Schäfer BW: Subtractive cloning and
characterization of DRAL, a novel LIM-domain protein down-regulated
in rhabdomyosarcoma. DNA Cell Biol. 16:433–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu J, Zhou J, Li MS, Ng CF, Ng YK, Lai PBS
and Tsui SKW: Transcriptional regulation of the tumor suppressor
FHL2 by p53 in human kidney and liver cells. PLoS One.
9:e993592014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gabriel B, Mildenberger S, Weisser CW,
Metzger E, Gitsch G, Schüle R and Müller JM: Focal adhesion kinase
interacts with the transcriptional coactivator FHL2 and both are
overexpressed in epithelial ovarian cancer. Anticancer Res.
24:921–927. 2004.PubMed/NCBI
|
|
17
|
Chan KK, Tsui SK, Ngai SM, Lee SM, Kotaka
M, Waye MM, Lee CY and Fung KP: Protein-protein interaction of
FHL2, a LIM domain protein preferentially expressed in human heart,
with hCDC47. J Cell Biochem. 76:499–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen D, Xu W, Bales E, Colmenares C,
Conacci-Sorrell M, Ishii S, Stavnezer E, Campisi J, Fisher DE,
Ben-Ze'ev A and Medrano EE: SKI activates Wnt/beta-catenin
signaling in human melanoma. Cancer Res. 63:6626–6634.
2003.PubMed/NCBI
|
|
19
|
Jin H, Lee K, Kim YH, Oh HK, Maeng YI, Kim
TM, Suh DS and Bae J: Scaffold protein FHL2 facilitates
MDM2-mediated degradation of IER3 to regulate proliferation of
cervical cancer cells. Oncogene. 35:5106–5118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin X, Jiao X, Jiao J, Zhang T and Cui B:
Increased expression of FHL2 promotes tumorigenesis in cervical
cancer and is correlated with poor prognosis. Gene. 669:99–106.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao CY, Mok SWF, Cheng VWS and Tsui SKW:
The FHL2 regulation in the transcriptional circuitry of human
cancers. Gene. 572:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brun J, Dieudonné FX, Marty C, Müller J,
Schüle R, Patiño-García A, Lecanda F, Fromigué O and Marie PJ: FHL2
silencing reduces Wnt signaling and osteosarcoma tumorigenesis in
vitro and in vivo. PLoS One. 8:e550342013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med
(Berl). 94:1313–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamidi H and Ivaska J: Every step of the
way: Integrins in cancer progression and metastasis. Nat Rev
Cancer. 18:533–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oudin MJ and Weaver VM: Physical and
chemical gradients in the tumor microenvironment regulate tumor
cell invasion, migration, and metastasis. Cold Spring Harb Symp
Quant Biol. 81:189–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nie H, Bu F, Xu J, Li T and Huang J: 29
immune-related genes pairs signature predict the prognosis of
cervical cancer patients. Sci Rep. 10:141522020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang FZ, Fei HR, Lian LH, Wang JM and Qiu
YY: Hepatitis B x-interacting protein induces HepG2 cell
proliferation through activation of the phosphatidylinositol
3-kinase/Akt pathway. Exp Biol Med (Maywood). 236:62–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Zhang Z, Zhou X, Li L, Liu Q, Wang
Z, Bai X, Zhao Y, Shi H, Zhang X and Ye L: The oncoprotein HBXIP
enhances migration of breast cancer cells through increasing
filopodia formation involving MEKK2/ERK1/2/Capn4 signaling. Cancer
Lett. 355:288–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Cui M, Cai X, Sun B, Liu F, Zhang
X and Ye L: The oncoprotein HBXIP up-regulates SCG3 through
modulating E2F1 and miR-509-3p in hepatoma cells. Cancer Lett.
352:169–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng K, He Z, Kitazato K and Wang Y:
Selective autophagy regulates cell cycle in cancer therapy.
Theranostics. 9:104–125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fei H, Zhou Y, Li R, Yang M, Ma J and Wang
F: HBXIP, a binding protein of HBx, regulates maintenance of the
G2/M phase checkpoint induced by DNA damage and enhances
sensitivity to doxorubicin-induced cytotoxicity. Cell Cycle.
16:468–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang C, Lv X, He C, Davis JS, Wang C and
Hua G: Four and a half LIM domains 2 (FHL2) contribute to the
epithelial ovarian cancer carcinogenesis. Int J Mol Sci.
21:77512020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hua G, He C, Lv X, Fan L, Wang C, Remmenga
SW, Rodabaugh KJ, Yang L, Lele SM, Yang P, et al: The four and a
half LIM domains 2 (FHL2) regulates ovarian granulosa cell tumor
progression via controlling AKT1 transcription. Cell Death Dis.
7:e22972016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shao C, Qiu Y, Liu J, Feng H, Shen S,
Saiyin H, Yu W, Wei Y, Yu L, Su W and Wu J: PARP12 (ARTD12)
suppresses hepatocellular carcinoma metastasis through interacting
with FHL2 and regulating its stability. Cell Death Dis. 9:8562018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bahrami A, Amerizadeh F, ShahidSales S,
Khazaei M, Ghayour-Mobarhan M, Sadeghnia HR, Maftouh M, Hassanian
SM and Avan A: Therapeutic potential of targeting wnt/beta-catenin
pathway in treatment of colorectal cancer: Rational and progress. J
Cell Biochem. 118:1979–1983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai T, Sun D, Duan Y, Qiu Y, Dai C, Yang J
and He W: FHL2 promotes tubular epithelial-to-mesenchymal
transition through modulating beta-catenin signalling. J Cell Mol
Med. 22:1684–1695. 2018. View Article : Google Scholar : PubMed/NCBI
|