Introduction
The cancer incidence rate and cancer-associated
death rate are increasing each year worldwide, with colorectal
cancer (CRC) being the second leading cause of cancer-related death
in 2020 (1). Surgery is one of the
most effective treatment options for patients with stage I–III CRC
and has been strongly recommended in several treatment guidelines
(2–4); however, the development of endoscopic
treatments for submucosal (SM) invasive CRC, including endoscopic
mucosal resection and endoscopic SM dissection, has led to the cure
of early CRC (5). T1 stage CRC [T1
CRC; staged using the Japanese Society for Cancer of the Colon and
Rectum (JSCCR) 2020 guidelines (4)]
is treated with endoscopic resection only, and T2-T4 CRC cases are
treated with surgery; however, the treatment of T1 CRC is
controversial. European and Japanese guidelines recommend
additional surgery following endoscopic resection to reduce local
recurrence based on pathological findings (4,6).
According to the JSCCR guidelines for the treatment of CRC
(4), additional surgery with lymph
node (LN) dissection is recommended for cases with an SM invasion
depth >1,000 µm, vascular invasion, a positive endoscopic
vertical margin, poorly differentiated adenocarcinoma (por),
signet-ring cell carcinoma (sig) or mucinous carcinoma (muc),
and/or grade 2/3 budding at the site of deepest invasion; however,
in T1 CRC, the probability of LN metastasis ranges from 7.4 to
46.9%, depending on the combination of these risk factors (7).
In a multi-institutional retrospective study of 758
patients of T1 CRC, there were 106 patients who, despite being at
high risk for LN metastasis, did not undergo LN dissection, and the
reported 5-year disease-free survival rates for these patients were
96.5% for 69 patients with T1 colon cancer and 77.7% for 37
patients with T1 rectal cancer (8).
In total, 5 and 2 patients had local recurrence and distant
metastases, respectively; however, the 5-year overall survival
rates were 98.3 and 96.2% for patients with T1 colon and rectal
cancer, respectively, with additional treatments, including surgery
and chemotherapy. Control of distant metastasis and local
recurrence may need to be considered separately; in other words,
additional surgery to minimize local recurrence for all patients
with current risk factors may be considered an overtreatment,
especially for patients with low risk of local recurrence and high
surgical risk.
In the present study, the aim was to develop a model
that accurately predicts the presence or absence of LN metastasis
beyond the risk factors recommended by the guidelines. In a
previous study, we retrospectively reported the risk factors for LN
metastasis and developed a nomogram as a prediction model (9); however, it lacked data on budding,
which has been considered a risk factor since 2009 according to
guidelines, and it was a single-institution study. The present
study aimed to evaluate risk factors, including new data on
budding, and to develop a universal predictive model to recommend
additional surgical resection in, to the best of our knowledge, the
largest dataset of patients with T1 CRC.
Materials and methods
Patients and datasets
In the present multi-institutional study, the
clinical records of 1,185 patients with T1 CRC who underwent
surgery at The Osaka University Hospital (Suita, Japan), Osaka
International Cancer Institute (Osaka, Japan), Japan Community
Health Care Organization Osaka Hospital (Osaka, Japan), Osaka Rosai
Hospital (Sakai, Japan), Minoh City Hospital (Minoh, Japan) and
Toyonaka Municipal Hospital (Toyonaka, Japan) between January 2008
and December 2020 were retrospectively reviewed. Patients with T1
CRC who underwent surgery with lymph node dissection were included
in the present study and those without lymph node dissection were
excluded. There were 684 males and 501 females, with a median age
of 68 (range 30–91) years. Data on primary CRC location, tumor
type, head invasion, SM invasion depth, histological grade,
lymphatic invasion, venous invasion, budding grade and LN
metastasis were collected from the medical records. All patients
underwent surgery with LN dissection after endoscopic resection or
surgery for primary CRC resection. The presence of LN metastases
was evaluated pathologically. SM invasion depth was evaluated as a
continuous variable according to the Japanese Classification of
Colorectal Carcinoma (9th edition in the Japanese version; 3rd
edition in the English version) (10). Vascular invasion was evaluated using
hematoxylin and eosin staining alone in 94 cases and
immunocytochemistry using D2-40 in the remaining cases. According
to the Japanese Classification of Colorectal Carcinoma 9th edition
guidelines, venous and lymphatic invasion was divided into four
categories: i) 0, no invasion; ii) 1a, slight invasion; iii) 1b,
moderate invasion; and iv) 1c, massive invasion. Budding was graded
into three categories: i) BD1, 0–4 buds; ii) BD2, 5–9 buds; and
iii) BD3, >10 buds. For those cases with missing values in the
medical records, but for which tissue sections were stored and
could be re-evaluated, the pathologist performed a re-evaluation to
supplement the missing values.
The minimum number of samples required for the
training set was calculated as 10 times the number of samples for
the explanatory variables used in the prediction model, and
patients were randomly assigned to training and validation sets per
institution. Random allocation was performed using the permuted
block method via Microsoft Excel for Mac 2019 (version 16.63.1;
Microsoft Corporation). In the training set, risk factors for LN
metastasis were analyzed, and a prediction model for LN metastasis
was developed using a logistic regression model. The prediction
model was validated using the validation set.
This study was performed in accordance with the
Declaration of Helsinki and was approved by the Osaka University
Ethics Committee (Suita, Japan; approval no. 17448-4) and the
ethics committees of all other institutions involved in this study.
Comprehensive informed consent for the use of their data for
research purposes was obtained from all patients in the form of the
opt-out method.
Statistical analysis
Categorical variables were analyzed using the
χ2 test and continuous variables were analyzed using the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference. Univariate and multivariate
logistic regression models were applied to calculate the odds
ratios (ORs) and 95% confidence intervals (CIs) to assess the
independent contributions of each risk factor for LN metastasis.
Nomograms for the prediction of LN metastasis were constructed
using significant variables, one with risk factors that were
significant in the univariate analysis and one with risk factors
that were significant in the multivariate analysis.
The predictive rates of the nomograms and the risk
factors for LN metastasis as defined in the JSCCR guidelines
(4) were assessed using the area
under the receiver operating characteristic curve (AUC). All
statistical analyses were performed using the JMP Pro 16.0.0
statistical software program (SAS Institute, Inc.). A nomogram was
constructed using R 3.6.3 (CRAN; The R Foundation for Statistical
Computing).
Results
Demographic and pathological
characteristics
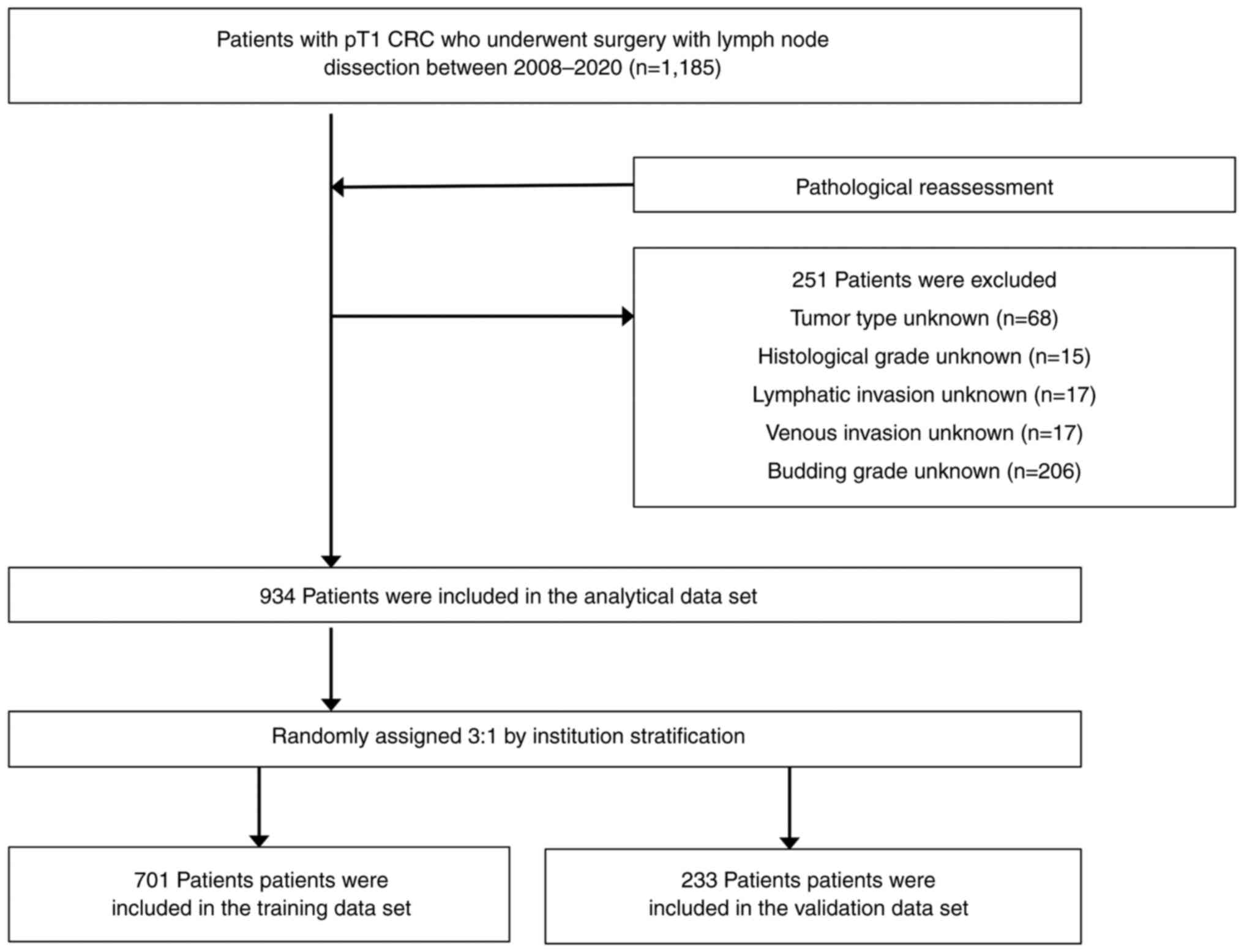
The characteristics of the 1,185 patients with T1
CRC are shown in Table SI. In
total, 251 patients who lacked any of the required
clinicopathological findings were excluded. Finally, 934 patients
were included in the analytic dataset (Table SII). LN metastasis was observed in
11.5% of patients, and the 934 patients were randomly assigned to
the training and validation sets, in a 3:1 ratio per institution,
and then analyzed together (701 and 233 patients were included in
the training and validation sets, respectively). A flow chart
presenting the recruiting and categorizing of patients in the study
is shown in Fig. 1. There were no
significant differences in clinicopathological factors between the
training and validation sets (Table
SII). In the training set, LN metastasis was evident in 87 out
of 701 patients (12.4%; Table I).
The mean ± SD SM invasion depth was 2,940±102 µm in patients
without metastasis and 3,396±268 µm in those with metastasis. In
the training set, among the patients in whom LN metastasis was
absent or present, lymphatic invasion was observed in 222 (36.2%)
and 65 (74.7%) patients, respectively, and venous invasion was
observed in 121 (19.7%) and 38 (43.7%) patients, respectively.
Budding grades BD2/3 were observed in 103 (16.8%) and 58 (66.7%)
patients, respectively. Histological grade was categorized into the
main histological and poor histological grades based on the degree
of differentiation, in the following order: Papillary
adenocarcinoma > well-differentiated tubular adenocarcinoma >
moderately differentiated tubular adenocarcinoma > por, sig or
muc. Left location (descending colon, sigmoid colon, rectosigmoid,
rectum and/or anal canal; P=0.004), deep SM invasion depth
(P=0.014), poor histological grade (por/sig/muc; P=0.016),
lymphatic invasion (P<0.001), venous invasion (P<0.001) and
BD2/3 (P<0.001) were all significant risk factors for LN
metastasis.
 | Table I.Analysis of risk factors for lymph
node metastasis in the training set. |
Table I.
Analysis of risk factors for lymph
node metastasis in the training set.
|
| Lymph node
metastasis |
|
|---|
|
|
|
|
|---|
| Factors | Absent (n=614) | Present (n=87) | P-value |
|---|
| Primary CRC location,
n |
|
| 0.004a |
|
Right | 224 | 18 |
|
| Left | 390 | 69 |
|
| Tumor type, n |
|
|
|
| Main
tumor typeb |
|
|
|
|
0-I | 340 | 50 | 0.731 |
|
0-II | 274 | 37 |
|
| All
elements of tumor typec |
|
|
|
|
Including
0-II | 221 | 29 | 0.722 |
|
Not including
0-II | 393 | 58 |
|
| Head invasion,
n |
|
| 0.338 |
|
Absent | 596 | 83 |
|
|
Present | 18 | 4 |
|
| Submucosal invasion
depth |
|
|
|
|
Measured valued, µm | 2,940 ±102 | 3,396±268 | 0.014a |
|
≥1,000 µm, n | 81 | 6 | 0.096 |
|
<1,000 µm,
n | 533 | 81 |
|
|
≥2,600 µm, n | 287 | 55 | 0.004a |
|
<2,600 µm,
n | 327 | 32 |
|
| Histological
grade |
|
|
|
| Main
histological grade, n |
|
| 0.787 |
|
Tub1, 2 | 586 | 84 |
|
|
Muc, por, sig | 28 | 3 |
|
| The
least differentiated histological grade, n |
|
| 0.016a |
|
Including muc,
por, sig | 22 | 8 |
|
|
Not including muc,
por, sig | 592 | 79 |
|
| Lymphatic invasion,
n |
|
|
<0.001a |
|
Ly0 | 392 | 22 |
|
| Ly1a,
b, c | 222 | 65 |
|
| Venous
invasion, n |
|
|
<0.001a |
|
V0 | 493 | 49 |
|
|
V1a, b, c | 121 | 38 |
|
| Budding grade,
n |
|
|
<0.001a |
|
BD1 | 511 | 29 |
|
| BD2,
3 | 103 | 58 |
|
Risk factors of lymph node
metastasis
The results of the univariate and multivariable
analyses of clinicopathological risk factors for LN metastasis in
the training set are shown in Table
II. The cut-off value for the SM invasion depth of 2,600 µm was
selected from Youden's index for the receiver operating
characteristic curve (11).
Left-sided CRC, deep LN invasion, poor histological grade,
lymphatic invasion, venous invasion and BD2/3 were all significant
risk factors for LN metastasis, and left-sided CRC (OR, 2.035; 95%
CI, 1.137-3.614; P=0.017), lymphatic invasion (OR, 3.812; 95% CI,
2.224-6.531; P<0.001), venous invasion (OR, 2.221; 95% CI,
1.332-3.670; P=0.002) and BD2/3 (OR, 1.969; 95% CI, 1.151-3.371;
P=0.013) were all independent risk factors.
 | Table II.Univariate and multivariable analyses
of lymph node metastasis in the training set. |
Table II.
Univariate and multivariable analyses
of lymph node metastasis in the training set.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Primary CRC
location (left/right) | 2.202 | 1.278-3.7938 | 0.003a | 2.035 | 1.137-3.614 | 0.017a |
| SM invasion depth,
µm (≥2,600/<2,600) | 1.959 | 1.232-3.114 | 0.005a | 1.574 | 0.955-2.596 | 0.075 |
| The least
differentiated histological grade |
|
|
|
|
|
|
| Muc,
por, sig/others | 2.275 | 1.173-6.328 | 0.020a | 1.954 | 0.774-4.934 | 0.156 |
| Muc,
por, sig/pap |
3.96×106 |
1.482-3.96×106 | 0.022a |
|
|
|
| Muc,
por, sig/tub1 | 3.273 | 1.277-7.741 | 0.015a |
|
|
|
| Muc,
por, sig/tub2 | 2.295 | 0.915-5.270 | 0.074 |
|
|
|
| Lymphatic invasion
(Ly1a, b, c/Ly0) | 5.217 | 3.131-8.694 |
<0.001a | 3.812 | 2.224-6.531 |
<0.001a |
| Venous invasion
(V1a, b, c/V0) | 3.160 | 1.979-5.045 |
<0.001a | 2.221 | 1.332-3.670 | 0.002a |
| Budding grade (BD2,
3/BD1) | 2.481 | 1.514-4.063 |
<0.001a | 1.969 | 1.151-3.371 | 0.013a |
Nomograms constructed using risk
factors
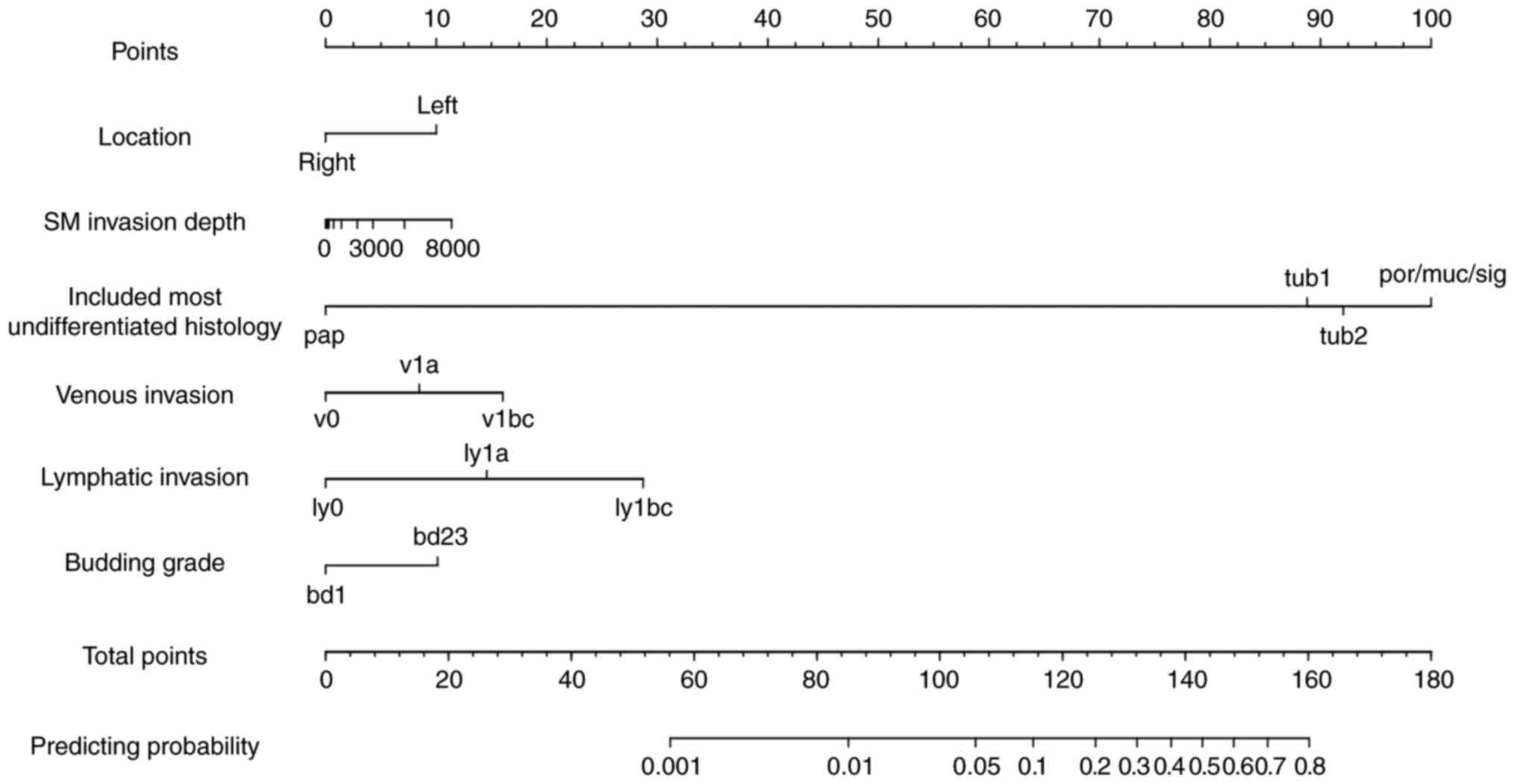
A predictive nomogram for LN metastasis in T1 CRC
was constructed using the six significant variables from the
univariate analysis, as shown in Fig.
2. The AUC of the training set was 0.786. The prediction model
was validated using the validation set, and the AUC was 0.721. The
calibration plots for both are shown in Fig. S1. A nomogram with the four
independent risk factors from the multivariable analysis was also
constructed (Figs. S2 and S3). The AUCs were 0.775 and 0.692 for the
training and validation sets, respectively. The AUC values were
higher in the nomogram with six factors than in the nomogram with
four factors. Therefore, the nomogram with six factors was
determined to be a good prediction model for LN metastasis in
patients with T1 CRC.
Next, the developed nomogram was compared with the
risk factors recommended for additional surgical resection after
endoscopic treatment. The data of the 934 included patients were
analyzed, and the AUC was 0.530 for poor histological grade, 0.525
for SM invasion depth (≥1,000 µm), 0.703 for vascular invasion and
0.586 for BD2/3 (Table III). The
nomogram utilizing all these factors together had a higher AUC
(0.779) than each risk factor alone. Risk is seen not only as
additive but also as synergistic. By combining the risk factors in
this way as a nomogram, it should be possible to produce a range of
low and high risks, which in turn produce an improved prediction.
Moreover, the sensitivity and specificity were calculated using
several cut-off values of the nomogram from the viewpoint of the
patient population for whom an operation should be recommended. A
total cut-off nomogram score of 114 points was determined as
optimal via Youden's index, yielding a sensitivity and specificity
of 0.882 and 0.637, respectively (Fig.
S4). Among the 560 patients with a score <114 points, 22 had
positive LNs (3.9%), and among the 374 patients with a score ≥114
points, 85 had positive LNs (22.7%). The cut-off value
corresponding to a sensitivity of 1.000 was 90 points; among the 40
patients with a nomogram score <90, none had positive LNs (0.0%)
(Table SIII; Fig S5).
 | Table III.List of the AUCs from the nomogram
and recommended risk factors for additional surgical resection
after endoscopic treatment in all datasets. |
Table III.
List of the AUCs from the nomogram
and recommended risk factors for additional surgical resection
after endoscopic treatment in all datasets.
|
Characteristics | AUC |
|---|
| Nomogram | 0.779 |
| Histological grade
(por, sig or muc) | 0.530 |
| Depth of invasion
(≥1,000 µm) | 0.525 |
| Vascular invasion
(venous and/or lymphatic invasion) | 0.703 |
| Venous
invasion | 0.608 |
| Lymphatic
invasion | 0.703 |
| Budding
(BD2/3) | 0.586 |
Discussion
With the development of various surgical instruments
and methods, surgery has become safer and has resulted in a lower
mortality rate in recent years; the mortality rate for CRC surgery
is ~3% (12,13), however, in recent years, older
patients and those with more severe complications have also
undergone surgery for cancer more frequently than before (14), and the survival gain is reportedly
smaller in older patients than in younger patients, with the
possibility of a subsequent increase in morbidity and mortality
rates (15). Authors of
population-based studies have warned that it is unclear whether
surgery is also the best option for elderly patients and those with
severe complications; therefore, the non-surgical treatment of
older patients with CRC has increased over time (16). Thus, the patient's background must
be considered and precision medicine suitable for each individual
case should be performed. As drug treatments for CRC have different
outcomes depending on tumor characteristics, such as its location
and gene mutations, it is important to evaluate the risk of LN
metastasis in T1 CRC.
Previously, a re-examination of risk factors for LN
metastasis in T1 CRC was reported to consider criteria for
additional surgical resection (17). A meta-analysis also identified that
an SM invasion depth >1,500 µm, vascular invasion, poorly
differentiated histology and tumor budding were significantly
associated with LN metastasis (18). To the best of our knowledge, the
present study includes the largest number of cases to date for this
topic in terms of being a complete dataset. Additionally, to the
best of our knowledge, the present study is the first to show
left-sided CRC as an independent significant risk factor for LN
metastasis in T1 CRC. A nomogram was developed that accurately
predicts the presence or absence of LN metastasis using significant
risk factors, and if a cut-off value of 90 points was selected, 40
out of 934 (4.3%) patients in the dataset could have avoided
undergoing surgery, with none of them having LN metastases.
Undoubtedly, even if the score was 90–100 points, the predicted
rate of LN metastasis was <5%, which is not considered
high-risk; however, the nomogram may help reduce overtreatment and
assess the benefits of surgery according to the individual's
surgical risk and risk of LN metastasis.
However, the present study did have certain
limitations in determining the best treatment for T1 CRC. The
present study focused on surgical treatment aimed to reduce local
recurrence and developed a nomogram by using existing
clinicopathological factors. BRAF mutation is reportedly
related to LN metastases (19);
however, the study data lack information on the genetic status.
Furthermore, although a high LN metastasis rate predicted by a
combination of transcriptomic biomarkers has been reported
(20,21), this issue was not examined in the
present study. Further combinations of these useful molecular
biological parameters may lead to an improvement in predictions. By
contrast, local recurrence at the resection site or regional LNs
can be prevented through surgical resection with LN dissection, but
not distant metastasis. There have been reports of cases where no
local recurrence or distant metastasis was observed in
endoscopically treated T1 CRC (8,22).
Distant metastases may be caused by other tumor characteristics
that influence mortality. The KRAS, NRAS, BRAF and
microsatellite stability statuses do not seem to be useful for
prognosis in T1 CRC cases (19) and
may differ from that in cases of advanced cancer. Further genetic
analysis in T1 CRC should improve prognosis. In addition,
information on the number of LNs dissected in this multi-center
study was not collected, since there was no significant association
between the number of retrieved LNs and the presence of LN
metastases in the data set of the previous study (9).
Despite these limitations, the new nomogram for the
prediction of LN metastasis in T1 CRC established in the present
study is a useful clinical tool that may help clinicians provide
personalized medical care, resulting in a reduction of
overtreatment.
In conclusion, left-sided CRC, SM invasion depth,
poor histological grade, lymphatic invasion, vascular invasion and
BD2/3 were significant factors for the prediction of LN metastasis
in T1 CRC cases. A nomogram using these variables may be able to
predict LN metastases with high accuracy.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SF, NM and CSGOCG were responsible for the study
conceptualization and design. MK, MY, MO, HO, YI, TS, MT, TT, KD,
YS, KM, HY and SN made substantial contributions to acquisition and
interpretation of data. KO and EM were responsible for pathological
examination. SF, NM, MK, MY, HO, TS and YS confirm the authenticity
of all the raw data. HT, MU, YD and HE were responsible for the
analysis and interpretation of data. SF was responsible for
drafting the manuscript. SF and NM were responsible for statistical
analysis. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was performed in accordance with the
Declaration of Helsinki and was approved by The Osaka University
Ethics Committee (Suita, Japan; approval no. 17448-4), The Minoh
City Hospital Ethics Committee (Minoh, Japan; R0203B92), The
Toyonaka Municipal Hospital Ethics Committee (Toyonaka, Japan;
2019-03-04-5), The Osaka International Cancer Institute Ethics
Committee (Osaka, Japan; 1607229069-3), The Japan Community Health
Care Organization Osaka Hospital Ethics Committee (Osaka, Japan;
2019-50) and The Osaka Rosai Hospital Ethics Committee (Sakai,
Japan; 2022-121).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the receiver operating
characteristic curve
|
|
CI
|
confidence interval
|
|
CRC
|
colorectal cancer
|
|
JSCCR
|
Japanese Society for Cancer of the
Colon and Rectum
|
|
LN
|
lymph node
|
|
muc
|
mucinous carcinoma
|
|
OR
|
odds ratio
|
|
por
|
poorly differentiated
adenocarcinoma
|
|
sig
|
signet-ring cell carcinoma
|
|
SM
|
submucosal
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Labianca R, Nordlinger B, Beretta GD,
Mosconi S, Mandalà M, Cervantes A and Arnold D; ESMO Guidelines
Working Group, : Early colon cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 24
(Suppl 6):vi64–vi72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glynne-Jones R, Wyrwicz L, Tiret E, Brown
G, Rödel C, Cervantes A and Arnold D: Rectal cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 29 (Suppl 4):iv2632018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomita N, Ishida H, Tanakaya K, Yamaguchi
T, Kumamoto K, Tanaka T, Hinoi T, Miyakura Y, Hasegawa H, Takayama
T, et al: Japanese society for cancer of the colon and rectum
(JSCCR) guidelines 2020 for the clinical practice of hereditary
colorectal cancer. Int J Clin Oncol. 26:1353–1419. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka S, Oka S and Chayama K: Colorectal
endoscopic submucosal dissection: Present status and future
perspective, including its differentiation from endoscopic mucosal
resection. J Gastroenterol. 43:641–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferlitsch M, Moss A, Hassan C, Bhandari P,
Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M,
Nalankilli K, et al: Colorectal polypectomy and endoscopic mucosal
resection (EMR): European society of gastrointestinal endoscopy
(ESGE) clinical guideline. Endoscopy. 49:270–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueno H, Hase K, Hashiguchi Y, Shimazaki H,
Yoshii S, Kudo SE, Tanaka M, Akagi Y, Suto T, Nagata S, et al:
Novel risk factors for lymph node metastasis in early invasive
colorectal cancer: A multi-institution pathology review. J
Gastroenterol. 49:1314–1323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ikematsu H, Yoda Y, Matsuda T, Yamaguchi
Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T,
et al: Long-term outcomes after resection for submucosal invasive
colorectal cancers. Gastroenterology. 144:551–559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujino S, Miyoshi N, Ohue M, Yasui M,
Sugimura K, Akita H, Takahashi H, Kobayashi S, Fujiwara Y, Yano M,
et al: A nomogram for predicting lymph node metastasis in
submucosal colorectal cancer. Int Surg. 102:102–108. 2017.
View Article : Google Scholar
|
|
10
|
Japanese Society for Cancer of the Colon
and Rectum, . Japanese classification of colorectal, appendiceal,
and anal carcinoma: The 3d english edition [secondary publication].
J Anus Rectum Colon. 3:175–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Youden WJ: Index for rating diagnostic
tests. Cancer. 3:32–35. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Nes LC, Hannink G, 't Lam-Boer J, Hugen
N, Verhoeven RH and de Wilt JHW; Dutch Colorectal Audit Group, :
Postoperative mortality risk assessment in colorectal cancer:
Development and validation of a clinical prediction model using
data from the Dutch ColoRectal Audit. BJS Open. 6:zrac0142022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vallance AE, Fearnhead NS, Kuryba A, Hill
J, Maxwell-Armstrong C, Braun M, van der Meulen J and Walker K:
Effect of public reporting of surgeons' outcomes on patient
selection, ‘gaming,’ and mortality in colorectal cancer surgery in
England: Population based cohort study. BMJ. 361:k15812018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueda Y, Shiraishi N, Kawasaki T, Akagi T,
Ninomiya S, Shiroshita H, Etoh T and Inomata M: Short- and
long-term outcomes of laparoscopic surgery for colorectal cancer in
the elderly aged over 80 years old versus non-elderly: A
retrospective cohort study. BMC Geriatr. 20:4452020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papamichael D, Audisio RA, Glimelius B, de
Gramont A, Glynne-Jones R, Haller D, Köhne CH, Rostoft S, Lemmens
V, Mitry E, et al: Treatment of colorectal cancer in older
patients: International society of geriatric oncology (SIOG)
consensus recommendations 2013. Ann Oncol. 26:463–476. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van der Vlies E, Vernooij LM, van Erning
FN, Vink GR, Bos WJW, Portielje JEA, Noordzij PG and Los M:
Survival of surgical and non-surgical older patients with
non-metastatic colorectal cancer: A population-based study in the
Netherlands. Eur J Surg Oncol. 47:3144–3150. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barel F, Cariou M, Saliou P, Kermarrec T,
Auffret A, Samaison L, Bourhis A, Badic B, Jézéquel J, Cholet F, et
al: Histopathological factors help to predict lymph node metastases
more efficiently than extra-nodal recurrences in submucosa invading
pT1 colorectal cancer. Sci Rep. 9:83422019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dykstra MA, Gimon TI, Ronksley PE, Buie WD
and MacLean AR: Classic and novel histopathologic risk factors for
lymph node metastasis in T1 colorectal cancer: A systematic review
and meta-analysis. Dis Colon Rectum. 64:1139–1150. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bourhis A, De Luca C, Cariou M, Vigliar E,
Barel F, Conticelli F, Marcorelles P, Nousbaum JB, Robaszkiewicz M,
Samaison L, et al: Evaluation of KRAS, NRAS and BRAF mutational
status and microsatellite instability in early colorectal
carcinomas invading the submucosa (pT1): Towards an in-house
molecular prognostication for pathologists? J Clin Pathol.
73:741–747. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wada Y, Shimada M, Murano T, Takamaru H,
Morine Y, Ikemoto T, Saito Y, Balaguer F, Bujanda L, Pellise M, et
al: A liquid biopsy assay for noninvasive identification of lymph
node metastases in T1 colorectal cancer. Gastroenterology.
161:151–162.e1. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyazaki K, Wada Y, Okuno K, Murano T,
Morine Y, Ikemoto T, Saito Y, Ikematsu H, Kinugasa Y, Shimada M and
Goel A: An exosome-based liquid biopsy signature for pre-operative
identification of lymph node metastasis in patients with
pathological high-risk T1 colorectal cancer. Mol Cancer. 22:22023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoda Y, Ikematsu H, Matsuda T, Yamaguchi
Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T,
et al: A large-scale multicenter study of long-term outcomes after
endoscopic resection for submucosal invasive colorectal cancer.
Endoscopy. 45:718–724. 2013. View Article : Google Scholar : PubMed/NCBI
|