Introduction
Neoadjuvant chemotherapy (NAC) is being increasingly
used for operable breast cancer (BC) which is either locally
advanced or has some risk factors (1–3). NAC
benefits include increased rate of breast-conserving surgery (BCS),
higher chance of tumor completion resection, and most importantly,
providing valuable data about tumor response to chemotherapy
(4–6). For example, randomized clinical trials
(RCTs) have previously reported that human epidermal growth factor
receptor 2 positive (HER2+) or triple negative BC (TNBC) patients
had a better prognosis as a result of the changes to the
chemotherapy strategy, based on the pathologic complete response
(pCR) status after NAC (7–9). However, the routine use of NAC instead
of adjunct chemotherapy (AC) in these patients with BC is still
controversial (10,11). Although overall survival (OS)
following NAC and AC were comparable in these trials, certain
concerns about NAC have been reported. For example, certain studies
have reported that NAC is associated with higher risk of
locoregional recurrence (LR), frequent inconsistent correlation
between pCR status and OS, and increased complexity of surgery
(4,6,12,13).
Some researchers have hypothesized that NAC may delay tumor surgery
and make it more conservative which can lead to a higher chance of
tumor recurrence in certain subgroups of patients with BC,
especially those with tumors insensitive to NAC (6,14).
In China, the use of NAC in practice has been slow.
Many Chinese patients with BC have low desire for BCS and are more
concerned about how NAC could delay surgery and increase tumor
progression (15). Furthermore,
chemotherapy side effects such as fatigue, nausea, vomiting,
leukopenia, emotional stress and unsatisfactory tumor reduction
could discourage the patient or surgeon from continuing the NAC
regime or completing the cycles as planned and then pursuing the
necessary AC after the surgery done. The aforementioned issues
indicate why the application of NAC in clinical practice is often
different from that in trials. As a result, a retrospective study
to characterize the possible different risk profiles of NAC vs. AC
vs. their combinative chemotherapy modes, on disease-free survival
(DFS) in patients with BC using real-world data was designed. After
considering the history of NAC administration, an analysis was
performed of all patients with BC at the first tumor recurrence
over an 11-year time period.
Patients and methods
Patients and study design
All women with unilateral primary TNM stage I–III BC
(7th edition, AJCC/UICC) and first tumor recurrence in 2008–2018 at
The Fourth Hospital of Hebei Medical University were enrolled in
the present study (16). Patient
inclusion criteria included: i) Age at diagnosis ≥18 years and
diagnosed in 1997 or later; and ii) BC diagnosed pathologically as
LR or presented clinically distant metastasis by computed
tomography, magnetic resonance, bone scintigraphy or positron
emission tomography imaging. In the present study, LR was defined
as tumor recurrence at contralateral or ipsilateral breast, chest
wall, or regional lymph node (LN) at axillary, supraclavicular,
infraclavicular and internal mammary regions. Exclusion criteria
included: i) Inflammatory BC; ii) contralateral BC diagnosed within
6 months following the primary BC diagnosis; iii) any recurrence
diagnosed before the completion of chemotherapy or RT; and iv) any
recurrence potentially from other cancers.
In the analysis, the primary BC diagnosis date was
defined as the baseline. The earliest date of all tumor recurrences
diagnosed in regular medical check-ups was regarded as the endpoint
date. DFS in days was calculated as the number of days between the
endpoint date and the baseline plus 1. Three time-to-event outcomes
of; any tumor recurrence, LR and organ metastasis were
analyzed.
The present study was approved by the Research
Ethics Committee of the Fourth Hospital of Hebei Medical University
(approval no. 2017-288). All participants provided written informed
consent. All sensitive health information of participants was
excluded from the study dataset.
Chemotherapy mode and regimen
While the National Comprehensive Cancer Network
(NCCN) guidelines for BC treatments were followed, the actual
timing and number of cycles of chemotherapy were at the discretion
of the individual physician. The most common regimens of
chemotherapy mainly consisted of the dose-dense doxorubicin and
cyclophosphamide followed by paclitaxel; docetaxel and
cyclophosphamide; cyclophosphamide, methotrexate and fluorouracil
epirubicin and cyclophosphamide; or docetaxel, doxorubicin and
cyclophosphamide. Trastuzumab was the only HER2 targeted therapy
drug available to be administered. Due to the high out-of-pocket
cost, trastuzumab was administered only in a small portion of HER2+
patients. Based on the timing of the chemotherapy cycles, patients
were assigned to one of four modes, as follows: ‘None’, ‘NAC only’,
‘NAC+AC’ or ‘AC only’.
Statistical methods
Continuous and categorical variables were analyzed
using descriptive statistics. Analysis of variance (ANOVA),
χ2 or Fisher's exact tests, were used to perform
statistical comparisons as appropriate. The Kaplan-Meier curve and
log-rank test were used to analyze DFS rates. The Cox survival
model was used to estimate the hazard ratio (HR), 95% confidence
interval (CI) and P-value. Two-sided P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SAS 9.4 for Windows (SAS Institute,
Inc.).
Results
Characteristics at baseline
Table I presented
the 637 patients who were enrolled after reviewing all hospital
admissions (n=16,891) of patients with BC. Among the enrolled
patients, 25.9% (n=165) received NAC. Many variables at baseline
were significantly associated with the choice of chemotherapy
modes. Because the number of chemotherapy cycles was not normally
distributed, its three-level category was created for analysis.
While the proportion of ‘1–4 cycles’ in ‘AC only’ patients was
23.1% (103/445), it was markedly lower, 7.9% (13/165) in combined
NAC patients. Furthermore, the proportion of ‘1–4 cycles’ was
significantly higher in ‘NAC only’ patients than in ‘NAC+AC’
patients [14.9% (7/47) vs. 5.1% (6/118), P=0.035]. The
aforementioned results indicated that NAC patients had either less
cycles of NAC alone or, more likely, did not attend for AC after
surgery.
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
|
|
| Chemotherapy
mode |
|
|---|
|
|
|
|
|
|---|
| Characteristic | All | None | NAC only | NAC+AC | AC only |
P-valuea |
|---|
| Patients (n,
%) | 637 (100) | 27 (4.2) | 47 (7.4) | 118 (18.5) | 445 (69.9) |
|
| Mean age ± SD,
years | 48.2±10.4 | 54.8±13.8 | 48.5±10.2 | 48.8±10.1 | 47.6±10.1 | 0.005 |
| Diagnosis
yearb, n (%) |
|
|
|
|
| <0.001 |
|
1997-2010 | 251 (39.4) | 10 (4.0) | 5 (2.0) | 41 (16.3) | 195 (77.7) |
|
|
2011-2016 | 386 (60.6) | 17 (4.4) | 42 (10.9) | 77 (19.9) | 250 (64.8) |
|
| ECOG grade, n
(%) |
|
|
|
|
| 0.040 |
| 0 | 617 (96.5) | 25 (4.0) | 46 (7.5) | 117 (19.0) | 429 (69.5) |
|
|
1-2 | 20 (3.5) | 2 (10.0) | 1 (5.0) | 1 (5.0) | 16 (80.0) |
|
| Tumor laterality, n
(%) |
|
|
|
|
| 0.230 |
|
Left | 351 (55.1) | 14 (4.0) | 24 (6.8) | 75 (21.4) | 238 (67.8) |
|
|
Right | 286 (44.9) | 13 (4.5) | 23 (8.0) | 43 (15) | 207 (72.4) |
|
| Tumor surgery, n
(%) |
|
|
|
|
| 0.053 |
|
Lumpectomy | 22 (3.5) | 3 (13.6) | 0 (0.0) | 2 (9.1) | 17 (77.3) |
|
|
Mastectomy | 615 (96.5) | 24 (3.9) | 47 (7.6) | 116 (18.9) | 428 (69.6) |
|
| LN procedure, n
(%) |
|
|
|
|
|
|
|
ALND | 579 (90.9) | 16 (2.8) | 47 (8.1) | 113 (19.5) | 403 (69.6) | <0.001 |
|
SLND | 22 (3.5) | 0 (0.0) | 3 (13.6) | 14 (63.6) | 5 (22.7) | <0.001 |
|
IMLND | 17 (2.7) | 0 (0.0) | 4 (23.5) | 5 (29.4) | 8 (47.1) | 0.134 |
| T stage, n (%) |
|
|
|
|
| <0.001 |
| T1 | 141 (22.1) | 6 (4.3) | 7 (5.0) | 10 (7.1) | 118 (83.7) |
|
| T2 | 338 (53.1) | 16 (4.7) | 10 (3.0) | 51 (15.1) | 261 (77.2) |
|
|
T3-4 | 114 (17.9) | 0 (0.0) | 30 (26.3) | 55 (48.2) | 29 (25.4) |
|
|
Unknown | 44 (6.9) | 5 (11.4) | 0 (0.0) | 2 (4.5) | 37 (84.1) |
|
| N stage, n (%) |
|
|
|
|
| <0.001 |
| N0 | 236 (37) | 16 (6.8) | 3 (1.3) | 14 (5.9) | 203 (86.0) |
|
| N1 | 167 (26.2) | 6 (3.6) | 9 (5.4) | 26 (15.6) | 126 (75.4) |
|
| N2 | 74 (11.6) | 1 (1.4) | 8 (10.8) | 14 (18.9) | 51 (68.9) |
|
| N3 | 141 (22.1) | 1 (0.7) | 27 (19.1) | 63 (44.7) | 50 (35.5) |
|
|
Unknown | 19 (3.0) | 3 (15.8) | 0 (0.0) | 1 (5.3) | 15 (78.9) |
|
| Tumor pathology, n
(%) |
|
|
|
|
| 0.345 |
|
IDC | 529 (83.0) | 21 (4.0) | 42 (7.9) | 102 (19.3) | 364 (68.8) |
|
|
Other | 108 (17.0) | 6 (5.6) | 5 (4.6) | 16 (14.8) | 81 (75.0) |
|
| Tumor grade, n
(%) |
|
|
|
|
| 0.070 |
| I | 8 (1.3) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 7 (87.5) |
|
| II | 393 (61.7) | 17 (4.3) | 34 (8.7) | 86 (21.9) | 256 (65.1) |
|
|
III | 140 (22.0) | 4 (2.9) | 8 (5.7) | 15 (10.7) | 113 (80.7) |
|
| Not
reported | 96 (15.1) | 6 (6.3) | 5 (5.2) | 16 (16.7) | 69 (71.9) |
|
| LVI, n (%) |
|
|
|
|
| <0.001 |
|
Positive | 152 (23.9) | 3 (2.0) | 5 (3.3) | 43 (28.3) | 101 (66.4) |
|
|
Negative | 164 (25.7) | 4 (2.4) | 8 (4.9) | 21 (12.8) | 131 (79.9) |
|
| Not
reported | 321 (50.4) | 20 (6.2) | 34 (10.6) | 54 (16.8) | 213 (66.4) |
|
| Subtype, n (%) |
|
|
|
|
| 0.036 |
| Luminal
A | 258 (40.5) | 11 (4.3) | 14 (5.4) | 43 (16.7) | 190 (73.6) |
|
| Luminal
B | 132 (20.7) | 6 (4.5) | 16 (12.1) | 21 (15.9) | 89 (67.4) |
|
|
HER2-enriched | 97 (15.2) | 2 (2.1) | 9 (9.3) | 21 (21.6) | 65 (67.0) |
|
|
TNBC | 86 (13.5) | 2 (2.3) | 8 (9.3) | 22 (25.6) | 54 (62.8) |
|
|
Unclassified | 64 (10.0) | 6 (9.4) | 0 (0.0) | 11 (17.2) | 47 (73.4) |
|
| Chemotherapy,
cycles, n (%) |
|
|
|
|
| <0.001 |
| 0 | 27 (4.2) | 27 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
1-4 | 116 (18.2) | 0 (0.0) | 7 (6.0) | 6 (5.2) | 103 (88.8) |
|
| ≥5 | 494 (77.6) | 0 (0.0) | 40 (8.1) | 112 (22.7) | 342 (69.2) |
|
| Systemic therapy, n
(%) |
|
|
|
|
|
|
| ET | 232 (36.4) | 7 (3.0) | 12 (5.2) | 33 (14.2) | 180 (77.6) | 0.015 |
| RT | 264 (41.4) | 1 (0.4) | 25 (9.5) | 75 (28.4) | 163 (61.7) | <0.001 |
|
Trastuzumab | 12 (1.9) | 0 (0.0) | 0 (0.0) | 5 (41.7) | 7 (58.3) | 0.158 |
Characteristics of tumor
recurrence
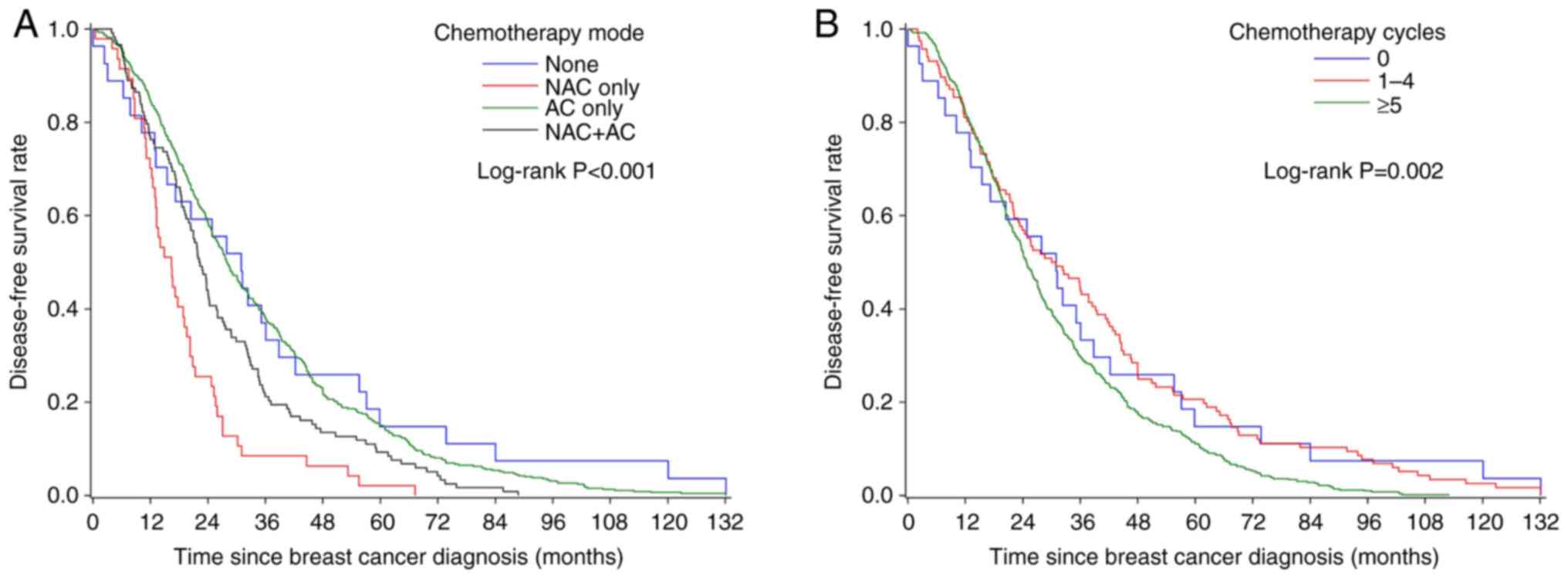
The overall median DFS was 25.9 months (Table II). The DFS rates were
significantly different among chemotherapy mode (P<0.001,
Fig. 1A) and cycle (P=0.002,
Fig. 1B) subgroups. Patients who
had ‘NAC only’ or had ≥5 chemotherapy cycles had the lowest DFS.
Given the selection of chemotherapy mode varied by factors such as
tumor size, LN status and BCS pursuit, as considered by the
oncologist, multivariate analysis was performed to verify the
independence of these observed links.
 | Table II.Characteristics of tumor recurrence
(n=637). |
Table II.
Characteristics of tumor recurrence
(n=637).
|
|
| Chemotherapy
mode |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Al | None | NAC only | NAC+AC | AC only |
P-valuea |
|---|
| Mean age ± SD,
years | 50.9±10.6 | 57.9±13.2 | 50.1±10.1 | 51.0±10.1 | 50.5±10.4 | 0.001 |
| Median DFS, months
(95%CI) | 25.9
(24.1-27.8) | 31.4
(13.3-39.4) | 16.6
(13.2-19.3) | 22.6
(19.7-24.7) | 28.4
(26.1-31.2) | <.001 |
| Recurrence site, n
(%) |
|
|
|
|
|
|
|
Ipsilateral region | 321 (50.4) | 16 (5.0) | 23 (7.2) | 51 (15.9) | 231 (72.0) | 0.293 |
|
Contralateral region | 123 (19.3) | 4 (3.3) | 18 (14.6) | 26 (21.1) | 75 (61.0) | 0.004 |
| Any
organ | 453 (71.1) | 20 (4.4) | 34 (7.5) | 82 (18.1) | 317 (70.0) | 0.959 |
| Recurrence organ, n
(%) |
|
|
|
|
|
|
|
Bone | 254 (39.9) | 11 (4.3) | 19 (7.5) | 37 (14.6) | 187 (73.6) | 0.218 |
|
Liver | 156 (24.5) | 3 (1.9) | 12 (7.7) | 33 (21.2) | 108 (69.2) | 0.331 |
|
Lung | 154 (24.2) | 9 (5.8) | 10 (6.5) | 30 (19.5) | 105 (68.2) | 0.652 |
|
Brain | 35 (5.5) | 1 (2.9) | 3 (8.6) | 9 (25.7) | 22 (62.9) | 0.675 |
|
Other | 107 (16.8) | 5 (4.7) | 5 (4.7) | 25 (23.4) | 72 (67.3) | 0.379 |
| Recurrence type, n
(%) |
|
|
|
|
|
|
| Region
only | 137 (21.5) | 6 (4.4) | 4 (2.9) | 23 (16.8) | 104 (75.9) | 0.074 |
| Organ
only | 315 (49.5) | 10 (3.2) | 24 (7.6) | 67 (21.3) | 214 (67.9) |
|
| Region
and organ | 185 (29.0) | 11 (5.9) | 19 (10.3) | 28 (15.1) | 127 (68.6) |
|
Cox survival models
Table III shows
the analysis results. The covariates were determined based on the
model building process and literature review. Compared with the ‘AC
only’ patients, the ‘NAC only’ patients were significantly
associated with higher risks of any recurrence (HR 1.452, P=0.037)
and organ metastasis (HR 2.675, P=0.003) after the adjustment of
covariates. No significant difference in the risk of any
recurrence, LR and organ metastasis were demonstrated among the
‘NAC+AC’ patients and the ‘AC only’ patients (all HR 0.945-1.141,
P=0.523-0.765). No difference in the risks linked to the ‘1–4
cycles’ (vs. ‘≥5 cycles’) of events was demonstrated by
multivariate analyses for any recurrence (HR 0.811, P=0.062), LR
(HR 0.824, P=0.207), and organ metastasis (HR 0.817, P=0.439) (data
not shown).
 | Table III.Cox proportional hazard regression
analysis of recurrence type. |
Table III.
Cox proportional hazard regression
analysis of recurrence type.
| A, Any
recurrence |
|---|
|
|---|
|
| Univariate |
Multivariatea |
|---|
| Chemotherapy | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| None | 0.835 | (0.56-1.245) | 0.376 | 1.182 | (0.682-2.047) | 0.551 |
| NAC only | 2.378 | (1.752-3.228) | <0.001 | 1.452 | (1.022-2.062) | 0.037 |
| NAC + AC | 1.388 | (1.131-1.703) | 0.002 | 1.085 | (0.845-1.394) | 0.523 |
| AC only | 1.000 |
| ref. | 1.000 |
| ref. |
|
| B, regional
recurrence |
|
|
|
Univariate |
Multivariatea |
|
|
|
|
|
Chemotherapy | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| None | 0.915 | (0.539-1.553) | 0.742 | 1.060 | (0.507-2.216) | 0.878 |
| NAC only | 2.278 | (1.474-3.522) | <0.001 | 1.448 | (0.867-2.419) | 0.157 |
| NAC + AC | 1.161 | (0.855-1.575) | 0.339 | 0.945 | (0.652-1.369) | 0.765 |
| AC only | 1.000 |
| ref. | 1.000 |
| ref. |
|
| C, Organ
metastasis |
|
|
|
Univariate |
Multivariatea |
|
|
|
|
|
Chemotherapy | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| None | 0.679 | (0.244-1.893) | 0.460 | 1.090 | (0.273-4.345) | 0.903 |
| NAC only | 5.960 | (3.510-10.122) | <0.001 | 2.675 | (1.392-5.139) | 0.003 |
| NAC + AC | 1.880 | (1.197-2.951) | 0.006 | 1.141 | (0.656-1.983) | 0.641 |
| AC only | 1.000 |
| ref. | 1.000 |
| ref. |
Stratified Cox analysis
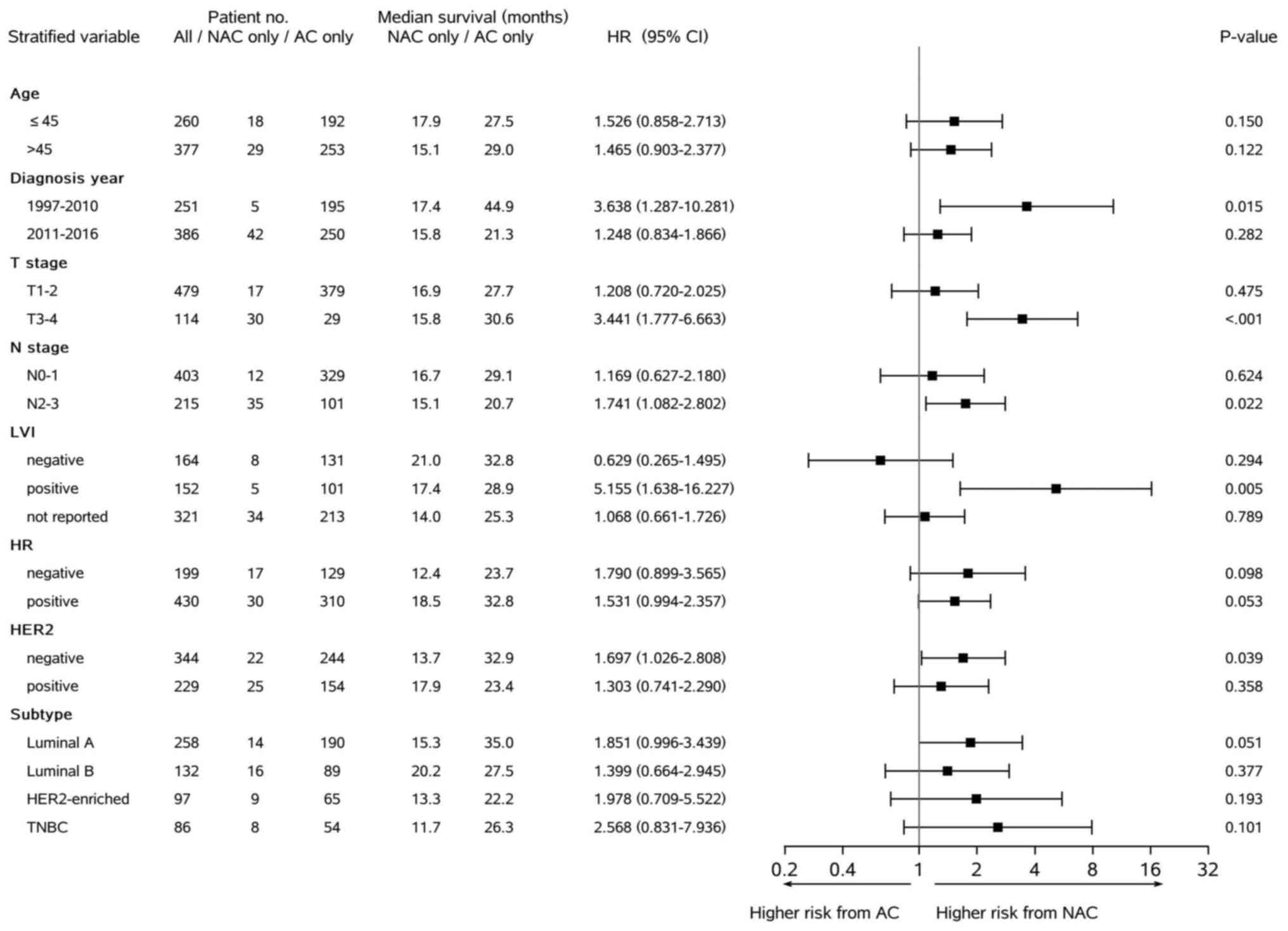
Fig. 2 demonstrated
that the significantly higher risk of any recurrence linked to the
‘NAC only’ mode compared with the ‘AC only’ mode was particularly
presented in certain subgroups of patients. Specifically, it was
present in patients with BC characterized by diagnosis and
treatment in earlier years of the study period (HR 3.638, P=0.015),
stage T3–4 (HR 3.441, P<0.001), stage N2–3 (HR 1.741, P=0.022),
lymphovascular invasion positive (LVI+) (HR 5.155, P=0.005) or
HER2-negative (HR 1.697, P=0.039). There were similar results from
the stratified Cox analysis of organ metastasis (data not
shown).
Discussion
For numerous reasons, such as different populations
and interventions, real-world data could provide risk profiles of
variables on tumor recurrence different to those generated in RCTs.
Through adjusted and stratified analyses, this retrospective study
demonstrated that, compared with the most commonly used ‘AC only’
mode, the ‘NAC only’ mode was associated with a significantly
higher risk of tumor recurrence, especially at distant organ. These
links were more specifically presented in high-risk BC subgroup
patients characterized by stage T3-4, stage N2-3, LVI+ or
HER2-negative. It could be hypothesized that the ‘inadequate’ total
cycles of chemotherapy in BC patients who had NAC could have played
a role. Another factor could possibly be the circumstances-these
‘NAC+AC’ patients would become ‘NAC only’ patients if they
abandoned the planned AC. Given the potential clinical value of
chemotherapy modes, more studies on the real-world data are needed
to confirm and explain this finding.
Many factors can influence the decision-making for
initiation, elongation, and termination of NAC in BC patients in
clinical practice. Under the NCCN guidelines, NAC is usually
recommended to patients with operable BC with larger tumors, more
metastatic LN, HER2+, TNBC or stronger desire for BCS (17). For most early TNBC patients, NAC is
currently used as the standard treatment while AC is only used for
some patients opting for upfront surgery (18,19).
The present study focused on patients with BC with first tumor
recurrence in the past. It was noted that, different from the
current guideline, the AC mode was demonstrated to be administered
markedly more often than the NAC mode 62.8% (54/86) compared with
9.3% (8/86) in TNBC patients (Table
I, Fig. 2). Furthermore, a
previous meta-analysis (9 studies, 2,109 patients) reported that
the inclusion of platinum in NAC regimens led to a significantly
higher rate of pCR (51.2% vs. 37.0%; OR 1.96, 95%CI 1.46-2.62) in
TNBC patients, but no significant improvement in OS (HR 0.86, 95%CI
0.46-1.63) (20). Many factors are
involved the decision-making of NAC against AC. Based on the
results of the present study, the tumor recurrence risk profiles of
chemotherapy modes should consider factors such as patient age,
performance status, tumor feature, tumor response, adverse effects
of prior chemotherapy and others. In the present study, data for
pCR and adverse effects of chemotherapy were lacking for additional
analysis. However, the consistent results of multivariate analyses
indicated that the covariates assessed could not fully account for
the study's results.
NAC and AC may have different drug mechanisms for
reducing risk of tumor recurrence in BC patients (6). While surgery can cure the primary
tumor, systemic treatments of NAC or AC are reported to be required
to eliminate the occult tumor cells (6,21).
Previous studies demonstrated that the effectiveness of AC varies
with BC subtypes, metastatic LN status, dosage and number of cycles
of chemotherapy regimens (22–25).
For example, only patients with estrogen receptor-positive (ER+)
and LN-negative BC with high 21-gene recurrence scores have been
reported to benefit from adding chemotherapy to ET based on
prognosis (26–28). In patients with ER-negative (ER-) or
HER2+ BC, only having AC over a certain dosage of its regimens and
number of cycles has been reported to lead to improved DFS and OS
(23,29). The present study was not able to
exclude the possibility that NAC and AC may have different
mechanisms and effectiveness in reducing the tumor recurrence risk
in certain BC subgroups.
Numerous recent studies have reported that the pCR
status after a certain number of cycles of NAC could provide
valuable guidance to the application of AC (9,30). For
example, the KATHERINE trial included 1,486 patients with early
HER2+ BC who had non-pCR after NAC and were randomized into the
trastuzumab armor T-DM1 arm in AC (9). The trial reported that there was an
11.3% absolute gain in 3-year DFS (HR 0.50; 95%CI 0.39-0.64) in the
T-DM1 arm, compared to the trastuzumab arm (9). The CREATE-X trial included 910
patients with early HER2-negative BC who had non-pCR after NAC
reported that adding capecitabine for 6–8 cycles significantly
improved the 5-year DFS (HR 0.70, 95%CI 0.53-0.92) and 5-year OS
(HR 0.59, 95%CI 0.39-0.90) (7).
This benefit was larger in the TNBC subgroup patients (n=286) with
5-year DFS (HR 0.58, 95% CI 0.39-0.87) and 5-year OS (HR 0.52, 95%
CI 0.30-0.90) (7). Given these
results, the adapted strategy of modifying AC based on the pCR
status after NAC has become more widely used in recent years
(18). In the present study,
trastuzumab, being the only anti-HER2 target agent used, was
administered to only a few HER2+ patients. As stated previously,
how the AC was discontinued or changed after NAC and surgery could
not be summarized for any possible analysis in the study. The ‘NAC
only’ patients had significantly less cycles of chemotherapy
compared with the ‘NAC+AC’ patients. Since the chemotherapy cycle
number was not normally distributed, the three levels were used one
covariate in subsequent analysis.
There are a limited number of published studies that
explore the relationship of NAC and AC with DFS and OS (6,13,14,31).
In 2008, one combined analysis of two RCTs [National Surgical
Adjuvant Breast and Bowel Project (NSABP) B-18, B-27] reported that
the NAC and AC approaches did not have different tumor recurrence
rates and OS in BC patients (13).
However, a 2005 meta-analysis of 9 RCTs (n=3,946) reported that
compared with the AC, the NAC approach was associated with a higher
LR [relative risk (RR) 1.22, P=0.015] and had similar rates of any
recurrence and BC-related mortality (14). An Early Breast Cancer Trialists
Collaborative Group meta-analysis which compared NAC and AC (10
RCTs, N=4,756, 9-year median follow-up) reported that NAC was
significantly associated with a higher BCS rate (65 vs. 49%) and
significantly higher LR rate (LRR; +5.5%; RR 1.37, 95% CI
1.17-1.61, P=0.0001) (6). To
interpret these results with caution, certain researchers believed
that it was the NAC-related more conservative surgery which
contributed to a higher LRR (6,11).
Recently, a retrospective analysis of19,151 stage II–III TNBC
patients from the National Cancer Database reported that the NAC
patients had a lower OS compared with the AC patients (73.6 vs.
76.8%, P<0.0001) (31).
Furthermore, the subgroup of patients with pCR after the NAC were
associated with a significantly improved 5-year OS compared with
the non-PCR patients (86.2 vs. 62.3%, P<0.0001) (31). The authors concluded that NAC may be
inferior to AC in TNBC for tumor control; however, the role of
patient selection bias could not be completely ruled out (31). In the present retrospective study, a
poorer PFS was associated with the ‘NAC only’ vs. ‘AC only’ groups
in TNBC patients as well.
The potential role of many factors in causation
meant it was challenging to explain the study findings. Numerous
patient, disease and physician factors can influence the choice and
continuity of NAC or AC in patients with BC. Through multivariate
analyses that adjust these factors, the present study assessed the
independent risk profiles of chemotherapy modes on DFS. This
analysis strategy along with stratifying the population in the
present study, demonstrated that compared with the traditional ‘AC
only’ approach, the ‘NAC only’ approach was associated with a
higher risk of tumor recurrence. Based on the results of the
present study it was hypothesized that an independent link of ‘NAC
only’ with a higher risk of recurrence in the real-world practice
exists. It was hypothesized that this could be due to the possible
unintentional use of ‘inadequate’ chemotherapy cycles in NAC
patients and unrecognized difference of drug mechanism of NAC vs.
AC regimen in medical and surgical practice. Furthermore, the
present study indicated that the aforementioned differences could
be more evident in subgroup patients with BC characterized as T2-3,
N2-3, LVI+ or HER2-negative.
The strengths of the present study included the
large sample size of real-world data about patients with BC with
first tumor recurrence, a large number of covariates for model
adjustment and the use of stratified analyses. The limitations of
the present study included being unable to infer a cause-effect
relationship from this retrospective observational study, the
possible existence of unadjusted confounders, the lack of pCR and
drug side effect data, and the lack of recorded reasons to add or
discontinue the NAC or AC for additional analysis. Furthermore, the
different BC populations and treatment administrations (e.g., BCS
procedure, inclusion of platinum in TNBC NAC, trastuzumab
availability, and pCR as guidance) mean it would be challenging to
simply compare and apply the findings of the present study to
patients with BC outside of China.
While the results of the present study require
further evaluation, oncologists should be aware that patients with
BC with a high risk of recurrence could benefit from the use of
full cycles of NAC or adding AC after ‘inadequate’ NAC for improved
tumor control.
NAC alone is potentially associated with a higher
risk of tumor recurrence in high-risk BC subgroup patients based on
real-world data from clinical practice. Patient selection of
chemotherapy mode was involved in practice but could not fully
explain this finding.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received
Availability of data and materials
The anonymised datasets used and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
ZSL designed and supervised this study. ZSL, YL and
YJL were responsible for the data analysis and manuscript writing.
YGS, YRZ, XHJ, HH and KYD participated in the data collection, data
quality control, data interpretation, and analysis discussion. YJL
and JZ contributed to the interpretation of data and study results
as well as providing critical discussion and revision of the
manuscript. ZSL and YL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of The Fourth Hospital of Hebei Medical University
(approval no. 2017-288). All patients provided written informed
consent before study enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
Authors' information
Dr Zhensheng Li: ORCID: 0000-0002-6928-0050. Dr
Yunjiang Liu: ORCID: 0000-0001-7202-2004.
Glossary
Abbreviations
Abbreviations:
|
AC
|
adjunct chemotherapy
|
|
ALND
|
axillary lymph node dissection
|
|
ANOVA
|
analysis of variance
|
|
BC
|
breast cancer
|
|
BCS
|
breast-conserving surgery
|
|
CI
|
confidence interval
|
|
DFS
|
disease-free survival
|
|
ECOG
|
Easter Cooperative Oncology Group
|
|
ER
|
estrogen receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
HR
|
hazard ratio
|
|
LN
|
lymph node
|
|
LR
|
locoregional recurrence
|
|
LRR
|
locoregional recurrence rate
|
|
LVI
|
lymphovascular invasion
|
|
NAC
|
neoadjuvant chemotherapy
|
|
NCCN
|
National Comprehensive Cancer
Network
|
|
OS
|
overall survival
|
|
pCR
|
pathologic complete response
|
|
RCT
|
randomized clinical trial
|
|
RR
|
relative risk
|
|
T-DM1
|
trastuzumab emtansine
|
|
TNBC
|
triple negative breast cancer
|
References
|
1
|
Mougalian SS, Soulos PR, Killelea BK,
Lannin DR, Abu-Khalaf MM, DiGiovanna MP, Sanft TB, Pusztai L, Gross
CP and Chagpar AB: Use of neoadjuvant chemotherapy for patients
with stage I to III breast cancer in the United States. Cancer.
121:2544–2552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caparica R, Brandão M and Piccart M:
Systemic treatment of patients with early breast cancer: Recent
updates and state of the art. Breast. 48 (Suppl 1):S7–S20. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fisher B, Brown A, Mamounas E, Wieand S,
Robidoux A, Margolese RG, Cruz AB Jr, Fisher ER, Wickerham DL,
Wolmark N, et al: Effect of preoperative chemotherapy on
local-regional disease in women with operable breast cancer:
Findings from national surgical adjuvant breast and bowel project
B-18. J Clin Oncol. 15:2483–2493. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moreno-Aspitia A: Neoadjuvant therapy in
early-stage breast cancer. Crit Rev Oncol Hematol. 82:187–199.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Long-term outcomes for neoadjuvant
versus adjuvant chemotherapy in early breast cancer: Meta-analysis
of individual patient data from ten randomised trials. Lancet
Oncol. 19:27–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES,
Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al: Adjuvant
capecitabine for breast cancer after preoperative chemotherapy. N
Engl J Med. 376:2147–2159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murphy BL, Day CN, Hoskin TL, Habermann EB
and Boughey JC: Neoadjuvant chemotherapy use in breast cancer is
greatest in excellent responders: Triple-negative and HER2+
subtypes. Ann Surg Oncol. 25:2241–2248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Minckwitz G, Huang CS, Mano MS, Loibl
S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A,
Redondo A, et al: Trastuzumab emtansine for residual invasive
HER2-positive breast cancer. N Engl J Med. 380:617–628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaidya JS, Massarut S, Vaidya HJ,
Alexander EC, Richards T, Caris JA, Sirohi B and Tobias JS:
Rethinking neoadjuvant chemotherapy for breast cancer. BMJ.
360:j59132018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reyal F, Hamy AS and Piccart MJ:
Neoadjuvant treatment: The future of patients with breast cancer.
ESMO Open. 3:e0003712018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fisher B, Bryant J, Wolmark N, Mamounas E,
Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux
A, et al: Effect of preoperative chemotherapy on the outcome of
women with operable breast cancer. J Clin Oncol. 16:2672–2685.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rastogi P, Anderson SJ, Bear HD, Geyer CE,
Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil
SR, et al: Preoperative chemotherapy: updates of national surgical
adjuvant breast and bowel project protocols B-18 and B-27. J Clin
Oncol. 26:778–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mauri D, Pavlidis N and Ioannidis JP:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: A
meta-analysis. J Natl Cancer Inst. 97:188–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Xiu BQ, Guo R, Yang BL, Zhang Q,
Su YH, Li L, Ji WR, Zhang YY, Cao AY, et al: Current trend of
breast cancer neoadjuvant treatment in China: A cross-sectional
study. Zhonghua Zhong Liu Za Zhi. 42:931–936. 2020.(In Chinese).
PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American joint
committee on cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Senkus E, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S and Cardoso
F; ESMO Guidelines Committee, . Primary breast cancer: ESMO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 26 (Suppl 5):v8–v30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Comprehensive Cancer Network
(NCCN), . Clinical Practice Guideline in Oncology. Breast Cancer
version 1.2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdfMarch
20–2021
|
|
19
|
Denduluri N, Chavez-MacGregor M, Telli ML,
Eisen A, Graff SL, Hassett MJ, Holloway JN, Hurria A, King TA,
Lyman GH, et al: Selection of optimal adjuvant chemotherapy and
targeted therapy for early breast cancer: ASCO clinical practice
guideline focused update. J Clin Oncol. 36:2433–2443. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poggio F, Bruzzone M, Ceppi M, Pondé NF,
La Valle G, Del Mastro L, de Azambuja E and Lambertini M:
Platinum-based neoadjuvant chemotherapy in triple-negative breast
cancer: A systematic review and meta-analysis. Ann Oncol.
29:1497–1508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Association of Breast Surgery at Baso
2009, . Surgical guidelines for the management of breast cancer.
Eur J Surg Oncol. 35 (Suppl 1):S1–S22. 2009. View Article : Google Scholar
|
|
22
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berry DA, Cirrincione C, Henderson IC,
Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB,
Norton L, et al: Estrogen-receptor status and outcomes of modern
chemotherapy for patients with node-positive breast cancer. JAMA.
295:1658–1667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pondé NF, Zardavas D and Piccart M:
Progress in adjuvant systemic therapy for breast cancer. Nat Rev
Clin Oncol. 16:27–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Increasing the dose intensity of
chemotherapy by more frequent administration or sequential
scheduling: A patient-level meta-analysis of 37 298 women with
early breast cancer in 26 randomised trials. Lancet. 393:1440–1452.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology, . American society of clinical
oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sparano JA and Paik S: Development of the
21-gene assay and its application in clinical practice and clinical
trials. J Clin Oncol. 26:721–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA
Jr, et al: Prospective validation of a 21-gene expression assay in
breast cancer. N Engl J Med. 373:2005–2014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cooke T, Reeves J, Lanigan A and Stanton
P: HER2 as a prognostic and predictive marker for breast cancer.
Ann Oncol. 12 (Suppl 1):S23–S28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caparica R, Lambertini M, Pondé N,
Fumagalli D, de Azambuja E and Piccart M: Post-neoadjuvant
treatment and the management of residual disease in breast cancer:
State of the art and perspectives. Ther Adv Med Oncol.
11:17588359198277142019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bagegni NA, Tao Y and Ademuyiwa FO:
Clinical outcomes with neoadjuvant versus adjuvant chemotherapy for
triple negative breast cancer: A report from the national cancer
database. PLoS One. 14:e02223582019. View Article : Google Scholar : PubMed/NCBI
|