Introduction
Medulloblastoma (MB) is one of the most common forms
of malignant tumor arising from the cerebellum and posterior fossa
with unknown etiology. MB occurs at the age of 3–7 years, but may
also be diagnosed in adolescents and young adults (1,2). Most
cases of MB have a sporadic occasion but there has been a certain
association with familial syndromes (3,4).
Despite the standard therapeutic options for primary care,
including chemo-/radiotherapy and even high-dose chemotherapy, the
10-year mortality rate of MB is 34,6% (5). Relapsed or refractory MBs in pediatric
patients are reported to have poor outcomes and, in most cases,
intensive chemotherapy and radiation are inappropriate treatment
options due to the high risk of adverse events, intolerance and low
effectiveness. Thus, there is a need to develop a less toxic and
more precise strategy with a modality of targeted therapy in order
to improve survival rates (6).
Metronomic chemotherapy (MCT), acting through
various mechanisms by producing direct and indirect effects on the
tumor cells and their microenvironment, was reported to be a
feasible and potentially effective approach for relapsed and
refractory central nervous system (CNS) tumors (7). A significant role in tumor progression
is assigned to neoangiogenesis, while antiangiogenic drugs in
combination are considered to be more promising (8). Thus, the phosphatidylinositol-3-kinase
(PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is known to
have a significant role in the development, progression and relapse
of various tumors, including MBs (9), and accordingly, the addition of
PI3K/Akt/mTOR inhibitor to the MCT regimen may be rational and has
been confirmed by various in-vitro and in-vivo studies (10–23).
The present study reported a successful case of
long-term stabilization of relapsed MB in a pediatric patient on
metronomic regimen chemotherapy along with mTOR inhibitor with
optimal tolerability. The literature review demonstrates the
mechanisms of MCT and mTOR inhibitors and underlines their
advantages for a selected group of patients.
Case report
A 3-year-old Caucasian male patient was admitted to
the Department of Pediatric Oncology of Almazov National Medical
Research Centre (St. Petersburg, Russia) in August 2017 with
complaints of acute headache and neck pain, as well as vomiting and
fatigue. CT scan of the brain indicated a tumor in the cerebellar
vermis and in the IV ventricle with a size of 42.6х43.7х47.5 mm.
Surgical resection of the tumor was performed and in the
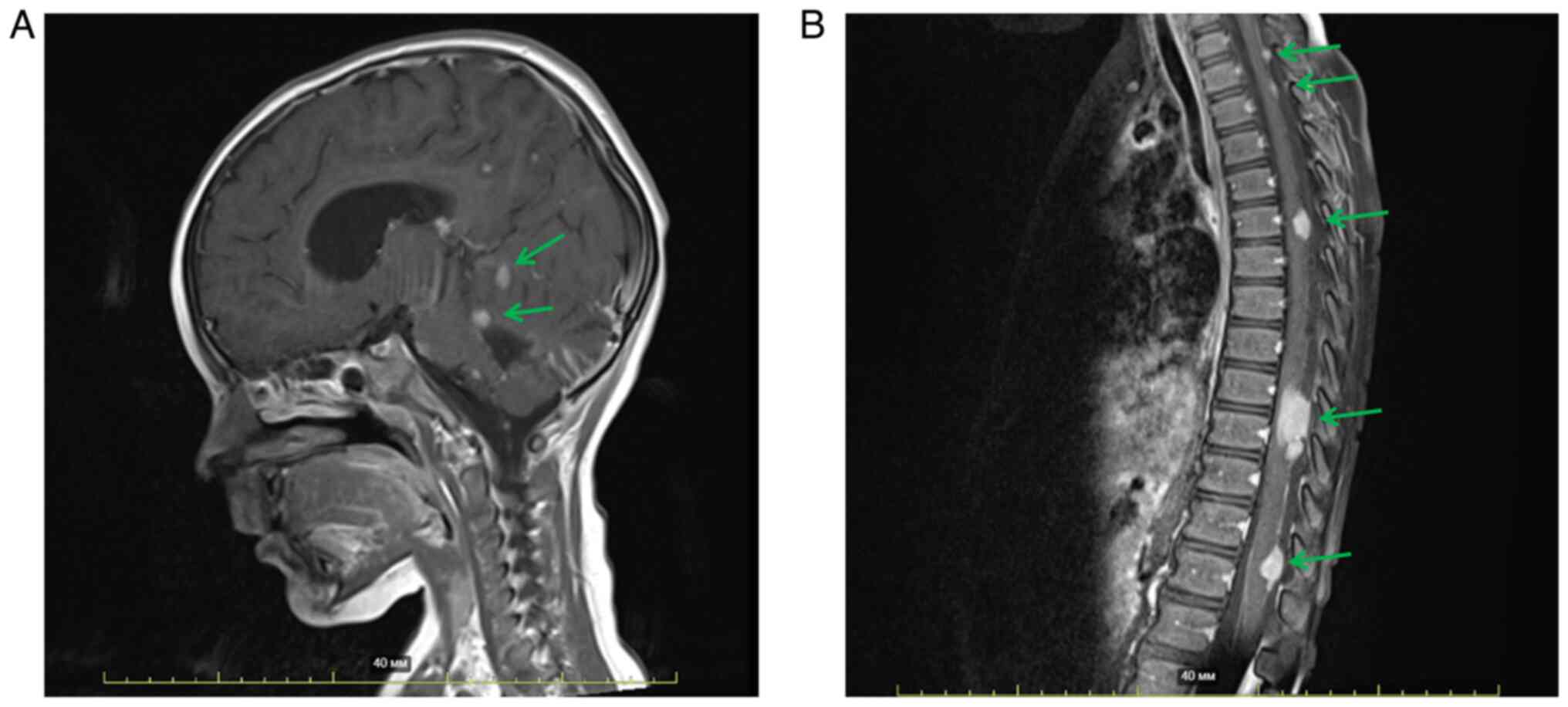
postoperative MRI of the brain with contrast enhancement (Fig. 1A), a residual tumor in the posterior
fossa was visualized. In addition, multiple metastatic lesions in
the meninges of the brain and spinal cord and signs of spinal
compression at the level of Th9-Th10 were observed (Fig. 1A and B). The patient did not have
any co-morbidities at the time of the initial diagnosis.
Histological examination (24) of the tumor sample revealed small
round undifferentiated cells with hyperchromatic nuclei, rosettes
and pseudorosettes, foci of necrosis and hemorrhages, as well as
pathologic mitoses. Based on the histological and
immunohistochemical (24) profile
(File S1) and radiological data,
the diagnosis of anaplastic MB of the posterior fossa, stage R+M3
was confirmed. There was no technological opportunity to provide
full molecular genetic testing to determine the subtype of MB.
After the surgery, the patient had neurological
symptoms, including cerebellar mutism syndrome, ataxia and
cognitive deficit. Considering the patient's age, histological type
of the tumor and stage of the disease, adjuvant treatment was
initiated. According to the HIT MED 2014 (version 4.0, 2017)
protocol, the patient was given 3 cycles of intensified induction,
including cisplatin, etoposide, high-dose methotrexate and
intraventricular methotrexate (25). The most common complications of the
chemotherapy in the patient of the present study were
myelosuppression, febrile neutropenia, toxic hepatitis,
catheter-related bloodstream infection and toxic dermatitis.
The subsequent MRI examination in October 2017
revealed a 50% decrease in tumor size. Next, two cycles of
high-dose chemotherapy (HDCT) with a conditioning regimen of
carboplatin/etoposide and thiotepa/cyclophosphamide followed by
autologous stem cell rescue (ASCR) were provided (25). Complications after HDCT with ASCR in
the patient of the present study were pancytopenia, mucositis grade
3, enterocolitis associated with Klebsiella pneumoniae and toxic
dermatitis. The median time to neutrophil and platelet recovery was
11 and 13 days, respectively.
After the HDCT with ASCR, the patient underwent
brain and spinal cord MRI, which revealed the positive clinical
effect of the therapy. There was a decrease in the size of the
metastatic lesions in the spinal meninges at the level of
Th10-Th11, L1-L2 and L5, but the number of lesions remained stable.
In the brain meninges, a decrease in both the size and the degree
of the contrast accumulation of the metastatic lesions was
observed.
As a result, the patient did not achieve complete
remission after surgery and chemotherapeutic treatment, and thus,
radiation therapy was provided by craniospinal irradiation with a
dose of 35.2 Gy with a boost on spinal metastases to 44.2 Gy and
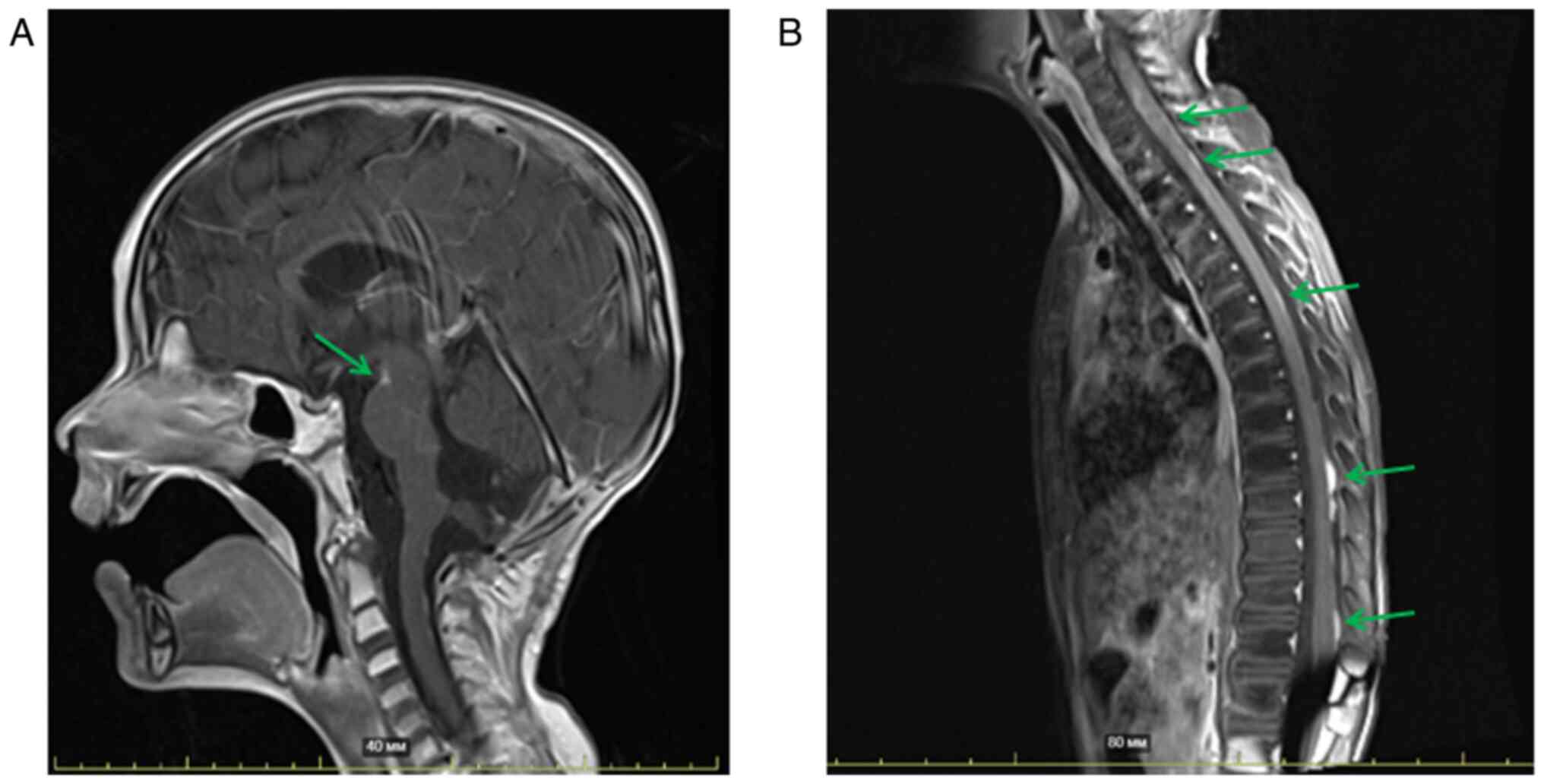
posterior fossa to 55 Gy. MRI was performed 6.5 weeks
post-radiation therapy, indicating postoperative changes with no
evidence of residual tumor in cerebellum and IV ventricle. The
metastases in the region of the interpeduncular cistern were
stable. Detectable spinal metastases remained with a slight
decrease in the degree of contrast enhancement. According to the
clinical and radiological data, the patient achieved stable disease
and continued to be monitored (Fig. 2A
and B). Considering the high risk of disease progression,
maintenance therapy with temozolomide at a dose of 150
mg/m2 was initiated. The patient tolerated the treatment
well and there were no reported adverse reactions.
Interim neuroimaging investigation revealed
stabilization of the disease and maintenance chemotherapy was
continued. The patient was given 6 cycles of temozolomide in total.
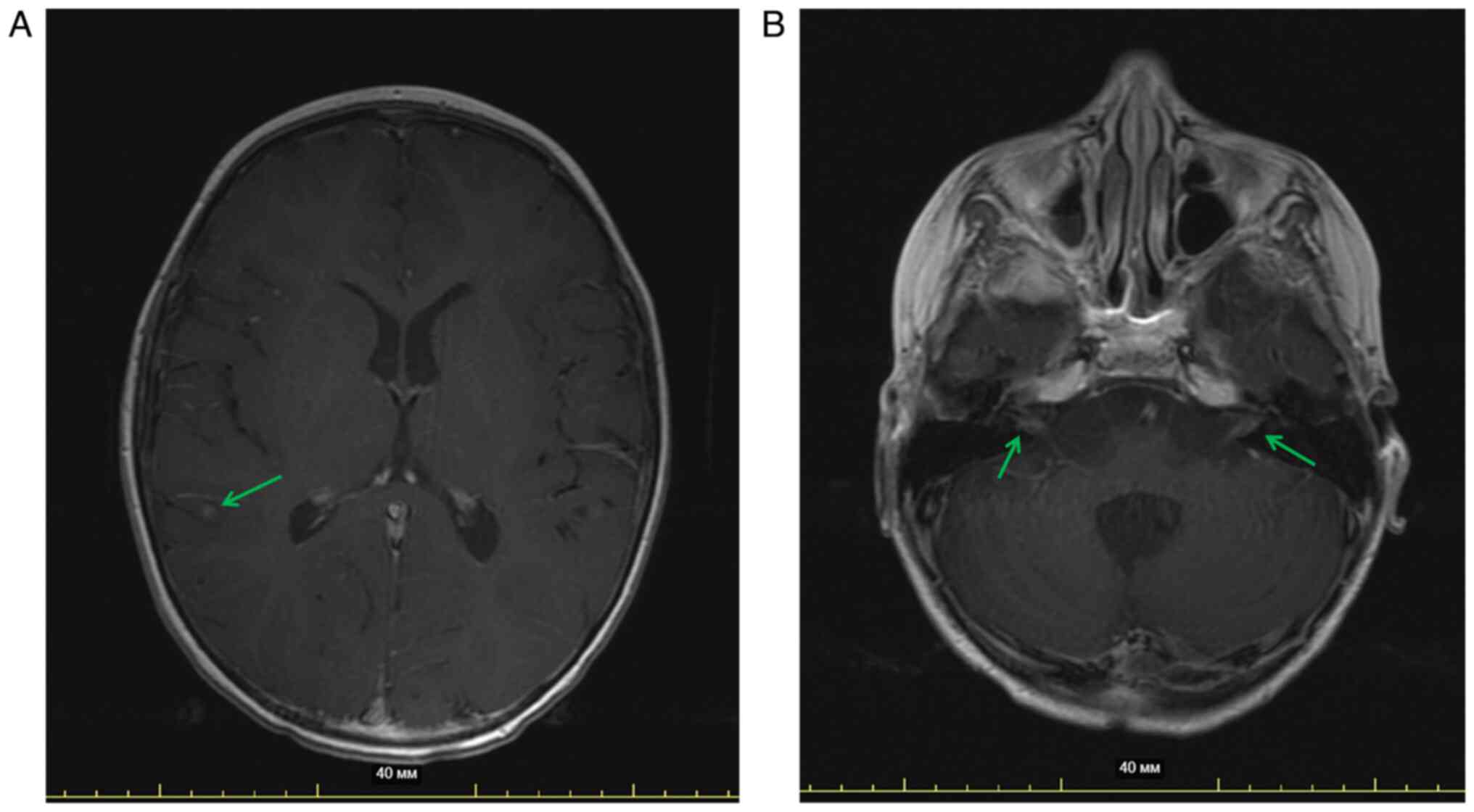
A month later, after the 6th cycle, the patient still had ataxia,
dysarthria and strabismus. MRI of the brain with contrast
enhancement indicated new leptomeningeal metastases in the brain,
suggesting continuous disease progression (Fig. 3A and B). Considering the scientific
data about the potential effectiveness of MCT with mTOR inhibitors
(17,26,27),
it was decided to prescribe this scheme. The treatment protocol was
approved by the institutional scientific committee (protocol no. 45
from September 2019). The patient was treated with celecoxib at a
dose of 100 mg twice a day, alternate cycles of etoposide 50
mg/m2/day for the first 3 weeks, followed by
cyclophosphamide 2.5 mg/kg/day from the 4th to the 6th week of each
cycle, along with sirolimus with an initial dose of 2
mg/m2/day. All medications were administered orally.
Side effects were monitored regularly according to criteria
(File S2) and the patient
exhibited grade 1–2 (not severe) recurrent episodes of neutropenia
and thrombocytopenia, but there were no indications for
antimicrobial therapy and hospitalization. After the maximum
cumulative dose of etoposide (2,100 mg/m2) was reached,
the drug was discontinued. During the metronomic therapy, the
clinical condition and MRI data of the patient remained stable. The
patient was given this therapy for 2 years and after finishing the
treatment, monitoring was continued.
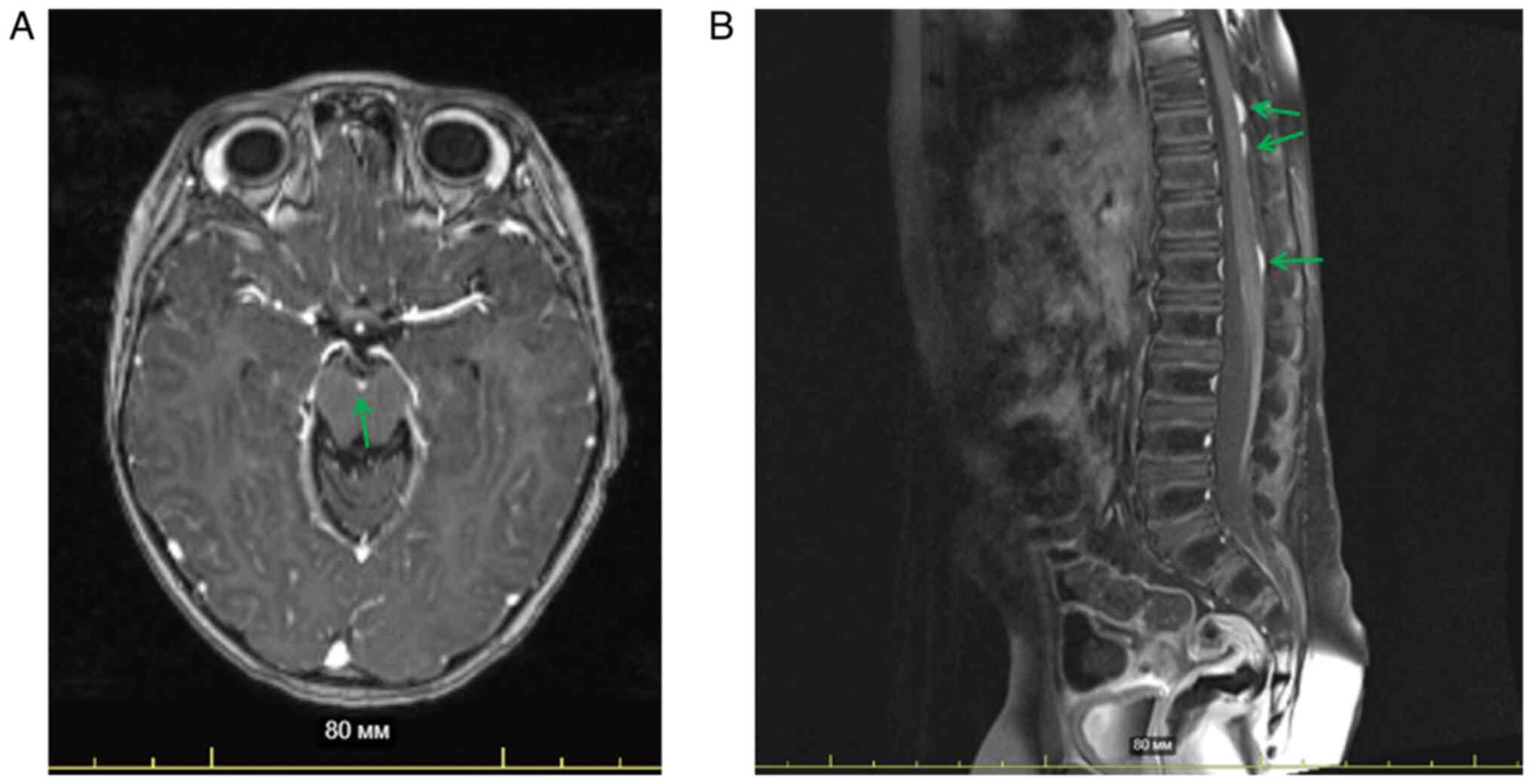
At 4 months after discontinuation of the metronomic
therapy, MRI was performed. The investigation revealed a decrease
in the size of leptomeningeal lesions at the level of Th5-Th6 to
4×1 mm (previously 6×2 mm), at the level of Th10 to 8×4 mm
(previously 20×4 mm) and at the level of Th12-L1 to 14×2 mm
(previously 16×2.5 mm), while the previously described metastatic
lesion at the level of Th1 was not detected. MRI data of the brain
were stable (Fig. 4A and B). At
eight months after discontinuation of the metronomic therapy, MRI
confirmed disease stabilization.
At the time of writing of this report, the patient
had been under surveillance for 14 months. The last MRI examination
was performed in June 2022, which indicated stabilization of the
disease.
Discussion
Despite the currently used multimodal therapy, the
prognosis for high-risk group patients remains dismal and almost
30% relapse during the 1st year of treatment completion (28,29).
Also von Bueren et al (30)
demonstrated poor survival rates for children with localized MB
with unfavorable histology.
Treatment modalities for recurrent MBs are limited
due to the tendency to develop resistance to conventional treatment
and high rate of toxicity as limiting factors for intensified
chemotherapy and repeated radiotherapy (28,29).
Novel strategies, including targeted therapy, are necessary in
order to improve overall and progression-free survival in these
patient groups (6).
The present results and previous reports (17,26,27,31–38)
confirm the effectiveness of MCT in pediatric patients with
refractory or relapsing CNS tumors with a satisfactory toxicity
profile. For the past 15 years, MCT regimens have been assessed for
replacing conventional regimens in miscellaneous cancers (39). While complete responses are rare,
MCT may lead to partial responses and long-term disease
stabilization with a significant improvement in the quality of life
of patients and their families (26,40,41).
Apparent advantages of MCT are cost-effectiveness and outpatient
treatment administration (39). In
cases of progressive disease and limited options for anticancer
therapy, this treatment remains available for palliative care.
Today, MCT remains empirical, but ongoing
preclinical and clinical studies define the best agents for use
according to tumor type, number, type and doses of drugs, and
timing of administration. It should be emphasized that childhood
malignancies may feature recurrent genomic alterations, but their
frequency is <10%. This is a limiting factor for designing and
conducting clinical trials of targeted therapy according to
revealed alterations and tumor histology (42).
Clear differences between MCT and conventional
cytotoxic chemotherapy have been defined and explain differences in
mechanisms of action. The MCT regimen usually consists of a
combination of various drugs with antiangiogenic, immunostimulatory
and apoptotic mechanisms (31). MCT
may be the most efficacious treatment for minimal residual disease
or significantly cytoreduced disease. Frequently used drugs are
cyclophosphamide, etoposide, methotrexate, vinblastine,
temozolomide, irinotecan and topotecan (32–34).
In preclinical studies, cyclo-oxygenase 2 (COX2) inhibitors have
demonstrated antitumor activity when given at subtherapeutic doses
due to inhibition of angiogenesis and cell cycle arrest (35). A significant reduction in tumor
invasion and proliferation was achieved via the COX-2 independent
pathway. Also, when given at a metronomic dose, celecoxib is free
from its well-established cardiovascular adverse events, which
otherwise limit its use (31).
One of the important points of the natural course of
malignancy is tumor dormancy, which occurs initially, during the
very early phase of cancer, and later, after completion of
anticancer treatment (remission phase), which is also regulated by
the initiation of angiogenesis (27). Thus, it may be assumed that
inhibition of the angiogenic switch by MCT may prevent the
progression of tumors and their metastases (27).
Further agents were described to be used in the MCT
regimen, including isotretinoin (inhibits cell proliferation and
induces differentiation), thalidomide (immunomodulator with
powerful antiangiogenic activity) (36), valproic acid (37), bevacizumab (37), rapamycin (17) and intraventricular liposomal
cytarabine-etoposide (38). mTOR
proteins have an essential role in the disease pathogenesis of MB
and the PI3K/AKT pathway is considered one of the major mechanisms
that are activated during MB development (26). Multiple drugs have been reported to
target mTOR through different binding sites or mechanisms, but the
importance of combination therapy in MB due to its highly resistant
nature is highlighted (26).
In the case of the present study, alternating
cyclophosphamide with etoposide and celecoxib, combined with the
mTOR inhibitor sirolimus, was used. It should be emphasized that
mTOR inhibitors were administered without the confirmation of the
presence of PIK3CA mutations, but the opportunity of tumor DNA
sequencing should be taken in order to predict the possible
effectiveness of treatment. Apart from targeting certain mutations,
PI3K/mTOR inhibitors exert their cytotoxic effects through other
mechanisms, as mentioned above. Concomitant administration of
molecular-targeted drug molecules on a daily basis may have immense
potential (26).
Despite the minimal associated toxicity of MCT,
there are several reports of secondary leukemia after prolonged
chemotherapy with temozolomide and etoposide (31,43).
Therefore, total doses of anticancer agents and treatment toxicity
should be closely monitored.
The current experience of MCT in combination with
mTOR inhibitors confirms the possibility of achieving long-term
survival in patients with MB refractory to previous treatment
options. In the present study, a good partial radiographic response
compared to the baseline measurement with the absence of
significant toxicity was obtained. Considering the duration of the
progression-free follow-up period, a favorable outcome may be
expected for the patient of the present study. Further
investigations of this strategy are necessary to define the
potential efficacy, toxicity and feasibility in similar treatment
groups.
Of note, the treatment strategy of the present study
cannot be considered a standard therapy at present, but may be
discussed as an option for second and subsequent lines of therapy
in heavily pretreated pediatric patients with relapsed or
refractory CNS tumors. Future investigations are warranted.
In conclusion, there is no doubt that multidrug MCT
in combination with mTOR inhibitors is a rational option for
heavily pretreated pediatric patients with recurrent or progressive
MBs with prolonged or persistent disease-free survival. The most
significant advantages of this option are no age restrictions, oral
low-dose form of cytotoxic drugs, absence of major toxicities and
potential effectiveness in different cancer types. It is necessary
to emphasize that in pediatric cancers, recurrent genomic
alterations occur at a frequency of <10%, making it difficult to
apply targeted therapy in most patients. Due to the mechanism of
action of MCT in combination with mTOR inhibitors, it appeared to
be a prospective anticancer regimen regardless of the
presence/absence of molecular targets. In general, MCT is a
palliative care option and is continued until the progression of
the disease, but there are plenty of case reports with complete
tumor response and long-term disease-free survival. Further cohort
investigations on this strategy are necessary to achieve an impact
on survival and long-term toxicity in definite cancer types.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was financially supported by the Ministry of Science
and Higher Education of the Russian Federation (agreement no.
075-15-2022-301).
Authors' contributions
YD determined the treatment strategy for the
patient. YD and AK were involved in the conceptualization of the
study. EL, AK, HP, YS, DM and YD were involved in acquisition of
data, wrote the text of the manuscript, analysed and interpreted
literature data and edited the manuscript. All authors participated
in group discussions and provided comments on drafts of the paper.
All authors read and approved the final manuscript. EL, DM, AH, HP,
YS and YD confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The treatment protocol of MCT with mTOR inhibitors
in children with relapsed or refractory CNS tumors was approved by
the Clinical and Research Board of Almazov National Medical
Research Centre (St Petersburg, Russia; protocol no. 45 from
06.09.2019). Individual usage of this treatment protocol was
permitted by the medical commission according to local
institutional practice. Written informed consent to the treatment
was obtained from the patient's guardian.
Patient consent for publication
Written informed consent was obtained from the legal
guardian of the patient for publication of any potentially
identifiable information/images or data included in this article in
an online open-access publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnston DL, Keene D, Strother D, Taneva
M, Lafay-Cousin L, Fryer C, Scheinemann K, Carret AS, Fleming A,
Afzal S, et al: Survival following tumor recurrence in children
with medulloblastoma. J Pediatr Hematol Oncol. 40:e159–e163. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Board PDQPTE, . Childhood medulloblastoma
and other central nervous system embryonal tumors treatment
(PDQ®). PDQ Cancer Information Summaries. National
Cancer Institute; Bethesda, MD: 2008
|
|
3
|
Khattab A and Monga DK: Turcot syndrome.
StatPearls. StatPearls Publishing; Treasure Island, FL: 2022
|
|
4
|
Onodera S, Nakamura Y and Azuma T: Gorlin
syndrome: Recent advances in genetic testing and molecular and
cellular biological research. Int J Mol Sci. 21:75592020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2011–2015. Neuro Oncol. 20 (Suppl 4):iv1–iv86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zapletalová D, André N, Deak L, Kyr M,
Bajciova V, Mudry P, Dubska L, Demlova R, Pavelka Z, Zitterbart K,
et al: Metronomic chemotherapy with the COMBAT regimen in advanced
pediatric malignancies: A multicenter experience. Oncology.
82:249–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panigrahy D, Kaipainen A, Butterfield CE,
Chaponis DM, Laforme AM, Folkman J and Kieran MW: Inhibition of
tumor angiogenesis by oral etoposide. Exp Ther Med. 1:739–746.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pasquier E, Kavallaris M and André N:
Metronomic chemotherapy: New rationale for new directions. Nat Rev
Clin Oncol. 7:455–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eckerdt F, Clymer J, Bell JB, Beauchamp
EM, Blyth GT, Goldman S and Platanias LC: Pharmacological mTOR
targeting enhances the antineoplastic effects of selective PI3Kα
inhibition in medulloblastoma. Sci Rep. 9:128222019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chaturvedi NK, Kling MJ, Coulter DW,
McGuire TR, Ray S, Kesherwani V, Joshi SS and Sharp JG: Improved
therapy for medulloblastoma: Targeting hedgehog and PI3K-mTOR
signaling pathways in combination with chemotherapy. Oncotarget.
9:16619–16633. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borders EB, Binova C and Medina PJ:
Mammalian target of rapamycin: Biological function and target for
novel anticancer agents. Am J Health Syst Pharm. 67:2095–2106.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown RE, Tan D, Taylor JS, Miller M,
Prichard JW and Kott MM: Morphoproteomic confirmation of
constitutively activated mTOR, ERK, and NF-kappaB pathways in high
risk neuro-blastoma, with cell cycle and protein analyte
correlates. Ann Clin Lab Sci. 37:141–147. 2007.PubMed/NCBI
|
|
13
|
Zenali MJ, Zhang PL, Bendel AE and Brown
RE: Morphoproteomic confirmation of constitutively activated mTOR,
ERK, and NF-kappaB pathways in Ewing family of tumors. Ann Clin Lab
Sci. 39:160–166. 2009.PubMed/NCBI
|
|
14
|
Mateo-Lozano S, Gokhale PC, Soldatenkov
VA, Dritschilo A, Tirado OM and Notario V: Combined transcriptional
and translational targeting of EWS/FLI-1 in Ewing's sarcoma. Clin
Cancer Res. 12:6781–6790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Houghton PJ, Morton CL, Kolb EA, Gorlick
R, Lock R, Carol H, Reynolds CP, Maris JM, Keir ST, Billups CA and
Smith MA: Initial testing (stage 1) of the mTOR inhibitor rapamycin
by the pediatric preclinical testing program. Pediatr Blood Cancer.
50:799–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnsen JI, Segerström L, Orrego A, Elfman
L, Henriksson M, Kågedal B, Eksborg S, Sveinbjörnsson B and Kogner
P: Inhibitors of mammalian target of rapamycin downregulate MYCN
protein expression and inhibit neuroblastoma growth in vitro and in
vivo. Oncogene. 27:2910–2922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vo KT, Karski EE, Nasholm NM, Allen S,
Hollinger F, Gustafson WC, Long-Boyle JR, Shiboski S, Matthay KK
and DuBois SG: Phase 1 study of sirolimus in combination with oral
cyclophosphamide and topotecan in children and young adults with
relapsed and refractory solid tumors. Oncotarget. 8:23851–23861.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Houghton PJ, Morton CL, Gorlick R, Lock
RB, Carol H, Reynolds CP, Kang MH, Maris JM, Keir ST, Kolb EA, et
al: Stage 2 combination testing of rapamycin with cytotoxic agents
by the pediatric preclinical testing program. Mol Cancer Ther.
9:101–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semela D, Piguet AC, Kolev M, Schmitter K,
Hlushchuk R, Djonov V, Stoupis C and Dufour JF: Vascular remodeling
and antitumoral effects of mTOR inhibition in a rat model of
hepatocellular carcinoma. J Hepatol. 46:840–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guba M, von Breitenbuch P, Steinbauer M,
Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S,
Anthuber M, et al: Rapamycin inhibits primary and metastatic tumor
growth by antiangiogenesis: Involvement of vascular endothelial
growth factor. Nat Med. 8:128–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qayed M, Cash T, Tighiouart M, MacDonald
T, Goldsmith KC, KeanPatty Church LS and Katzenstein HM: A phase I
study of sirolimus in combination with metronomic therapy in
children with recurrent and refractory solid/CNS tumors. J Clin
Oncol. May 20–2015.(Epub ahead of print). View Article : Google Scholar
|
|
22
|
Morgenstern DA, Marzouki M, Bartels U,
Irwin MS, Sholler GL, Gammon J, Yankanah R, Wu B, Samson Y and
Baruchel S: Phase I study of vinblastine and sirolimus in pediatric
patients with recurrent or refractory solid tumors. Pediatr Blood
Cancer. 61:128–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cash T: Sirolimus in combination with
metronomic chemotherapy in children with recurrent and/or
refractory solid and CNS tumors (AflacST1502). ClinicalTrials.govIdentifier: NCT02574728. 2022.
|
|
24
|
Putnam AR and Wallentine JC: Diagnostic
Pathology: Pediatric Neoplasms. Salt Lake City, UT: pp.
pp9842012
|
|
25
|
Ole Juhnke B, Mynarek M, von Hoff K,
Klagges S, Kortmann RD and Rutkowski S: HIT-MED guidance for
patients with newly diagnosed medulloblastoma ependymoma CNS
embryonal tumour and pineoblastoma. Version 4.0-02. 2017.
|
|
26
|
Alammar H, Nassani R, Alshehri MM,
Aljohani AA and Alrfaei BM: Deficiency in the treatment description
of mTOR inhibitor resistance in medulloblastoma, a systematic
review. Int J Mol Sci. 23:4642021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wen J and Hadden MK: Medulloblastoma drugs
in development: Current leads, trials and drawbacks. Eur J Med
Chem. 215:1132682021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar V, Kumar V, McGuire T, Coulter DW,
Sharp JG and Mahato RI: Challenges and recent advances in
medulloblastoma therapy. Trends Pharmacol Sci. 38:1061–1084. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
von Bueren AO, von Hoff K, Pietsch T,
Gerber NU, Warmuth-Metz M, Deinlein F, Zwiener I, Faldum A,
Fleischhack G, Benesch M, et al: Treatment of young children with
localized medulloblastoma by chemotherapy alone: Results of the
prospective, multicenter trial HIT 2000 confirming the prognostic
impact of histology. Neuro Oncol. 13:669–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bahl A and Bakhshi S: Metronomic
chemotherapy in progressive pediatric malignancies: Old drugs in
new package. Indian J Pediatr. 79:1617–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bowers DC, Aquino VM, Leavey PJ, Bash RO,
Journeycake JM, Tomlinson G, Mulne AF, Haynes HJ and Winick NJ:
Phase I study of oral cyclophosphamide and oral topotecan for
children with recurrent or refractory solid tumors. Pediatr Blood
Cancer. 42:93–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sterba J, Pavelka Z, Andre N, Ventruba J,
Skotakova J, Bajciova V, Bronisova D, Dubska L and Valik D: Second
complete remission of relapsed medulloblastoma induced by
metronomic chemotherapy. Pediatr Blood Cancer. 54:616–617. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peyrl A, Chocholous M, Kieran MW, Azizi
AA, Prucker C, Czech T, Dieckmann K, Schmook MT, Haberler C, Leiss
U and Slavc I: Antiangiogenic metronomic therapy for children with
recurrent embryonal brain tumors. Pediatr Blood Cancer. 59:511–517.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida S, Amano H, Hayashi I, Kitasato H,
Kamata M, Inukai M, Yoshimura H and Majima M: COX-2/VEGF-dependent
facilitation of tumor-associated angiogenesis and tumor growth in
vivo. Lab Invest. 83:1385–1394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Amato RJ, Loughnan MS, Flynn E and
Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl
Acad Sci USA. 91:4082–4085. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carcamo B and Francia G: Cyclic metronomic
chemotherapy for pediatric tumors: Six case reports and a review of
the literature. J Clin Med. 11:28492022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Slavc I, Peyrl A, Gojo J, Holm S, Blomgren
K, Sehested AM, Leblond P and Czech T: MBCL-43. Reccurent
medulloblastoma-long-term survival with a ‘MEMMAT’ based
antiangiogenic approach. Neuro Oncol. 22 (Suppl 3):iii3972020.
View Article : Google Scholar
|
|
39
|
Simsek C, Esin E and Yalcin S: Metronomic
chemotherapy: A systematic review of the literature and clinical
experience. J Oncol. 2019:54837912019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stempak D, Gammon J, Halton J, Moghrabi A,
Koren G and Baruchel S: A pilot pharmacokinetic and antiangiogenic
biomarker study of celecoxib and low-dose metronomic vinblastine or
cyclophosphamide in pediatric recurrent solid tumors. J Pediatr
Hematol Oncol. 28:720–728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sterba J, Valik D, Mudry P, Kepak T,
Pavelka Z, Bajciova V, Zitterbart K, Kadlecova V and Mazanek P:
Combined biodifferentiating and antiangiogenic oral metronomic
therapy is feasible and effective in relapsed solid tumors in
children: Single-center pilot study. Onkologie. 29:308–313.
2006.PubMed/NCBI
|
|
42
|
Allen CE, Laetsch TW, Mody R, Irwin MS,
Lim MS, Adamson PC, Seibel NL, Parsons DW, Cho YJ and Janeway K;
Pediatric MATCH Target and Agent Prioritization Committee, . Target
and agent prioritization for the children's oncology group-national
cancer institute pediatric MATCH trial. J Natl Cancer Inst.
109:djw2742017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
De Vita S, De Matteis S, Laurenti L,
Chiusolo P, Reddiconto G, Fiorini A, Leone G and Sica S: Secondary
Ph+ acute lymphoblastic leukemia after temozolomide. Ann Hematol.
84:760–762. 2005. View Article : Google Scholar : PubMed/NCBI
|