Introduction
Breast cancer is the most common cancer in women,
and its incidence is increasing in many parts of the world
including Japan. There were about 2.2 million new cases of breast
cancer, and estimated 685,000 women died from breast cancer in 2020
worldwide (1). A further
development of treatments is essential for improving the patient
outcome. Since breast cancer is a heterogeneous disease consisting
of various subtypes which show a different response to the various
treatments and lead to the different clinical outcome, it is
important to implement the precision medicine where treatment is
individually conducted according to the subtype. Breast cancer
subtyping has been done by clinical tumor features and histology
with immunohistochemical examination (2). However, in order to improve the
accuracy of subtyping so that it will reflect the biological
characteristics of tumors (malignancy and response to treatment
etc.) more precisely and thus it will be more useful for precision
medicine, many multigene profiling assays have been developed which
include intrinsic subtyping (3,4) and
multigene classifiers (MGCs) for early-stage hormone
receptor-positive and human epidermal growth factor receptor 2
(HER2)-negative (HR+/HER2-) breast cancer as mentioned below.
Considering the clinical importance of prognostic
prediction, especially in guiding adjuvant systemic therapy, many
MGCs have been developed for early-stage HR+/HER2- breast cancer.
Among them, Oncotype DX (ODX) has the best evidence that is
outstanding. Large-scale prospective studies (5–7) have
proven that Oncotype DX is useful in determining the indications
for adjuvant chemotherapy in patients with HR+/HER2-/node negative
(n0) and HR+/HER2-/nodes 1-3-positive breast cancer and is now
widely used in daily practice.
We have also been developing the ‘Curebest 95GC
breast (95GC)’ MGC through an approach different from ODX. The ODX
approach includes 16 genes selected from 250 candidate genes on the
basis of their prognostic ability (8), but we constructed 95GC by taking
advantage of the public datasets with comprehensive gene expression
(DNA microarray) data on primary estrogen receptor (ER)+ breast
cancer and its prognosis. First, the genes related to recurrence
were extracted, and a prognostic prediction model (95GC) was
developed using between-group analysis (9). The 95GC model has been confirmed to be
useful in the prognostic prediction of ER+/HER2/n0 breast cancer
through retrospective studies (10–13).
Since the 95GC assay uses an Affymetrix DNA microarray, the
comprehensive gene expression data are simultaneously obtained and
can be used for determining other MGCs as well as development of a
new MGC.
The genes included in 95GC are related to cell
proliferation, transcription, and apoptosis (9), and interestingly, there is no overlap
of the classifier genes between 95GC and ODX, indicating that a
combination of the two MGCs may improve prediction accuracy. In
fact, we reported that 95GC could further classify the ODX
intermediate risk group into the low-risk and high-risk groups
(10,13), suggesting that the combination of
ODX and 95GC enables a more detailed prognostic prediction and
subsequently a more personalized treatment.
In this paper, to validate the prognostic prediction
ability of 95GC, we report the results of a multiinstitutional
registry study on the prognosis of patients with (ER+/HER2-/n0)
invasive breast cancer who underwent the 95GC assay.
Materials and methods
Patients
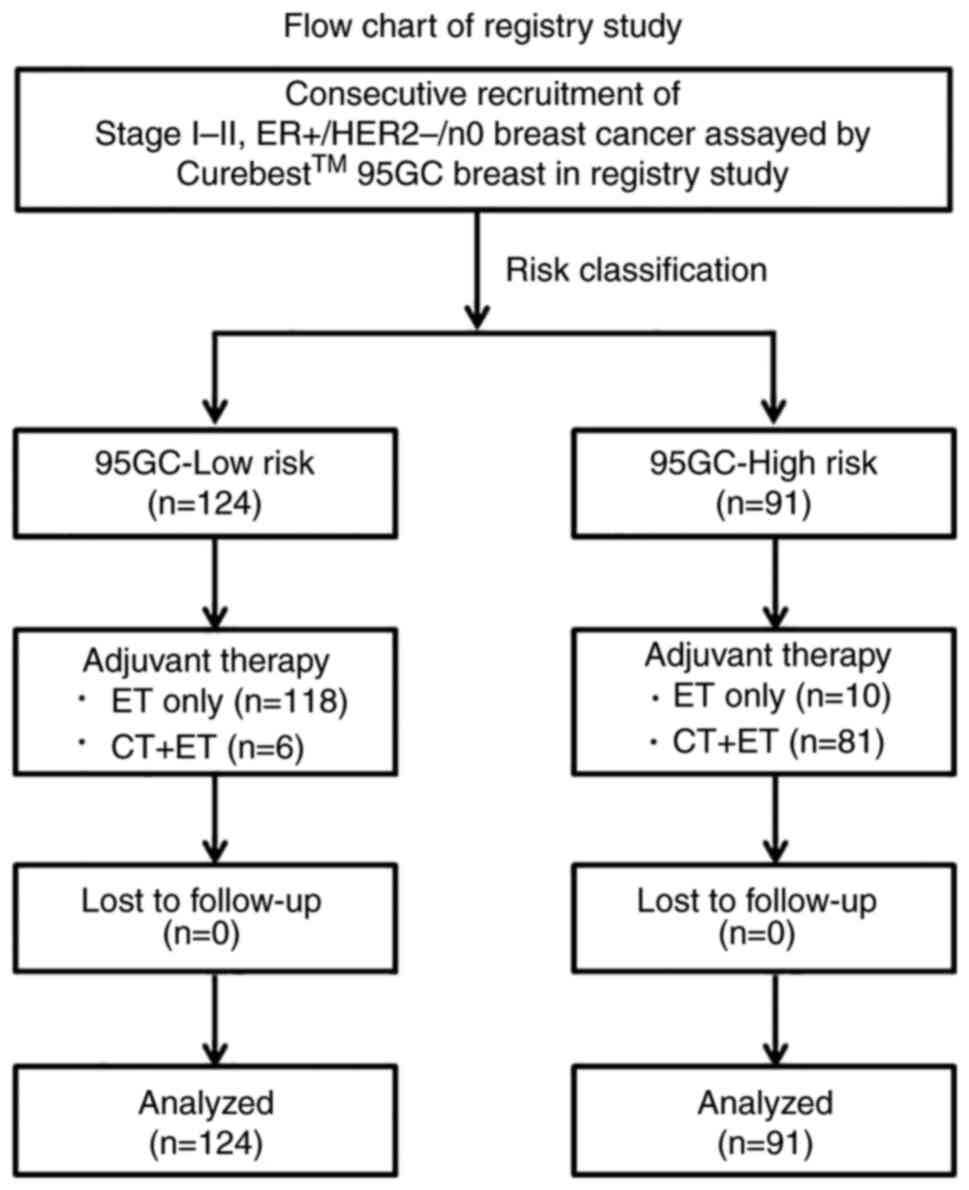
Registry study: Every patient (n=215) with
ER+/HER2-/n0 invasive breast cancer who had undergone the 95GC
assay in seven hospitals between December 2014 and March 2019 were
consecutively recruited in this registry study conducted by the
Japanese Association for Theranostics at various postoperative
times. These patients were treated with breast-conserving surgery
followed by radiation therapy or mastectomy. As an adjuvant
systemic therapy, endocrine therapy alone or chemo-endocrine
therapy was administered based on the physician's discretion and
patient's preference for treatment. No patients had disease
recurrence at the time of recruitment, and thereafter the patients
were prospectively followed up with a median of 62 (range, 6–91)
postoperative months (Fig. 1).
Historical control: The historical control
group included the 77 patients with ER+/HER2-/n0 invasive breast
cancer all of which were classified into the high-risk group by
95GC. The controls were treated with breast-conserving surgery
followed by radiation therapy or mastectomy and with adjuvant
endocrine therapy alone in Osaka University Hospital from 1995 to
2017 with a median follow-up period of 87 (range, 12–190) months
from the surgery. This historical control group is composed of the
same patients as previously reported (11). The registry study has been approved
by the Ethics Committees of All Participating Hospitals, and the
historical control study has been approved by the Ethics Committee
of Osaka University Hospital.
95GC assay
A tumor sample (4 mm in diameter ×10 mm in depth)
was taken from the primary tumor using a biopsy punch, after
surgical resection, stored in RNAlater® solution (4°C),
and sent to the Sysmex company. Hematoxylin and eosin section was
created from both sides of the sample to confirm the presence of
cancer cells (tumor cellularity ≥10%). Next, all gene expressions
underwent microarray analysis (Affymetrix U133 plus 2.0; Thermo
Fisher Scientific, Waltham, MA). The actual method of the assay,
the high/low-risk determination method using a 95GC-dedicated
algorithm, and the calculation method of the 95GC recurrence score
are the same as previously reported (9,13,14).
In some cases in the registry study and all of the historical
controls, 95GC was assayed using frozen (−80°C) tumor samples
(11).
Histological examination
ER, progesterone receptor (PR), and Ki67 were
assessed by immunohistochemistry and HER2 was assessed by
fluorescence in situ hybridization and/or immunohistochemistry in
local hospitals/laboratories. The cutoff values were 10% for both
ER and PR. The ASCO/CAP 2013 guideline was used to determine
HER2.
Statistical analysis
All statistical analyses were performed using R
statistical software (version 3.5.1; http://www.r-project.org/). Fisher's exact test was
used to compare 2×2 groups. All statistical analyses were
two-sided, and P<0.05 was considered to be indicative of
statistical significance. Distant recurrence-free survival (DRFS)
was defined as the time from surgery to distant recurrence or death
from any cause, whichever occurred first. DRFS was calculated by
the Kaplan-Meier method.
Results
Clinicopathological characteristics of
patients with breast cancer recruited in this study according to
95GC category
In total, 215 patients with ER+/HER2-/n0 invasive
breast cancer were recruited in this registry study, and 124 were
classified into the 95GC low-risk group and 91 into the 95GC
high-risk group (Table I). The
high-risk group was significantly correlated with high Ki67
(P<0.001) and high histological grade (P<0.001)
and showed a tendency (P=0.077) toward larger tumor
size.
 | Table I.Clinicopathological characteristics of
patients recruited in the registry study. |
Table I.
Clinicopathological characteristics of
patients recruited in the registry study.
|
|
| 95GC |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total | Low | High | P-value |
|---|
| No. of patients | 215 | 124 | 91 |
|
| Age |
|
|
| 0.894 |
|
≤50 | 98 | 57 | 41 |
|
|
>50 | 117 | 67 | 50 |
|
| Menopausal
status |
|
|
| 0.786 |
|
Premenopausal | 111 | 65 | 46 |
|
|
Postmenopausal | 104 | 59 | 45 |
|
| Tumor size |
|
|
| 0.077 |
|
T1 | 139 | 88 | 51 |
|
|
T2 | 74 | 35 | 39 |
|
|
T3 | 2 | 1 | 1 |
|
| Histological
typea |
|
|
| 0.502 |
|
Invasive
ductal | 186 | 108 | 78 |
|
|
Special type | 28 | 16 | 12 |
|
|
Unknown | 1 | 0 | 1 |
|
| Histological
grade |
|
|
| 0.001 |
|
1 | 74 | 59 | 15 |
|
|
2 | 96 | 54 | 42 |
|
|
3 | 44 | 11 | 33 |
|
| Estrogen
receptor |
|
|
| NA |
|
Positive | 215 | 124 | 91 |
|
|
Negative | 0 | 0 | 0 |
|
| Progesterone
receptor |
|
|
| 0.467 |
|
Positive | 195 | 114 | 81 |
|
|
Negative | 19 | 9 | 10 |
|
|
Unknown | 1 | 1 | 0 |
|
| Ki67 index |
|
|
| 0.001 |
|
<20% | 90 | 69 | 21 |
|
|
≥20% | 122 | 53 | 69 |
|
|
Unknown | 3 | 2 | 1 |
|
Effect of 95GC on choice of adjuvant
therapy
The adjuvant therapy recommended by the St. Gallen
2013 guideline was compared with the therapy actually given to the
patients for the purpose of evaluating the effect of 95GC on the
choice of adjuvant therapy (Table
II). The guideline recommended adjuvant chemo-endocrine therapy
for the 155 patients, of whom 80 patients were in the 95GC
high-risk group and 75 were in the 95GC low-risk group.
Seventy-five (93.8%) patients in the 95GC high-risk group and four
(5.3%) in the 95GC low-risk group were treated with adjuvant
chemo-endocrine therapy (Table
II). On the other hand, adjuvant endocrine therapy alone was
recommended for the 57 patients by the guideline, of whom 10 were
in the 95GC high-risk group and 47 were in the 95GC low-risk group.
Five (50%) in the 95GC high-risk group and two (4.3%) in the 95GC
low-risk group were treated with adjuvant chemo-endocrine therapy.
All of the other patients were treated with adjuvant endocrine
therapy alone. According to the St. Gallen 2013 guideline, 155
(73.1%) patients were recommended to receive adjuvant
chemo-endocrine therapy; however, 86 (40.6%) patients were treated
with adjuvant chemo-endocrine therapy because of the implementation
of 95GC.
 | Table II.Effect of 95GC on the choice of
adjuvant chemo-endocrine therapy. |
Table II.
Effect of 95GC on the choice of
adjuvant chemo-endocrine therapy.
|
| Adjuvant therapy
recommended by the St. Gallen Guideline 2013a |
|
|---|
|
|
|
|
|---|
| 95GC risk
category | CT + ET | ET | Total |
|---|
| High risk | 93.8%b (75/80)c | 50.0% (5/10) | 88.9% (80/90) |
| Low risk | 5.3% (4/75) | 4.3% (2/47) | 4.9% (6/122) |
| Total | 51.0% (79/155) | 12.3% (7/57) | 40.6% (86/212) |
Prognosis according to 95GC
category
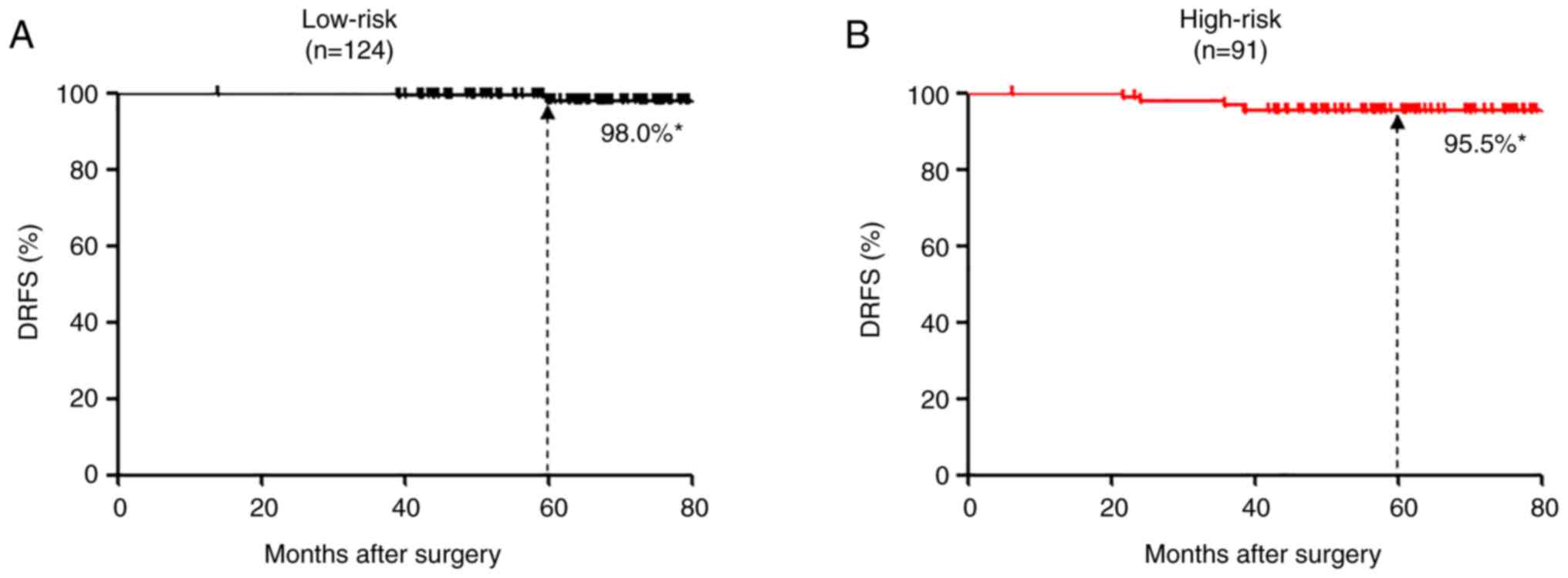
The median postoperative follow-up period was 62
(range: 6–91) months, and 123 patients were followed for >5
years. Of the 124 patients in the 95GC low-risk group, 118 received
adjuvant endocrine therapy alone and only 6 received adjuvant
chemo-endocrine therapy. Only two patients who received adjuvant
endocrine therapy alone developed distant recurrences in this
group, and the 5-year DRFS was as high as 98.0% (Fig. 2). Of the 91 patients in the 95GC
high-risk group, 81 received adjuvant chemo-endocrine therapy and
10 received adjuvant endocrine therapy alone. Four patients
developed distant recurrences in this group, resulting in the
5-year DRFS of 95.5% (Fig. 2). The
regimens for adjuvant therapy are summarized in Table SI according to the 95GC risk
group.
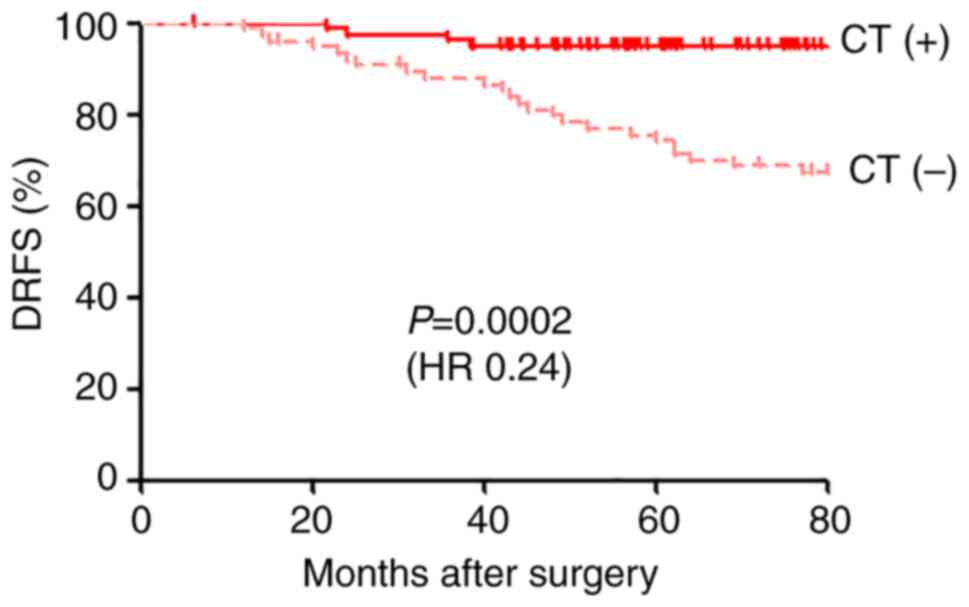
Among all patients classified as 95GC high risk, to
estimate the therapeutic benefit of adjuvant chemotherapy, we
compared the prognosis of the 81 patients treated with adjuvant
chemo-endocrine therapy with that of the 77 patients treated with
adjuvant endocrine therapy alone [historical control group
(11)]. The patients in these two
groups were found to have similar backgrounds (Table III), and the regiments for
adjuvant endocrine therapy were also similar between the two groups
(Table SII). Prognosis was
significantly better for the patients treated with adjuvant
chemo-endocrine therapy than for those treated with adjuvant
endocrine therapy alone (P=0.0002, HR 0.24) (Fig. 3).
 | Table III.Clinicopathological characteristics
of patients with invasive breast cancer at 95GC high risk and
treated with adjuvant chemo-endocrine therapy or endocrine therapy
alone. |
Table III.
Clinicopathological characteristics
of patients with invasive breast cancer at 95GC high risk and
treated with adjuvant chemo-endocrine therapy or endocrine therapy
alone.
|
| Adjuvant
therapy |
|
|---|
|
|
|
|
|---|
| Characteristic |
Endocrinea |
Chemo-endocrine | P-value |
|---|
| No. patients | 77 | 81 |
|
| Menopausal
status |
|
| 0.867 |
|
Premenopausal | 37 | 40 |
|
|
Postmenopausal | 40 | 41 |
|
| Tumor size |
|
| 0.721 |
|
T1 | 44 | 44 |
|
|
T2 | 33 | 37 |
|
| Histological
classificationb |
|
| 0.132 |
|
Invasive
ductal | 74 | 72 |
|
|
Special type | 3 | 9 |
|
| Histological
grade |
|
| 0.163 |
|
Grade 1 | 16 | 10 |
|
|
Grade 2 + 3 | 61 | 70 |
|
|
unknown | 0 | 1 |
|
| Estrogen
receptor |
|
| NA |
|
Positive | 77 | 81 |
|
|
Negative | 0 | 0 |
|
| Progesterone
receptor |
|
| 0.685 |
|
Positive | 61 | 62 |
|
|
Negative | 16 | 19 |
|
| Human epidermal
growth factor receptor 2 |
|
| NA |
|
Positive | 0 | 0 |
|
|
Negative | 77 | 81 |
|
Discussion
This is the first multiinstitutional registry study
in which the patients were prospectively followed up to investigate
the effect of 95GC on the prognosis of patients with ER+/HER2-/n0
breast cancer. Only two of 124 patients at 95GC low-risk had
distant recurrences, and their 5-year DRFS was as high as 98.0%.
This result is consistent with the previous retrospective studies
(10–13), suggesting that 95GC is useful in
selecting patients who show an excellent prognosis with adjuvant
endocrine therapy alone and thus can forgo adjuvant chemotherapy.
In addition, when the therapeutic effect of adjuvant chemotherapy
was evaluated in the patients in the 95GC high-risk group, their
prognosis was significantly improved by adding adjuvant
chemotherapy relative to that of the historical controls treated
with adjuvant endocrine therapy alone (Fig. 3). This result is also consistent
with our previous observation that 95GC high-risk tumors were more
sensitive to neoadjuvant chemotherapy than 95GC low-risk tumors
(15).
One important requirement for MGC is that it has a
significant effect on the de-escalation of adjuvant chemotherapy in
patients with ER+/HER2-/n0 breast cancer. Therefore, we evaluated
MGC by comparing the frequency of adjuvant chemo-endocrine therapy
actually given to the patients with that recommended by the St.
Gallen 2013 guideline (2).
According to the guideline, 155 (73.1%) patients were recommended
to receive adjuvant chemo-endocrine therapy. Actually; however, 86
(40.6%) patients were treated with adjuvant chemo-endocrine therapy
because of the implementation of 95GC (Table II), indicating a significant
de-escalation in adjuvant chemotherapy from 73.1% to 40.6%. The
excellent prognosis of the 95GC low-risk group (n=124), even though
it included the 71 (75–4) patients
who were recommended to receive adjuvant chemo-endocrine therapy by
the guideline but actually were treated with adjuvant endocrine
therapy alone, suggests that 95GC is useful in a safe de-escalation
of adjuvant chemotherapy.
The TAILORx trial showed no benefit of adding
adjuvant chemotherapy to endocrine therapy for HR+/HER2-/n0 breast
cancer with ODX recurrence score (RS) of 11–25, but the exploratory
analyses indicated that adjuvant chemotherapy was associated with
some benefit for women ≤50 years old who had an RS of 16–25
(5,6). In addition, the recent RxPonder trial
in patients with HR+/HER2-/n1-3 breast cancer and an ODX RS ≤ 25
showed a significant benefit of adjuvant chemotherapy in
premenopausal but not postmenopausal patients (7). Recently, we showed that patients with
breast cancer and an ODX RS of 11–25 could be classified into
low-risk and high-risk groups by 95GC (10). This further classification by 95GC
might be useful since the low-risk group could be treated with
adjuvant endocrine therapy alone and the high-risk group could be
treated with adjuvant chemo-endocrine therapy or adjuvant endocrine
therapy with ovarian suppression. Thus, 95GC can potentially
provide a more individualized treatment for patients with breast
cancer and an ODX RS ≤ 25 (11).
Since the 95GC assay is performed using microarray,
the expression data of all genes are available for each tumor. One
advantage of 95GC is that by utilizing such data, it is possible to
simultaneously analyze multiple MGCs including those developed for
prediction of chemosensitivity (anthracycline/taxane) such as 23GC
(16) and 155GC (17). Additional information on
chemosensitivity might be helpful in the selection of adjuvant
therapeutic regimens. Besides, we are conducting a registry study
to collect not only clinical information but also gene expression
data (DNA microarray CEL files) from patients subjected to the 95GC
assay. We believe that this registry will facilitate not only
further validation of 95GC but also the development of a new MGC
through the ecosystem proposed in Fig.
S1.
One limitation of this study is that it is a
retrospective study including a relatively small number of patients
who underwent the 95GC assay, which might have introduced survival
bias. However, to minimize this bias, we consecutively recruited
every patient with ER+/HER2-/n0 breast cancer who underwent the
95GC assay from each institution. Another limitation is that this
was an observational study in which the decision on adjuvant
therapy was at the physician's discretion and patient's preference
for treatment and not according to a protocol. However, the fact
that a percentage of the patients who were at 95GC low risk and
treated with adjuvant chemo-endocrine therapy accounted for only
4.8% is unlikely to compromise our hypothesis that patients at 95GC
low risk will have an excellent prognosis when treated with
adjuvant endocrine therapy alone.
In conclusion, the excellent prognosis of patients
with ER+/HER2-/n0 invasive breast cancer classified as 95GC low
risk could be validated in this registry study, indicating that
such patients can forgo adjuvant chemotherapy. However, to
establish the clinical utility of 95GC, it would be necessary to
conduct a prospective study in a large number of patients with
long-term follow-up.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by Sysmex.
Availability of data and materials
The raw data analyzed for the current study is not
publicly available due to the study protocol, but might be
available from the corresponding author on reasonable request under
the permission of the Japanese Association for Theranostics.
Authors' contributions
YN conceived and designed the study, acquired and
analyzed the data and wrote and edited the original manuscript. RT,
KS, MO, SI, HK, YK, KI and MS acquired and analyzed the data and
edited the original manuscript. TO, TK, AS, SNa and HT conceived
and designed the study and edited the original manuscript. SNo
conceived and designed the study, acquired and analyzed the data
and wrote and edited the original manuscript. YN and SNo confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The registry study has been approved by the ethics
committees of all participating hospitals, and the historical
control study has been approved by the Ethics Committee of Osaka
University Hospital (approval nos. 19510-3 and 14106-10).
Patient consent for publication
Because this study is a retrospective registry
(observational) study carried out by the opt-out method, informed
consent was not obtained.
Competing interests
Yasuto Naoi has received research funding from
Sysmex and AstraZeneca; he has received honoraria from AstraZeneca,
Pfizer, Eli Lilly, and Chugai outside the submitted work; and he
holds joint patents with Sysmex including CurebestTM
95GC Breast (JP.5725274.B2) and has received patent royalties
outside the submitted work. Kenzo Shimazu has received honoraria
from Sysmex and research funding to institution from Sysmex. Tomo
Osako received honoraria from Diaceutics and Daiichi Sankyo, and
consulting fee from Chiba Cytopathology Laboratory outside the
submitted work. Seigo Nakamura/Showa University received a research
grant from Sysmex Corporation. Hitoshi Tsuda received research
grant from Roche Diagnostics, Goryo Chemical and Scholarship
donation from Chugai Pharmaceutical. Kazuhiro Ishihara received
lecture fees from Nippon Kayaku, Kyowa Kirin, Daiichi Sankyo and
Eisai. Shinzaburo Noguchi has received consulting fees and research
funding from Sysmex; he has received consulting fees from
AstraZeneca and Nittobo outside the submitted work; he has received
honoraria from AstraZeneca, Pfizer, Eli Lilly, and Chugai outside
the submitted work; and he holds joint patents with Sysmex
including Curebest™ 95GC Breast (JP.5725274.B2) and has received
patent royalties outside the submitted work.
Glossary
Abbreviations
Abbreviations:
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
DRFS
|
distant recurrence-free survival
|
|
TAM
|
Tamoxifen
|
|
pCR
|
pathological complete response
|
|
GC
|
gene classifier
|
|
MGC
|
multigene classifier
|
|
FFPE
|
formalin fixed and paraffin
embedded
|
|
FF
|
fresh-frozen
|
|
NAC
|
neo-adjuvant chemotherapy
|
|
ODX
|
Oncotype DX
|
|
BGA
|
between-group analysis
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members.
Personalizing the treatment of women with early breast cancer:
Highlights of the St gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parker JS, Mullins M, Cheang MC, Leung S,
Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al:
Supervised risk predictor of breast cancer based on intrinsic
subtypes. J Clin Oncol. 27:1160–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA
Jr, et al: Prospective validation of a 21-gene expression assay in
breast cancer. N Engl J Med. 373:2005–2014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sparano JA, Robert JGray, Gray RJ, Makower
DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz
MP, et al: Adjuvant chemotherapy guided by a 21-gene expression
assay in breast cancer. N Engl J Med. 379:111–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalinsky K, Barlow WE, Gralow JR,
Meric-Bernstam F, Albain KS, Hayes DF, Lin NU, Perez EA, Goldstein
LJ, Chia SKL, et al: 21-gene assay to inform chemotherapy benefit
in node-positive breast cancer. N Engl J Med. 385:2336–2347. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naoi Y, Kishi K, Tanei T, Tsunashima R,
Tominaga N, Baba Y, Kim SJ, Taguchi T, Tamaki Y and Noguchi S:
Development of 95-gene classifier as a powerful predictor of
recurrences in node-negative and ER-positive breast cancer
patients. Breast Cancer Res Treat. 128:633–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujii T, Masuda H, Cheng YC, Yang F, Sahin
AA, Naoi Y, Matsunaga Y, Raghavendra A, Sinha AK, Fernandez JRE, et
al: A 95-gene signature stratifies recurrence risk of invasive
disease in ER-positive, HER2-negative, node-negative breast cancer
with intermediate 21-gene signature recurrence scores. Breast
Cancer Res Treat. 189:455–461. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naoi Y, Tsunashima R, Shimazu K and
Noguchi S: The multigene classifiers 95GC/42GC/155GC for precision
medicine in ER-positive HER2-negative early breast cancer. Cancer
Sci. 112:1369–1375. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsukamoto F, Arihiro K, Takahashi M, Ito
KI, Ohsumi S, Takashima S, Oba T, Yoshida M, Kishi K, Yamagishi K
and Kinoshita T: Multicenter retrospective study on the use of
Curebest™ 95GC Breast for estrogen receptor-positive and
node-negative early breast cancer. BMC Cancer. 21:10772021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naoi Y, Kishi K, Tsunashima R, Shimazu K,
Shimomura A, Maruyama N, Shimoda M, Kagara N, Baba Y, Kim SJ, et
al: Comparison of efficacy of 95-gene and 21-gene classifier
(Oncotype DX) for prediction of recurrence in ER-positive and
node-negative breast cancer patients. Breast Cancer Res Treat.
140:299–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naoi Y, Saito Y, Kishi K, Shimoda M,
Kagara N, Miyake T, Tanei T, Shimazu K, Kim SJ and Noguchi S:
Development of recurrence risk score using 95gene classifier and
its application to formalinfixed paraffinembedded tissues in
ERpositive, HER2negative and nodenegative breast cancer. Oncol Rep.
42:2680–2685. 2019.PubMed/NCBI
|
|
15
|
Tsunashima R, Naoi Y, Kishi K, Baba Y,
Shimomura A, Maruyama N, Nakayama T, Shimazu K, Kim SJ, Tamaki Y
and Noguchi S: Estrogen receptor positive breast cancer identified
by 95-gene classifier as at high risk for relapse shows better
response to neoadjuvant chemotherapy. Cancer Lett. 324:42–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sota Y, Naoi Y, Tsunashima R, Kagara N,
Shimazu K, Maruyama N, Shimomura A, Shimoda M, Kishi K, Baba Y and
Kim SJ: Construction of novel immune-related signature for
prediction of pathological complete response to neoadjuvant
chemotherapy in human breast cancer. Ann Oncol. 25:100–106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsunashima R, Naoi Y, Kagara N, Shimoda M,
Shimomura A, Maruyama N, Shimazu K, Kim SJ and Noguchi S:
Construction of multi-gene classifier for prediction of response to
and prognosis after neoadjuvant chemotherapy for estrogen receptor
positive breast cancers. Cancer Lett. 365:166–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuda H; General Rule Committee of the
Japanese Breast Cancer Society, . Histological classification of
breast tumors in the General Rules for clinical and pathological
recording of breast cancer (18th edition). Breast Cancer.
27:309–321. 2020. View Article : Google Scholar : PubMed/NCBI
|