Introduction
Colorectal cancer (CRC) has been ranked as the
second most lethal and the third most prevalent type of cancer
throughout the world (1). According
to GLOBOCAN statistics in 2020, >935,000 deaths and 1.9 million
new cases were registered as CRC, which accounted for ~10% of
cancer deaths and new cases in the world (1). Unfortunately, the number of new CRC
cases is likely to increase to ~2.5 million by 2035 (2). Currently, the standard treatment for
patients with CRC is radical surgery combined with adjuvant
chemotherapy, which has positive effects on early cases (2). Benefiting from the rapid development
of more effective screening methods and improved treatments, the
death rate of CRC declined by ~50% in 2016 compared with that in
1970 according to a clinical study in the United States (2). However, the 5-year survival rate for
CRC is only ~64%, which drops to 12% for metastatic CRC (3). Furthermore, current CRC chemotherapy
has certain limitations, including systemic toxicity, suboptimal
response rate, acquired drug resistance and low tumor-specific
selectivity (4,5). Therefore, it is necessary to search
for novel efficient compounds with good stability, safety and
efficacy for the treatment of CRC.
Natural products are a key source of new
antitumorigenic agents, as 80–83% of approved anticancer drugs are
natural compounds or their derivatives (6). Flavonoids, an important group of
natural molecules, are found in a variety of dietary plants, such
as fruits and vegetables. Based on their chemical structure,
flavonoids can be classified as isoflavonoids, flavanones,
flavanols, flavonols, flavones and anthocyanidins (7). Flavonoids, well known for their
chemopreventive and chemotherapeutic activities in multiple cancer
types, work by arresting the cell cycle, suppressing cell
proliferation, inducing apoptosis, modulating reactive oxygen
species (ROS)-scavenging enzyme activities and inhibiting
invasiveness (8). Notably, early
epidemiological reports have indicated that flavonoids can reduce
the risk of CRC (9,10). Hence, flavonoids have received a
great deal of interest in CRC research.
For example, oroxylin A, one of the main bioactive
flavonoids of Scutellariae Radix, can suppress the growth of CRC by
reprogramming HIF1α-modulated fatty acid metabolism (11). Tectochrysin, one of the major
flavonoids of Alpinia oxyphylla Miquel, can markedly
suppress the proliferation of SW480 and HCT-116 human colon cancer
cell lines, and lead to apoptotic cell death through regulating
death receptor expression and NF-κB activity (12). Additionally, 6-C-(E-phenylethenyl)
naringenin, a type of flavonoid from naringenin-fortified fried
beef, has been shown to suppress tumor cell proliferation, and
induce necrotic cell death and autophagy in CRC cells (13). However, these flavonoid compounds
are still in preclinical trials. Notably, ~90% of drug candidates
fail during the various phases of clinical trials and drug approval
(14). In order to accelerate the
development of new drugs, it is necessary to search for more
potential natural flavonoids for CRC treatment.
Shuteria involucrata (Wall.) Wight & Arn,
has long been used for the treatment of cold, chronic bronchitis,
cough, sore throat, pharyngitis, and tonsillitis in China (15). Recently, the natural flavanone,
involucrasin A (C21H22O5), was
isolated from S. involucrata (Wall.) Wight & Arn by our
team (15). However, the anticancer
activity of involucrasin A has not been reported in detail.
Furthermore, the mechanisms underlying the potential
antitumorigenic effects of involucrasin A remain elusive.
The Akt/murine double minute 2 homologue (MDM2)/p53
signaling pathway has significant roles in regulation of the cell
cycle, proliferation and apoptosis (16). Akt, a classic intracellular
signaling axis, is associated with tumorigenesis through a number
of mechanisms (17). The malignancy
of numerous types of cancer, such as CRC, lung cancer, breast
cancer, endometrial cancer and liver cancer, is induced by
increasing abnormal phosphorylation levels of Akt. As an important
downstream target of Akt, MDM2 can be phosphorylated and
translocated to the nucleus following Akt activation, thereby
suppressing the bioactivity of the tumor suppressor protein, p53
(18). Bax, a pro-apoptotic
regulator of Bcl2 family members, has been shown to be involved in
p53-mediated apoptosis (19). Upon
apoptotic stimuli, the expression of Bax is upregulated by p53
(19,20). Furthermore, p53 can regulate the
transition of G2/M phase (21), and p53-dependent G2
arrest is associated with a decrease in cyclin A2 level (22). Therefore, novel therapeutic
compounds inhibiting the Akt/MDM2/p53 signaling pathway are
intended as a strategy to eliminate cancerous cells. For example,
germacrone and saponin have been reported to induce cancer cell
apoptosis and cell cycle arrest by suppressing the Akt/MDM2/p53
signaling pathway, and may be potential therapeutic agents for the
treatment of cancer (16,23).
In the present study, the antitumorigenic activities
of involucrasin A in HCT-116 CRC cells were investigated and its
regulatory effects on the Akt/MDM2/p53 pathway were examined.
Materials and methods
Reagents and antibodies
The chemical structure of involucrasin A
(C21H22O5) is shown in Fig. 1A. Involucrasin A was isolated as a
white amorphous powder from S. involucrata (Wall.) Wight
& Arn by our team (15). The
whole plant of S. involucrata (Wall.) Wight & Arn was
identified and collected from Pu'er district (Yunnan, China) by
Professor Zi-Gang Qian (Yunnan University of Chinese Medicine,
Kunming, China). Involucrasin A was dissolved in dimethyl sulfoxide
(Thermo Fisher Scientific, Inc.) and stored at −40°C.
Sulforhodamine B (SRB), trichloroacetic acid (TCA), puromycin, Tris
base and crystal violet were purchased from MilliporeSigma.
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were obtained from Gibco; Thermo Fisher Scientific, Inc. The
Annexin V-FITC Apoptosis Detection Kit I was purchased from BD
Biosciences.
In this study, all primary antibodies were used at a
dilution of 1:1,000. Furthermore, the secondary antibodies were
used at a dilution of 1:10,000. Primary antibodies against human
proteins included anti-β-actin (cat. no. A5441; mouse monoclonal;
MilliporeSigma); anti-cyclin A2 (cat. no. AF6624; rabbit
polyclonal; Beyotime Institute of Biotechnology); anti-Bax (cat.
no. 2772; rabbit polyclonal), anti-cleaved caspase-9 (cat. no.
9505; rabbit polyclonal), anti-cleaved caspase-6 (cat. no. 9761;
rabbit polyclonal), anti-phosphorylated (p)-Akt (cat. no. 2965;
rabbit monoclonal) and anti-Akt (cat. no. 9272; rabbit polyclonal)
(all from Cell Signaling Technology, Inc.); anti-p53 (cat. no.
sc-126; mouse monoclonal; Santa Cruz Biotechnology, Inc.);
anti-p-MDM2 (cat. no. ab170880; rabbit monoclonal) and anti-MDM2
(cat. no. ab16895; mouse monoclonal) (both from Abcam). The
anti-rabbit (cat. no. 211-035-109) and anti-mouse (cat. no.
715-035-150) secondary antibodies were obtained from Jackson
ImmunoResearch Laboratories, Inc.
Cell lines and culture
Colorectal cancer cell lines (HCT-116, Caco-2 and
CT26), breast cancer cell lines (BT-549, SKBR3, HS578T, MDA-MB-436
and MDA-MB-453) and a liver cancer cell line (HepG2) were obtained
from the American Type Culture Collection and grown in DMEM
supplemented with 10% FBS and 1% antibiotics (100 U/ml penicillin
and 0.1 mg/ml streptomycin), and incubated at 5% CO2 and
37°C. CT26 is a murine CRC cell line from a BALB/c mouse. The
HCT-116 p53 KO cell line was provided by Dr. Bert Vogelstein (Johns
Hopkins University, Baltimore, MD, USA) and was cultured in DMEM
supplemented with 10% FBS and 1% antibiotics, and incubated at 5%
CO2 and 37°C (24). To
disrupt the two p53 alleles in HCT116 cells, two promoterless
targeting vectors were used, each containing a hygromycin- or
geneticin-resistance gene in place of the p53 genomic sequences
(24).
Colony formation assay
To assess the effects of involucrasin A on the
clonogenic capacity of HCT-116 cells, cells were plated into
12-well plates (1×103 cells/well) or 24-well plates (500
cells/well) and allowed to adhere overnight. Cells were then
treated with three concentrations of involucrasin A (12.5, 25 and
50 µM) for 10 days, and incubated at 5% CO2 and 37°C.
Colonies were washed with PBS and stained with 0.2% (w/v) crystal
violet in buffered formalin for 20 min at room temperature. A
colony was defined as consisting of at least 50 cells and was
counted manually. Colony images were captured using an Epson
Perfection V700 Photo (Epson Corporation).
Cell viability assay
Cell viability was determined using the SRB assay.
Briefly, HCT-116 cells were seeded in 96-well plates at a density
of 5×103 cells/well overnight. After incubation, fresh
media containing different concentrations (0, 3.12, 6.25, 12.5, 25,
50, 100, and 200 µM) of involucrasin A was added, and further
incubated at 5% CO2 and 37°C for 48 or 72 h. The cells
were fixed with 50 µl ice-cold 50% (w/v) TCA at 4°C for 1 h. Then,
wells were carefully rinsed with deionized water five times. After
air drying, cells were subsequently stained with 0.4% (w/v) SRB for
30 min and rinsed with 1% acetic acid at room temperature. Finally,
adhered cells were solubilized with 200 µl 10 mM Tris base solution
(pH 10.5), and plates were agitated for 5 min before absorbance was
measured using a SpectraMax paradigm microplate reader (Molecular
Devices, LLC) at a wavelength of 515 nm.
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry.
Briefly, HCT-116 cells were plated in 6-well plates at a density of
2×105 cells/well and treated with involucrasin A (0, 25,
50 and 100 µM) in DMEM supplemented with 10% FBS and 1%
antibiotics, and incubated at 5% CO2 and 37°C for 72 h.
Subsequently, cells were washed with 1X PBS before trypsinization
(3 min), centrifugation (300 × g for 5 min at room temperature),
and fixation with 70% ethanol at −20°C for 2 h. Then, cells were
stained with 500 µl propidium iodide (PI) (50 µg/ml)/RNase A (200
µg/ml; Nanjing KeyGen Biotech, Co., Ltd.) for 30 min at room
temperature. Finally, cells were washed with 1X PBS, centrifuged at
room temperature (300 × g for 5 min), and resuspended in 1X PBS,
and their fluorescence was detected using a FACS Aria II flow
cytometer (BD Biosciences). Data analysis was performed using
FlowJo software (V.10.4.1; FlowJo LLC).
Flow cytometric analysis of
apoptosis
The evaluation of apoptosis induction was performed
using a FITC Annexin V Apoptosis Detection Kit I (BD Biosciences)
according to the manufacturer's instructions. HCT-116 cells
(2×105 cells/well) in a 6-well plate were treated with
involucrasin A (0, 25, 50 and 100 µM) and incubated at 5%
CO2 and 37°C for 48 or 72 h. Then, cells were washed
with cold 1X PBS (pH 7.4) before trypsinization (3 min),
centrifugation (300 × g for 5 min at room temperature),
resuspension in 100 µl 1X binding buffer, staining with 2.5 µl FITC
Annexin V and 2.5 µl PI, and incubation for 15 min in the dark at
room temperature. The cellular analysis was performed using a flow
cytometer (Beckman Coulter, Inc.).
Lentivirus transduction for gene
overexpression and knockdown
Three different genes encode the isoforms of Akt,
including Akt1, Akt2 and Akt3. Akt3 is expressed mainly in the
testes and brain, Akt2 is highly expressed in insulin-responsive
tissue, and Akt1 is ubiquitously expressed (25). Notably, Akt1 is involved in the
cellular survival pathway through suppressing the apoptotic
process, and has an important role in numerous types of cancer
(26). Therefore, the
constitutively active Akt (pCDH-puro-myr-HA-Akt1; CA-Akt) was
exogenously expressed in the present study. The CA-Akt plasmid was
obtained from Addgene, Inc. (cat. no. 46969). Empty plasmid was
used as the control. The short hairpin RNA (shRNA) control plasmid
(sh007) was from MilliporeSigma (cat. no. SHC007; insert sequence,
5′-CCGGCGCTGAGTACTTCGAAATGTCCTCGAGGACATTTCGAAGTACTCAGCGTTTTT-3′).
For Bax knockdown, the following double-strand oligonucleotides
were cloned into a pLKO.1 plasmid (cat. no. 10878; Addgene, Inc.):
shBax,
F-5′-CCGGGACGAACTGGACAGTAACATGCTCGAGCATGTTACTGTCCAGTTCGTCTTTTTG-3′,
R-5′-AATTCAAAAAGACGAACTGGACAGTAACATGCTCGAGCATGTTACTGTCCAGTTCGTC-3′.
Lentivirus was produced using the 293T cells transduced with 0.5 µg
of the aforementioned construct, 0.375 µg psPAX2 (cat. no. 12260;
Addgene, Inc.) and 0.125 µg pMD2.G (cat. no. 12259; Addgene, Inc.)
in 50 µl Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) with 3 µl
FuGENE HD (Promega Corporation). The cells were incubated in a
humidified incubator at 5% CO2, 37°C for 16 h, and the
medium containing the transfection mixture was replaced with fresh
medium. After an additional 48 h, the medium was collected and
filtered using a 0.45-µM filter unit, and directly added to target
HCT116 cells. HCT-116 cells were incubated at 5% CO2 and
37°C with lentivirus for 24 h before selection with puromycin (cat.
no. A1113803; Thermo Fisher Scientific, Inc.) for 3 days. The
concentrations of puromycin used for selection and maintenance were
10 and 1 µg/ml, respectively.
Western blot analysis
Western blotting was performed to detect the protein
expression levels extracted from HCT-116 cells. Firstly, the
protein samples were collected in RIPA buffer (Beijing Solarbio
Science & Technology Co., Ltd.) with phosphatase inhibitor
(Roche Diagnostics GmbH) and protease inhibitor cocktail (Roche
Diagnostics GmbH). Next, a BCA Protein Assay Kit (Beyotime
Institute of Biotechnology) was used to determine the concentration
of the protein samples. Samples containing 20 µg of protein were
separated by SDS-PAGE on 10–15% gels and transferred to PVDF
membranes. Membranes were blocked with 3% skimmed milk in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at
room temperature. The blocked membranes were then incubated with
primary antibodies diluted in TBST containing 5% bovine serum
albumin (Beyotime Institute of Biotechnology) overnight at 4°C.
Membranes were washed three times with TBST for 1 h at room
temperature and then incubated with the corresponding horseradish
peroxidase-conjugated secondary antibodies diluted in 5% skimmed
milk in TBST for 1 h at room temperature. Membranes were washed
three times with TBST and visualized using SuperSignal West Pico
PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.)
via an Amersham Imager 800 (Cytiva). ImageJ software (version 1.48;
National Institutes of Health) was used to semi-quantify protein
levels after western blotting.
Statistical analysis
SPSS version 20.0 (IBM Corp.) was used for
statistical analysis. All data are from at least three independent
experiments. Data are presented as the mean ± standard deviation.
Comparisons between two groups were performed using an unpaired
Student's t-test; for comparisons of three or more groups, one-way
ANOVA followed by Tukey's multiple comparison test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Involucrasin A effectively inhibits
HCT-116 cell proliferation
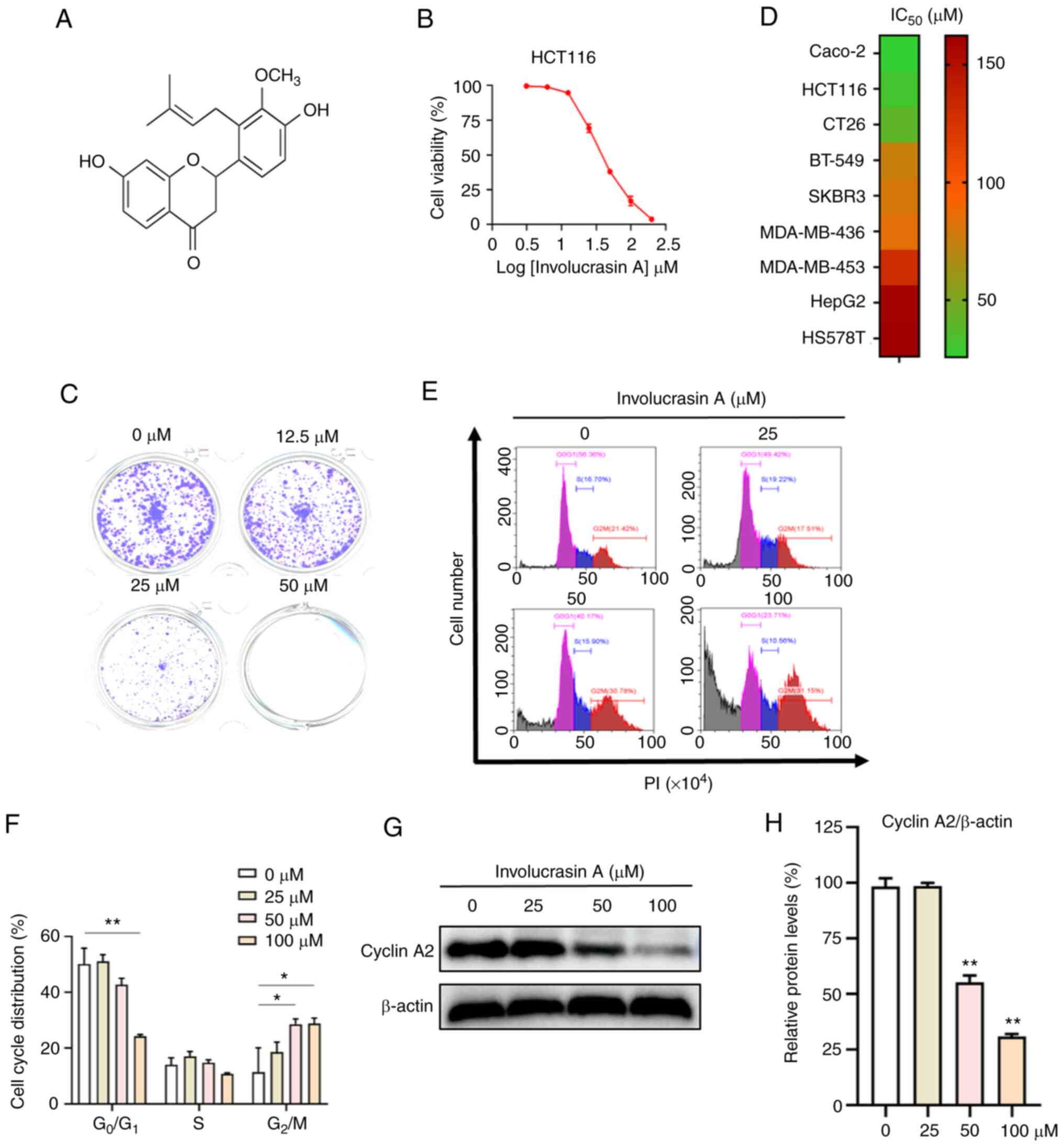
To determine the effects of involucrasin A on CRC
viability, HCT-116 cells were treated with involucrasin A at
various doses for 72 h, and cell viability was analyzed by SRB
assay. The results suggested that involucrasin A effectively
inhibited the viability of the CRC cell line HCT-116 in a
dose-dependent manner with an IC50 value of 37.92 mM
(Fig. 1B). This was confirmed by a
long-time colony formation assay (Fig.
1C). Furthermore, involucrasin A exhibited the most potent
anti-proliferative activity in the colorectal cancer cell lines
(HCT-116, Caco-2 and CT26) compared with other types of cancer cell
lines (Fig. 1D). Therefore, the
anticancer activities and mechanisms of involucrasin A in CRC cells
became the focus of the present study.
In cancer, cancer cell proliferation is generally
induced by a dysregulated cell cycle (27,28).
Thus, the cell cycle distribution of HCT-116 cells treated with
involucrasin A was analyzed. After 72 h, a significant increase in
the G2/M fraction and a decrease in the
G0/G1 fraction was observed in cells treated
with 50 and 100 µM involucrasin A compared with in the untreated
cells (Fig. 1E and F). These
results indicated that involucrasin A could induce HCT-116 cell
cycle arrest at G2/M phase. Cell cycle progression is
regulated by several key proteins, such as cyclin A2 and cyclin D1
(28). Cyclin A2 is a crucial
enzyme that promotes the start of cell mitosis, and depletion of
cyclin A2 leads to an arrest in G2 phase (29). In line with its effect on cell cycle
distribution, involucrasin A significantly downregulated the
expression levels of cyclin A2 in HCT-116 cells (Fig. 1G and H).
Involucrasin A effectively induces
HCT-116 cell apoptosis
A close relationship exists between cell cycle
progression, proliferation and apoptosis in cancer cells (27). Therefore, whether involucrasin A can
induce apoptosis was analyzed. After treatment for 72 h, 50 and 100
µM involucrasin A markedly induced HCT-116 cell apoptosis compared
with the control group (Fig. 2A).
Induced by the apoptotic signal, Bax promotes the release of
cytochrome c from mitochondria, and cytochrome c
subsequently activates the caspase-9 apoptotic pathway (30,31).
Once activated, caspase 9 activates downstream ‘effector caspases’
(i.e., caspase 6), and upregulates the levels of cleaved-caspase 6
(32). In the present study, the
protein expression levels of cleaved-caspase 9 and cleaved-caspase
6 were significantly upregulated with involucrasin A treatment
(Fig. 2B and C).
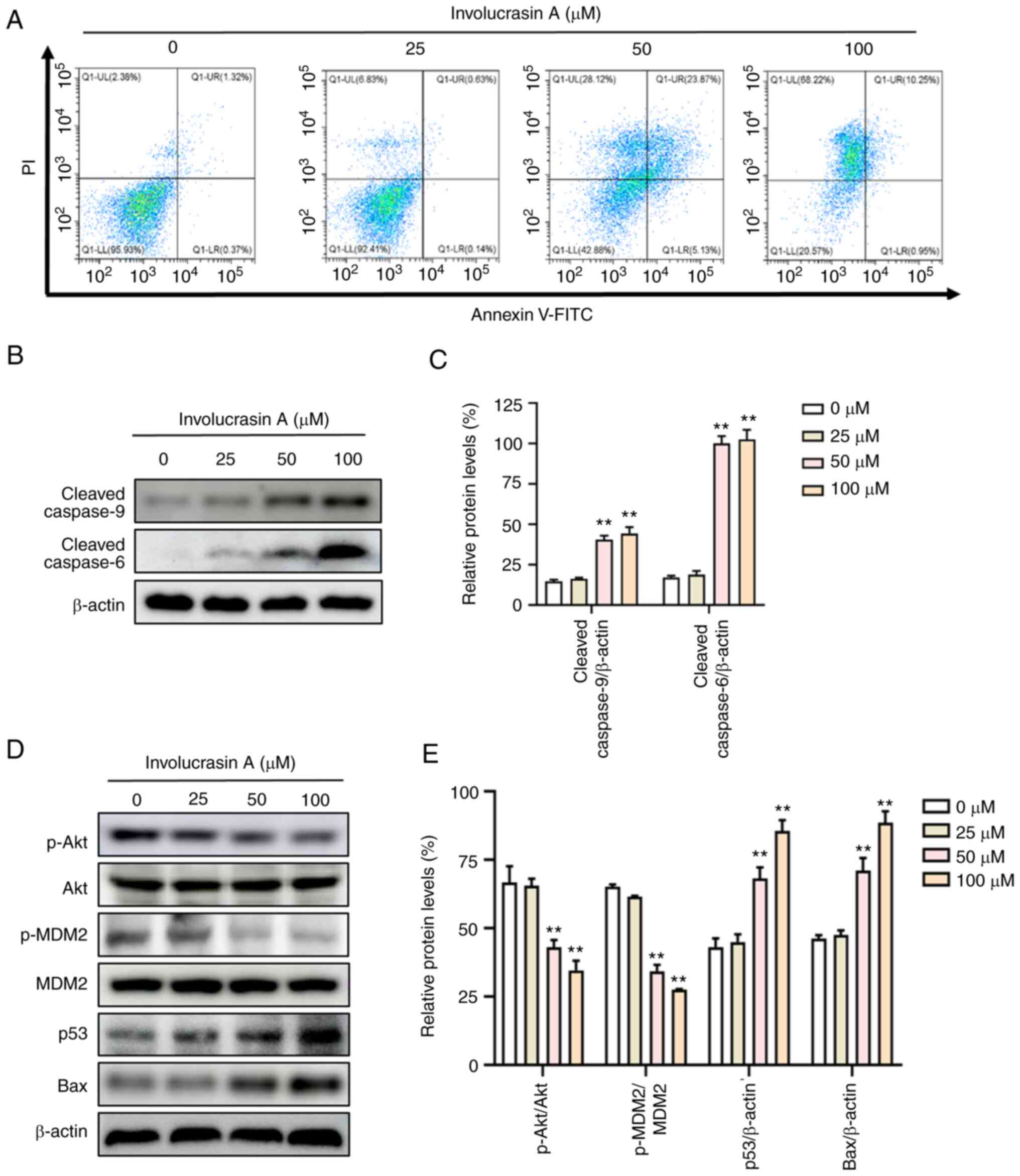 | Figure 2.Involucrasin A effectively induces
HCT-116 cell apoptosis. HCT-116 cells were treated with
involucrasin A (0, 25, 50 and 100 µM) for 72 h. (A) HCT-116 cells
were stained with Annexin V-FITC/PI, and apoptosis was quantified
by flow cytometry. (B-E) Protein expression levels of
cleaved-caspase 9, cleaved-caspase 6, p-Akt, Akt, p-MDM2, MDM2, p53
and Bax were examined via western blotting. β-actin served as a
loading control. All data are from at least three independent
experiments. **P<0.01 vs. control (0 µM). PI, propidium iodide;
p, phosphorylated; MDM2, murine double minute 2 homologue. |
Involucrasin A exerts anticancer
activities through modulating the Akt/MDM2/p53 pathway
The Akt/MDM2/p53 signaling pathway is a well-known
modulator of cell proliferation and apoptosis (16). To investigate whether this signaling
pathway was affected by involucrasin A, the present study examined
the related protein expression levels by western blotting. Notably,
the phosphorylation levels of Akt and MDM2 were significantly
decreased with involucrasin A treatment in a dose-dependent manner
(Fig. 2D and E). MDM2 is a key
downstream protein of Akt, which can be phosphorylated by p-Akt
(33). p-MDM2 translocates to the
nucleus, promoting ubiquitin-dependent degradation of p53 (34). As expected, p53 was upregulated by
involucrasin A in HCT-116 cells. Notably, p53 can upregulate the
expression levels of Bax, leading to programmed cell death
(20). Therefore, the protein
expression levels of Bax were also analyzed via western blotting.
In the present study, Bax was shown to be upregulated by
involucrasin A.
CA-Akt attenuates involucrasin
A-induced inhibition of proliferation and apoptosis
To confirm that involucrasin A exerts
antitumorigenic functions by modulating the Akt/MDM2/p53 pathway,
CA-Akt was exogenously expressed in HCT-116 cells. As shown in
Fig. 3A and B, the protein
expression levels of p-Akt and Akt were significantly increased in
cells transfected with CA-Akt. The anti-proliferative and
pro-apoptotic effects of involucrasin A on HCT-116 cells were
reversed by CA-Akt (Fig. 3C-E).
Consequently, the involucrasin A-induced expression levels of
apoptotic proteins, cleaved-caspase 9 and cleaved-caspase 6, were
attenuated in CA-Akt-expressing cells (Fig. 3F and G). Accordingly, the
involucrasin A-induced changes in the protein expression levels of
p-Akt, p-MDM2, p53 and Bax were blunted in CA-Akt cells compared
with in the control cells (Fig. 3H and
I). All results indicated that CA-Akt reversed involucrasin
A-induced anti-proliferative and pro-apoptotic effects.
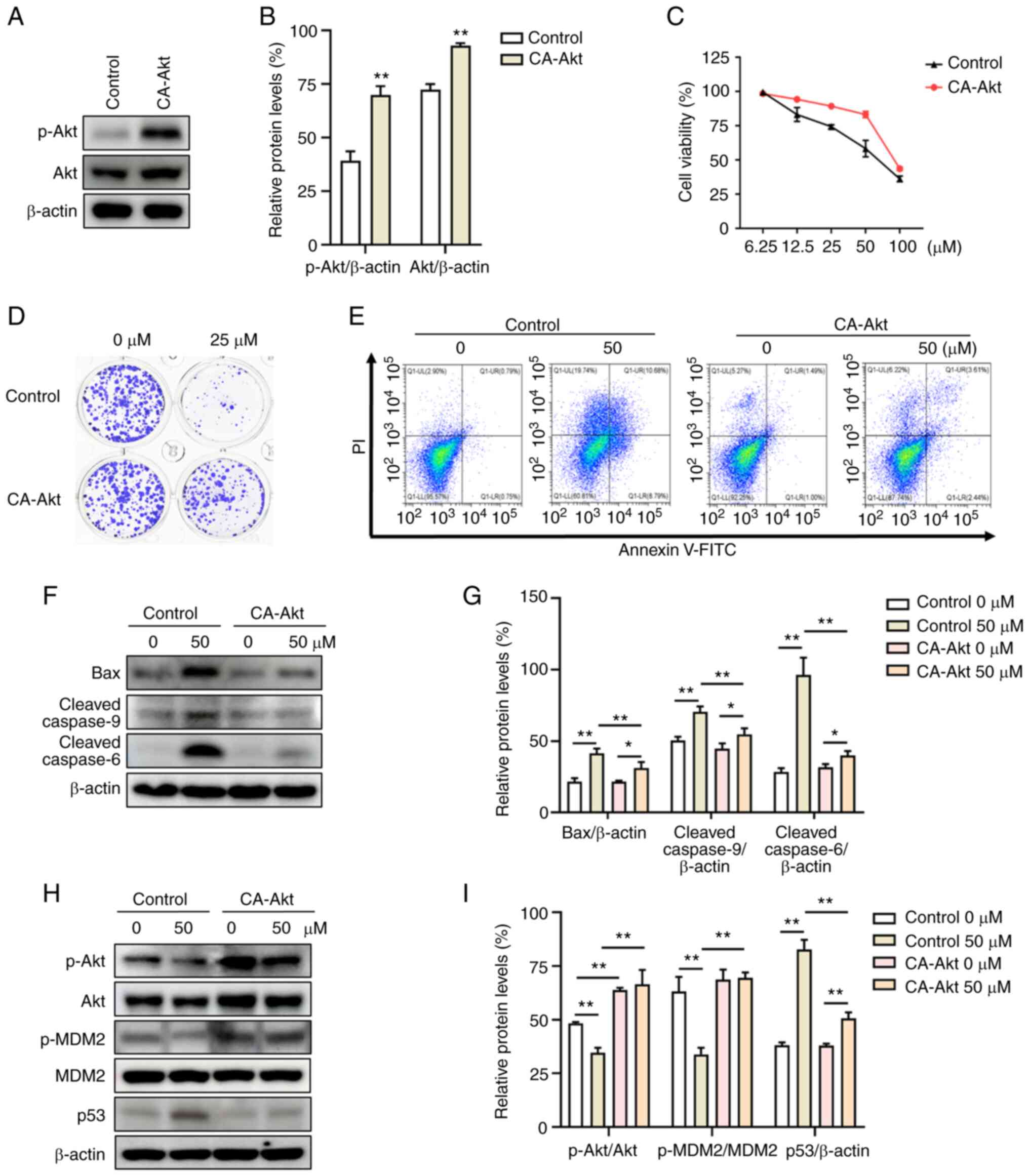 | Figure 3.CA-Akt attenuates involucrasin
A-induced inhibition of proliferation and apoptosis. (A and B) In
cells with CA-Akt, the protein expression levels of p-Akt and Akt
were significantly increased. HCT116 cells transduced with the
empty vector alone as the negative control. (C) HCT-116 cells were
treated with involucrasin A at the indicated concentrations for 48
h. Cell viability was analyzed by sulforhodamine B assay. (D)
Colony formation of control and CA-Akt HCT-116 cells treated with
involucrasin A (0 or 25 µM) for 10 days. Cells were treated with
involucrasin A (0 or 50 µM) for 48 h. (E) Apoptosis was quantified
by flow cytometry assay. (F) Western blotting was used to analyze
the protein expression levels of cleaved-caspase 9, cleaved-caspase
6 and Bax. β-actin served as a loading control. (G) Relative
protein expression levels of Bax, cleaved-caspase 9,
cleaved-caspase 6 were semi-quantified. (H and I) The protein
levels of p53, p-Akt, Akt, p-MDM2 and MDM2 were analyzed by western
blotting. All data are from at least three independent experiments.
*P<0.05, **P<0.01 as indicated or vs. control. CA-Akt,
constitutively active Akt; PI, propidium iodide; p, phosphorylated;
MDM2, murine double minute 2 homologue. |
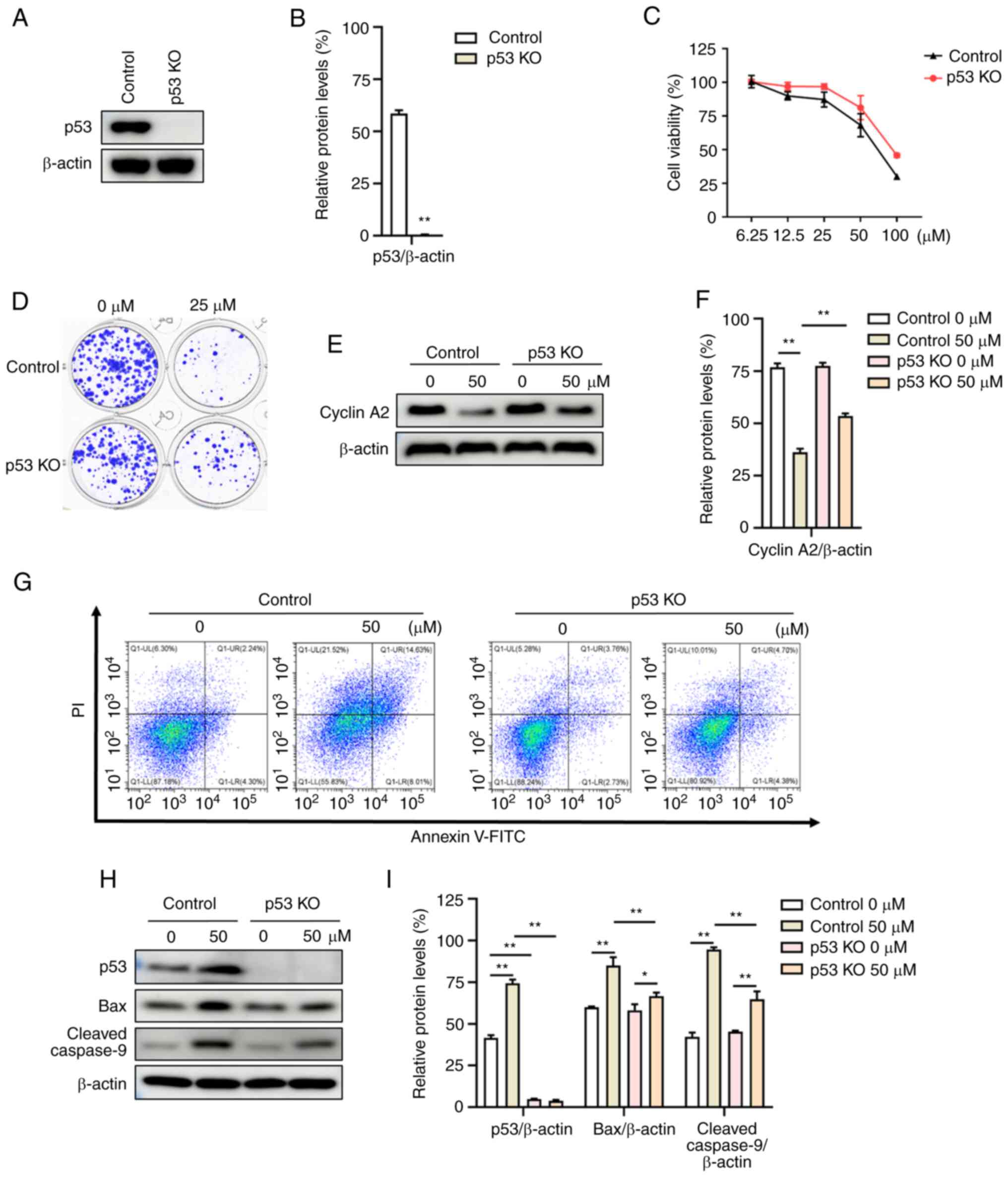
p53 KO attenuates the anticancer
functions of involucrasin A
To further investigate the dependency of p53 in
involucrasin A-induced anti-proliferative and pro-apoptotic
effects, the present study took advantage of the isogenic p53 KO
HCT-116 cell line. In cells with p53 KO, the protein expression
levels of p53 were significantly decreased compared with those of
the control group (parental HCT-116 cells) (Fig. 4A and B). Depletion of p53 led to the
reversal of involucrasin A-induced proliferation inhibition
(Fig. 4C and D). Additionally, the
survival and proliferation of p53 KO and control HCT116 cells were
almost the same when not treated with involucrasin A (Fig. 4D). Activation of p53 can induce
G2 arrest, which is associated with transcriptional
downregulation of cell cycle genes, such as cyclin A2 (22). After treatment with involucrasin A,
the protein expression levels of cyclin A2 in p53 KO cells were
increased significantly compared with those of the control cells
(Fig. 4E and F). Furthermore,
involucrasin A-induced apoptosis was attenuated in p53 KO cells
(Fig. 4G). The induction of
cleaved-caspase 9 and Bax were also reversed in involucrasin
A-treated p53 KO cells, compared with control cells (Fig. 4H and I). These data suggested that
involucrasin A-induced anti-proliferation and pro-apoptotic effects
were p53 dependent.
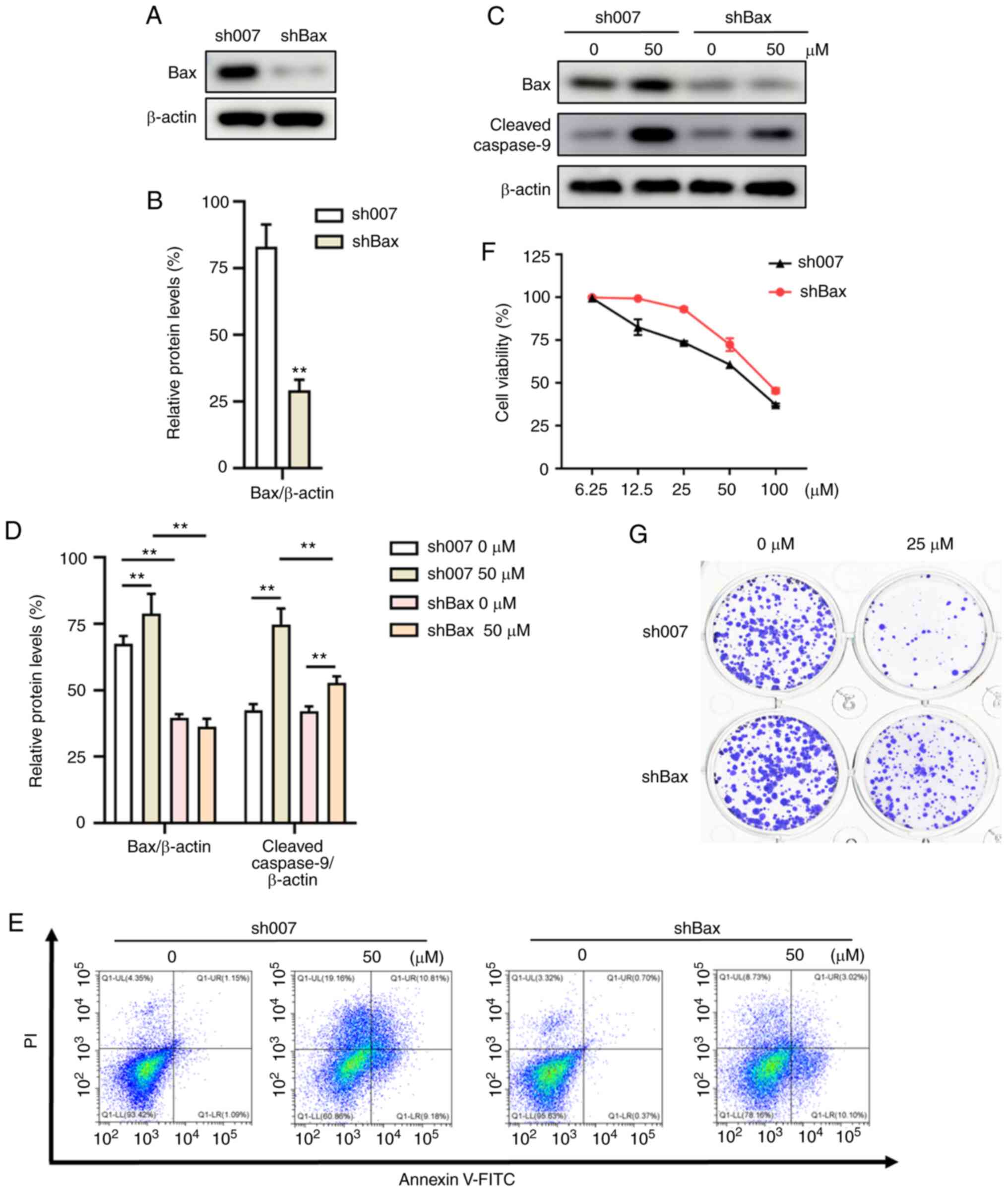
Bax knockdown attenuates the
anticancer functions of involucrasin A
To confirm the involvement of Bax in involucrasin
A-induced cytotoxic effects on HCT-116 cells, the present study
knocked down Bax using lentivirus-mediated RNA interference. In
cells transduced with shBax, the protein expression levels of Bax
were significantly decreased (Fig. 5A
and B). Notably, Bax knockdown significantly attenuated the
induction of cleaved-caspase 9 following treatment with
involucrasin A (Fig. 5C and D). As
expected, Bax knockdown markedly inhibited involucrasin A-induced
apoptosis (Fig. 5E). Consequently,
the SRB assay and the colony formation assay revealed that Bax
knockdown markedly decreased involucrasin A-induced proliferation
inhibition (Fig. 5F and G).
Discussion
Although chemotherapy is the preferred treatment
method for human CRC, adverse side effects and chemoresistance
remain major hurdles to successful treatment. Natural flavonoids
are a rich repository for colon cancer drug discovery (35). Involucrasin A, a novel natural
flavonoid isolated from S. involucrata (Wall.) Wight &
Arn, exhibited anti-proliferative activities on cancer cells
(15). This finding stimulated our
interest in further investigating the effects and mechanisms of
involucrasin A on CRC cells.
Proliferation is a crucial part of cancer
development and progression. Cancer therapy involving cytotoxic
drugs kills cells that have a high basal level of proliferation.
Therefore, excellent anticancer agents, such as vinblastine,
homoharringtonine, etoposide, teniposide, docetaxel and
camptothecin derivatives, can block proliferation, resulting in
cell cycle arrest and apoptosis (36). To determine the effects of
involucrasin A on CRC cell proliferation, SRB and colony formation
assays were performed. The results suggested that involucrasin A
could efficiently inhibit the proliferation of human CRC cells.
Furthermore, cell cycle analysis showed that involucrasin A
significantly increased G2/M arrest and downregulated
the expression levels of cyclin A2 in HCT-116 cells. The present
study also analyzed whether apoptosis was affected by involucrasin
A. After treatment for 48 h, involucrasin A markedly induced
apoptotic cell death. In addition, involucrasin A significantly
upregulated apoptotic protein expression levels of cleaved-caspase
9 and cleaved-caspase 6.
According to a previous report, flavonoids induce
apoptosis by increasing levels of ROS in cancer cells (37). However, ROS levels were not assessed
in the present study. The Akt/MDM2/p53 signaling pathway in cancer
has been studied by our team for a long time. Importantly, a
preliminary experiment by our team found that the protein
expression levels of p53 and Bax were notably increased by
involucrasin A. These results suggested that the Akt/MDM2/p53
signaling pathway may have a key role in the anticancer function of
involucrasin A. The Akt protein kinase has important roles in
several interconnected molecular signaling axes related to
proliferation, angiogenesis, apoptosis and cell metabolism. Thus,
Akt represents a therapeutic target, especially in colon cancer and
triple-negative breast cancer (TNBC), where the Akt signaling axis
is largely hyper-activated (38).
For example, a clinical trial showed that the Akt inhibitor
ipatasertib plus paclitaxel could improve progression-free survival
of patients with locally advanced/metastatic TNBC (39). Additionally, the constitutive
activation of the Akt pathway generally induces chemotherapy
resistance (17). p53 inhibits
cancer development through transcriptionally activating related
genes, and the functional link between Akt and p53 has been
reported (40,41). MDM2, a key downstream protein of
Akt, can be phosphorylated by p-Akt. p-MDM2 can translocate to the
nucleus and modulate p53 levels by targeting p53 for protein
degradation (42). Therefore, the
Akt/MDM2/p53 signaling pathway serves a key role in regulation of
the cell cycle, proliferation and apoptosis. Novel agents that can
inhibit this pathway are an emerging strategy to treat cancer.
In the present study, the results suggested that
involucrasin A could significantly decrease the phosphorylation
levels of Akt and MDM2, and upregulate p53 protein expression in
HCT-116 cells. To confirm the involvement of the Akt/MDM2/p53
pathway in the antitumorigenic functions of involucrasin A,
overexpressed CA-Akt and p53 KO in HCT-116 cells were assessed. The
results showed that CA-Akt and p53 KO attenuated involucrasin
A-induced anti-proliferative and pro-apoptotic effects. Bax has
been verified to be involved in p53-mediated apoptosis (19). Upon apoptotic stimuli, the protein
expression levels of Bax are upregulated directly by p53, thereby
stimulating programmed cell death (20).
Notably, the present study found that the expression
levels of Bax were upregulated by involucrasin A. However, p53 KO
attenuated involucrasin A-induced Bax expression. These results
suggested that involucrasin A induced cancer cell apoptosis by
p53-modulated Bax signaling. To confirm this hypothesis, Bax
knockdown in HCT-116 cells was performed. The results showed that
Bax knockdown attenuated involucrasin A-induced pro-apoptotic
effects and proliferation inhibition. During apoptosis, Bax induces
the release of cytochrome c from the mitochondria, and
subsequently stimulates the caspase-9 pathway (30,31).
This process is a classical signaling pathway, more attention was
therefore focused on the protein levels of cleaved-caspase 9 rather
than cytochrome c in the present study. Notably, Bax
knockdown significantly attenuated the induction of cleaved-caspase
9 following treatment with involucrasin A.
In summary, the present study indicated that
involucrasin A may exert a potent anticancer effect on colon cancer
cells through inducing apoptosis and cell cycle arrest.
Mechanistically, involucrasin A exhibited anticancer functions by
modulating the Akt/MDM2/p53 pathway. For the present study,
involucrasin A was isolated from S. involucrata (Wall.)
Wight & Arn by our team. Unfortunately, it was too difficult to
extract enough involucrasin A for use in animal studies. Based on
these results, involucrasin A is a promising natural compound for
treating colon cancer, and worthy of further exploration in
preclinical and clinical trials.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Science and Technology
Development Fund, Macau SAR, P.R. China (grant. nos. 0105/2022/A2,
0036/2020/A1 and 0013/2019/A1), the National Natural Science
Foundation of China (grant. no. 81960778), the Applied Basic
Research Project of Yunnan Province (grant. no. 2019FF002-009), and
the Reserve Talents Project for Young and Middle-Aged Academic and
Technical Leaders of Yunnan Province (grant. no.
202205AC160039).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, ZY and WM conceived and designed the
experiments. JD, YS, ZiW, QL, FZ, WL, ZhW and JC performed the
experiments and analyzed the results. All authors agree to be
accountable for all aspects of work ensuring integrity and
accuracy. CW and WM wrote the manuscript. CW and WM confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrelli F, Ardito R, Ghidini A, Zaniboni
A, Ghidini M, Barni S and Tomasello G: Different toxicity of
cetuximab and panitumumab in metastatic colorectal cancer
treatment: A systematic review and meta-analysis. Oncology.
94:191–199. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marin JJG, Romero MR, Blazquez AG, Herraez
E, Keck E and Briz O: Importance and limitations of chemotherapy
among the available treatments for gastrointestinal tumours.
Anticancer Agents Med Chem. 9:162–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aiello P, Consalvi S, Poce G, Raguzzini A,
Toti E, Palmery M, Biava M, Bernardi M, Kamal MA, Perry G and
Peluso I: Dietary flavonoids: Nano delivery and nanoparticles for
cancer therapy. Semin Cancer Biol. 69:150–165. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kopustinskiene DM, Jakstas V, Savickas A
and Bernatoniene J: Flavonoids as anticancer agents. Nutrients.
12:4572020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossi M, Negri E, Talamini R, Bosetti C,
Parpinel M, Gnagnarella P, Franceschi S, Dal Maso L, Montella M,
Giacosa A and La Vecchia C: Flavonoids and colorectal cancer in
Italy. Cancer Epidemiol Biomarkers Prev. 15:1555–1558. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang ZJ, Ohnaka K, Morita M, Toyomura K,
Kono S, Ueki T, Tanaka M, Kakeji Y, Maehara Y, Okamura T, et al:
Dietary polyphenols and colorectal cancer risk: The Fukuoka
colorectal cancer study. World J Gastroenterol. 19:2683–2690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni T, He Z, Dai Y, Yao J, Guo Q and Wei L:
Oroxylin A suppresses the development and growth of colorectal
cancer through reprogram of HIF1α-modulated fatty acid metabolism.
Cell Death Dis. 8:e28652017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park MH, Hong JE, Park ES, Yoon HS, Seo
DW, Hyun BK, Han SB, Ham YW, Hwang BY and Hong JT: Anticancer
effect of tectochrysin in colon cancer cell via suppression of
NF-kappaB activity and enhancement of death receptor expression.
Mol Cancer. 14:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y, Fan D, Ru B, Cheng KW, Hu S, Zhang
J, Li ETS and Wang M: 6-C-(E-phenylethenyl)naringenin induces cell
growth inhibition and cytoprotective autophagy in colon cancer
cells. Eur J Cancer. 68:38–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dowden H and Munro J: Trends in clinical
success rates and therapeutic focus. Nat Rev Drug Discov.
18:495–496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu L, Li XH, Ma XX, Tan WH, Ma WZ, Shen
YF, Khan A, Zhou ZH and Yang ZY: (±)-Involucrasins A and B, two
pairs of flavanone enantiomers from Shuteria involucrata and
their inhibitory effects on the proliferation of various cancer
cell lines. J Asian Nat Prod Res. 24:641–647. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Cai J, Shi K, Li H, Du J, Hu D,
Liu Z and Wang W: Germacrone induces lung cancer cell apoptosis and
cell cycle arrest via the Akt/MDM2/p53 signaling pathway. Mol Med
Rep. 23:4522021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta A, Shah K, Oza MJ and Behl T:
Reactivation of p53 gene by MDM2 inhibitors: A novel therapy for
cancer treatment. Biomed Pharmacother. 109:484–492. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
20
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Badie C, Bourhis J, Sobczak-Thépot J,
Haddada H, Chiron M, Janicot M, Janot F, Tursz T and Vassal G:
p53-dependent G2 arrest associated with a decrease in cyclins A2
and B1 levels in a human carcinoma cell line. Br J Cancer.
82:642–650. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tu Y, Chen L, Ren N, Li B, Wu Y, Rankin
GO, Rojanasakul Y, Wang Y and Chen YC: Standardized saponin extract
from baiye No.1 tea (camellia sinensis) flowers induced S phase
cell cycle arrest and apoptosis via AKT-MDM2-p53 signaling pathway
in ovarian cancer cells. Molecules. 25:35152020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bunz F, Dutriaux A, Lengauer C, Waldman T,
Zhou S, Brown JP, Sedivy JM, Kinzler KW and Vogelstein B:
Requirement for p53 and p21 to sustain G2 arrest after DNA damage.
Science. 282:1497–1501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol
K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al: Growth
retardation and increased apoptosis in mice with homozygous
disruption of the Akt1 gene. Genes Dev. 15:2203–2208. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matthews HK, Bertoli C and de Bruin RAM:
Cell cycle control in cancer. Nat Rev Mol Cell Biol. 23:74–88.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oakes V, Wang W, Harrington B, Lee WJ,
Beamish H, Chia KM, Pinder A, Goto H, Inagaki M, Pavey S and
Gabrielli B: Cyclin A/Cdk2 regulates Cdh1 and claspin during late
S/G2 phase of the cell cycle. Cell Cycle. 13:3302–3311. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh P and Lim B: Targeting apoptosis in
cancer. Curr Oncol Rep. 24:273–284. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peña-Blanco A and García-Sáez AJ: Bax, Bak
and beyond-mitochondrial performance in apoptosis. Febs J.
285:416–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang H, Luo J and Lei H: The roles of
mouse double minute 2 (MDM2) oncoprotein in ocular diseases: A
review. Exp Eye Res. 217:1089102022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zafar A, Wang W, Liu G, Xian W, McKeon F,
Zhou J and Zhang R: Targeting the p53-MDM2 pathway for
neuroblastoma therapy: Rays of hope. Cancer Lett. 496:16–29. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Afshari K, Haddadi NS, Haj-Mirzaian A,
Farzaei MH, Rohani MM, Akramian F, Naseri R, Sureda A, Ghanaatian N
and Abdolghaffari AH: Natural flavonoids for the prevention of
colon cancer: A comprehensive review of preclinical and clinical
studies. J Cell Physiol. 234:21519–21546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Butler MS: Natural products to drugs:
Natural product-derived compounds in clinical trials. Nat Prod Rep.
25:475–516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Slika H, Mansour H, Wehbe N, Nasser SA,
Iratni R, Nasrallah G, Shaito A, Ghaddar T, Kobeissy F and Eid AH:
Therapeutic potential of flavonoids in cancer: ROS-mediated
mechanisms. Biomed Pharmacother. 146:1124422022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hua H, Zhang H, Chen J, Wang J, Liu J and
Jiang Y: Targeting Akt in cancer for precision therapy. J Hematol
Oncol. 14:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dent R, Oliveira M, Isakoff SJ, Im SA,
Espié M, Blau S, Tan AR, Saura C, Wongchenko MJ, Xu N, et al: Final
results of the double-blind placebo-controlled randomized phase 2
LOTUS trial of first-line ipatasertib plus paclitaxel for
inoperable locally advanced/metastatic triple-negative breast
cancer. Breast Cancer Res Treat. 189:377–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bykov VJN, Eriksson SE, Bianchi J and
Wiman KG: Targeting mutant p53 for efficient cancer therapy. Nat
Rev Cancer. 18:89–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duffy MJ, Synnott NC and Crown J: Mutant
p53 as a target for cancer treatment. Eur J Cancer. 83:258–265.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lai CW, Xie C, Raufman JP and Xie G:
Targeting post-translational regulation of p53 in colorectal cancer
by exploiting vulnerabilities in the p53-MDM2 axis. Cancers
(Basel). 14:2192022. View Article : Google Scholar : PubMed/NCBI
|