Introduction
Bronchogenic carcinoma is the development of
malignant tumors from the respiratory epithelium; it involves
>90% of primary lung tumors (1).
Pathologically, it is categorized into two types: Small cell lung
cancer (SCLC) and non-SCLC (NSCLC). SCLC is known as oat cell
cancer owing to the packed nature of the small dense cells. SCLCs
represent ~20% of all lung cancer cases; they tend to metastasize
to the lymph nodes, but are very responsive to chemotherapy
(2). NSCLC consists of several
types of cancer, including adenocarcinoma (the most common type),
squamous carcinoma, large cell undifferentiated carcinoma,
bronchioalveolar carcinoma and neuroendocrine tumors (2).
Smoking is the most common risk factor, accounting
for ~85% of lung cancer cases (3).
Radon gas is regarded as the second most common cause of lung
cancer, which is generated by the breakdown of radioactive radium
(3). Other factors, like asbestos
exposure, air pollution, genetic abnormalities and pre-existing
lung diseases, also play an important role in the occurrence of
lung cancer (4,5). Cough, the most common symptom, is
associated with almost 90% of lung cancer cases and is followed by
dyspnea. Pleuritic pain, local chest wall pain, swelling of the
head and arms, weakness, seizures and confusion are all among the
abundant symptoms that can be associated with lung cancer (6). Despite the significant increase in
lung cancer incidence in recent decades in Iraq, there are only a
few small sample-sized studies focused on the profile of
bronchogenic carcinoma (1,7).
The present study on a single-center experience
aimed to estimate the profile of Iraqi patients with bronchogenic
carcinoma and assess the cancer resectability in newly diagnosed
patients.
Patients and methods
Study design
This is a single-center retrospective review with
longitudinal follow-up conducted over 5 years (January 1, 2015, to
October 1, 2019). The present study was approved by the Ethical
Committee of the University of Sulaimani (Sulaimani, Iraq).
Participants
The records of 800 patients with bronchogenic
carcinoma were collected from the Department of Thoracic and
Vascular Surgery, Ghazzi Al-Hariri Hospital for Surgical
Specialties (Baghdad, Iraq). Patients with bronchogenic carcinoma
were mostly differentiated using either cytological examination or
histopathological diagnosis.
Inclusion and exclusion criteria
All the patients with proven bronchogenic carcinoma
were included. Patients with a carcinoid tumor, metastatic nodules
regardless of size, and suspicion of bronchogenic carcinoma with
negative cytological and histopathological samples were all
excluded from the study.
Preoperative preparation
A detailed clinical assessment was conducted for the
patients by taking the patients' history, performing physical
examinations, and observing radiological imaging such as chest
radiography (CXR), computed tomography (CT) and magnetic resonance
imaging. Sputum analysis, cytological examination of pleural
effusion and bronchoscopic examination using either flexible or
rigid bronchoscopy were performed.
Lymph node biopsy, minimally invasive procedures
[mediastinoscopy and video-assisted thoracoscopic surgery (VATS)],
Tru-cut biopsy or fine-needle aspiration (FNA) were used to obtain
the samples for diagnosis. Patients with a resectable mass should
have undergone a positron emission tomography (PET) scan to exclude
lymph node metastasis. If the PET scan was clear, the patients were
scheduled for surgery. The surgeries (lobectomy and pneumonectomy)
included the removal of the masses in addition to lymph node
dissection.
Sputum analysis
After collecting the samples (sputum in the early
morning), the samples were preserved and fixed for 30 min at 25°C
using a saccomano fixative solution (BioGnost, Ltd.). A mucolytic
agent such as methyl cysteine HC1 (Cytoclair) (MilliporeSigma) 2%
solution in normal saline was used to obtain cell suspensions in
samples at a temperature of 37°C for up to 18 h. The samples were
centrifuged at 7,168 × g for ~10 min at 25°C and the precipitates
were then immersed in absolute alcohol (99%) for 15 min at 37°C for
further fixation and dehydration. The precipitations were put in
clearing agents such as xylene or acetone for 30 min before
preparing cell blocks using the Leukhardt paraffin box. For each
sample, a 4-µm thick section was taken using a conventional
microtome and stained with hematoxylin and eosin for 2 min at 37°C.
A light microscope was used for the valuation and examination of
the section with or without using the immunocytochemical staining
method for confirmation of the histogenesis of the cells.
Cytological examination
After taking FNA samples, the samples were preserved
and fixed for 30 min at 25°C using a saccomano fixative solution
(BioGnost, Ltd.). The samples were centrifuged at 7,168 × g) for
~10 min at 25°C and the precipitates were then immersed in absolute
alcohol (99%) for 15 min at 37°C for further fixation and
dehydration. The precipitations were put in clearing agents such as
xylene or acetone for 30 min before preparing cell blocks using the
Leukhardt paraffin box. For each sample, a 4-µm thick section was
taken using a conventional microtome and stained with hematoxylin
and eosin for 2 min at 37°C. A light microscope was used for the
valuation and examination of the section with or without using the
immunocytochemical staining method for confirmation of the
histogenesis of the cells.
Data collection and analysis
A data form was designed to collect and organize the
information of the patients. The form included introductory
information (age, sex and smoking history), symptoms,
investigations, imaging findings, bronchoscopic data, surgical
operation data and the final histological or cytological
diagnosis.
The patients' information was taken either
prospectively during their visit to the outpatient clinic or at
admission when they were prepared for the procedures. In some other
cases, the data was obtained retrospectively from the patients'
medical files or surgeon's notes. Final histopathological
information was obtained from the pathology laboratory.
Microsoft Excel (Microsoft Corporation) was used to
collect and organize the data. SPSS version 25 (IBM Corp.) was used
for encoding and descriptive analysis of the data.
Surgical procedures
Lobectomy
The surgeries were conducted under general
anesthesia with a double endotracheal lumen and lateral position
(right or left) according to the mass location. An anterior
thoracotomy was performed through the 5th or 6th intercostal space
according to the mass location. After accessing the pleural space,
adhesions were released (if present). A hemoclip was used to ligate
the vein of the affected lobe, followed by the dissection of the
fissure between the lobes to identify the arterial supply of the
affected lobe. The bronchi were isolated and a bronchial clamp was
applied. The lung was inflated to ensure the clamping of the
affected bronchus alone, and a linear stapler was used to ligate
the bronchus and separate it. A single layer of non-absorbable
sutures, such as polypropylene sutures, secured the bronchus, vein
and artery. The stump of the vein, artery, and bronchus were
checked for hemostasis and air leakage. A single chest drain was
inserted, with the wound closed in multiple layers.
Pneumonectomy
The surgeries were conducted under general
anesthesia with a double endotracheal lumen and lateral position
(right or left) according to the mass location. A classical
posterolateral thoracotomy was performed through the 5th or 6th
intercostal space according to the mass location. After accessing
the pleural space, adhesions were released (if present). The
inferior pulmonary ligament was released. The pulmonary vein was
identified by swab dissection between the lung and pericardium, and
then ligated using a hemoclip. An apical dissection was performed
to identify the main pulmonary artery, which was ligated using a
hemoclip. No inflation of the lung following the clamping of the
bronchus indicated a perfect closure. A linear stapler was applied
and divided the main bronchus. A single layer of non-absorbable
sutures, such as polypropylene sutures, secured the bronchus, vein
and artery. The stump of the vein, artery and bronchus were checked
for hemostasis and air leakage. A single chest drain was inserted
and removed after 48 h, with the wound closed in multiple
layers.
Results
The present study included a total of 800 patients
with bronchogenic carcinoma. The age range was between 22 and 87
years, with a mean age of 62.95 years. With a ratio of 3.8:1, the
dominant sex was male (636 patients, 79.5%). Almost half of the
patients were in their sixth to seventh decades of life (373
patients, 46.6%) (Table I). Most of
the patients were smokers or ex-smokers (84.3%); 87.6% of the males
and 71.3% of the females were active or ex-smokers. The most common
symptom was a cough (544 patients, 68.0%), followed by dyspnea (380
patients, 47.5%) (Table I). The CXR
presented a suspicious mass in 699 patients (87.4%), which in most
of the cases was located in the right lung. Other findings were
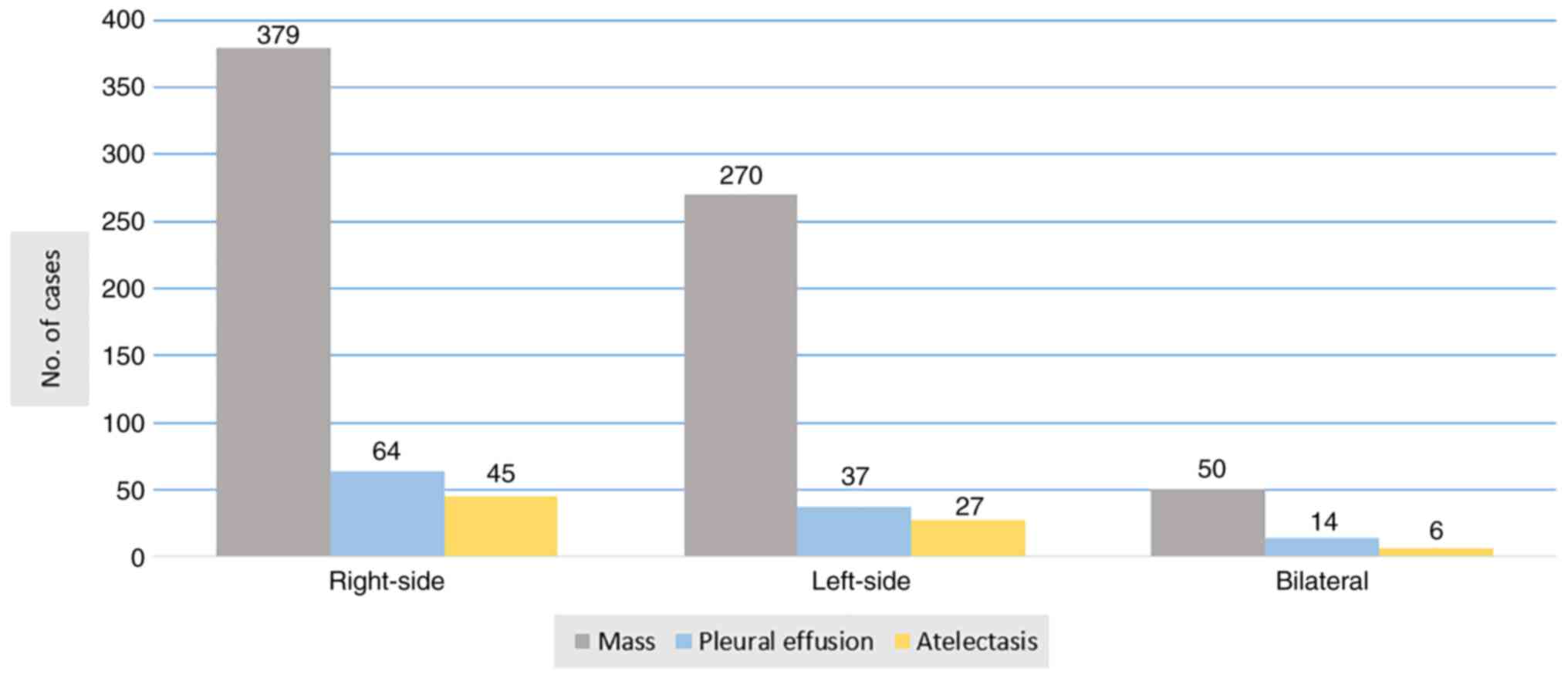
pleural effusion (14.4%) and atelectasis (9.8%) (Fig. 1). A high-resolution CT of the chest
and upper abdomen was performed for all of the patients. The most
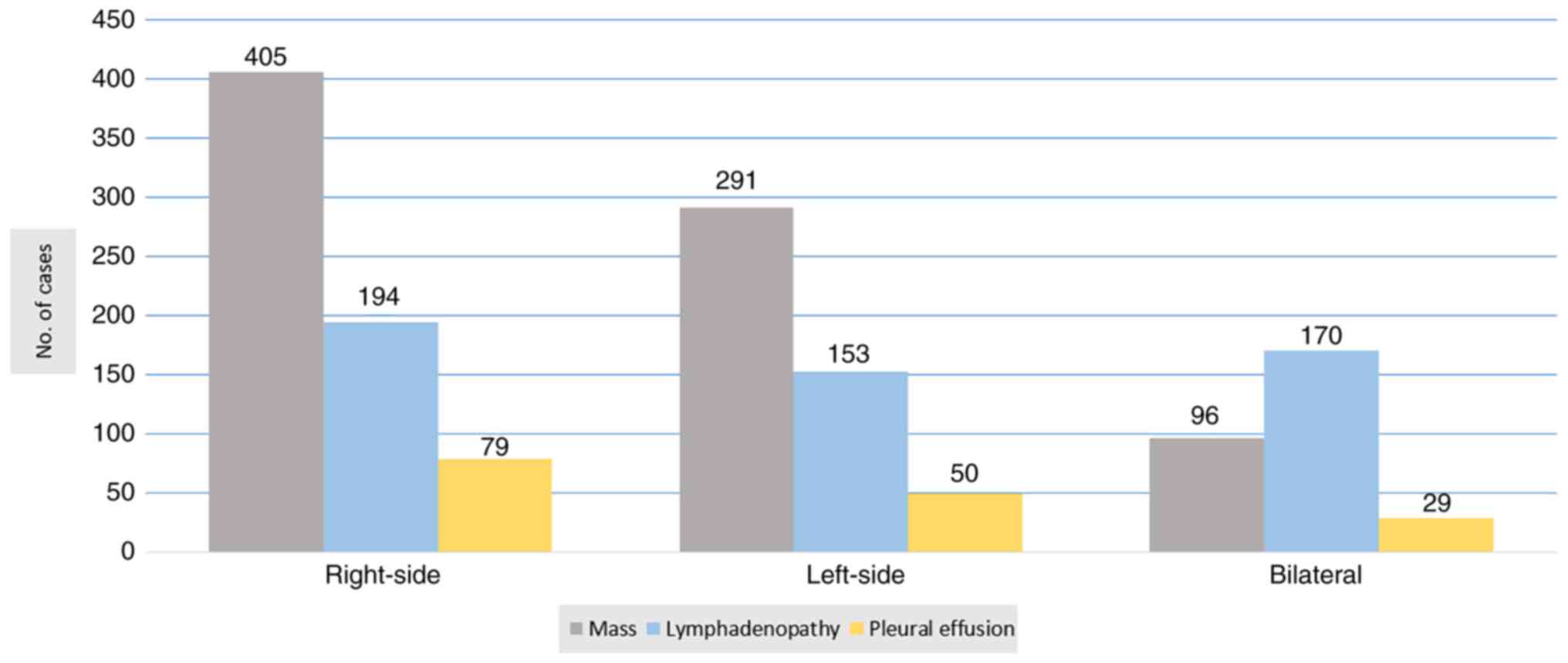
common findings were a mass (99.0%), lymphadenopathy (64.6%) and
pleural effusion (19.8%) (Fig. 2).
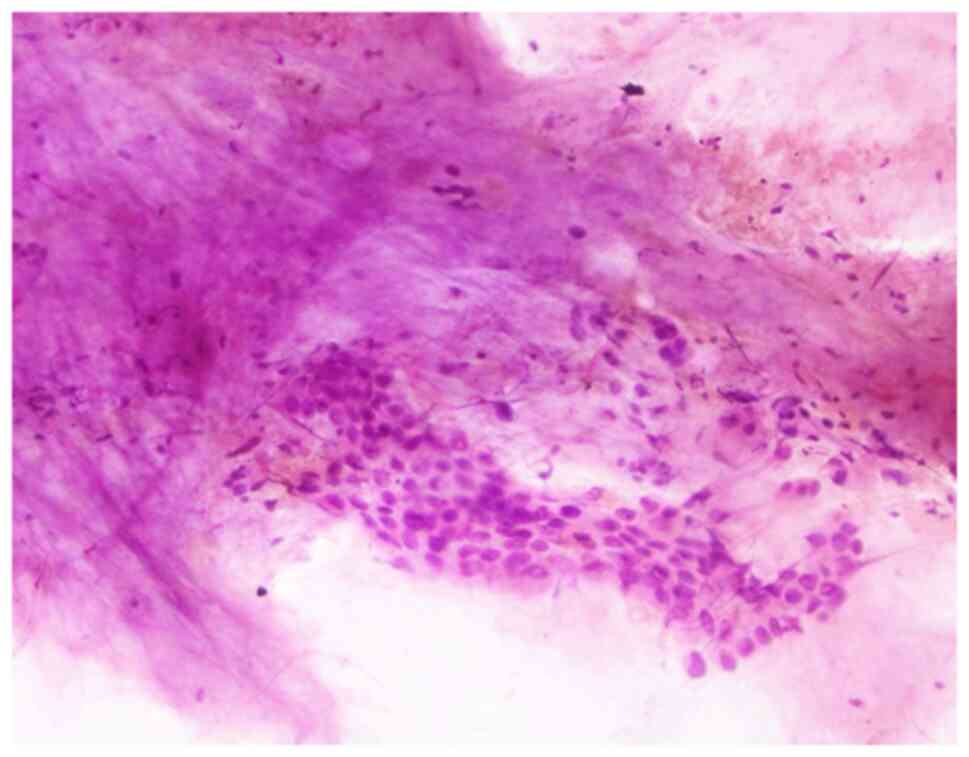
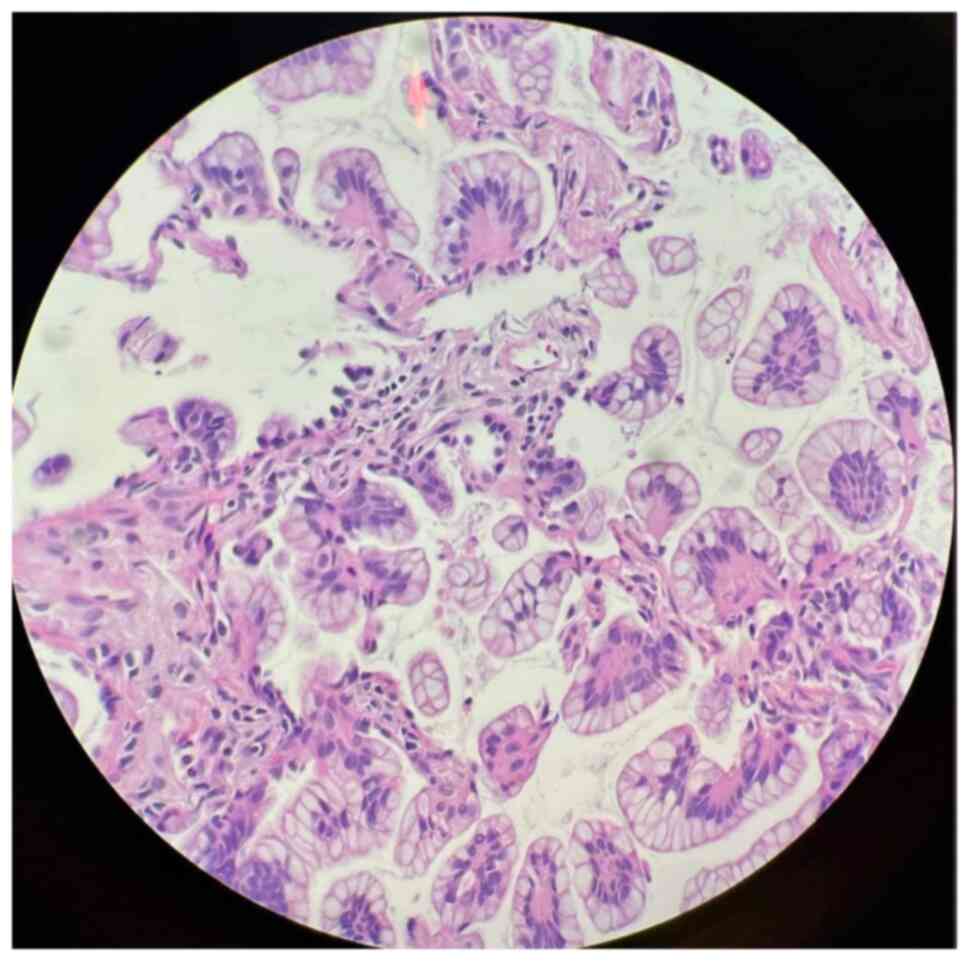
Cytological examination of the pleural effusions showed cancer
cells in 90 patients (57.0%) (Fig.
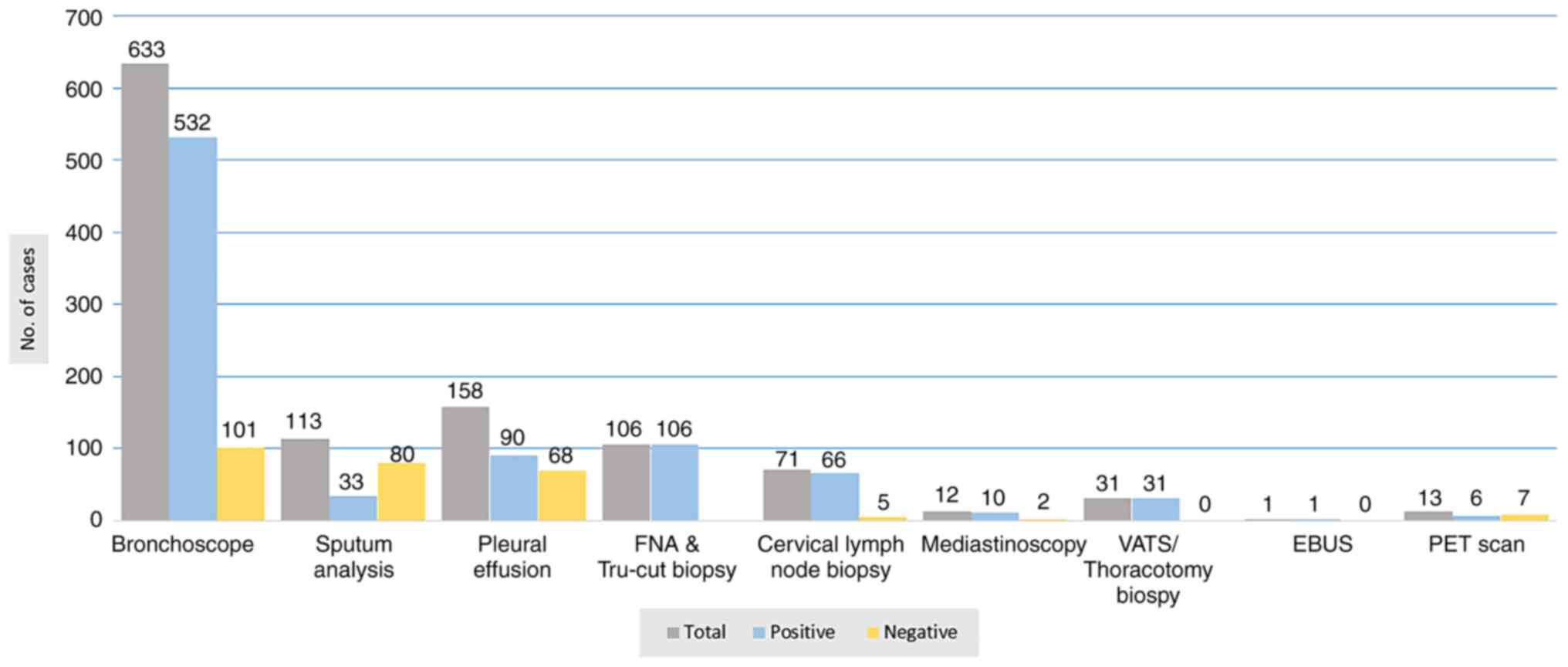
3). Bronchoscopic evaluations were performed for the majority
of the patients (633 patients, 79.1%). Fiberoptic bronchoscopy
(FOB) was performed under local anesthesia for 569 patients
(71.1%). Endobronchial masses and other suggestive malignancy
findings were present in 473 of these patients (83.1%), while
normal findings were observed in 96 patients (16.9%). Rigid
bronchoscopy was used only for 64 non-cooperative patients under
general anesthesia, and 59 of these patients (92.2%) had abnormal
findings. Cytological and/or histopathological samples of 581
(91.8%) of the patients who underwent bronchoscopic evaluation were
positive (Figs. 4 and 5). VATS and open lung biopsy were
performed for 23 and 8 patients, respectively, with cancer detected
in all of them. The findings of diagnostic modalities are all
summarized in Fig. 6.
 | Table I.Characteristics of the included
participants. |
Table I.
Characteristics of the included
participants.
| Characteristic | No. of cases | Percentage |
|---|
| Sex |
|
|
|
Male | 636 | 79.50 |
|
Female | 164 | 20.50 |
| Age, years |
|
|
|
20-30 | 4 | 0.50 |
|
30-40 | 7 | 0.88 |
|
40-50 | 62 | 7.75 |
|
50-60 | 219 | 27.38 |
|
60-70 | 373 | 46.62 |
|
70-80 | 101 | 12.62 |
|
>80 | 34 | 4.25 |
|
Smoker/ex-smoker | 674 | 84.25 |
|
Male | 557 | 87.58a |
|
Female | 117 | 71.34a |
| Symptoms |
|
|
|
Cough | 544 | 68.00 |
|
Dyspnoea | 380 | 47.50 |
|
Hemoptysis | 289 | 36.13 |
| Weight
loss | 169 | 21.13 |
| Chest
pain | 137 | 17.13 |
|
Hoarseness of voice | 38 | 4.75 |
| SVC
obstruction | 31 | 3.88 |
|
Neurological symptoms | 24 | 3.00 |
| Type of
operation |
|
|
|
Non-operable | 772 | 96.50 |
|
Lobectomy | 23 | 2.88 |
|
Pneumonectomy | 5 | 0.62 |
| Histopathology |
|
|
|
SCLC | 38 | 4.75 |
|
NSCLC | 762 | 95.25 |
| NSCLC subtypes |
|
|
|
Squamous cell carcinoma | 402 | 50.25 |
|
Adenocarcinoma | 345 | 43.12 |
| Large
cell carcinoma | 15 | 1.88 |
| Post-operative
complications |
|
|
| Wound
infection | 2 | 7.14b |
| Air
leakage | 2 | 7.14b |
|
Arrhythmia | 1 | 3.57b |
SCLC was present in 38 patients (4.75%) and NSCLC
was detected in 762 patients (95.25%) (Table I).
Tumor-Node-Metastasis (TNM) staging (8) was conducted for most of the cases
(95.3%) depending on their workup results, and most were in stages
III (55.4%) and IV (31.8%). All stage I and 24% of stage II tumors
were resectable (Table II).
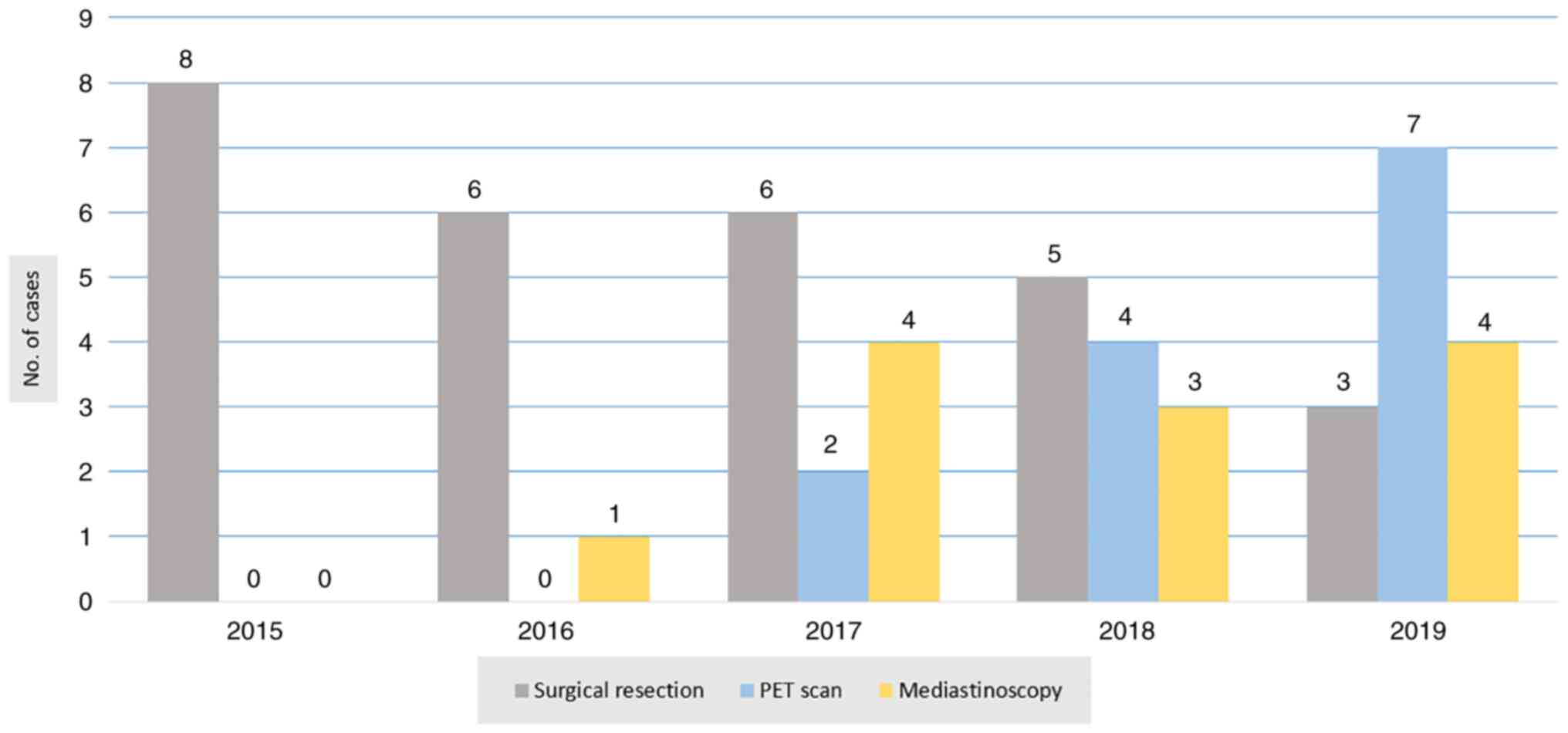
Regarding surgical resectability, only 28 patients (3.5%) were
candidates for surgery with radical lymph node dissection (Table III). Most of the cases were
investigated by PET scan and mediastinoscopy before making a
surgical decision. The ratio of using PET scan and mediastinoscopy
to aid in surgical resectability is presented in Fig. 7. Regarding sex, 20 of the surgical
candidates were male and the other 8 were female (Table IV). Lobectomy was the main surgical
procedure performed for 23 of the patients (82.1%), and
pneumonectomy was performed for 5 of the patients (17.9%) (Table III). Regarding morbidity, 5 of the
patients (17.9%) developed postoperative complications (Table I). There were no mortalities.
 | Table II.Stages of bronchogenic carcinoma in
non-small cell lung cancer cases. |
Table II.
Stages of bronchogenic carcinoma in
non-small cell lung cancer cases.
|
Tumor-Node-Metastasis staging | No. of cases | Percentage | Operability, % |
|---|
| I | 6 | 0.8 | 100.0 |
| II | 92 | 12.1 | 24.0 |
| III | 422 | 55.4 | 0.0 |
| IV | 242 | 31.8 | 0.0 |
 | Table III.Type of resections and resectability
rate. |
Table III.
Type of resections and resectability
rate.
| Year | No. of
patients | Resectability rate,
% | No. of
operations | Type of
resection | No. of
resections |
|---|
| 2015 | 167 | 4.70 | 8 | Lobectomy | 5 |
|
|
|
|
| Pneumonectomy | 3 |
| 2016 | 163 | 3.60 | 6 | Lobectomy | 4 |
|
|
|
|
| Pneumonectomy | 2 |
| 2017 | 169 | 3.50 | 6 | Lobectomy | 6 |
| 2018 | 161 | 3.10 | 5 | Lobectomy | 5 |
| 2019 | 140 | 2.10 | 3 | Lobectomy | 3 |
 | Table IV.Resectable patients according to sex,
age and histopathology. |
Table IV.
Resectable patients according to sex,
age and histopathology.
|
Characteristics | No. of cases | Percentage |
|---|
| Sex |
|
|
|
Male | 20 | 71.4 |
|
Female | 8 | 28.6 |
| Age, years |
|
|
|
20-40 | 0 | 0.0 |
|
40-50 | 4 | 14.3 |
|
50-60 | 11 | 39.3 |
|
60-70 | 12 | 42.9 |
|
>70 | 1 | 3.6 |
| Histopathology |
|
|
|
SCLC | 0 | 0.0 |
|
NSCLC | 28 | 100.0 |
| NSCLC type |
|
|
|
Squamous carcinoma | 14 | 50.0 |
|
Adenocarcinoma | 14 | 50.0 |
Discussion
Alongside breast cancer, lung cancer is known as a
major cause of cancer-related deaths worldwide (9). Despite the advancements in diagnostic
modalities and management techniques, lung cancer still has a high
incidence with a poor prognosis. The overall 5-year survival rates
are <20% (10). In the UK,
~40,000 new cases are recorded annually. From the time of
diagnosis, a large proportion of the patients die within 1 year,
and only 5% of the patients have a chance of 5-year survival. This
high mortality rate makes lung cancer one of the most common causes
of cancer-related death (5). In
Iraq, lung cancer is regarded as the most common cancer in men and
one of the five most common cancer types in women (7).
It has been estimated that smoking is the major
cause of lung cancer in 85–90% of all cases. In total, over 40
carcinogenic agents have been identified in cigarette smoke. Even
non-smokers are susceptible to being affected by lung cancer when
there is a high rate of exposure to tobacco smoke (11). In reviewing the results of the
present study, smoking was the major risk factor for bronchogenic
carcinoma. This is comparable with the result of the study by
Al-Kadhimi et al (7).
The majority of patients in the present study were
between 60 and 70 years old, with a mean age of 62.95 years. This
is consistent with the studies by Westermann et al (12), De Perrot et al (13) and Boffa et al (14). The long-term carcinogenic effect of
smoking may be the reason for these findings. In the current study,
most of the patients were male, with a ratio of 3.8:1, which is not
consistent with the previous studies. Boffa et al (14) reported a ratio of 1.5:1 and the
results of Al-Kadhimi et al (7) were significantly different, with a
reported male-to-female ratio of 5.4:1.
The development of smoking habits in the female sex
may be responsible for this increase in incidence of bronchogenic
carcinoma in Iraq. A cough was the most common presenting symptom
in the present study. This is consistent with the local studies by
Al-Rahim (1) and Al-Kadhimi et
al (7). This commonality could
be due to the strong association between bronchogenic carcinoma and
smoking habits.
TNM staging based on clinical examination,
mediastinoscopic examination, bronchoscopic examination and
radiography has a vital role in deciding the resectability in most
cases (15). In the present study,
TNM staging was conducted for most of the cases depending on their
work-up results. The CXR in a posterolateral view was taken for the
patients in this study. The vast majority of abnormal radiological
findings consisted of the presence of a mass, with a sensitivity of
87.3%, followed by the presence of effusion and atelectasis. This
result agreed with that in the study by Gupta et al
(16). Contrast CT of the chest and
abdomen was performed for all the patients who had abnormal
radiological findings on CXR, for confirmation of the diagnosis and
staging of the tumor, and the disease was found in 792 patients.
The lesions were distributed as follows: 405 on the right side, 291
on the left side and 96 bilateral masses. The second finding was of
mediastinal lymphadenopathy (LAP) in 517 patients. Pleural effusion
was found in 158 patients. These findings were consistent with the
data obtained in the study by Gupta et al (16), in which a bronchogenic mass was
reported in 96.7% of patients and LAP in 79.1%.
PET/CT imaging has been increasingly used in the
last decade in the assessment of patients with lung cancer
(17). In the present study, the
high sensitivity of PET scans was similar to that found in the
study by Hussein et al (18). A PET/CT scan was used in the last 2
years for 13 patients. Out of those 13 patients, only 6 patients
were operated on due to clear mediastinal LAP. The other 7 patients
had N2 disease at the time of examination, so surgical intervention
was canceled. Sputum cytology is a rapid test used for the
diagnosis and screening of bronchogenic carcinoma (19). This was performed for only 113
(14.1%) patients in the present study, while another local study
performed sputum cytology in almost 79.5% of the cases (1). The reduction in the use of sputum
cytology in the present study is due to the availability of more
accurate tests for cytological examinations, such as bronchoscopy
and FNA.
Despite the progress in cancer treatment, the
management of malignant pleural effusion (MPE) remains palliative,
with median survival ranging from 3 to 12 months (20). In the present study, 158 patients
(19.8%) had pleural effusion. Cytological examination revealed
positive results in 90 patients (57.0%), and this may be due to the
advancement of cytological techniques and the more common use of CT
scans that can detect even a small amount of MPE. In addition, the
present results corresponded with those of the study by Asciak and
Rahman (21), which reported
positive results in 60% of the patients.
The rapid evolution in imaging technology makes
bronchoscopes more flexible with a smaller diameter, a finer
resolution of view and a wider working channel. This provides a
facility for endobronchial ultrasound (EBUS), biopsy sampling and
therapeutic intervention (22,23).
In the present series, rigid and flexible bronchoscopies were
performed for 633 patients (79.1%). Abnormal findings were found in
59 patients (92.2%) out of 64 cases that had undergone rigid
bronchoscopy. FOB was performed for the other 569 patients, with
abnormal bronchoscopic findings in 473 patients (83.1%) and
non-specific findings in 96 patients (16.9%). However, in the study
by Al-Rahim (1), bronchoscopy was
performed in ~84% of the cases, with an abnormal finding in 64% of
them.
Thoracoscopy is considered a standard diagnostic
modality, but less invasive techniques have emerged as potential
alternatives, including transthoracic needle aspiration and EBUS
with needle aspiration (24). In
the present study, in the 106 cases (13.3%) that were unable to
undergo surgical intervention due to medical contraindications or
advanced disease, FNA and Tru-cut biopsy were conducted to obtain
tissue for histological diagnosis.
The use of an open cervical and scalene lymph node
biopsy to confirm bronchogenic carcinoma has decreased with the
advancement of other modalities. Recently, it has only been
preserved for patients with palpable lymph nodes and those who have
experienced the failure of less invasive techniques to confirm the
diagnosis. In the present study experience, the results of the
lymph node biopsy were almost the same as those of the study by
Al-Rahim (1). Cervical
mediastinoscopy was conducted for only 1.5% of the patients in the
present study, with a positive histopathological finding in 83.3%
of them. However, in the study by Bousema et al (25), it was performed for 29.5% of the
patients. The cause of this low application in the present study is
the late introduction of video-assisted mediastinoscopy in the
Department of Thoracic and Vascular Surgery, Ghazzi Al-Hariri
Hospital for Surgical Specialties. Furthermore, the data were
obtained from a single center while the data collected in the study
by Bousema et al (25) were
from six Dutch thoracic surgery centers.
VATS represents a new developing technique for
diagnosing patients with bronchogenic carcinoma; it greatly
improves a patient's prognosis and significantly reduces morbidity
and mortality rates (26). In the
present series, VATS was performed for 23 patients, and open lung
biopsy for 8 patients. In a previous study, a biopsy was required
for 22 patients, and all of these were performed by open thoracic
surgery (1).
Regarding the histological types of lung cancer, the
most common type was squamous cell carcinoma, followed by
adenocarcinoma in the present study. This result agreed with that
found in the study by Al-Rahim (1)
and differed from that found in the study by Strand et al
(27), where the results showed
that adenocarcinoma was the most common type compared with squamous
cell carcinoma.
The first lobectomy for lung cancer was conducted by
Hugh Davies in 1912 (28). The
patient died with empyema 8 days after the resection. Surgical
resection for lung cancer has become prevalent with the advancement
of the water-sealed drainage system and anesthetic techniques. In
1933, Graham and Singer reported the first successful one-stage
pneumonectomy (29). Since then,
the standard operation for lung cancer has become pneumonectomy.
Around the same time, Barney and Churchill (30) described an experience of lobectomies
with hilar dissections. The vast majority of surgical procedures in
the present study were lobectomies in 23 patients. Pneumonectomy
was performed in only 5 cases.
Other studies used the segmentectomy technique
alongside lobectomy and pneumectomy in a specific portion of the
cases (14,27). The absence of segmentectomy in the
present study can be explained by the lack of an intraoperative
frozen section facility, which is necessary to confirm a
cancer-free margin. The morbidity rate in the present study was
17.9%, without any mortality. In a study by Boffa et al
(14), the morbidity rate was 32%,
with a mortality rate of 2.5%. As most of the patients at the time
of diagnosis were non-operable, the rate of surgical resectability
in the present study was 3.5%. This result was lower than the
percentage of resectability in the global studies by Strand et
al (27) and Boffa et al
(14).
In conclusion, bronchogenic carcinoma is an
aggressive tumor with various presentations. The incidence of
bronchogenic carcinoma is rapidly increasing without a predilection
for either sex in the Iraqi population. Advanced preoperative
staging and investigation tools are required to determine the rate
of resectability.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MMM provided major contributions to the study
concept and final approval of the manuscript. SNJ and OFA performed
the operations. FHK was involved in the conception of the study,
literature review and drafting the manuscript. BJHA, SHT, AMS, SHA,
RKA, RJR, SHM, SMM, RAA and HMR were involved in critically
revising the manuscript, data analysis and revision of the tables
and figures. All authors read and approved the final manuscript.
MMM, FHK and OFA confirm the authenticity of all the raw data. All
authors agreed to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
Sulaimani University (Sulaimani, Iraq). Written informed consent
was obtained from all patients.
Patient consent for publication
The patients provided written consent for the
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al-Rahim YA: Lung Cancer in a sample of
Iraqi patients. Al-Kindy Col Med J. 4:53–59. 2007.
|
|
2
|
Kumar V, Abbas AK and Aster JC: Robbins
Basic Pathology. 9th edition. Elsevier Saunders; Philadelphia, USA:
pp. 5052013
|
|
3
|
Broaddus VC, Mason RJ, King TE Jr, Lazarus
SC, Murray JF, Nadal JA, Slutsky AS and Gotway MB: Epidemiology of
Lung Cancer. Murray & Nadel's Textbook of Respiratory Medicine.
6th edition. Saunders Elsevier; Philadelphia, USA: pp. 9272016
|
|
4
|
O'Reilly KM, Mclaughlin AM, Beckett WS and
Sime PJ: Asbestos-related lung disease. Am Fam Physician.
75:683–688. 2007.PubMed/NCBI
|
|
5
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Epidemiology of lung cancer'. Fishman's Pulmonary Diseases and
Disorders. Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI,
Senior RM and Siegel MD: 5th edition. McGraw-Hill; NY: pp.
16732015
|
|
6
|
Brunicardi F, Andersen D, Billiar T, Dunn
D, Hunter J, Matthews J, et al: Schwartz's principles of surgery.
McGraw-Hill; NY: 19. pp. 9802014
|
|
7
|
M Al-Kadhimi H, H Al-Azzawi Q and K Salih
A: Bronchogenic Carcinoma in Patients Younger Than 40 Years.
Kerbala J Med. 10:2785–2791. 2017.
|
|
8
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Institutes of Health, . National
Cancer Institute, Surveillance Epidemiology and End Results Program
(SEER). SEER Stat Fact Sheets: Liver and Intrahepatic Bile Duct
Cancer. 2010.https://seer.cancer.gov/statfacts/html/livibd.htmlApril
1–2023
|
|
11
|
Ettinger DS: Lung cancer and other
pulmonary neoplasms. Goldman's Cecil Medicine. 24th edition.
Elsevier Inc; pp. 1264–1272. 2011
|
|
12
|
Westermann CJ, van Swieten HA, Brutel de
la Rivière A, van den Bosch JM and Duurkens VA: Pulmonary resection
after pneumonectomy in patients with bronchogenic carcinoma. J
Thorac Cardiovasc Surg. 106:868–874. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Perrot M, Licker M, Reymond MA, Robert
J and Spiliopoulos A: Influence of age on operative mortality and
long-term survival after lung resection for bronchogenic carcinoma.
Eur Respir J. 14:419–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boffa DJ, Allen MS, Grab JD, Gaissert HA,
Harpole DH and Wright CD: Data from The Society of Thoracic
Surgeons General Thoracic Surgery database: The surgical management
of primary lung tumors. J Thorac Cardiovasc Surg. 135:247–254.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ali MK, Mountain CF, Ewer MS, Johnston D
and Haynie TP: Predicting loss of pulmonary function after
pulmonary resection for bronchogenic carcinoma. Chest. 77:337–342.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta R, Chowdhury I and Singh P:
Clinical, radiological and histological profile of Primary Lung
Carcinomas. JK Science. 17:1462015.
|
|
17
|
Laking G and Price P:
18-Fluorodeoxyglucose positron emission tomography (FDG-PET) and
the staging of early lung cancer. Thorax. 56 (Suppl 2):ii38–ii44.
2001.PubMed/NCBI
|
|
18
|
Hussein O, Sherity SE and Omar Y: Contrast
computed tomography versus PET/CT in the assessment of bronchogenic
carcinoma. Egypt Pharmaceut J. 18:135–140. 2019.
|
|
19
|
Schreiber G and McCrory DC: Performance
characteristics of different modalities for diagnosis of suspected
lung cancer. Summary of published evidence. Chest. 123 (1
Suppl):115S–128S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Psallidas I, Kalomenidis I, Porcel JM,
Robinson BW and Stathopoulos GT: Malignant pleural effusion: From
bench to bedside. Eur Respir Rev. 25:189–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asciak R and Rahman NM: Malignant pleural
effusion: From diagnostics to therapeutics. Clin Chest Med.
39:181–193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arroliga AC and Matthay RA: The role of
bronchoscopy in lung cancer. Clin Chest Med. 14:87–98. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herth F, Becker HD, Manegold C and Drings
P: Endobronchial ultrasound (EBUS)-assessment of a new diagnostic
tool in bronchoscopy for staging of lung cancer. Onkologie.
24:151–154. 2001.PubMed/NCBI
|
|
24
|
Galluccio G, Palazzolo M, Battistoni P,
Dello Iacono R, Batzella S, Caterino U and Lucantoni G: Role of
transbronchial needle core biopsy in the diagnosis of mediastinal
diseases. J Bronchology Interv Pulmonol. 25:239–244. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bousema JE, van Dorp M, Hoeijmakers F,
Huijbregts IA, Barlo NP, Bootsma GP, van Boven WP, Claessens NJM,
Dingemans AC, Hanselaar WE, et al: Guideline adherence of
mediastinal staging of non-small cell lung cancer: A multicentre
retrospective analysis. Lung Cancer. 134:52–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sellke F, Del Nido P and Swanson S:
Sabiston & Spencer surgery of the chest. Saunders Elsevier;
Philadelphia, PA: Chapter 17. 9th edition. pp. 2532010
|
|
27
|
Strand TE, Rostad H, Møller B and Norstein
J: Survival after resection for primary lung cancer: A
population-based study of 3211 resected patients. Thorax.
61:710–715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meyer JA: Hugh morriston davies and
lobectomy for cancer, 1912. Ann Thorac Surg. 46:472–474. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baue AE: Landmark perspective Evarts A:
Graham and the first pneumonectomy. JAMA. 251:260–264. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barney JD and Churchill EJ: Adenocarcinoma
of the kidney with metastasis to the lung: Cured by nephrectomy and
lobectomy. J Urol. 42:269–276. 1939. View Article : Google Scholar
|