Introduction
Mucosal Malignant Melanoma (MMM) originates from the
malignant transformation of neuroectodermal melanocytes. It is a
very rare tumor. It only accounts for less than 2% of malignant
melanoma in white people. Its common sites are head and neck (nasal
mucosa and tongue bottom mucosa), female reproductive tract
(vagina, vulva) and digestive tract (esophagus, anus and rectum),
This may be related to melanocytes in squamous epithelium mucosa of
these parts (1). The morphology of
melanoma cells varies from epithelioid to spindle shaped
differentiation, including various cytoplasmic morphologies
(2). Among them, signet ring cell
malignant melanoma (SRCMM) is a rare subtype of melanoma (3–6).
Malignant melanoma has a broad spectrum of
histological characteristics, which can imitate epithelial tissue,
hematopoietic tissue, neural tissue and mesenchymal tissue.
Melanocytes can also form various structural features, including
nest like, trabecular, glandular, whirlpool like, rose like and
papillary structures (2). It can
differentiate into a variety of cells, including Schwann cells,
fibroblasts, myofibroblasts, rhabdomyoid cells, osteoid cells,
chondroid cells, ganglion cells and smooth muscle cells. Cell
morphology varies from epithelioid to spindle shaped
differentiation, including various cytoplasmic morphology, such as
signet ring cells, clear cells, balloon like cells, rhabdomyoid and
plasma cell like appearance (2).
The proportion of signet ring cell morphology in melanoma is
extremely low 0.5% (7), which was
first proposed by Sheibani and Battifora in 1988 (3). Because signet ring cell morphology can
appear in a variety of tumors, including adenocarcinoma, squamous
cell carcinoma, basal cell carcinoma, lymphoma and sarcoma,
immunohistochemistry has become the initial means to distinguish it
from other malignant tumors (4).
These immunohistochemical markers include nerve growth factor and
receptor, as well as related signal molecules regulating melanocyte
differentiation and proliferation. Despite the increasing number of
immunohistochemical markers, S-100 is still the most sensitive to
melanocytosis. However, some other markers, such as HMB-45,
Melan-A, and tyrosinase, have certain specificity, but are less
sensitive than S-100 (8).
Melanomas are malignant tumors arising from
neuroectoderm melanocytes. Mucosal melanomas arise from melanocytes
located in mucosal membranes lining respiratory tract,
gastrointestinal tract including anorectum, and urogenital tract
etc. They can arise in any part of mucosal membranes, but the
incidence of primary mucosal melanoma originating in the digestive
tract is extremely rare. Melanoma can show a broad-spectrum
morphologic differentiation from epithelioid to spindled cells with
variable cytoplasmic features such as clear cell, rhabdoid cell,
giant cell, signet-ring shape and plasmacytoid appearance (1). Signet-ring cell melanoma is a rare
variant of malignant melanoma. To the best of our knowledge, there
are only 22 previous cases that have been described in the English
literature (2–18). This rare morphologic variant of
malignant melanoma was firstly described by Sheibani and Battifora
in 1988 (3). Skin is the primary
site for the majority of reported signet-ring melanomas and there
have been only 2 previously reported signet-ring melanomas in the
digestive tract. We herein report a primary anorectal signet-ring
cell melanoma without evidence of cutaneous or ocular malignant
melanoma. We also reviewed the literature on this rare variant of
mucosal melanoma and discuss the differential diagnosis with other
rectum tumors such as signet-ring cell carcinoma and lymphoma
etc.
Case report
A 64-year-old Asian woman presented with a 2-week
history of rectal bleeding and tenesmus. She denied any other
discomforts. The rectoscope showed an elevated lesions less than 2
cm from the anus. The magnetic resonance imaging (MRI) revealed
suspicious wall thickening in the distal rectum. The abdomen of
computerized tomography (CT) scan showed no hepatic or peritoneal
cavity metastases or enlarged lymph nodes. A biopsy was performed
in an outside hospital and the mass was initially diagnosed as
signet-ring cell carcinoma. However, after further histologic and
immunohistochemical review of the biopsy, this tumor turned out to
be a malignant melanoma with signet-ring cell morphology. The
patient then came to our hospital for further surgical treatment.
In the resection sample, the tumor cells diffusely infiltrated the
rectum mucosa with focal hyperpigmentation, with most cells being
spindled but with some signet-ring cells as well. In considering
prior consultation opinion on the biopsy material, additional
immunohistochemical stains were performed. The clinical examination
did not reveal skin melanoma. After a comprehensive analysis of the
histomorphology and immunohistochemical results, the final
diagnosis of signet-ring cell melanoma was made. Further imaging
analysis revealed no tumor in the internal organs. The patient has
no history of cutaneous melanoma or melanoma in another site, and
therefore we classified this tumor as a primary mucosal melanoma of
signet-ring cell subtype.
Serial 4 µm thick sections from the whole
formalin-fixed paraffin-embedded (FFPE) samples of mucosal melanoma
were prepared for immunohistochemical staining with an automated
DAKO immunostainer. Heat-induced epitope retrieval for
immunohistochemical analysis was performed and standardized for
each antibody. The immunostains with the following antibodies were
performed: pan-cytokeratin (ZSGB-BIO; clone 35βH11), cytokeratin 7
(ZSGB-BIO; clone EP16), cytokeratin 8/18 (ZSGB-BIO; clone Zym5.2),
EMA (ZSGB-BIO; clone GP1.4), S-100 protein (ZSGB-BIO; clone
15E2E2+4C4.9),vimentin (ZSGB-BIO; clone EP21), human melanoma
black-45 or HMB-45 (M2-7C10 + M2-9E3), Melan-A (ZSGB-BIO; clone
A103) and Rabbit antihuman antibodies to Cytokeratin 20(ZSGB-BIO;
clone EP23). Special stains including mucicarmin, PAS and Alcian
blue/periodic acid Schiff staining diastase (AB/PAS) were also
performed.
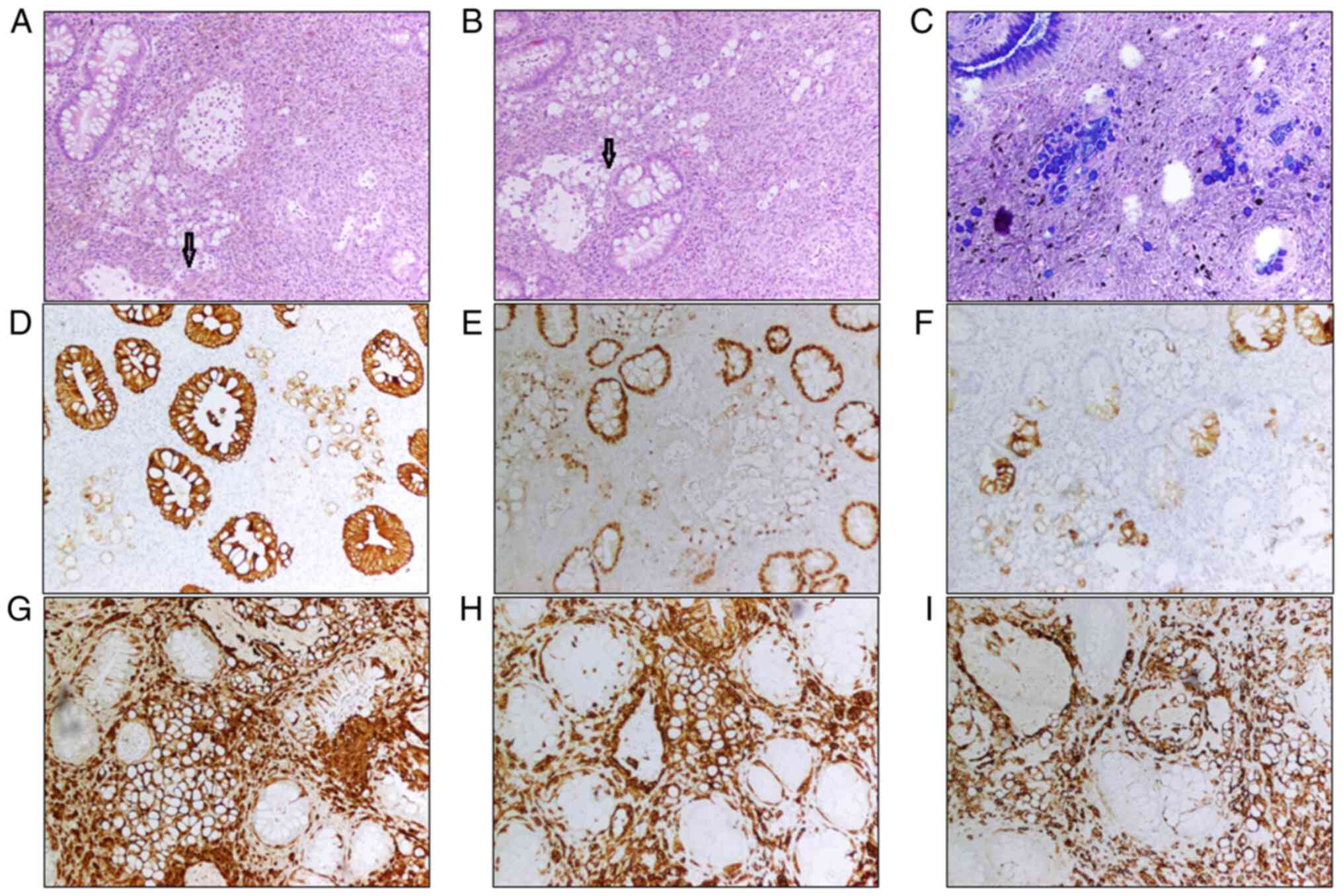
In the resection sample, the rectal mucosa is
infiltrated by spindled cells and signet-ring cells. There was a
gray-black protuberant mass adjacent to the dentate line with a
maximum diameter of 2.3 cm, 0.8 cm above the mucosa. The cut
surface was gray-black, solid and hard. Moreover, the melanin
pigment can be seen in the area with spindled tumor cells. The
spindle cells infiltrated to the deep layer of muscularis propria,
while the signet-ring cells were located in the mucosa. There were
also some lymphoid cells in the mucosa, and they were focally
admixed with signet-ring cells. The signet-ring cells demonstrated
abundant clear cytoplasm compressing the nuclei to the periphery
(Fig. 1A and B). The cytoplasm of
the signet-ring cells was positive for staining with Alcian
blue/periodic acid Schiff staining (AB/PAS) (Fig. 1C), while the spindle cells were
negative (Fig. 1C). The signet-ring
cells were focally and weak positive for pan-CK (Fig. 1D), CDX2 (Fig. 1E), and CK20 (Fig. 1F) while the spindled tumor cells
were negative for these three markers. The tumor cells of both
components (spindled and signet-ring cells) were stained strongly
positive for S-100 protein (Fig.
1G), MelanA (Fig. 1H) and HMB45
(Fig. 1I). In combining the
histologic morphology with immunohistochemical findings, the
diagnosis of the signet-ring cell melanoma was established.
Next-generation sequencing (NGS) with multiplex
amplification of targets and AmpliSeq strategy was performed using
the ION TORRENT PGM including a set of 74 exons of 31 genes
including ABL1 exons 4, 5, and 6; AKT1 exons 4; BRAF exons 11, and
15; CTNNB1 exons 3; DDR2 exons 18; EGFR exons 18, 19, 20, and 21;
ERBB4 exons 7, 15, and 23; FBXW7 exons 9 and 10; FGFR1 exons 12;
FGFR2 exons 7, 9, and 12; FGFR3 exons 7 and 14; GNA11 exons 5; GNAQ
exons 5; GNAS exons 8 and 9; HER2 exons 19, 20, and 21; HRAS exons
2, 3, and 4; IGH1 exons 4; IGH2 exons 4; JAK2 exons 14; KIT exons
9, 11, 13, 14, and 17; KRAS exons 2, 3, and 4; MAP2K1 exons 2 and
3; MET exons 14, 16, and 19; NOTCH1 exons 27; NRAS exons 2, 3, and
4; PDGFRA exons 12, 14, and 18; PIK3CA exons 10 and 21; PTEN exons
1, 5, 6, and 7; SMAD4 exons 9 and 10; STK11 exons 4, 6, and 8; TP53
exons 2, 4, 5, 6, 7, 8, and 10. TP53 missense mutation was
identified and no mutation was seen in other genes in the panel.
Studies have reported that colorectal melanoma has a poor
prognosis, because local lymph nodes and distant metastasis often
occur at the time of diagnosis (9).
Interestingly, BRAF and NRAS mutations were less frequent than skin
melanoma. In contrast, more than 30% of cases reported activated
KIT mutations (10). In these
patients, imatinib has shown promising activity (11). Other mutations of anorectal melanoma
were found in BRCA1, HRAS, MLH1, NF1, PDGFRA and SF3B1 (12).
The differential diagnosis also includes metastatic
melanoma. Primary melanomas originating from the alimentary tract
is extremely rare (13). In
addition, several studies have reported that the signet-ring cells
are occasionally present only in metastatic sites. In a study of
comparing expression of immunohistochemical markers between primary
and metastatic malignant melanomas, it was found that epithelial
marker reactivity was more common in the metastases of melanomas
(14). The diagnostic criteria for
primary malignant melanoma in the GI tract include: no evidence of
metastatic spread on physical examination, no history of resection
of melanoma or cutaneous melanoma, lack of gastrointestinal
epithelium and its adjacent areas lentiginous lesions in situ. The
criterion was proposed by Blecker with his colleges and other
people (15,16). Based on the above criteria, primary
anorectal melanoma was made for our case.
The melanoma in our case showed two distinct
populations of tumor cells: spindled and signet-ring cells, raising
the differential diagnosis of sarcomatoid carcinoma arising in
association with signet-ring cell carcinoma. Immunohistochemical
markers are useful for clarifying this issue as showed by our
case.
We have presented a case of signet-ring cell
melanoma accompanied by spindle cell areas, a rare variant of
mucosal melanoma, in an uncommon location, the anorectum. To our
best knowledge, this is the third report of signet-ring melanoma in
the digestive mucosa. The diagnosis of signet-ring cell melanoma
can be confirmed by the strongly positivity for melanocytic markers
in the signet ring tumor cells, which also showed focally weak
staining for epithelial markers such as pan-CK.
The patient was given paclitaxel/carboplatin) +
bevacizumab chemotherapy and TC bevy regimen. At present, there is
no clear treatment guideline for this disease, so we first treat it
according to the treatment plan of melanoma.
Discussion
Signet-ring cell melanoma is a rare subtype of
melanoma and poses some diagnostic challenges if without
immunohistochemical staining. In this case, the differential
diagnose is challenging and includes signet-ring carcinoma. Indeed,
the initial pathologic diagnosis in the biopsy was signet ring cell
carcinoma. In the resection specimen, we have done several
additional epithelial markers including pan-CK, CK7, CK20, CK8/18
and EMA to address the differential diagnosis. Interestingly, all
these markers were focally weakly positive in the signet ring
cells. Previously, only one case of signet ring cell melanoma
(17) was reported to show focal
and weak positive staining for CK. In our case, the special stains
including mucicarmin, PAS, Alcian blue/periodic acid Schiff
staining (AB/PAS) were also positive in the signet ring cells.
Although the PAS-stain can be detected in malignant melanoma, the
PAS-D-stain was proved positive in only two (3,18) of
the previous reports. Just based on the immunohistochemical
results, one cannot exclude the diagnosis of signet-ring cell
carcinoma. However, the usual melanocytic markers including S-100
protein, Melan-A and HMB-45 were also detected in the signet-ring
cells in our case. From Fig. 1, we
found that the signet-ring cells showed weak positivity for these
markers, while such positivity also demonstrated in the fundus of
enterocytes, which may indicate that the so-called signet-ring
cells might originate from the intestinal epithelial stem cells.
Moreover, the spindle cells were negative for the epithelial
markers, then a hypothesis can be made that the two components were
different in their origin. Thus, after making a comprehensive
analysis, the diagnosis of signet-ring cell melanoma was
established.
In this case, the spindle cell component is easy to
overlook, and it is not difficult to ascertain its nature through a
combination of immunohistochemical applications, bearing in mind
that the rectum is a predilection site for malignant melanoma in
the digestive system. The special feature of this case is its
signet ring cell-like morphology, which not only showed
immunohistochemical differentiation towards melanoma, but also
showed expression of epithelial components, which could easily be
misdiagnosed as Signet ring cell carcinoma, therefore, the
comprehensive application of immunohistochemistry and comprehensive
interpretation of the results is very important. We report a rare
case, the mechanism of which is not clear. We need to collect more
cases for further study.
Acknowledgements
Not applicable.
Funding
The authors received financial support for the research,
authorship and/or publication of this article from the Beijing
Municipal Education Commission (grant no. KZ201910025033) and
Beijing Municipal Administration of Hospitals Clinical Medicine
Development of Special Funding (grant no. ZYLX201814).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HM and HL were responsible for the research idea,
reviewing the literature and drafting the manuscript. ZZ and SS
confirmed the authenticity of all raw data. HM acquired and
analyzed patient data. HM and HL contributed to study conception,
overall design and quality control. ZZ and SS collated and analyzed
the pathological data of patients. All authors critically reviewed
the manuscript and approved submission. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Cangzhou People's Hospital (approval no. AF/SC-07/02.0).
Patient consent for publication
The patient provided written informed consent to
publish the case.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gutman M, Inbar M, Chaitchik S, Merhav A,
Pausner D, Skoznik Y, Ilie B, Rozin RR and Klausner JM: Malignant
melanoma of the mucus membranes. Eur J Surg Oncol. 18:307–312.
1992.PubMed/NCBI
|
|
2
|
Banerjee SS and Harris M: Morphological
and immunophenotypic variations in malignant melanoma.
Histopathology. 36:387–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheibani K and Battifora H: Signet-ring
cell melanoma. A rare morphologic variant of malignant melanoma. Am
J Surg Pathol. 12:28–34. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rutten A, Huschka U, Requena C,
Rodriguez-Peralto JL and Requena L: Primary cutaneous signet-ring
cell melanoma: A clinico-pathologic and immunohistochemical study
of two cases. Am J Dermatopathol. 25:418–422. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kacerovska D, Sokol L, Michal M and
Kazakov DV: Primary cutaneous signet-ring cell melanoma with
pseudoglandular features, spindle cells and oncocytoid changes. Am
J Dermatopathol. 31:81–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bastian BC, Kutzner H, Yen T and LeBoit
PE: Signet-ring cell formation in cutaneous neoplasms. J Am Acad
Dermatol. 41:606–613. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakhleh RE, Wick MR, Rocamora A, Swanson
PE and Dehner LP: Morphologic diversity in malignant melanomas. Am
J Clin Pathol. 93:731–740. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohsie SJ, Sarantopoulos GP, Cochran AJ and
Binder SW: Immunohistochemical characteristics of melanoma. J Cutan
Pathol. 35:433–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang AE, Karnell LH and Menck HR: The
National Cancer Data Base report on cutaneous and noncutaneous
melanoma: A summary of 84,836 cases from the past decade. The
American College of Surgeons Commission on Cancer and the American
Cancer Society. Cancer. 83:1664–1678. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo J, Si L, Kong Y, Flaherty KT, Xu X,
Zhu Y, Corless CL, Li L, Li H, Sheng X, et al: Phase II,
open-label, single-arm trial of imatinib mesylate in patients with
metastatic melanoma harboring c-Kit mutation or amplification. J
Clin Oncol. 29:2904–2909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreira A, Heinzerling L, Bhardwaj N and
Friedlander P: Current melanoma treatments: Where Do We Stand?
Cancers. 13:2212021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang HM, Hsiao SJ, Schaeffer DF, Lai C,
Remotti HE, Horst D, Mansukhani MM and Horst BA: Identification of
recurrent mutational events in anorectal melanoma. Mod Pathol.
30:286–296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kocovski L and Alowami S: Signet-ring cell
melanoma: A potential diagnostic pitfall. Am J Dermatopathol.
36:985–988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ben-Izhak O, Stark P, Levy R, Bergman R
and Lichtig C: Epithelial markers in malignant melanoma. A study of
primary lesions and their metastases. Am J Dermatopathol.
16:241–246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blecker D, Abraham S, Furth EE and Kochman
ML: Melanoma in the gastrointestinal tract. Am J Gastroenterol.
94:3427–3433. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khalid U, Saleem T, Imam AM and Khan MR:
Pathogenesis, diagnosis and management of primary melanoma of the
colon. World J Surg Oncol. 9:142011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
LiVolsi VA, Brooks JJ, Soslow R, Johnson
BL and Elder DE: Signet cell melanocytic lesions. Mod Pathol.
5:515–520. 1992.PubMed/NCBI
|
|
18
|
Bonetti F, Colombari R, Zamboni G and
Chilosi M: Signet ring melanoma, S-100 negative. Am J Surg Pathol.
13:522–523. 1989. View Article : Google Scholar : PubMed/NCBI
|