Introduction
Renal cell carcinoma (RCC) is a predominantly solid
tumor of the kidneys, representing ~90% of all kidney malignancies
and 2–3% of all human cancer cases (1). The most common histological types of
RCC are clear cell (70%), papillary (10–15%), chromophobe (5%) and,
collecting duct carcinoma (2).
Clear cell RCC and papillary RCC likely originate from the proximal
tubules, whereas chromophobe RCC is considered to originate from
distal portions of the nephron (3).
Urothelial carcinoma (UC) is the most common cancer
of the urinary tract that can develop anywhere along the upper part
(renal calyx, renal pelvis or ureter) or the lower part (bladder or
urethra) of the urinary tract (4).
Renal pelvis and upper ureter carcinomas are less common than renal
parenchymal tumors and represent ~5% of all urothelial malignancies
(5,6). Additionally, the majority of UC cases
are unilateral, with bilateral disease occurring in ~1.6% of all
cases (7).
Concurrence of RCC and UC in the same kidney is rare
(8,9). Reported cases have revealed a slight
male preponderance, with the peak incidence being at 60–70 years of
age (7). Synchronous ipsilateral
renal malignancies are more difficult to diagnose and have a poorer
prognosis than solitary tumors. It is critical to research this
uncommon condition in order to prevent a delay in diagnosis and
improve its prognosis (10,11).
The present report aims to describe a rare case of
synchronous RCC and UC of the ipsilateral renal pelvis and ureter
in a 71-year-old male patient.
Case report
Patient information
A 71-year-old male patient presented to Sulaymaniyah
General Teaching Hospital (Sulaimani, Iraq) in February 2022 with a
3-month history of intermittent attacks of left loin pain and frank
hematuria. Weight loss of 5 kg was also reported during the same
time period. The patient had been a chronic heavy smoker with a
history of smoking 1.5 packs a day for more than 45 years (67.5
pack-years), but he had ceased smoking 3 years before
presentation.
Clinical findings
The patient's vital signs were stable. Palpation of
the abdomen revealed a mobile, non-tender mass in the left upper
quadrant.
Diagnostic approach
Blood investigations showed the following results:
White blood cells (WBCs), 17×1010 (reference range,
4–11×109/l); hemoglobin, 12 g/dl (reference range,
13.0-18.0 g/dl); blood urea, 63 mg/dl (reference range, 8–23
mg/dl); serum creatinine, 1.4 mg/dl (reference range, 0.6-1.2
mg/dl); glucose, 121 mg/dl (reference range, 70–110 mg/dl); and
C-reactive protein, 25 mg/dl (reference range, <5.0 mg/l). An
ultrasound scan of the abdomen and pelvis revealed moderate to
severe left hydronephrosis with decreased renal cortical thickness
and hydroureter to the level of a 54×41-mm hypoechoic mass located
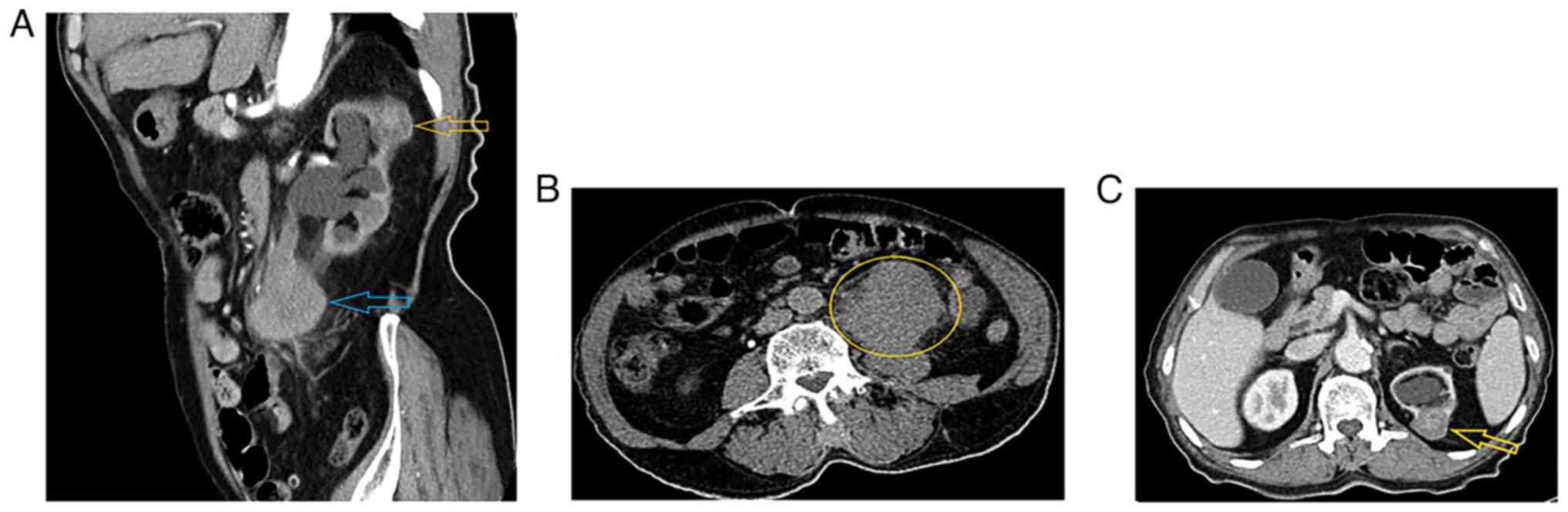
4 cm away from the left ureteropelvic junction. A contrast computed
tomography (CT) scan of the abdomen and pelvis demonstrated two
masses. A 60×50×48-mm mass was identified at the left upper and mid
ureter, showing heterogeneous enhancement, causing proximal
ureteric and pelvicalyceal system dilatation with parenchymal
thinning and moderate to severe hydronephrosis. The disease had
spread from the ureter wall to the retroperitoneum, with no
surrounding organ invasion. There were only a few 7-mm para-aortic
lymph nodes, but without definite pathological features. Overall,
the mass was suggestive of ureteric UC. The second mass was located
in the upper pole of the left kidney, measured 37×27×20 mm, was
partially exophytic and exhibited a hyperdense central region with
no enhancement that was probably a hemorrhage. The peripheral
hypodense part showed enhancement in a delayed-phase scan, and was
not associated with perinephric or peritumoral pseudocapsule
invasion. Overall, it was suggestive of RCC T1a (a stage 1 tumor ≤4
cm in size according to the TNM staging system) (12) (Fig.
1). Cystoscopy was performed during surgery, and the bladder
urothelium appeared normal, however multiple bladder biopsies were
taken. Metastatic workup was performed, including contrast CT scans
of the chest, abdomen and pelvis. There were no suspicious bone
lesions found in the CT scan, and laboratory tests, such as
alkaline phosphatase and serum calcium tests, were normal.
Additionally, the patient had no syptoms of bone pain. According to
guidelines (13,14), upper UC and RCC do not require a
bone scan, so this was not performed.
CT was performed on a Siemens SOMATOM Definition AS
64-slice scanner (Siemens AG). The CT scan parameters and contrast
enhancement settings used were the following: 5 mm-thick, native,
corticomedullary, nephrographic and excretory phases, each with
1-mm reconstruction, were reviewed in the axial, coronal and
sagittal planes.
Therapeutic intervention
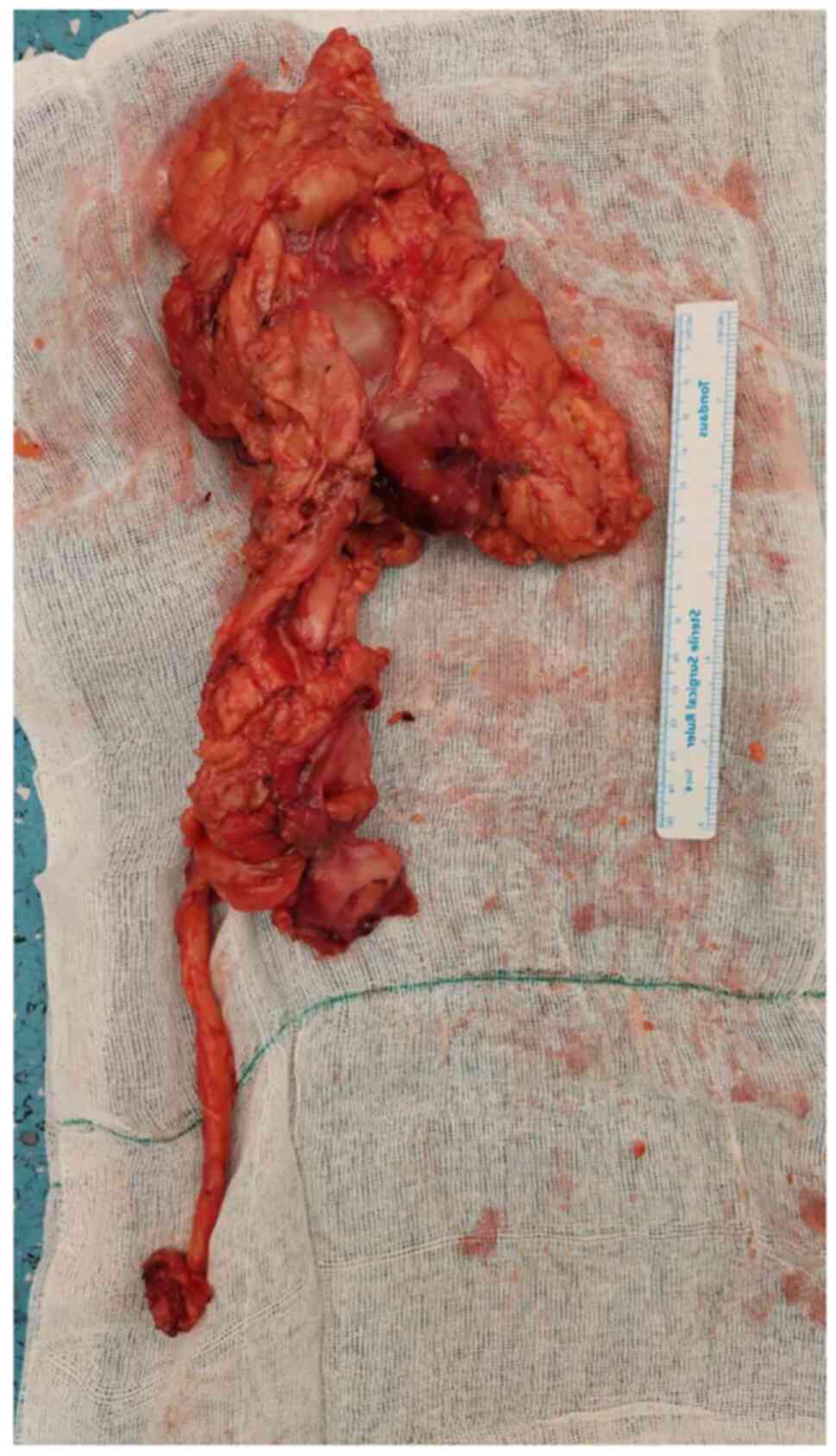
A left nephroureterectomy with the removal of a
bladder cuff was performed under general anesthesia and through
left anterior subcostal and left Gibson incisions (Fig. 2). Several para-aortic and pelvic
lymph nodes were removed. Open surgical intervention was preferred
over robotic and laparoscopic surgery due to the lack of a robotic
surgery facility and the researcher experience in performing major
surgeries and extensive lymph node dissections. The post-operative
period was uneventful; the patient was discharged on the third
post-operative day with a Foley catheter, to be removed 10 days
later.
The specimen was fixed in 10% neutral buffered
formalin at room temperature for 24 h prior to grossing. Gross
examination revealed a 35-mm diameter, well-defined, variegated,
soft mass in the upper pole of the kidney, with areas of necrosis
and hemorrhage, without invasion beyond the renal capsule or into
the renal hilum. In addition, there were two ill-defined, firm,
white masses in the renal pelvis (52 mm in diameter) and upper
ureter (56 mm in diameter), which were not connected to each other
or to the mass in the upper pole. The ureteric mass was found to be
invading the ureter wall and reaching the soft-tissue margin.
Sections taken from tumors, adjacent tissues and margins were
placed into tissue cassettes. The tissue cassettes were then
processed with the DiaPath Donatello automated processor using a
standard 11-h processing protocol with alcohol, xylene and
paraffin. Following embedding in paraffin and trimming, the blocks
were sectioned (4–6 µm) onto regular glass slides, kept in an oven
at 60°C overnight, and then stained with the DiaPath Giotto (1% for
10 min) automated stainer for hematoxylin and eosin (H&E) using
Gill II hematoxylin. The slides were then dried, and coverslips
were applied.
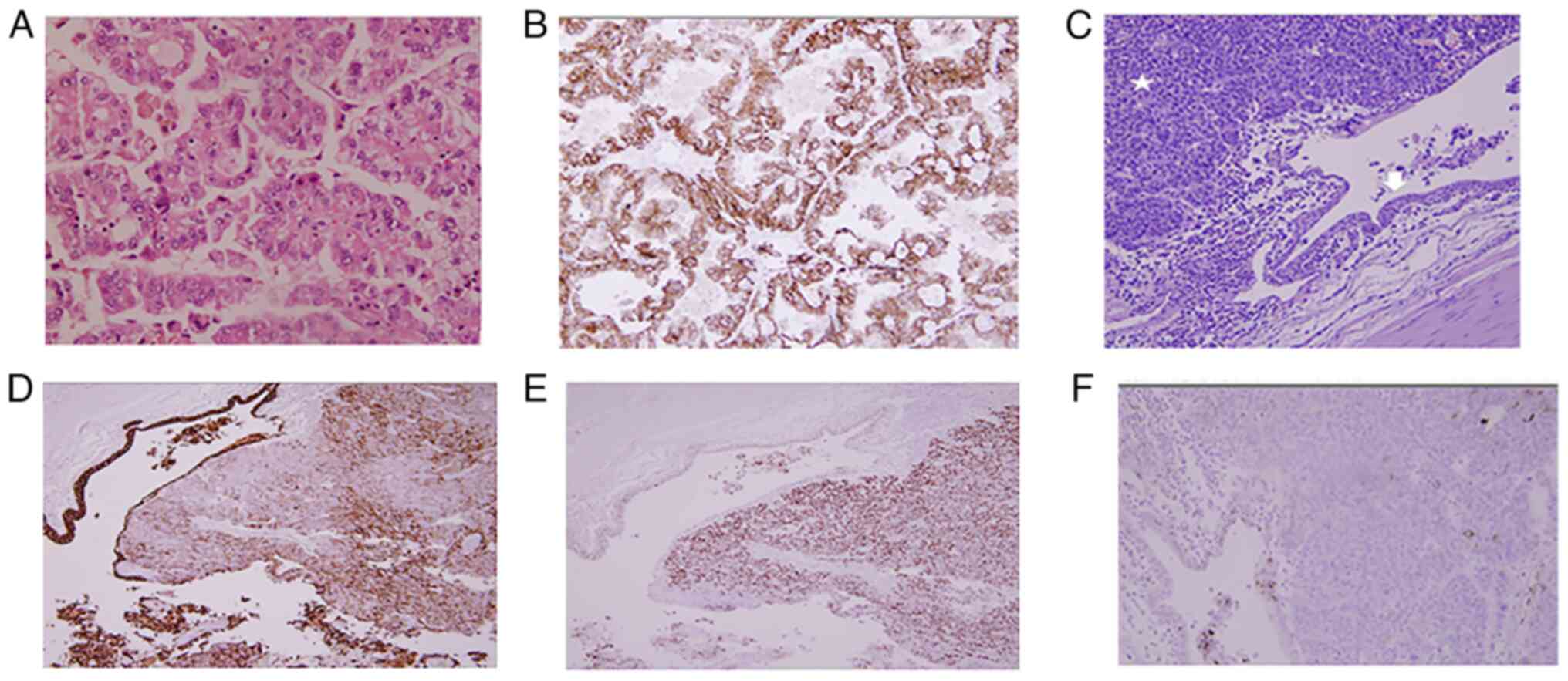
Microscopic examination revealed a classic type I
papillary RCC in the upper pole with well-formed papillae lined by
cuboidal cells with moderate amphophilic cytoplasm, grade 1 to
grade 2 nuclear features, areas of necrosis (<50%) and
hemorrhage. The tumor was low risk, staged as pT1a-pN0-pMx
(12). The other masses had the
histology of UC (Fig. 3), all
composed of nests and solid sheets of large polygonal cells with
abundant eosinophilic cytoplasm, moderate to high nuclear atypia
and lymphovascular invasion. Metastatic UC was identified from one
of the para-aortic lymph nodes. The highest stage identified for
these tumors was pT3-pN1-pMx. No invasive or in situ UC was
identified from the bladder mucosa and distal cuff margin.
For immunohistochemistry, the paraffin blocks were
sectioned (4–6 µm) onto charged glass slides and kept overnight in
an oven at 60°C. Antigen retrieval was achieved through boiling at
100°C for 5–10 min using the Dako PT Link (Agilent Technologies,
Inc.) with a solution of pH 6.0 or pH 9.0, depending on the target
antibody. The slides were then washed with buffer solution (20 ml)
[0.05 mol/l Tris/HCl, 0.15 mol/l NaCl, 0.05% Tween 20 (pH 7.6)] for
15 min at room temperature and welled using the Dako Pen (Agilent
Technologies, Inc.), followed by blocking endogenous peroxidase
using 3% hydrogen peroxide. The primary antibodies were then
applied at room temperature for 80 min, followed by the secondary
antibody (horseradish peroxidase) and the chromogen
(diaminobenzidine) at room temperature for 15 min. Counterstaining
was achieved using hematoxylin Gill II for 30 sec at room
temperature, followed by drying and applying coverslips.
Immunohistochemical examination showed diffuse and
strong positivity for CK7 (clone OV-TL 12/30; pH 9.0; dilution 1:1;
Dako; Agilent Technologies, Inc.) (LOT#: 20038751) and GATA-3
(clone EP368; pH 9.0; dilution 1:1.3; Bio SB, Inc.) (LOT#:
3333PKE11), with negativity for CD10 (clone EP195; pH 9.0; dilution
1:1.5; Bio SB, Inc.) (LOT#: 6434XKI28) in the renal pelvic mass
(UC), while the upper pole mass showed diffuse and strong granular
cytoplasmic staining for AMACR (clone 13H4; pH 9; dilution 1:1; Bio
SB, Inc.) (LOT#: 5062JKC24) (papillary RCC). Since the histological
image of both tumor types on routine H&E was sufficiently
straightforward for establishing the diagnosis, this limited
immunohistochemistry panel was to be performed for confirmation,
and a wider panel was not deemed necessary.
Follow-up and outcome
On the 14th post-operative day, the patient was in a
good general condition; however, no imaging assessment was
performed and the patient was referred to an oncology center in
Hiwa Hospital (Sulaimani, Iraq) for further management. After
surgery, the patient received six cycles of adjuvant chemotherapy
that consisted of gemcitabine (1,000 mg/m2 on days 1, 8
and 15) and cisplatin (70 mg/m2 on day 2). Each cycle
lasted 3 weeks. At the time of writing this paper, the patient was
being monitored by an oncologist every 3 to 6 months. At 6 months
after the surgery, imaging scans showed no signs of local
recurrence or distant metastases. The second post-operative imaging
scan in February 2023 was normal.
Discussion
RCC is one of the 10 most commonly detected
malignancies worldwide, and especially in Western countries, its
incidence is rising. RCC comprises of histologically defined
subtypes that differ in pathophysiology, clinical course,
therapeutic response and prognosis (15). Approximately 40% of patients with
localized RCC have distant metastases after surgery (6). The liver, lungs, bones and lymph nodes
are the common sites for RCC metastasis, and have a high growth
pattern (16). The main factors
contributing to the increase in RCC incidence include the
development of diagnostic techniques and the public awareness of
the importance of periodic health screening. Consequently, there is
an increase in the number of patients being diagnosed in the early
stages. Due to early diagnosis and treatment, the mortality rate of
RCC has markedly decreased over the past three decades, and the
total incidence rate has increased, especially in developed
countries (16). Papillary RCC
comprises 10–15% of all cases of RCC and is associated with a
better prognosis than clear cell RCC (6). Papillary RCC can be histologically
subclassified as either type I, defined by small cells arranged in
a single layer, scant basophilic cytoplasm and oval nuclei, or type
II, characterized by large cells with eosinophilic cytoplasm,
spherical nuclei, prominent nucleoli and pseudostratification
(17).
Upper urinary tract UC is a relatively unusual
condition and the prognosis has primarily been associated with
tumor stage and grade. Similar to bladder cancer, upper urinary
tract UC types include muscle-invasive and non-muscle-invasive
diseases (18). The concurrence of
ipsilateral RCC and UC of the upper urinary tract is an unusual
event, with only a few small series and case reports being
available in the literature (5). To
the best of our knowledge, Graves and Templeton (19) reported the first case in 1921, and
the most recent case was reported by Symeonidis et al
(9) in 2022. A review of 47
reported cases showed that the prognosis was comparable to that
reported for patients with solitary tumors (5).
Several etiological factors, including lifestyle
parameters such as smoking, obesity, hypertension and pathological
agents (such as chronic irritation, hydronephrosis, hyperkalemia
and exposure to renal carcinogens) have been associated with
primary renal pelvis neoplasms (1).
Smoking is also a salient risk factor for RCC (8). While no definitive risk factors have
been identified for the concurrence of different types of renal
neoplasms, one review reported that 24% of the patients were
smokers (1). The present case was
that of a 71-year-old male who had been a chronic smoker for >45
years.
There are no differences in clinical presentation
between patients with isolated primary renal pelvis neoplasms and
those with synchronous RCC and UC. Approximately 10% of patients
with RCC and UC are asymptomatic and incidentally diagnosed while
undergoing abdominal imaging for other reasons (5). Some RCC patients still present with
clinical symptoms such as gross haematuria, flank pain and a
palpable abdominal mass (the classical triad), metastatic symptoms,
such as lung nodules or bone pain, or paraneoplastic syndromes such
as unexplained fever, erythrocytosis or wasting syndrome (20). While hematuria is one of the common
symptoms of RCC, it is usually associated with advanced disease.
Additionally, upper urinary tract UC may be randomly diagnosed or
as a result of certain symptoms, mainly visible or non-visible
hematuria (70–80%) and flank pain (20%). Systemic symptoms of upper
urinary tract UC, such as anorexia, weight loss, malaise,
exhaustion, fever, night sweats or a cough, are associated with
metastases and a worse prognosis (20).
A CT scan is crucial for a detailed evaluation of
the flank mass and for obtaining staging information regarding
lymph nodes, renal veins and inferior vena cava involvement
(4). For patients presenting with
unexplained visible hematuria, CT urography is recommended as the
first-line diagnostic test. Ultrasonography of the renal tract is a
low-cost, non-ionizing method of evaluating the kidneys and bladder
in low-risk, young individuals with non-visible hematuria. However,
ultrasound sensitivity is only 26% for renal lesions <1 cm in
size, and it is insufficient when examining the collecting systems
(21). CT urography provides the
highest diagnostic precision for upper urinary tract UC. A
meta-analysis of 13 studies with 1,233 patients found that CT
urography had a pooled sensitivity and specificity of 92 and 95%,
respectively (20). Although MR
urography has the advantage of a higher soft tissue contrast than
CT and carries no radiation burden, it is limited by MR
contraindications and scanner availability, as well as being prone
to motion artifacts (21). MR
urography may be reserved for problematic cases in pregnancy and
renal failure, or for patients with an allergy to iodinated
contrast agent (20). In the
present case, a CT scan of the abdomen and pelvis revealed a left
upper ureteric enhancing mass with an enhancing ipsilateral upper
pole renal mass, suggestive of RCC. Qi et al (22) have reported two cases that were
initially diagnosed with isolated RCC and one patient who was
initially diagnosed with isolated cancer of the ureter.
An accurate pre-operative diagnosis of RCC with
synchronous ipsilateral UC of the renal pelvis is critical for the
selection of the surgical method. As this scenario is rare, there
is a high rate of misdiagnosis (23). The standard treatment for small RCC
is radical nephrectomy or partial nephrectomy. This modality,
however, is not sufficient for upper tract UC, due to its poorer
prognosis compared to RCC. The gold-standard treatment for upper
tract UC is radical nephroureterectomy with excision of a bladder
cuff (17). Segmental and
endoscopic interventions are now widely accepted options based on
the tumor's location, size and histological parameters (7). Lwin et al (7) showed that a higher number of
endoscopic and nephron-sparing treatments should be employed to
treat individuals with ureteral UC.
The prognosis for a patient with dual malignancies
is determined by the aggressiveness rate of the two tumors
(5). Upper urinary tract UC is more
commonly diagnosed in advanced stages compared to bladder cancer.
Secondary bladder cancer is ~10-fold more likely to occur after
primary upper urinary tract UC, with a risk of 20–50% (18). In the majority of the reported
cases, the tumor stages at diagnosis were reported as being pT2 or
above (24). The prognostic impact
of tumor location has been discussed but is controversial. After
adjusting for stage, some studies have shown that ureteral tumors
have a worse prognosis than pelvic malignancies; however, this was
not confirmed in a study by Zigeuner and Pummer (18). In the study by Leelamma et al
(24) the Fuhrman nuclear grade of
RCC was 3, with no renal vein invasion or a pT1b lesion, and no
signs of metastatic disease. Similar to the present case, the UC in
this case had a high grade. The patient in the present study had
RCC with a pathological stage of pT1 and no evidence of metastatic
disease, combined with a high-grade UC of the renal pelvis and
ureter with a pathological stage of pT3. Dutta et al
(25) reported a better survival
rate for patients with primary renal pelvis tumors than ureteral
tumors. Holmäng and Johansson (26)
reported a dismal 25% survival rate for high-grade pT3 upper tract
UC. One of the limitations of the present study was the short
follow-up period.
In conclusion, the occurrence of synchronous RCC and
UC of the same kidney is a rare event. Adjuvant therapy should be
given in accordance with the stage and pathological grade of the
RCC. Radical nephroureterectomy with bladder cuff removal may be
curative, especially in low-grade tumors.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RB was the surgeon who performed the operation and
literature review, and gave final approval of the manuscript. FHK
was a major contributor to the study idea, performed the literature
review, drafted the manuscript and accepted the final version of
the manuscript to be published. RMA was the pathologist who
examined the specimens, was a major contributor to the idea for the
study and revised the manuscript. BAA and DMH critically revised
the manuscript, accepted the final version of the manuscript to be
published and analyzed the patient data. SHM, DHKR, SSF and MHB
were involved in the literature review, the design of the study,
revision of the manuscript and in the processing of the figures.
SHT was the radiologist who performed the assessment of the case
and gave final approval of the manuscript. RB and SHT were major
contributors to the study idea. RB and FHK confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient has provided written informed consent
for the publication of the data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Atilgan D, Uluocak N and Parlaktas BS:
Renal cell carcinoma of the kidney with synchronous ipsilateral
transitional cell carcinoma of the renal pelvis. Case Rep Urol.
2013:1941272013.PubMed/NCBI
|
|
2
|
Mucciardi G, Galì A, D'Amico C, Muscarà G,
Barresi V and Magno C: Transitional cell carcinoma of the renal
pelvis with synchronous ipsilateral papillary renal cell carcinoma:
Case report and review. Urol Case Rep. 3:93–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Büttner F, Winter S, Rausch S, Reustle A,
Kruck S, Junker K, Stenzl A, Agaimy A, Hartmann A, Bedke J, et al:
Survival prediction of clear cell renal cell carcinoma based on
gene expression similarity to the proximal tubule of the nephron.
Eur Urol. 68:1016–1020. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carlson P and McGary CT: Educational case:
Renal cell and urothelial carcinoma. Acad Pathol.
7:23742895209563632020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leveridge M, Isotalo PA, Boag AH and
Kawakami J: Synchronous ipsilateral renal cell carcinoma and
urothelial carcinoma of the renal pelvis. Can Urol Assoc J.
3:64–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Rourke D: Renal Cell and Renal
Pelvis/Ureter Carcinoma. Boyle D and Allen D: Histopathology
Reporting. Springer; Cham, Switzerland: pp. 347–361. 2020,
View Article : Google Scholar
|
|
7
|
Lwin AA, Hsu CH and Chipollini J:
Urothelial carcinoma of the renal pelvis and ureter: Does location
make a difference? Clin Genitourin Cancer. 18:45–49.e1. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alhusban M, Alhamss S, Alzumaili B and
Al-Daghmin A: Ipsilateral synchronous clear and papillary renal
cell carcinoma: A case report and review of the literature. Urol
Case Rep. 16:110–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Symeonidis A, Tsikopoulos I, Symeonidis
EN, Tsifountoudis I, Michailidis A, Tsantila I, Gkekas C,
Georgiadis C, Malioris A and Papathanasiou M: More than meets the
eye: A case of synchronous ipsilateral clear cell renal cell
carcinoma and urothelial carcinoma of the pelvicalyceal system and
literature review. Acta Biomed. 92:e20213802022.PubMed/NCBI
|
|
10
|
Lee JW, Kim MJ, Song JH, Kim JH and Kim
JM: Ipsilateral synchronous renal cell carcinoma and transitional
cell carcinoma. J Korean Med Sci. 9:466–470. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JY, Kwak EK and Park TI: Ipsilateral
synchronous renal cell carcinoma and transitional cell carcinoma: A
Case Report. Korean J Pathol. 36:429–432. 2002.
|
|
12
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Escudier B, Porta C, Schmidinger M,
Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S and
Horwich A; ESMO Guidelines Committee, : Renal cell carcinoma: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 30:706–720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abu-Ghanem Y, Powles T, Capitanio U,
Beisland C, Järvinen P, Stewart GD, Gudmundsson EO, Lam TB, Marconi
L, Fernandéz-Pello S, et al: The impact of histological subtype on
the incidence, timing, and patterns of recurrence in patients with
renal cell carcinoma after surgery-results from RECUR Consortium.
Eur Urol Oncol. 4:473–482. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Büttner FA, Winter S, Stühler V, Rausch S,
Hennenlotter J, Füssel S, Zastrow S, Meinhardt M, Toma M, Jerónimo
C, et al: A novel molecular signature identifies mixed subtypes in
renal cell carcinoma with poor prognosis and independent response
to immunotherapy. Genome Med. 14:1052022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bahadoram S, Davoodi M, Hassanzadeh S,
Bahadoram M, Barahman M and Mafakher L: Renal cell carcinoma: An
overview of the epidemiology, diagnosis, and treatment. G Ital
Nefrol. 39:2022–vol3. 2022.PubMed/NCBI
|
|
17
|
Yang X, Li P, Deng X, Dong H, Cheng Y,
Zhang X, Yang C, Tang J, Yuan W, Xu X, et al: Perioperative
treatments for resected upper tract urothelial carcinoma: A network
meta-analysis. Oncotarget. 8:3568–3580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zigeuner R and Pummer K: Urothelial
carcinoma of the upper urinary tract: Surgical approach and
prognostic factors. Eur Urol. 53:720–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Graves RC and Templeton ER: Combined
tumors of the kidney. J Urol. 5:517–538. 1921. View Article : Google Scholar
|
|
20
|
Rouprêt M, Babjuk M, Burger M, Capoun O,
Cohen D, Compérat EM, Cowan NC, Dominguez-Escrig JL, Gontero P,
Hugh Mostafid A, et al: European Association of Urology guidelines
on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol.
79:62–79. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rossi SH, Prezzi D, Kelly-Morland C and
Goh V: Imaging for the diagnosis and response assessment of renal
tumours. World J Urol. 36:1927–1942. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi N, Chen Y, Gong K and Li H: Concurrent
renal cell carcinoma and urothelial carcinoma: Long-term follow-up
study of 27 cases. World J Surg Oncol. 16:162018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu Q, Zhuang J and Guo H: Renal cell
carcinoma with synchronous ipsilateral urothelial carcinoma of the
renal pelvis. Oncol Lett. 13:4521–4525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leelamma JP, Pothen L and Ramachandran NP:
Synchronous urothelial carcinoma of renal pelvis and ipsilateral
renal cell carcinoma. Saudi J Health Sci. 6:110–112. 2017.
View Article : Google Scholar
|
|
25
|
Dutta G, Silver D, Oliff A and Harrison A:
Synchronous renal malignancy presenting as recurrent urinary tract
infections. Case Rep Urol. 2011:8326732011.PubMed/NCBI
|
|
26
|
Holmäng S and Johansson SL: Urothelial
carcinoma of the upper urinary tract: Comparison between the
WHO/ISUP 1998 consensus classification and WHO 1999 classification
system. Urology. 66:274–278. 2005. View Article : Google Scholar : PubMed/NCBI
|