Introduction
Glioblastomas are the primary tumors of the central
nervous system that cause the most deaths and therefore have the
highest fatality rate. The median survival time of patients with
this disease is <15 months, and this statistic has not changed
for more than two decades (1).
Glioblastoma cells' high migration and invasion capacity into areas
of the brain parenchyma adjacent to the tumor, even reaching the
contralateral hemisphere, makes it impossible to perform a complete
surgical resection, resulting in tumor recurrence (2).
Proto-oncogene tyrosine-protein kinase Src (cSrc) is
a non-receptor tyrosine kinase that participates in diverse
biological activities, including cell migration. Furthermore, it
has been broadly associated with controlling the expression of
metalloproteinases (MMPs) and tumor invasiveness (3). cSrc and focal adhesion kinase (Fak)
kinase activity inhibition in the breast cancer MCF-7 cell line,
was reported to have blocked MMP-9 secretion (4). Gautam et al (5) reported that, in triple-negative
MDA-MB-231 breast cancer cells, the inhibition of cSrc activity
decreased MMP-9 levels and cell invasion. Within the high molecular
complexity of the mechanisms that regulate invasion in
glioblastomas, MMPs serve a fundamental role in degrading the
extracellular matrix (ECM). Upregulation of MMP-2 and −9 in
glioblastomas is associated with poor prognosis (6). The involvement of MMP-2 and −9 in the
progression of glioblastomas has been studied for >20 years. In
1999, Forsyth et al (7)
reported that the levels and activity of MMP-2 and −9 were higher
in glioblastomas than in normal brain tissue. The expression level
of MMP-9 also showed a strong correlation with the tumor grade in
gliomas. Later, Kondraganti et al (8) reported that impaired expression of
MMP-9 reduced glioblastoma cells' invasiveness. More recently, in
2019, Zhou et al (6)
reported that MMP-2 and −9 expression was higher in recurrent
glioma than in primary glioma.
Progesterone (P4) has been associated with increased
invasiveness capacity in numerous malignant processes (9–11). In
breast cancer cells, P4 increases the formation of the protrusions
associated with focal adhesion complex, and migration and invasion
processes through cSrc kinase activation (12). Although glioblastoma is not a cancer
of the reproductive system, it is sex-dependent since there is a
higher prevalence in men than in women, as evidenced by
epidemiological data (13). In the
context of P4, the results of previous studies indicate that this
hormone induces glioblastoma progression when administered at 10–50
nM (14,15). Notably, in terms of glioblastoma,
Piña-Medina et al (16)
reported that P4 increased the number of invasive and migrating
cells. More recently, Bello-Alvarez et al (15) demonstrated that P4 activates cSrc
kinase and Fak, which are fundamental components of focal adhesion
complexes. It has also been reported that 3α-tetrahydroprogesterone
(3α-THP), an active P4 reduced metabolite, increased cell
proliferation of glioblastoma cells. In addition, finasteride, an
inhibitor of 5α-reductase, the rate-limiting enzyme for the P4
transformation to 3α-THP, partially inhibited this effect (17). Moreover, 3α-THP regulates migration
in glioblastoma cells, and this effect is dependent on the
activation of cSrc (18). In rat
Schwann cells, the 3α-THP induction of cell migration is regulated
by cSrc and Fak (19). However, it
is not known whether P4 and 3α-THP regulate MMP-2 and −9 expression
through cSrc activity and whether this effect is reflected in the
migration and invasiveness of glioblastoma cells.
Materials and methods
Cell culture and treatments
U251 (astrocytoma cell line) and U87 (HTB-14;
glioblastoma of unknown origin) cells were purchased from the
American Type Culture Collection (ATCC) and were previously
authenticated by STR profiling using an AmpFISTR®
Identifiler™ kit (cat. no. 4322288) and Genetic Analyzer 3130×l
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Mycoplasma
contamination was routinely monitored using a Universal Mycoplasma
Detection Kit (cat. no. 30-1012K; ATCC). Cells were plated in 35 mm
culture dishes and maintained in DMEM medium (Vitro SA)
supplemented with 10% fetal bovine serum (FBS; Biowest), 1 mM
pyruvate, 2 mM glutamine, 0.1 mM MEM Non-Essential Amino Acids
Solution (Gibco; Thermo Fisher Scientific) and 1 mM antibiotic
(streptomycin 10 g/l; penicillin G 6.028 g/l; and amphotericin B
0.025 g/l, Biowest, cat. no. L0010) at conditions of 37°C and 5%
CO2. Culture medium was replaced by DMEM phenol red-free
(Biowest) and supplemented with 10% charcoal-stripped serum FBS
(sFBS; HyClone; Cytiva), to avoid additional hormone
supplementation, ~24 h before treatment. On the day of treatment,
cells were treated with 50 nM P4 (0.001% DMSO vehicle; cat. no.
P-8783; MilliporeSigma) or 100 nM 3α-THP (0.01% ethanol vehicle;
cat. no. 195886; MP Biomedicals, LLC) for 3 or 6 h at 37°C to
assess the protein expression levels of MMP-2 and MMP-9.
Cell migration (wound healing
assays)
For evaluating cell migration, wound healing assays
were performed. A total of 3.5×105 U251 or U87 cells
were seeded per well in 6-well plates and cultured as
aforementioned. After 24 h of culture conditions free of phenol red
and 10% sFBS (20), a scratch wound
was made in the 90% confluent cells with a fine pipette tip. After
washing the detached cells with 1X PBS (137 mM NaCl, 2.7 mM KCl,
1.8 mM KH2PO4 and 10 mM NaHPO4),
the cell cultures were treated with 10 µM cytosine
β-D-arabinofuranoside (Ara-C; Sigma-Aldrich; Merck KGaA) to inhibit
cell proliferation 1 h before adding either 50 nM P4 (0.001% DMSO
vehicle), 1 µM 1-tert-Butyl-3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]
pyrimidin-4-amine (PP2, a Src family kinase inhibitor; 0.001% DMSO
vehicle), P4 + PP2 at the aforementioned concentrations, 100 nM
3α-THP (0.01% ethanol vehicle) or 3α-THP + PP2 at the
aforementioned concentrations. To determine the percentage of cell
migration, images of four fields of view per treatment condition
were taken at 0, 6, 12 and 24 h with an Infinity 1–2C camera
attached to an Olympus CKX41 inverted microscope. Image processing
was performed using the macro-MRI Wound Healing Tool in ImageJ
1.45S software (National Institutes of Health).
Invasion assays
Cells were grown according to the aforementioned
method. The invasion assays were performed using Transwell inserts
(8.0 µm membrane; Corning, Inc.) and 6-well plates with Matrigel (2
mg/ml; Engelbreth-Holm-Swarn murine sarcoma extract;
MilliporeSigma) diluted in DMEM phenol red-free without FBS or
antibiotics. This dilution was incubated in the inserts at
conditions of 37°C and 5% CO2 atmosphere for 2 h. In the
upper insert, 3×104 cells suspended in DMEM without
phenol red, FBS or antibiotics but with 10 µM Ara-C were seeded.
DMEM supplemented with 10% FBS (Biowest) as a chemoattractant was
added to the lower wells. Cells in the upper Transwell inserts
received the following steroid treatments: 50 nM P4, 100 nM 3α-THP
or 1 µM PP2. The incubation conditions were 37°C and 5%
CO2 atmosphere for 24 h. To evaluate the specific role
of cSrc on cell invasion, cSrc expression was first downregulated
as described in the small interfering (siRNA) transfection
subsection of this manuscript, followed by vehicle, P4 or 3α-THP
treatments. After incubation, the non-invading cells were washed
from the upper surface of the insert. Invasive cells at the
membrane were fixed, stained, visualized and quantified as
described previously by Piña-Medina et al (16).
Senescence assays
Based on preliminary results, growth pattern and
cell size, a total of 5×104 U87 cells or
3×104 U251 cells were plated per well in 6-well plates
with phenol red-free DMEM and 10% sFBS for 24 h at 37°C. After 24
h, the following treatments were added: 50 nM P4, 1 µM PP2, 50 nM
P4 + 1 µM PP2, 100 nM 3α-THP, 100 nM 3α-THP + 1 µM PP2 or vehicle
(0.01% DMSO for PP2 and P4 or 0.01% ethanol for 3α-THP). After a
further 24 h, cells were washed with PBS and senescence was
evaluated using the Senescent Cells Staining Kit (cat. no.
CS0030-1KT; MilliporeSigma), according to the manufacturer's
instructions, to determine the expression of β-galactosidase in
senescent cells. Incubation with β-galactosidase staining solution
was performed overnight at 37°C. A total of three independent
experiments were performed for each cell line. After staining,
images of four fields of view per treatment condition were taken
with an Infinity 1–2C camera attached to an Olympus CKX41 inverted
microscope. ImageJ 1.45S software (National Institutes of Health)
was used to perform the image processing and counting of
β-galactosidase positive cells to determine the proportion of
senescent cells.
siRNA transfection
A total of 2.5×105 U251 cells were plated
in 35-mm culture dishes with DMEM and 10% FBS for 24 h. After
incubation, the medium was replaced with DMEM without phenol red,
FBS, or antibiotics. Transfection was performed with 100 nM
commercial cSrc siRNA (cat. no. 4392420; ID, s13412; Thermo Fisher
Scientific, Inc.) or with 100 nM control siRNA (Silencer Select
Negative Control #1; cat. no. 4390844; Thermo Fisher Scientific,
Inc.), an aleatory sequence that did not recognize a specific
target in the cell, using Lipofectamine RNAiMAX (Thermo Fisher
Scientific, Inc.) for 48 h at 37°C. This commercial cSrc siRNA was
also used to evaluate the effect of P4 on MMP-2 and MMP-9
expression promoted by cSrc. After transfection with the cSrc siRNA
or control siRNA, the medium was refreshed for 12 h. Cells were
harvested for protein extraction to evaluate transfection
efficiency 48 h after the addition of siRNAs, and the wound healing
assays were performed. Commercial siRNA against progesterone
receptor (PR) (Ambion, PGR Silencer Select, Pre-designed; cat. no.
4392420) and control siRNA (Silencer Select Negative Control #1;
cat. no. 4390844) (both Thermo Fisher Scientific, Inc.) was used to
evaluate the effect of PR on MMP-2 and −9 expression 48 h after the
addition of siRNAs. The transfection protocol was performed as
previously described (15,18).
Protein extraction and western
blotting
The protein expression levels of MMP-2, MMP-9, cSrc
and PR were determined by western blotting. After any hormone
treatment or transfection procedure described above was performed,
cells were homogenized in RIPA buffer with protease inhibitors
(cat. no. P8340; Sigma-Aldrich; Merck KGaA). Proteins were obtained
as described by Bello-Alvarez et al (15). For protein electrophoresis, 40 µg of
total extracted proteins were loaded on an 8.5% SDS-PAGE gel.
Proteins were transferred to nitrocellulose membranes in semi-dry
conditions (Bio-Rad Laboratories, Inc.) at 25 V for 1 h (MMP-2,
MMP-9 and cSrc) or 2 h (PR). Membranes were blocked with 5% bovine
serum albumin (cat. no. A-420-100; Gold Biotechnology) in
Tris-buffered saline with 0.1% Tween, at 37°C for 2 h. Membranes
were then incubated with primary antibodies against MMP-2 (cat. no.
4042; Cell Signaling Technology, Inc.), MMP-9 (cat. no. 3852; Cell
Signaling Technology, Inc.), cSrc (cat. no. 2108; Cell Signaling
Technology, Inc.), PR (cat. no. B-30 sc-811; Santa Cruz
Biotechnology, Inc.) or the α-tubulin loading control (cat. no.
sc-398103; Santa Cruz Biotechnology, Inc.). The dilution of all
primary antibodies was 1:1,000 and antibodies were incubated for 24
h at 4°C. Secondary antibodies were against rabbit (cat. no.
1858415; Thermo Fisher Scientific, Inc.) or mouse (cat. no.
sc-516102; Santa Cruz Biotechnology, Inc.), and were incubated at a
dilution of 1:10,000 at room temperature for 45 min. Finally the
signal was detected with Super Signal West Femto Maximum
Sensitivity Substrate (Thermo Fisher Scientific, Inc.) (15). Densitometric analysis was performed
with ImageJ 1.45S software (National Institutes of Health). To
determine changes in the protein expression levels of MMP-2 and
MMP-9, the same membrane was re-probed three times, alongside the
α-tubulin loading control.
Statistical analysis
All statistical analyses were performed using Graph
Pad Prism 5 software (GraphPad Software; Dotmatics). A one-way
ANOVA and Bonferroni post hoc test, or unpaired Student's t-test
were used to analyze differences between comparable groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
P4 and 3α-THP enhance cell migration
through activation of the Src family of kinases
The impact of the Src kinase family on the actions
of P4 and 3α-THP in cell migration using the kinase inhibitor, PP2,
which has a high affinity for many members of the Src kinase
family, was evaluated. Both progestins (P4 and 3α-THP) induced cell
migration (Figs. 1 and 2). The effect of P4 in the U251 and U87
cell lines was significative compared to the vehicle following 24 h
of treatment (Figs. 1A-C and
S1). 3α-THP also promoted cell
migration but significance compared with vehicle was only
demonstrated at 12 and 24 h of treatment in both cell lines
(Figs. 2A-C and S2). The inhibitor PP2 completely blocked
the effect of P4 in U251 cells and significantly blocked the effect
of P4 in U87 cells (Fig. 1A-C). PP2
significantly, partially blocked the effect of 3α-THP at 12 and 24
h of treatment in U251 cells (Fig. 2A
and B); whereas in U87 cells, PP2 completely blocked its effect
(Fig. 2C). No marked changes in
cell migration were observed for any treatments at 6 h (Figs. 1, 2,
S1 and S2).
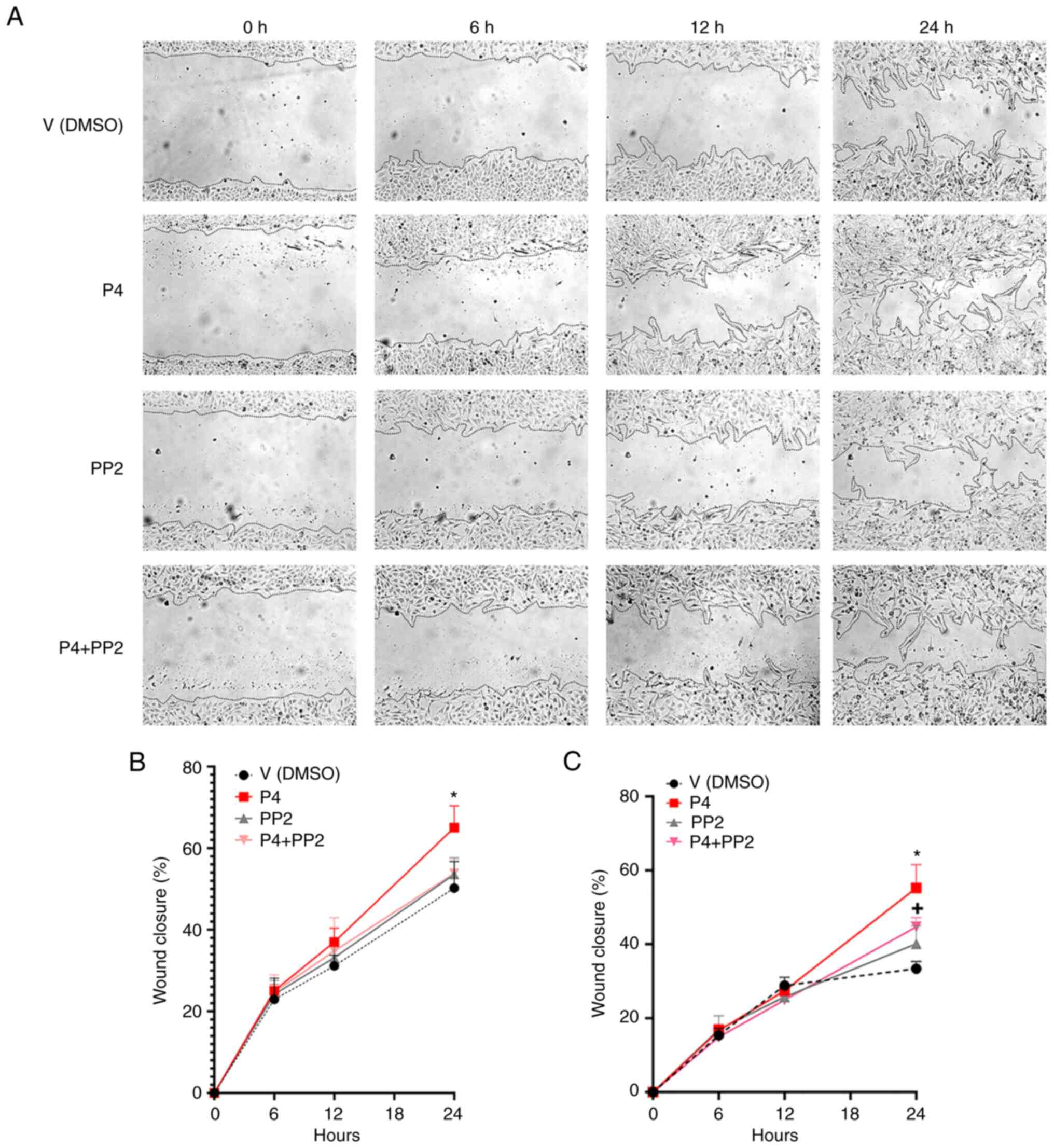 | Figure 1.The pharmacological inhibition of
cSrc interferes with the effect of P4 on glioblastoma cell
migration. (A) Representative images of U251 cells treated with P4
(50 nM), PP2 (1 µM) and the P4 + PP2 conjunct treatment at 0, 6, 12
and 24 h. Percentage cell migration graphs of (B) U251 (*P<0.05,
P4 vs. V at 24 h) and (C) U87 cells (*P<0.05, P4 vs. all other
treatments at 24 h; +P<0.05, P4 + PP2 vs. V at 24 h).
Each point represents the mean ± SEM, n=4. All images were taken
using a 10X magnification lens. P4, progesterone; V, vehicle; PP2,
1-tert-Butyl-3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]
pyrimidin-4-amine. |
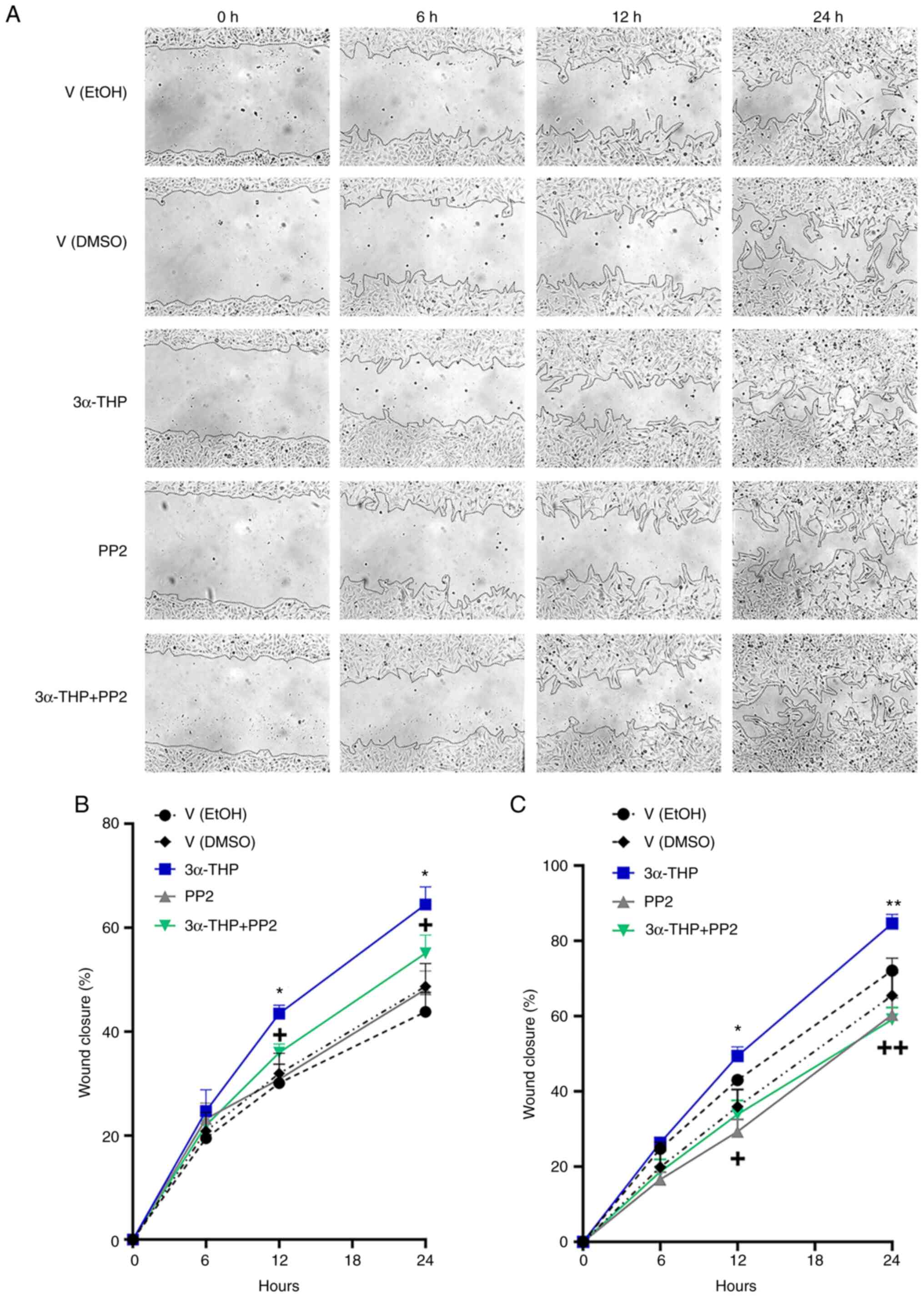 | Figure 2.cSrc inhibition blocks the effect of
3α-THP on glioblastoma cell migration. (A) Representative images of
U251 cells treated with 3α-THP (100 nM), PP2 (1 µM) and the 3α-THP
+ PP2 conjunct treatment at 0, 6, 12 and 24 h. Percentage migration
graph of (B) U251 cells [*P<0.05, 3α-THP vs. all other
treatments; +P<0.05, 3α-THP + PP2 vs. V (EtOH); n=3]
and (C) U87 cells [*P<0.05, 3α-THP vs. V (DMSO 0.001%), 3α -THP
+ PP2, and PP2; **P<0.05, 3α-THP vs. all other treatments;
+P<0.05, PP2 and 3α-THP + PP2 vs. V (EtOH 0.01%);
++P<0.05, PP2 vs. V (EtOH), and 3α-THP + PP2 vs. V
(EtOH)]; n=4. Each point represents the mean ± SEM. EtOH, ethanol;
V, vehicle; PP2, 1-tert-Butyl-3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]
pyrimidin-4-amine; 3α-THP, allopregnanolone. |
Furthermore, the potential of any of the treatment
conditions to induce senescence was evaluated in both cell lines
using a β-galactosidase staining assay (β-galactosidase catalyzes
the hydrolysis of β-galactosidase only in senescent cells). In U251
cells, the proportion of β-galactosidase positive cells was 0% for
any treatment (Figs. S3 and
S4). In U87 cells, the proportion
of β-galactosidase positive cells in each treatment was 0.88-1.5%.
However, no significant differences between treatments were
demonstrated (Figs. S5 and
S6).
cSrc activity participates in the
invasion induced by P4 and 3α-THP in human glioblastoma-derived
cells
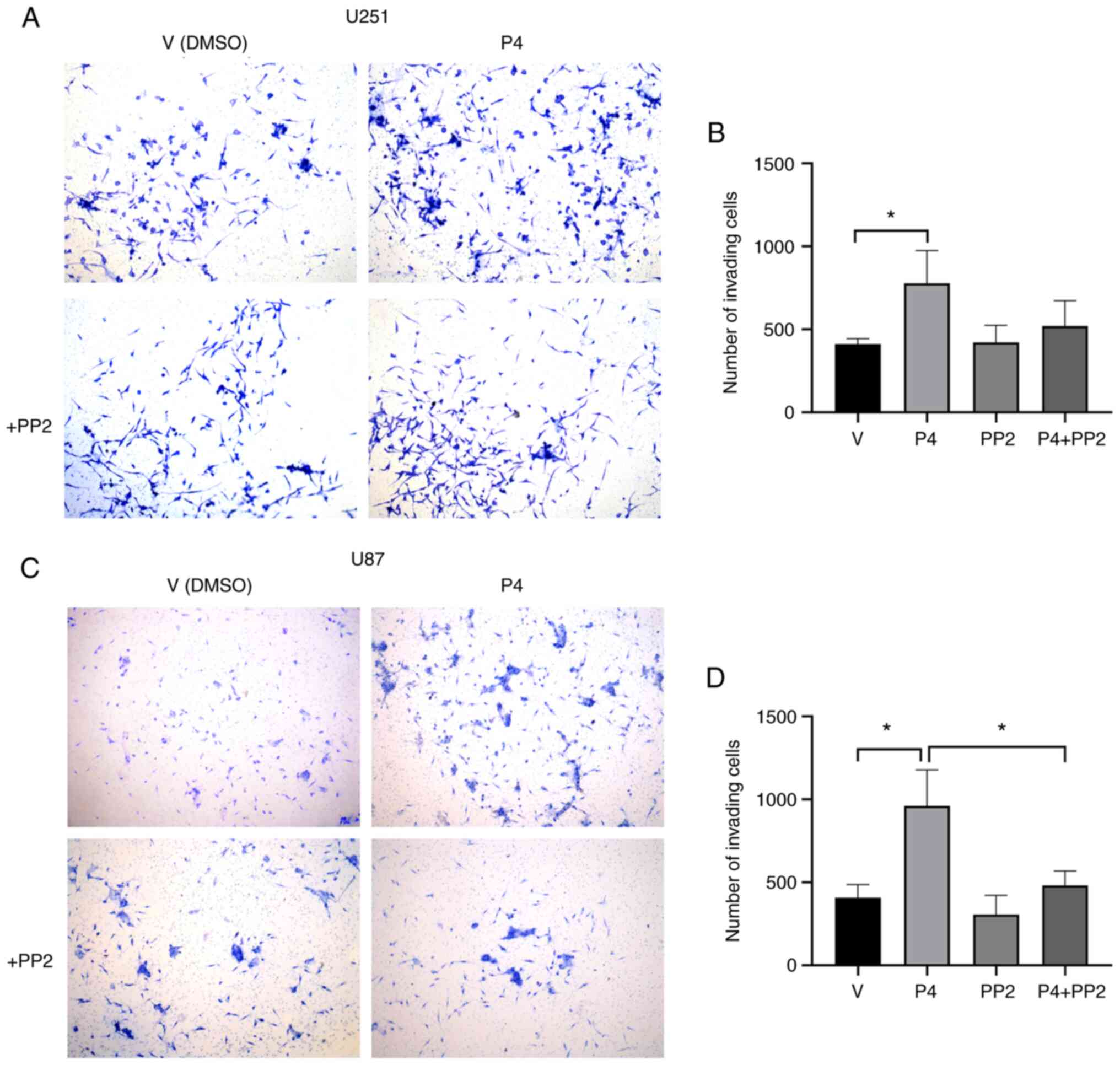
Considering that the addition of P4 increased cell
migration, the effect of cSrc on the invasiveness of P4-stimulated
human glioblastoma-derived cells was evaluated. A Transwell
invasion assay was performed to determine whether inhibiting Src
kinase family activity with PP2 altered the invasion triggered by
P4 in U251 and U87 cells. In P4-treated cells, a significant
enhancement of invasion compared with the vehicle group was
observed at 24 h in both U251 (Fig. 3A
and B) and U87 cells (Fig. 3C and
D). This enhancement was significantly suppressed by PP2 in U87
cells (Fig. 3D). In U251 cells, the
effect of PP2 on invasiveness was not significant; however, there
was a marked trend towards a decrease in invasion (Fig. 3B). This result demonstrated that the
Src kinase family modulates the invasion of glioblastoma cells
triggered by P4.
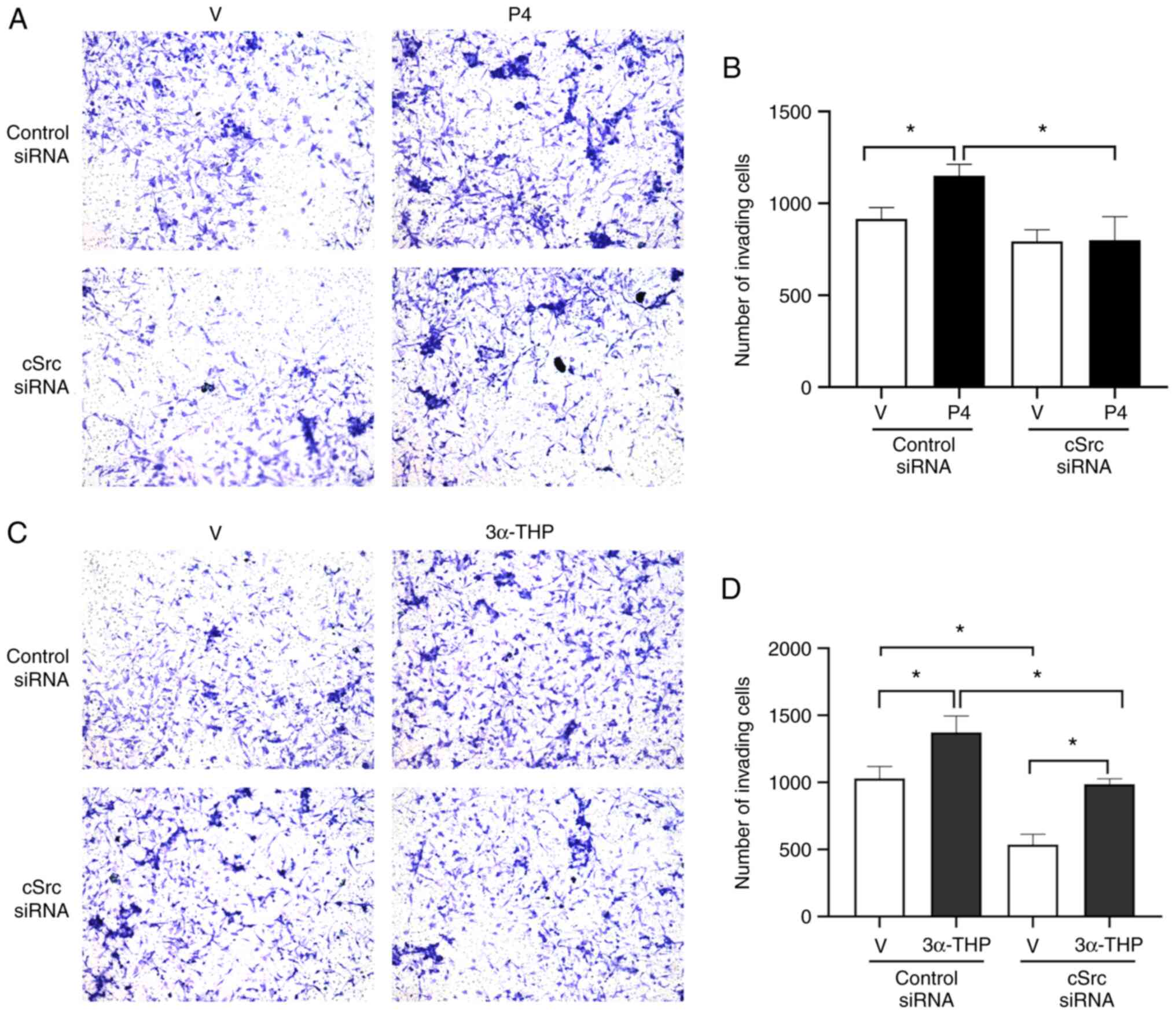
The effect of cSrc silencing on the invasiveness of
U251 cells after treatment with P4 or 3α-THP was also assessed.
Silencing in U87 was not performed due to limited siRNA
availability). As with the PP2 experiments, P4 significantly
induced U251 cell invasion compared with the untreated group and
this effect was significantly reduced by cSrc silencing (Fig. 4A and B) compared with the siRNA
control. 3α-THP also significantly promoted cell invasion compared
with the untreated group and this effect decreased significantly
when cSrc was silenced compared with the siRNA control (Fig. 4C and D). Notably, marked inhibition
of cell invasion by cSrc silencing was evident in vehicle-treated
cells (Fig. 4D). When treatment
with 3α-THP was administered to the cells, invasion returned to
basal levels.
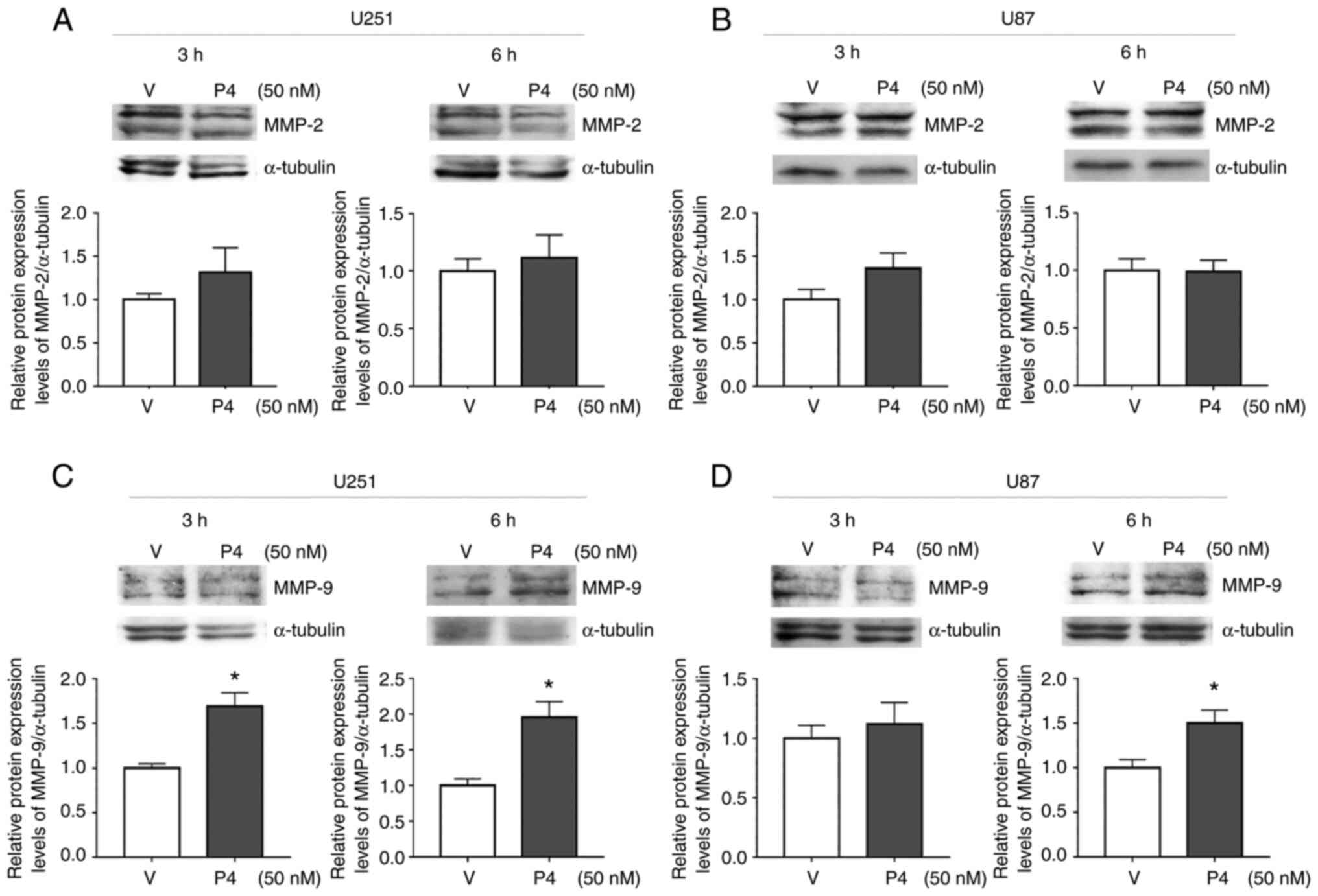
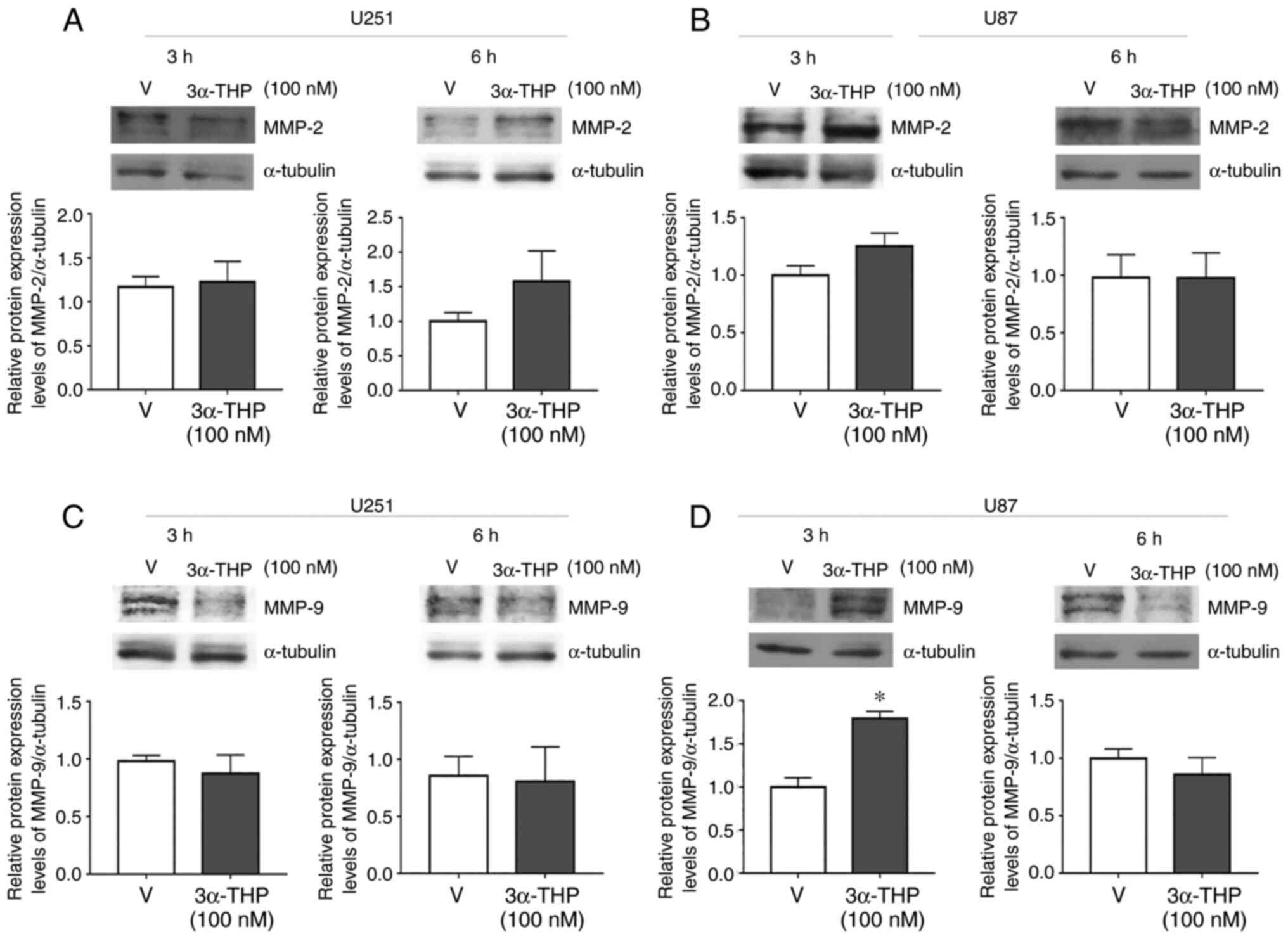
P4 and 3α-THP increase the protein
expression level of MMP-9 in human glioblastoma-derived cells
MMPs perform a vital role in ECM degradation to
promote cell invasion and are frequently upregulated in malignant
tumors (21). MMP-2 and MMP-9 are
upregulated in glioblastomas and are associated with poor prognosis
(6). Therefore, the effect of P4
and 3α-THP on MMP-2 and MMP-9 protein expression levels in human
glioblastoma-derived cell lines was evaluated. U87 and U251 cells
were treated with 50 nM P4 and 100 nM 3α-THP for 3 or 6 h (Figs. 5 and 6). P4 and 3α-THP did not significantly
increase MMP-2 protein expression levels compared with the control
in U251 and U87 cells (Figs. 5A and
B, and 6A and B). However, P4
significantly increased MMP-9 protein expression levels compared
with the control at 3 and 6 h in U251 cells and at 6 h in U87 cells
(Fig. 5C and D). 3α-THP
significantly increased MMP-9 protein expression levels compared
with the control in U87 cells at 3 h (Fig. 6D).
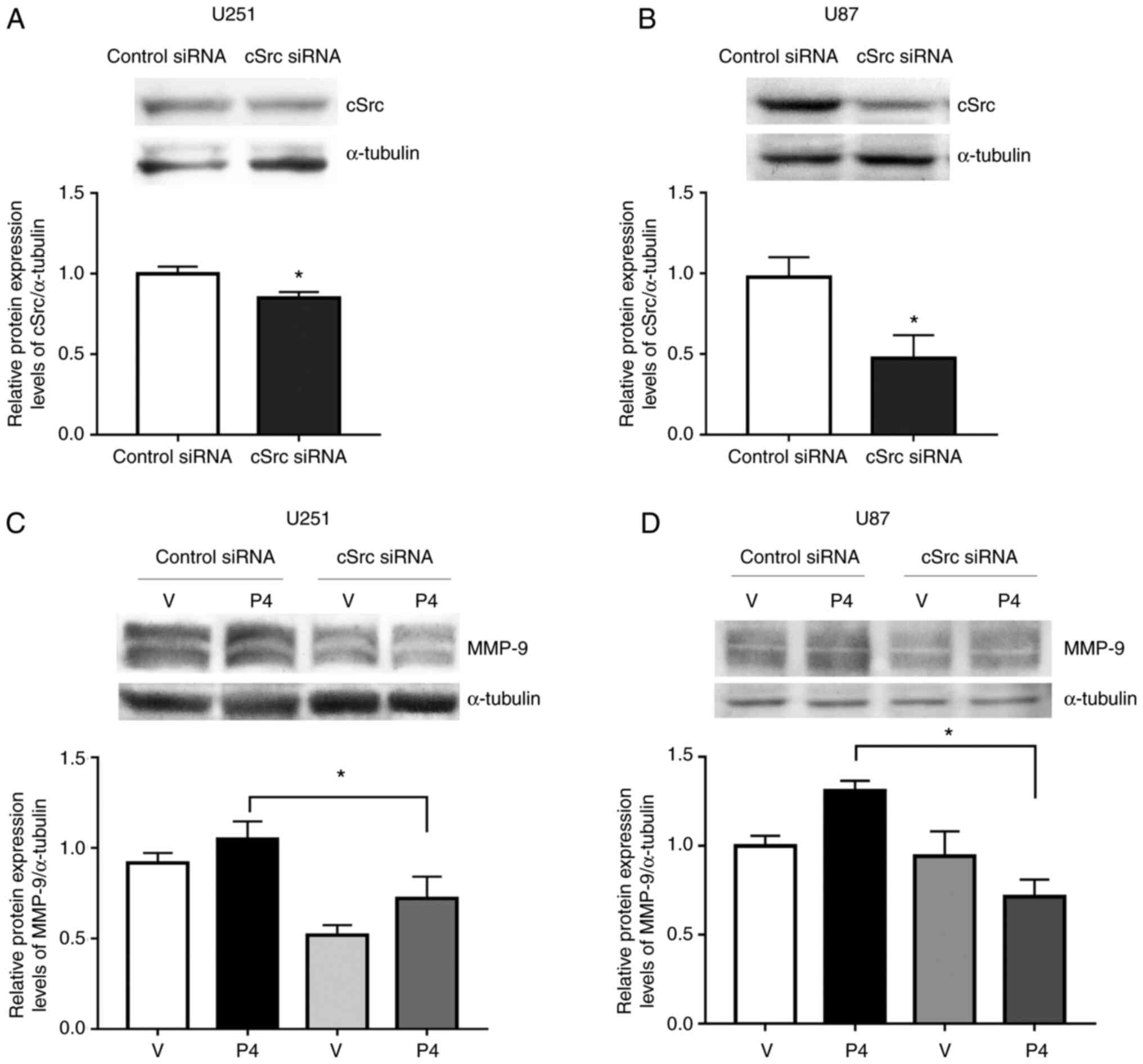
cSrc is involved in the regulation of
MMP-9 expression in human glioblastoma-derived cells treated with
P4
It has previously been reported that the
inactivation of cSrc inhibits triple-negative breast cancer
progression through the downregulation of MMP-9 expression
(5). Therefore, the role of cSrc in
the expression of MMP-9 in P4-treated glioblastoma cells was
evaluated. A commercial siRNA against cSrc or a control siRNA were
used to transfect U251 and U87 cells. After transfection, cells
were treated with 100 nM 3α-THP or 50 nM P4 for 6 h, respectively
(Fig. 7). cSrc silencing caused a
significant reduction in cSrc protein expression levels compared
with the siRNA control (Fig. 7A and
B). In cells transfected with siRNA against cSrc, the increase
in MMP-9 expression triggered by P4 was significantly reduced
compared with cells that received the control siRNA. This result
suggested that, at least partially, cSrc participated in the
induction of MMP-9 expression by P4 (Fig. 7C and D).
PR regulates the expression of
MMP-9
Bello-Alvarez et al (15) previously reported that P4 promoted
interaction between the PR and cSrc, which led to the activation of
cSrc. To assess the effect of PR on MMP-2 and MMP-9 expression,
cells were transfected with an siRNA against PR (Fig. 8). MMP-9 protein expression levels
were significantly reduced upon PR silencing compared with control
siRNA, whereas no significant change was demonstrated for MMP-2
(Fig. 8B and C).
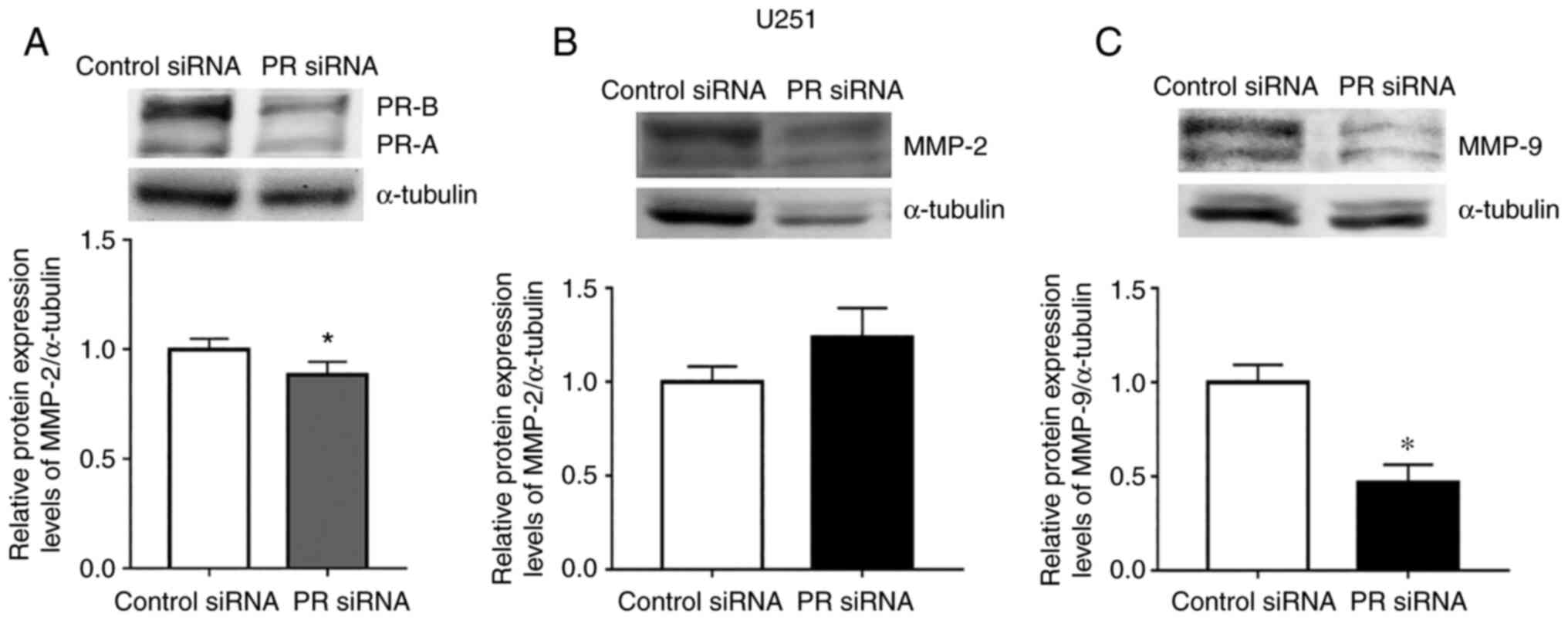 | Figure 8.PR silencing reduces the protein
expression levels of MMP-9 but not MMP-2. (A) U251 cells were
transfected with a PR siRNA and a control siRNA (100 nM). (B) MMP-2
and (C) MMP-9 protein expression levels were determined in cells
transfected with PR siRNA at 6 h. The upper panels show the
representative western blots for PR (PR-B 110 kDa, 90 PR-A kDa),
MMP-2 (proenzyme, 72 kDa; active enzyme, 64 kDa), MMP-9 (proenzyme,
92 kDa; active enzyme, 84 kDa), and α-tubulin (55 kDa, loading
control). The lower panels show the densitometric analysis. Data
were normalized with respect to control siRNA and are presented as
the mean ± SEM; n=3. *P<0.05. MMP, metalloproteinase; P4,
progesterone; PR, progesterone receptor; siRNA, small interfering
RNA; V, vehicle. |
Discussion
According to the Global Cancer Observatory database
(https://gco.iarc.fr/) (22), central nervous system tumors are the
13th leading cause of mortality among all known types of cancer.
Glioblastoma is the most malignant and frequent entity among adult
brain tumors (1). Due to
glioblastoma's migratory and invasive nature, the treatments
available are insufficient and tumor recurrence is invariably
manifested (23). The migration and
subsequent degradation of the ECM is one of the critical events for
a malignant cell to colonize areas distant from the tumor's site of
origin (24). Proteases are members
of the group of molecules that, in a coordinated manner,
orchestrate this event to gain cancer aggressiveness (25). MMPs are among the most relevant
families of proteases in glioblastoma invasion. The expression of
11 members (MMP-1, −2, −7, −8, −9, −10, −11, −14, −15, −19 and −23)
of this group have been reported to be elevated in glioblastomas
(26). However, according to the
results of several studies (6,27–29),
MMP-2 and MMP-9 are essential in the progression of glioblastomas.
The expression of these proteins are increased in recurrent gliomas
compared with primary gliomas (6).
In addition, the expression of both of these MMPs correlates with
the tumor grade (7). In the case of
MMP-9, its low expression is related to an improved prognosis and
response to Temozolomide (30).
The role of P4 at physiological concentrations
(10–100 nM) in the development of glioblastomas has been
extensively reported. In addition to inducing glioblastoma cell
proliferation (23–25), P4 promotes migration and invasion in
both in vitro and in vivo models (15,16,31–33).
P4 is one of the most prominent examples of the hormesis effect, a
dose-response phenomenon in which stimulation occurs at low
concentrations and inhibition at high concentrations (34). In the context of glioblastomas, it
has been reported that P4 concentrations >20 µM promoted
apoptosis and decreased tumor growth, while concentrations <10
µM promoted the opposite effect (35). Previous results published by our
laboratory support this observation as concentrations of 10 and 50
nM promoted glioblastoma cell progression by increasing
proliferation, migration and invasion (14–16).
The previous study by Piña Medina et al
(16), from our laboratory,
reported that PR was partially implicated in the induction of
migration and invasion processes in glioblastoma cells, which
suggested alternative activation of other regulatory mechanisms
mediated by P4 or its metabolites. In that study, when P4 were
added to cells treated with antisense oligonucleotides against PR
expression, the P4-induced effect was partially reduced. Recently,
Bello-Alvarez et al (15)
reported that, in glioblastoma cells, P4 promoted PR-cSrc
interaction, which led to cSrc autophosphorylation and subsequent
Fak activation and migration.
Notably, 3α-THP possesses different mechanisms of
action than those reported for P4. One of the main differences
between these progestins is that 3α-THP lacks affinity for the
classical PR (36). This metabolite
induces rapid cellular changes by activating membrane PR (mPR) δ
and mPRα (37,38). At the genomic level, 3α-THP
activates the pregnane X receptor, which is a transcription factor
(39,40). The progestin 3α-THP also modulates
the action of neurotransmitters due to its interaction with their
receptors, such as the ionotropic channel receptor,
GABAAR (41). It has
recently been reported that 3α-THP promotes the migration of
glioblastoma cells independently of the oxidation of other P4
metabolites with high binding affinity from the PR. In addition,
the activation of cSrc kinase by 3a-THP has been reported (18,42).
Melfi et al (19) reported
that, in rat Schwann cells, induced cell migration was dependent on
cSrc activation. An additional study reported that 3α-THP promoted
cSrc activation at the ventromedial hypothalamus, although the
mechanism involved in this activation has not been fully elucidated
(43). In the present study, an
increase in glioblastoma cell migration induced by P4 is reported.
This effect was completely blocked with the addition of the cSrc
kinase family inhibitor, PP2, in U251 cells and partially blocked
in U87 cells. Glioblastomas are among the tumors with the greatest
intra- and inter-tumor heterogeneity (44,45).
Therefore, it can be assumed that the established cell lines
derived from these tumors have significant differences in their
genetic signature. The U87 cell line has a neuronal-like phenotype
with a high proliferative capacity, while the U251 cell line has a
mesenchymal-like phenotype with a lower proliferative activity
(46). The results in the present
study demonstrated a difference in the migration rate of both cell
lines, which may be related to the aforementioned differences.
Considering that, despite the differences in migration rate, the
results showed the same trend in both cell lines, it can be
hypothesized that the effects of P4, in general, not cell
line-dependent but pathology-dependent.
Regarding 3α-THP, in the present study, an increase
in cell migration partially blocked by PP2 in U251 and U87 cells
was demonstrated. Considering that PP2 is not a specific inhibitor
of cSrc, but of the entire Src family, this result suggested that,
in addition to cSrc, other members of the Src kinase family may
interact with P4 or 3α-THP effectors. For example, the high
expression of Lyn, a member of cSrc kinase family, has been
reported in glioblastoma cells and is linked to increased tumor
progression (47).
The invasiveness of glioblastoma cells depends on
the dynamic activation of at least two essential processes: Cell
migration and ECM degradation. Until recently, no information on
the role of cSrc in the regulation of proteins directly involved in
ECM degradation in glioblastoma cells treated with P4 or any of its
metabolites was available. In the present study, the effect of P4
and 3α-THP on the expression of MMP-2 and MMP-9 was evaluated. The
results demonstrated that in P4-treated cells, the protein
expression level of MMP-9 was increased in U251 and U87 cells, and
in 3α-THP-treated cells, the protein expression level of MMP-9 was
increased in U87 cells. These results indicated that increased
MMP-9 expression may be one of the mechanisms implicated in P4- and
3α-THP-induced invasion of glioblastoma cells. MMP-2 upregulation
is also associated with poor prognosis and progression of
glioblastoma (48). Notably, no
significant changes in MMP-2 expression were demonstrated with P4
and 3α-THP treatments. In fibroblast cells, the increase in the
protein expression levels of MMP-9 but not MMP-2 were reported to
be dependent on the activation of the cSrc-Fak signaling pathway
(49). In MCF-7 breast cancer
cells, cSrc and Fak activity inhibition blocked MMP-9 secretion
(4). A recent article reported the
anti-inflammatory property of quercetin that, through the
inhibition of TNF-α, decreased MMP-9 expression and activity in the
gastric mucosa epithelial GES-1 cell line. This study also reported
that the addition of PP1 (a Src family kinase inhibitor) decreased
the activity of MMP-9 but not MMP-2 (50). The occurrence of this phenomenon in
cells of different lineages suggests that it is independent of
cellular context and that it is regulated by highly conserved
molecular mechanisms.
Interactions between cSrc and Fak have been widely
reported. It is known that cSrc phosphorylates residues Y576 and
Y577 of Fak, which are indispensable for full activation of the
latter (51,52). Sex steroid receptors are primarily
known for their function as transcription factors. However, PR and
the androgen receptor are also known to function in the activation
of signaling cascades in the cytoplasm through the interaction of
their polyproline motifs with the SH3 domains of cytoplasmic
molecules, such as cSrc (53). In a
recent publication by Bello-Alvarez et al (15), it was demonstrated that P4 activates
cSrc kinase through the PR, for the first time in
glioblastoma-derived cells. This, in turn, induces the
phosphorylation of Fak at residues Y397, Y576 and Y577, which
provided evidence of the non-genomic function of the PR in this
brain tumor.
In the present study to assess the role of cSrc in
the regulation of MMP-9 expression by P4 and 3α-THP, an siRNA
against cSrc expression was used. The silencing of cSrc blocked the
increase in MMP-9 protein expression levels induced by P4 in U251
and U87 cells. Only a partial effect was demonstrated in U251
cells, possibly due to lower efficiency of the silencing. These
results indicated that cSrc mediates P4-induced MMP-9 expression
and suggest that, in the context of glioblastoma cells, P4
treatment activates the cSrc-Fak signaling pathway, which modifies
the expression of MMP-9 but not that of MMP-2.
Notably, Liu et al (54) reported that the PR−/−
condition in zebrafish follicular cells decreased the expression of
MMP-9 but not MMP-2. In the present study, transfection with an
siRNA against the PR decreased MMP-9 but not MMP-2 protein
expression levels, which suggested that the difference in the
effect of P4 on the regulation of MMP-9 and MMP-2 also involved the
PR. However, another study reported that, in a rat model of
blood-brain barrier breakdown, P4 and 3α-THP downregulated the
expression of MMP-9 and MMP-2 (55); this could be related to differences
in the progestin concentration. At the level of transcriptional
regulation, these differences could be related to the composition
of the MMP-2 and MMP-9 promoters (56).
In the present study, PP2 was used to evaluate the
effect of the cSrc family of kinases on P4-induced invasion in
human glioblastoma-derived cells. The results of the present study
demonstrated that inhibition of cSrc activity decreased
invasiveness in both U251 and U87 cell lines, as measured using a
Matrigel Boyden chamber assay. A similar effect of 3α-THP has also
been recently reported by our laboratory (18).
In conclusion, the results of the present study
suggest that the effect of P4 and 3α-THP on the invasion of human
glioblastoma-derived cells involves the activation of cSrc and its
regulatory role in MMP-9 expression. Experimentation on cell lines
is an important limitation of this study since it implies a study
model that is far from reality. However, the results obtained
broaden the knowledge for the future development of targeted
therapies against migration and invasion processes in an
underestimated area in the study of glioblastomas, namely,
signaling through sex hormones and their receptors.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Programa de Apoyo a
Proyectos de Investigación e Innovación Tecnológica (PAPIIT;
project no. PAPIIT IN217120), DGAPA-UNAM, México, and Programa de
Apoyo a la Investigación y el Posgrado (PAIP) 5000-9107.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CBA, CJZS and ICA conceptualized the study. CBA,
CJZS and KMPG performed the experiments. CBA and CJZS prepared the
first draft of the manuscript and ICA contributed to the revision
and editing of the manuscript. ICA obtained the funding. CBA, CJZS,
KMPG and ICA confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2011–2015. Neuro Oncol. 20:iv1–iv86. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel A, Sabbineni H, Clarke A and
Somanath PR: Novel roles of Src in cancer cell
epithelial-to-mesenchymal transition, vascular permeability,
microinvasion and metastasis. Life Sci. 157:52–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cortes-Reynosa P, Robledo T, Macias-Silva
M, Wu SV and Salazar EP: Src kinase regulates metalloproteinase-9
secretion induced by type IV collagen in MCF-7 human breast cancer
cells. Matrix Biol. 27:220–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gautam J, Banskota S, Lee H, Lee YJ, Jeon
YH, Kim JA and Jeong BS: Down-regulation of cathepsin S and matrix
metalloproteinase-9 via Src, a non-receptor tyrosine kinase,
suppresses triple-negative breast cancer growth and metastasis. Exp
Mol Med. 50:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou W, Yu X, Sun S, Zhang X, Yang W,
Zhang J, Zhang X and Jiang Z: Increased expression of MMP-2 and
MMP-9 indicates poor prognosis in glioma recurrence. Biomed
Pharmacother. 118:1093692019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forsyth PA, Wong H, Laing TD, Rewcastle
NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM,
Sutherland G and Edwards DR: Gelatinase-A (MMP-2), gelatinase-B
(MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are
involved in different aspects of the pathophysiology of malignant
gliomas. Br J Cancer. 79:1828–1835. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondraganti S, Mohanam S, Chintala SK, Kin
Y, Jasti SL, Nirmala C, Lakka SS, Adachi Y, Kyritsis AP, Ali-Osman
F, et al: Selective suppression of matrix metalloproteinase-9 in
human glioblastoma cells by antisense gene transfer impairs
glioblastoma cell invasion. Cancer Res. 60:6851–6855.
2000.PubMed/NCBI
|
|
9
|
Trabert B, Sherman ME, Kannan N and
Stanczyk FZ: Progesterone and breast cancer. Endocr Rev.
41:320–344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grindstad T, Richardsen E, Andersen S,
Skjefstad K, Khanehkenari MR, Donnem T, Ness N, Nordby Y, Bremnes
RM, Al-Saad S and Busund LT: Progesterone receptors in prostate
cancer: Progesterone receptor B is the isoform associated with
disease progression. Sci Rep. 8:1–11. 2018. View Article : Google Scholar
|
|
11
|
Bello-Alvarez C and Camacho-Arroyo I:
Impact of sex in the prevalence and progression of glioblastomas:
The role of gonadal steroid hormones. Biol Sex Differ. 12:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu XD, Goglia L, Sanchez AM, Flamini M,
Giretti MS, Tosi V, Genazzani AR and Simoncini T: Progesterone
receptor enhances breast cancer cell motility and invasion via
extranuclear activation of focal adhesion kinase. Endocr Relat
Cancer. 17:431–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grochans S, Cybulska AM, Simińska D,
Korbecki J, Kojder K, Chlubek D and Baranowska-Bosiacka I:
Epidemiology of glioblastoma multiforme-literature review. Cancers
(Basel). 14:24122022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Germán-Castelán L, Manjarrez-Marmolejo J,
González-Arenas A and Camacho-Arroyo I: Intracellular progesterone
receptor mediates the increase in glioblastoma growth induced by
progesterone in the rat brain. Arch Med Res. 47:419–426. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bello-Alvarez C, Moral-Morales AD,
González-Arenas A and Camacho-Arroyo I: Intracellular progesterone
receptor and cSrc protein working together to regulate the activity
of proteins involved in migration and invasion of human
glioblastoma cells. Front Endocrinol (Lausanne). 12:6402982021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piña-Medina AG, Hansberg-Pastor V,
González-Arenas A, Cerbón M and Camacho-Arroyo I: Progesterone
promotes cell migration, invasion and cofilin activation in human
astrocytoma cells. Steroids. 105:19–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zamora-Sánchez CJ, Hansberg-Pastor V,
Salido-Guadarrama I, Rodríguez-Dorantes M and Camacho-Arroyo I:
Allopregnanolone promotes proliferation and differential gene
expression in human glioblastoma cells. Steroids. 119:36–42. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zamora-Sánchez CJ, Bello-Alvarez C,
Rodríguez-Dorantes M and Camacho-Arroyo I: Allopregnanolone
promotes migration and invasion of human glioblastoma cells through
the protein tyrosine kinase c-Src activation. Int J Mol Sci.
23:49962022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melfi S, Guevara MM, Bonalume V, Ruscica
M, Colciago A, Simoncini T and Magnaghi V: Src and phospho-FAK
kinases are activated by allopregnanolone promoting Schwann cell
motility, morphology and myelination. J Neurochem. 141:165–178.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pirkmajer S and Chibalin AV: Serum
starvation: Caveat emptor. Am J Physiol Cell Physiol.
301:C272–C279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31:177–183.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Global Cancer Observatory, .
|
|
23
|
van Linde ME, Brahm CG, de Witt Hamer PC,
Reijneveld JC, Bruynzeel AME, Vandertop WP, van de Ven PM,
Wagemakers M, van der Weide HL, Enting RH, et al: Treatment outcome
of patients with recurrent glioblastoma multiforme: A retrospective
multicenter analysis. J Neurooncol. 135:183–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gkretsi V and Stylianopoulos T: Cell
adhesion and matrix stiffness: Coordinating cancer cell invasion
and metastasis. Front Oncol. 8:1452018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quintero-Fabián S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V,
Lara-Riegos J, Ramírez-Camacho MA and Alvarez-Sánchez ME: Role of
matrix metalloproteinases in angiogenesis and cancer. Front Oncol.
9:13702019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Quesnel A, Karagiannis GS and Filippou PS:
Extracellular proteolysis in glioblastoma progression and
therapeutics. Biochim Biophys Acta Rev Cancer. 1874:1884282020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai HP, Wang J, Xi SY, Ni XR, Chen YS, Yu
YJ, Cen ZW, Yu ZH, Chen FR, Guo CC, et al: Tenascin-c mediated
vasculogenic mimicry formation via regulation of MMP2/MMP9 in
glioma. Cell Death Dis. 10:1–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mirabdaly S, Komi DE, Shakiba Y, Moini A
and Kiani A: Effects of temozolomide on U87MG glioblastoma cell
expression of CXCR4, MMP2, MMP9, VEGF, anti-proliferatory cytotoxic
and apoptotic properties. Mol Biol Rep. 47:1187–1197. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen CJ, Shang HS, Huang YL, Tien N, Chen
YL, Hsu SY, Wu RSC, Tang CL, Lien JC, Lee MH, et al:
Bisdemethoxycurcumin suppresses human brain glioblastoma multiforme
GBM 8401 cell migration and invasion via affecting NF-κB and MMP-2
and MMP-9 signaling pathway in vitro. Environ Toxicol.
37:2388–2397. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Q, Chen B, Cai J, Sun Y, Wang G, Li Y,
Li R, Feng Y, Han B, Li J, et al: Comparative analysis of matrix
metalloproteinase family members reveals that MMP9 predicts
survival and response to temozolomide in patients with primary
glioblastoma. PLoS One. 11:e01518152016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
González-Agüero G, Gutiérrez AA,
González-Espinosa D, Solano JD, Morales R, González-Arenas A,
Cabrera-Muñoz E and Camacho-Arroyo I: Progesterone effects on cell
growth of U373 and D54 human astrocytoma cell lines. Endocrine.
32:129–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hernández-Hernández OT, González-García TK
and Camacho-Arroyo I: Progesterone receptor and SRC-1 participate
in the regulation of VEGF, EGFR and cyclin D1 expression in human
astrocytoma cell lines. J Steroid Biochem Mol Biol. 132:127–134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
González-Arenas A, Valadez-Cosmes P,
Jiménez-Arellano C, López-Sánchez M and Camacho-Arroyo I:
Progesterone-induced blocking factor is hormonally regulated in
human astrocytoma cells, and increases their growth through the
IL-4R/JAK1/STAT6 pathway. J Steroid Biochem Mol Biol. 144:463–470.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Strom JO, Theodorsson A and Theodorsson E:
Hormesis and female sex hormones. Pharmaceuticals (Basel).
4:726–740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Atif F, Yousuf S and Stein DG: Anti-tumor
effects of progesterone in human glioblastoma multiforme: Role of
PI3K/Akt/mTOR signaling. J Steroid Biochem Mol Biol. 146:62–73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Diviccaro S, Cioffi L, Falvo E, Giatti S
and Melcangi RC: Allopregnanolone: An overview on its synthesis and
effects. J Neuroendocrinol. 34:e129962022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thomas P and Pang Y: Membrane progesterone
receptors: Evidence for neuroprotective, neurosteroid signaling and
neuroendocrine functions in neuronal cells. Neuroendocrinology.
96:162–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pang Y, Dong J and Thomas P:
Characterization, neurosteroid binding and brain distribution of
human membrane progesterone receptors δ and {epsilon} (mPRδ and
mPR{epsilon}) and mPRδ involvement in neurosteroid inhibition of
apoptosis. Endocrinology. 154:283–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lamba V, Yasuda K, Lamba JK, Assem M,
Davila J, Strom S and Schuetz EG: PXR (NR1I2): Splice variants in
human tissues, including brain, and identification of neurosteroids
and nicotine as PXR activators. Toxicol Appl Pharmacol.
199:251–265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Frye CA, Koonce CJ and Walf AA: The
pregnane xenobiotic receptor, a prominent liver factor, has actions
in the midbrain for neurosteroid synthesis and behavioral/neural
plasticity of female rats. Front Syst Neurosci. 8:602014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Belelli D and Gee KW: 5 alpha-pregnan-3
alpha,20 alpha-diol behaves like a partial agonist in the
modulation of GABA-stimulated chlride ion uptake by
synaptoneurosomes. Eur J Pharmacol. 167:173–176. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zamora-Sánchez CJ, Hernández-Vega AM,
Gaona-Domínguez S, Rodríguez-Dorantes M and Camacho-Arroyo I:
5alpha-dihydroprogesterone promotes proliferation and migration of
human glioblastoma cells. Steroids. 163:1087082020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
González-Flores O, Beyer C, Gómora-Arrati
P, García-Juárez M, Lima-Hernández FJ, Soto-Sánchez A and Etgen AM:
A role for Src kinase in progestin facilitation of estrous behavior
in estradiol-primed female rats. Horm Behav. 58:223–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bergmann N, Delbridge C, Gempt J,
Feuchtinger A, Walch A, Schirmer L, Bunk W, Aschenbrenner T,
Liesche-Starnecker F and Schlegel J: The intratumoral heterogeneity
reflects the intertumoral subtypes of glioblastoma multiforme: A
regional immunohistochemistry analysis. Front Oncol. 10:4942020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Becker AP, Sells BE, Haque SJ and
Chakravarti A: Tumor heterogeneity in glioblastomas: From light
microscopy to molecular pathology. Cancers (Basel). 13:1–25. 2021.
View Article : Google Scholar
|
|
46
|
Motaln H, Koren A, Gruden K, Ramšak Ž,
Schichor C and Lah TT: Heterogeneous glioblastoma cell cross-talk
promotes phenotype alterations and enhanced drug resistance.
Oncotarget. 6:40998–41017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stettner MR, Wang W, Nabors LB, Bharara S,
Flynn DC, Grammer JR, Gillespie GY and Gladson CL: Lyn kinase
activity is the predominant cellular Src kinase activity in
glioblastoma tumor cells. Cancer Res. 65:5535–5543. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ramachandran RK, Sørensen MD,
Aaberg-Jessen C, Hermansen SK and Kristensen BW: Expression and
prognostic impact of matrix metalloproteinase-2 (MMP-2) in
astrocytomas. PLoS One. 12:e01722342017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hsia DA, Mitra SK, Hauck CR, Streblow DN,
Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, et al:
Differential regulation of cell motility and invasion by FAK. J
Cell Biol. 160:753–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hsieh HL, Yu MC, Cheng LC, Chu MY, Huang
TH, Yeh TS and Tsai MM: Quercetin exerts anti-inflammatory effects
via inhibiting tumor necrosis factor-α-induced matrix
metalloproteinase-9 expression in normal human gastric epithelial
cells. World J Gastroenterol. 28:1139–1158. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yeatman TJ: A renaissance for SRC. Nat Rev
Cancer. 4:470–480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-Src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Boonyaratanakornkit V and Edwards DP:
Receptor mechanisms mediating non-genomic actions of sex steroids.
Semin Reprod Med. 25:139–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu DT, Carter NJ, Wu XJ, Hong WS, Chen SX
and Zhu Y: Progestin and nuclear progestin receptor are essential
for upregulation of metalloproteinase in zebrafish preovulatory
follicles. Front Endocrinol (Lausanne). 9:5172018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ishrat T, Sayeed I, Atif F, Hua F and
Stein DG: Progesterone and allopregnanolone attenuate blood-brain
barrier dysfunction following permanent focal ischemia by
regulating the expression of matrix metalloproteinases. Exp Neurol.
226:183–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yan C and Boyd DD: Regulation of matrix
metalloproteinase gene expression. J Cell Physiol. 211:19–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|