Introduction
Lung cancer is one of the most fatal and common
types of cancer globally, accounting for ~11.4% of new cancer cases
and 18.0% of cancer-related deaths in 2020. Notably, non-small cell
lung cancer (NSCLC) accounts for the majority of lung cancer cases
(1–3). To date, chemotherapy remains the most
common therapeutic approach for patients with NSCLC. However, a
proportion of patients with NSCLC develop chemoresistance during
treatment, resulting in a less favorable survival profile (4–7).
Cancer stemness is considered the most crucial factor contributing
to chemoresistance (8). Therefore,
exploring the mechanism underlying chemoresistance and stemness in
NSCLC is of great importance.
Transient receptor potential canonical 1 (TRPC1)
mediates the influx of extracellular Ca2+ and plays a
critical role in cell proliferation, differentiation, apoptosis,
and migration (9,10). The regulatory role of TRPC1 in
chemoresistance in solid carcinomas has been well established
(11–13). A previous study showed that TRPC1
could promote hypoxia-associated epithelial-mesenchymal transition
(EMT), subsequently contributing to chemoresistance in endometrial
carcinoma (11,13). Another study demonstrated that TRPC1
attenuated the sensitivity of breast cancer cells to chemotherapy
(12). However, the role of TRPC1
in NSCLC chemoresistance to cisplatin (CDDP, one of the most widely
used and effective compounds in cancer treatment, which functions
by binding to the DNA bases in the nucleus and inhibits DNA
replication and transcription to exhibit its anti-tumor efficacy)
and stemness remains unclear. Therefore, the current study aimed to
evaluate the regulatory effect of TRPC1 on NSCLC chemoresistance
and stemness, as well as its underlying mechanism of action.
Materials and methods
Cell culture
A549 and H460 cells were purchased from BeNa Culture
Collection. A549 and H460 cells resistant to
cis-Diamminedichloroplatinum (cisplatin/CDDP, MilliporeSigma;
A549/CDPP and H460/CDDP respectively) were established by gradually
exposing parental cells to increasing concentrations of CDDP as
described previously (14). All
cells were maintained in RPMI-1640 medium (Lonza Pharma &
Biotech) supplemented with 10% FBS (Lonza Pharma & Biotech) and
1% penicillin/streptomycin solution (Beijing Solarbio Science &
Technology Co., Ltd.) at 37°C in a humidified incubator supplied
with 5% CO2. To maintain resistance, A549/CDDP and
H460/CDDP cells were cultured in the presence of 2 and 4 µM CDDP,
respectively. The mRNA and protein expression levels of TRPC1 in
A549, A549/CDDP, H460, and H460/CDDP cells were detected by reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis,
respectively.
CDDP sensitivity assay
The sensitivity of A549, A549/CDDP, H460, and
H460/CDDP cells to CDDP was assessed using Cell Counting Kit-8
assays (CCK-8; MedChemExpress). Briefly, cells were seeded into
96-well plates at a density of 2×103 cells/well.
Subsequently, A549 and H460 cells were treated with 0, 0.25, 0.5,
1, 2, 4 or 8 µM CDDP for 48 h. Additionally, A549/CDDP and
H460/CDDP cells were treated with 0, 2, 4, 8, 16, 32, or 64 µM CDDP
for 48 h. Following treatment, cells were incubated for 2 h in the
presence of CCK-8 reagent. The absorbance values were subsequently
detected at a wavelength of 450 nm using a plate reader (Molecular
Devices, LLC). The half-maximal inhibitory concentration
(IC50) of CDDP was evaluated using a sigmoidal
dose-response curve, as previously described (15).
Cell transfection
The small interfering RNA (siRNA) constructs
targeting TRPC1 (si-TRPC1-1, si-TRPC1-2, and si-TRPC1-3) and the
corresponding negative control (si-NC) were obtained from Shanghai
GenePharma Co., Ltd. A549/CDDP and H460/CDDP cells were plated into
6-well plates at a density of 2×106 cells/well and then
transfected with the above siRNAs using Lipofectamine™
3000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. The knockdown
efficiency of siRNAs was detected by RT-qPCR and western blot
analysis. Since si-TRPC1-2 exhibited the most potent knockdown
effect on TRPC1 expression, this siRNA clone was used for the
subsequent interference experiments. Further assays, including CDDP
sensitivity, sphere formation assay, and western blot analysis were
performed after transfection. The sequences of the siRNAs used were
as follows: For siRNA-1 sense, CGAUCAUCAAGACCAACUAUA and antisense,
UAGUUGGUCUUGAUGAUCGUU; siRNA-2 sense, GGAAGUCUCUUUAAUGCAAUG and
antisense, UUGCAUUAAAGAGACUUCCUA); siRNA-3 sense,
GCUUUCAGUUGAUAGCAAAUC and antisense, UUUGCUAUCAACUGAAAGCUU); and
si-NC sense, UUCUCCGAACGUGUCACGUTT and antisense,
ACGUGACACGUUCGGAGAATT.
Cell treatment with 740 Y-P
A549/CDDP and H460/CDDP cells were seeded into
6-well plates and transfected as described above. Subsequently, to
evaluate the regulatory effect of TRPC1 on PI3K/AKT signaling,
cells were treated with 25 µg/ml 740 Y-P (a PI3K/AKT activator;
MedChemExpress) as described previously (16). Following cell treatment with 740 Y-P
for 48 h, western blot analysis was performed. Additionally, CDDP
sensitivity and sphere formation assays were also performed in
cells treated with 740 Y-P for 48 h.
RNA isolation and RT-qPCR
Total RNA was isolated from A549, A549/CDDP, H460,
and H460/CDDP cells using Beyozol (Beyotime Institute of
Biotechnology) and subsequently reverse transcribed into cDNA using
the QuantiNova RT Kit according to the manufacturer's protocol
(Qiagen GmbH). qPCR was performed using the SYBR® Premix
DimmerEraser™ kit (Takara Bio, Inc.). The thermocycling
conditions were: 95°C for 30 sec; followed by 40 cycles of 95°C for
5 sec and 61°C for 30 sec. The expression of TRPC1 was assessed
using the 2−ΔΔCq method and GAPDH was used as the
internal control (17). The
sequences of the primers were: TRPC1 forward,
5′-ACCTTCCATTCGTTCATTGG-3′ and reverse, 5′-TGGTGAGGGAATGATGTTGA-3′;
and GAPDH forward, 5′-GAGTCCACTGGCGTCTTCAC-3′ and reverse,
5′-ATCTTGAGGCTGTTGTCATACTTCT-3′.
Western blot analysis
Total protein was extracted from cells using RIPA
lysis reagent supplemented with 1% PMSF (both from Wuhan Servicebio
Technology Co., Ltd.). The protein concentration was measured using
a BCA kit according to the manufacturer's protocol (Beyotime
Institute of Biotechnology). Subsequently, 40 µg total protein was
loaded per a lane on a BeyoGel™ Plus Precast PAGE Gel (Beyotime
Institute of Biotechnology), resolved using SDS-PAGE, and then
transferred to a nitrocellulose membrane (Wuhan Servicebio
Technology Co., Ltd.). Following blocking with 5% nonfat milk
(Beyotime Institute of Biotechnology), the membranes were incubated
with primary and secondary antibodies for 1 h at 37°C,
successively. Signals were visualized using the ECL-PLUS reagent
(Beyotime Institute of Biotechnology). The following antibodies
were used: Anti-TRPC1 (1:500; cat. no. DF12783; Affinity
Biosciences), anti-AKT (dilution, 1:500; cat. no. AF6261;
Affbiotech), anti-PI3K (1:500; cat. no. AF6241, Affbiotech),
anti-CD133 (1:1,000; cat. no. 51917; Cell Signaling Technology,
Inc.), anti-CD44 (1:1,000; cat. no. 37259; Cell Signaling
Technology, Inc.), anti-phospho (p)-PI3K (1:2,000; cat. no.
ab182651; Abcam), anti-p-AKT (1:1,000; cat. no. ab38449; Abcam),
anti-GAPDH (1:5,000; cat. no. GB15004; Wuhan Servicebio Technology
Co., Ltd.), and goat anti-rabbit secondary antibody (1:10,000; cat.
no. GB23303; Wuhan Servicebio Technology Co., Ltd.).
Sphere formation assay
Sphere formation assays in A549/CDDP and H460/CDDP
cells were performed 48 h after transfection. Briefly, cells at a
density of 1×103 cells/well were seeded into 6-well
ultra-low attachment plates (Corning, Inc.) and were then cultured
in spheroid medium, composed of DMEM/Nutrient Mixture F-12
(DMEM-F12; Gibco; Thermo Fisher Scientific, Inc.) containing
B-27™ Supplement (Thermo Fisher Scientific, Inc.), 20
ng/ml epidermal growth factor (EGF, MedChemExpress), 20 ng/ml basic
fibroblast growth factor (MedChemExpress), and 1%
penicillin/streptomycin solution (Beijing Solarbio Science &
Technology Co., Ltd.). Following 10 days of culture, the number of
formed spheres (diameter, >50 µm) was counted, and images were
captured under a light microscope with a magnification of ×200.
Statistical analysis
All data were compared using an unpaired Student's
t-test or a one-way ANOVA followed by a Tukey's post hoc test in
GraphPad Prism 7 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
TRPC1 is upregulated in chemoresistant
NSCLC cells
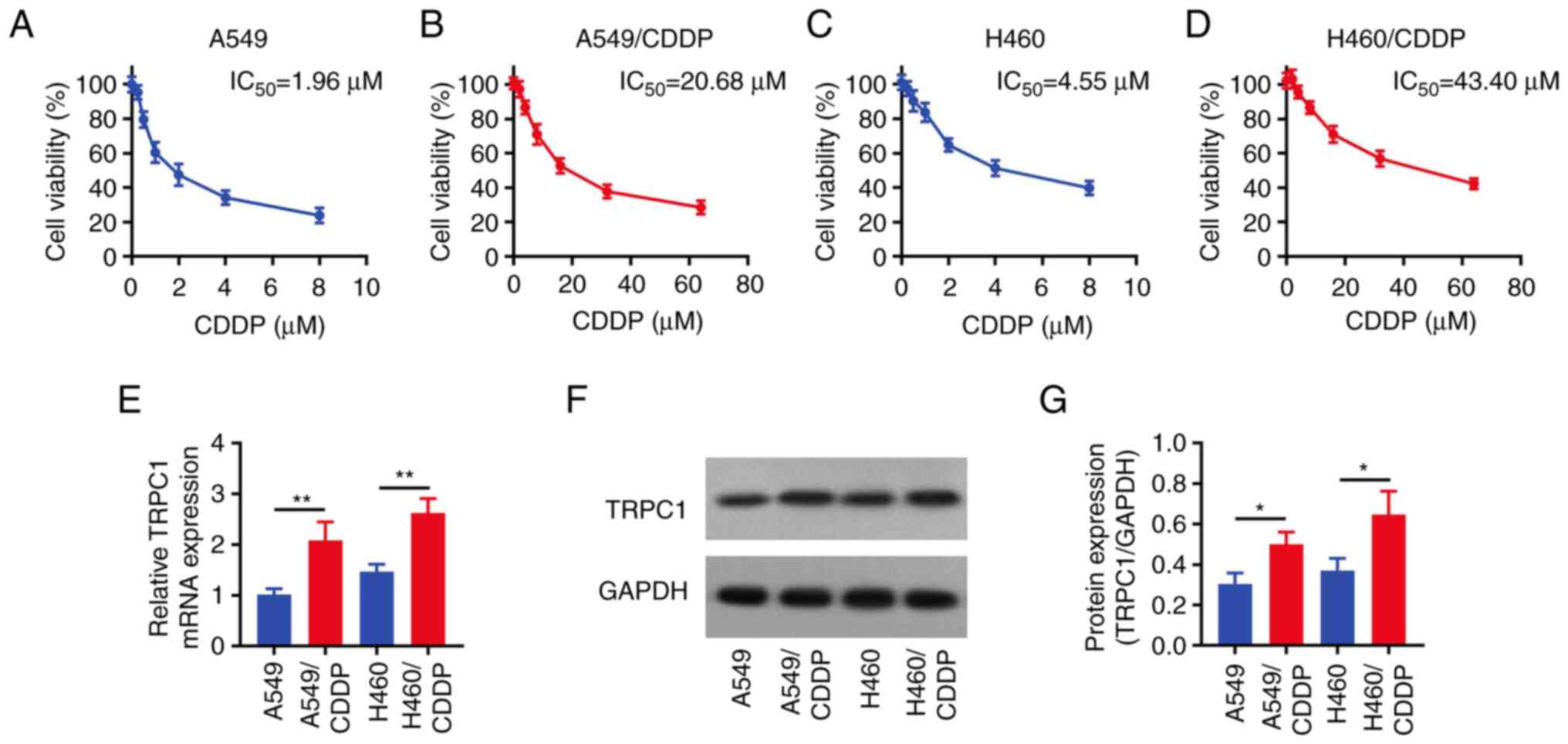
The IC50 values of CDDP were 1.96
(Fig. 1A), 20.68 (Fig. 1B), 4.55 (Fig. 1C), and 43.40 µM (Fig. 1D) in parental A549 cells, A549/CDDP
cells, parental H460 cells, and H460/CDDP cells, respectively, thus
confirming that CDDP-resistant NSCLC cells were successfully
established. Subsequently, TRPC1 expression was detected in the
above cells and the results showed that the mRNA and protein
expression levels of TRPC1 were notably increased in A549/CDDP
cells compared with the parental A549 cells. Consistently, the same
trend was observed in H460/CDDP cells compared with the parental
H460 cells (all P<0.05; Fig.
1E-G).
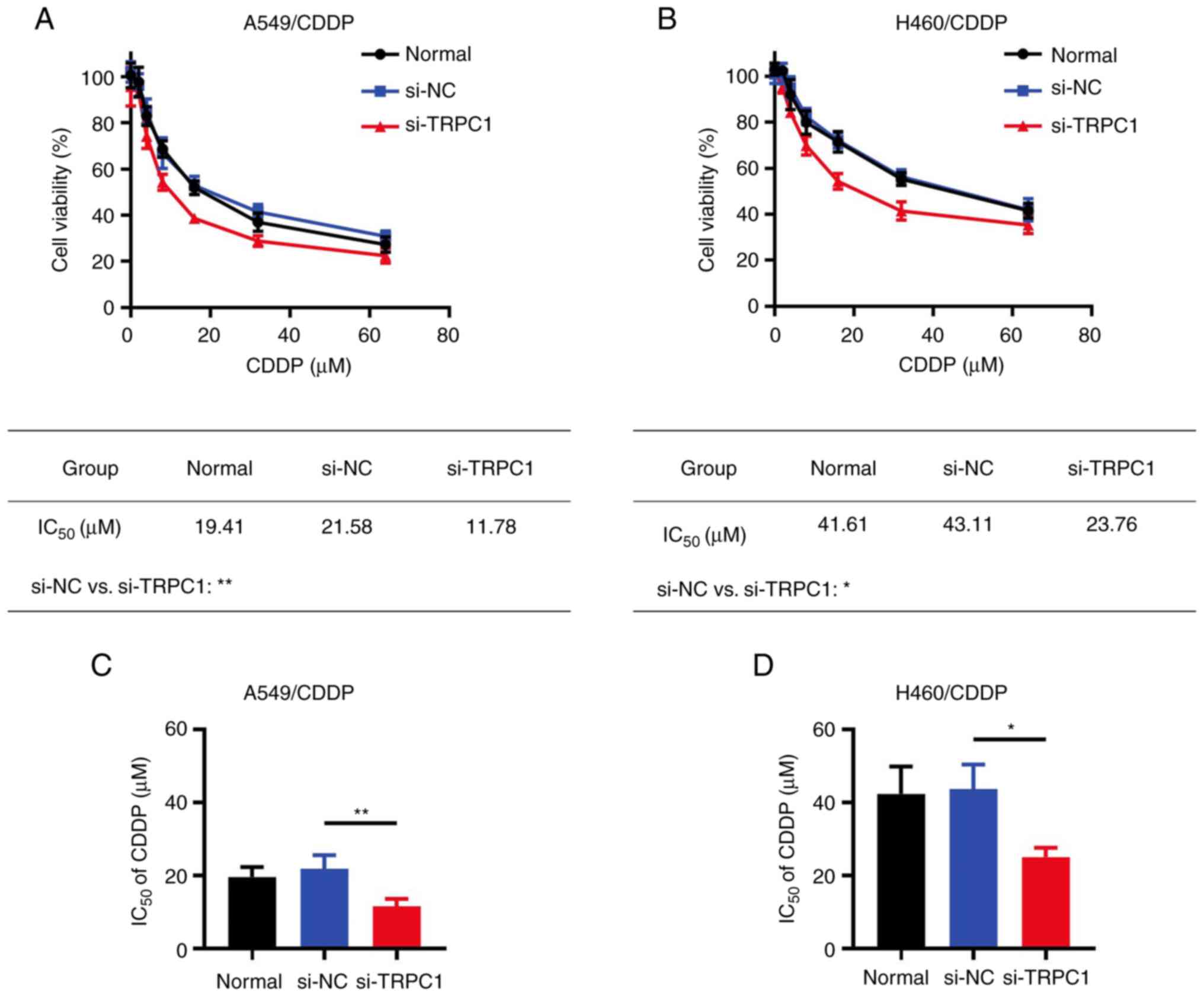
TRPC1 knockdown attenuates the
chemoresistance of NSCLC cells to CDDP
To further investigate the effect of TRPC1 knockdown
on NSCLC chemoresistance, A549/CDDP and H460/CDDP cells were
transfected with one of three siRNAs targeting TRPC1. RT-qPCR
(Fig. 2A and B) and western blot
analysis (Fig. 2C-E) revealed that
the si-TRPC1-2 construct exhibited the highest TRPC1 knockdown
activity in both A549/CDDP and H460/CDDP cells. Therefore,
si-TRPC1-2 was chosen for the subsequent experiments. The
IC50 value of CDDP was significantly reduced in
TRPC1-knockdown A549/CDDP cells compared with the si-NC group
(11.78 vs. 21.58 µM; P<0.01); this trend was also observed in
H460/CDDP cells (23.76 vs. 43.11 µM; P<0.05; Fig. 3A-D).
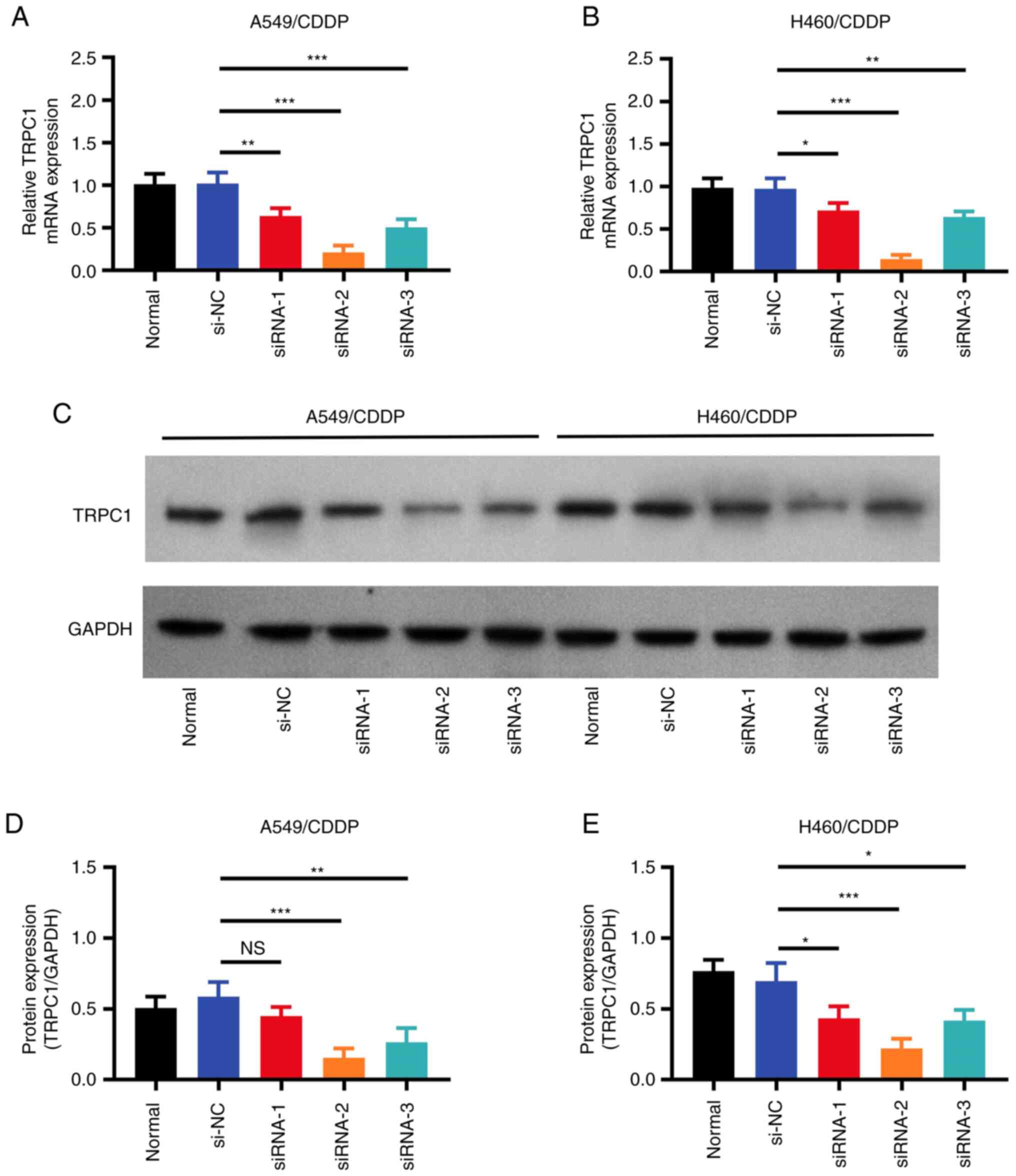 | Figure 2.Transfection efficiency of siRNAs.
Relative TRPC1 mRNA expression in the untransfected, si-NC, and
si-TRPC1 transfected (A) A549/CDDP and (B) H460/CDDP cells. (C)
Representative western blots of TRPC1 expression in the
untransfected, si-NC, and si-TRPC1 transfected NSCLC cells. (D)
Densitometry analysis of TRPC1 protein expression in the
untransfected, si-NC, and si-TRPC1 transfected A549/CDDP and (E)
H460/CDDP cells. *P<0.05, **P<0.01, ***P<0.001. NS, not
significant; TRPC1, transient receptor potential canonical 1; CDDP,
cis-Diamminedichloroplatinum; si, small interfering; NC, negative
control; NSCLC, non-small cell lung cancer. |
TRPC1 knockdown reduces NSCLC
stemness
The number of spheres formed was markedly decreased
in both the TRPC1-knockdown A549/CDDP (P<0.01; Fig. 4A and B) and H460/CDDP (P<0.05;
Fig. 4A and C) cells compared with
the respective si-NC group. Furthermore, in A549/CDDP cells, the
expression levels of CD133 (P<0.01) and CD44 (P<0.05) were
reduced in the si-TRPC1 group compared with the si-NC group
(Fig. 4D and E). However, only
CD133 expression was significantly downregulated in TRPC1-depleted
H460/CDDP cells (the transfection efficiency of si-TRPC1
transfected H460/CDDP cells was not sufficient) (P<0.05;
Fig. 4D and F).
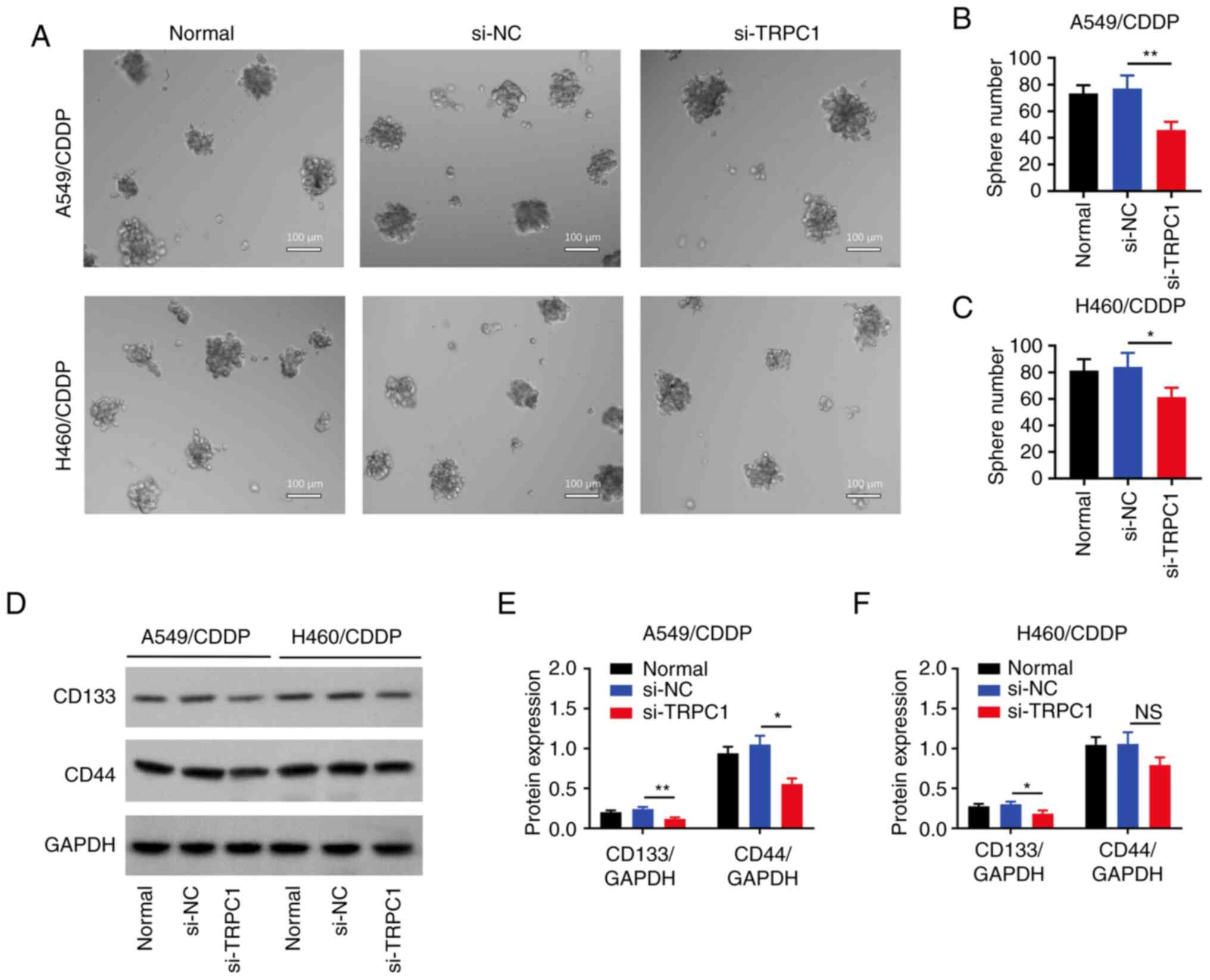 | Figure 4.Effect of si-TRPC1 on stemness. (A)
Representative images of the sphere formation assay in the
untransfected, si-NC, and si-TRPC1 transfected NSCLC cells. Number
of spheres formed in the untransfected, si-NC, and si-TRPC1
transfected (B) A549/CDDP and (C) H460/CDDP cells. (D)
Representative blots of CD133, CD44, and GAPDH expression in the
untransfected, si-NC, and si-TRPC1 transfected NSCLC cells.
Densitometry analysis of CD133 and CD44 expression in the
untransfected, si-NC, and si-TRPC1 transfected (E) A549/CDDP and
(F) H460/CDDP cells. *P<0.05, **P<0.01. TRPC1, transient
receptor potential canonical 1; CDDP, cis-Diamminedichloroplatinum;
si, small interfering; NC, negative control. |
TRPC1 knockdown reduces PI3K/AKT
signaling
TRPC1 knockdown reduced the p-PI3K/PI3K and
p-AKT/AKT ratios in both A549/CDDP (both P<0.05; Fig. 5A and B) and H460/CDDP cells (both
P<0.01; Fig. 5A and C) compared
with the respective si-NC group.
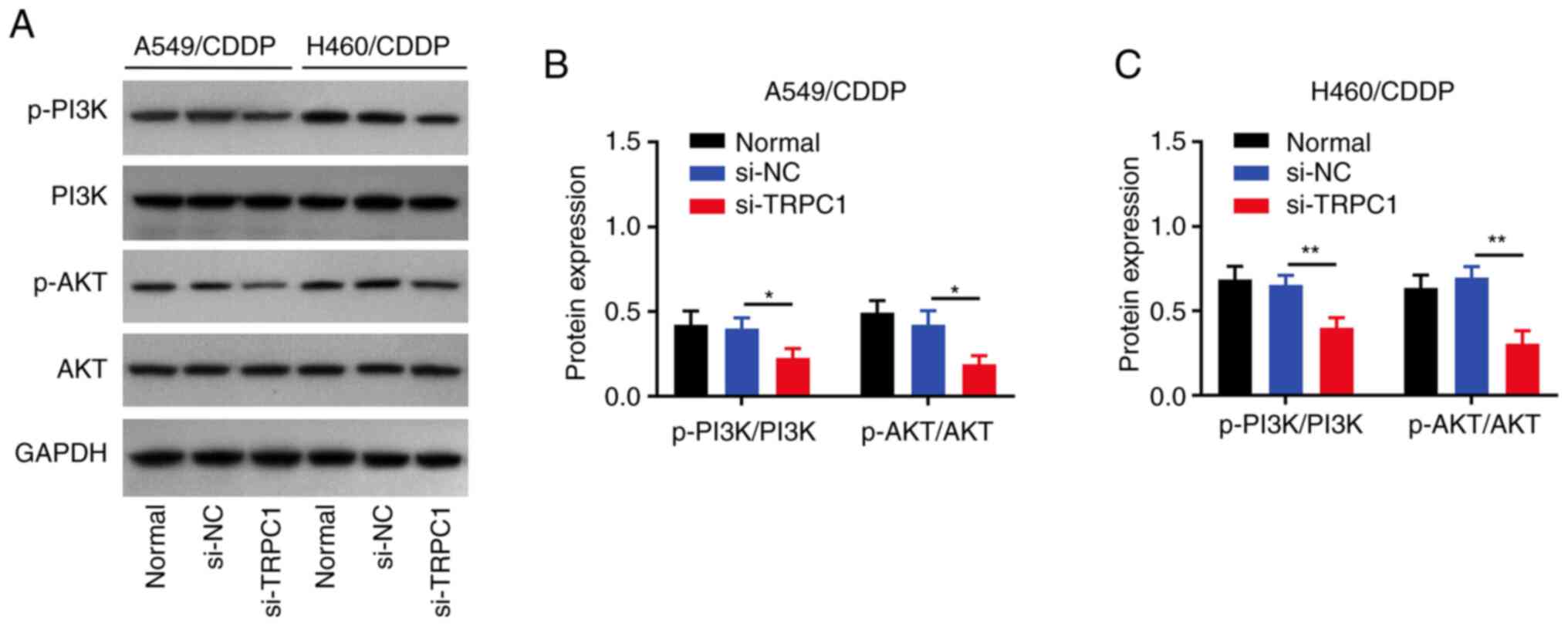 | Figure 5.Effect of si-TRPC1 on PI3K/AKT
signaling. (A) Representative blots of p-PI3K, PI3K, p-AKT, AKT,
and GAPDH expression in the untransfected, si-NC, and si-TRPC1
transfected NSCLC cells. Densitometry analysis of the p-PI3K/PI3K
and p-AKT/AKT ratios in the untransfected, si-NC, and si-TRPC1
transfected (B) A549/CDDP and (C) H460/CDDP cells (C). *P<0.05,
**P<0.01. TRPC1, transient receptor potential canonical 1; CDDP,
cis-Diamminedichloroplatinum; si, small interfering; NC, negative
control; p-, phospho; NSCLC, non-small cell lung cancer. |
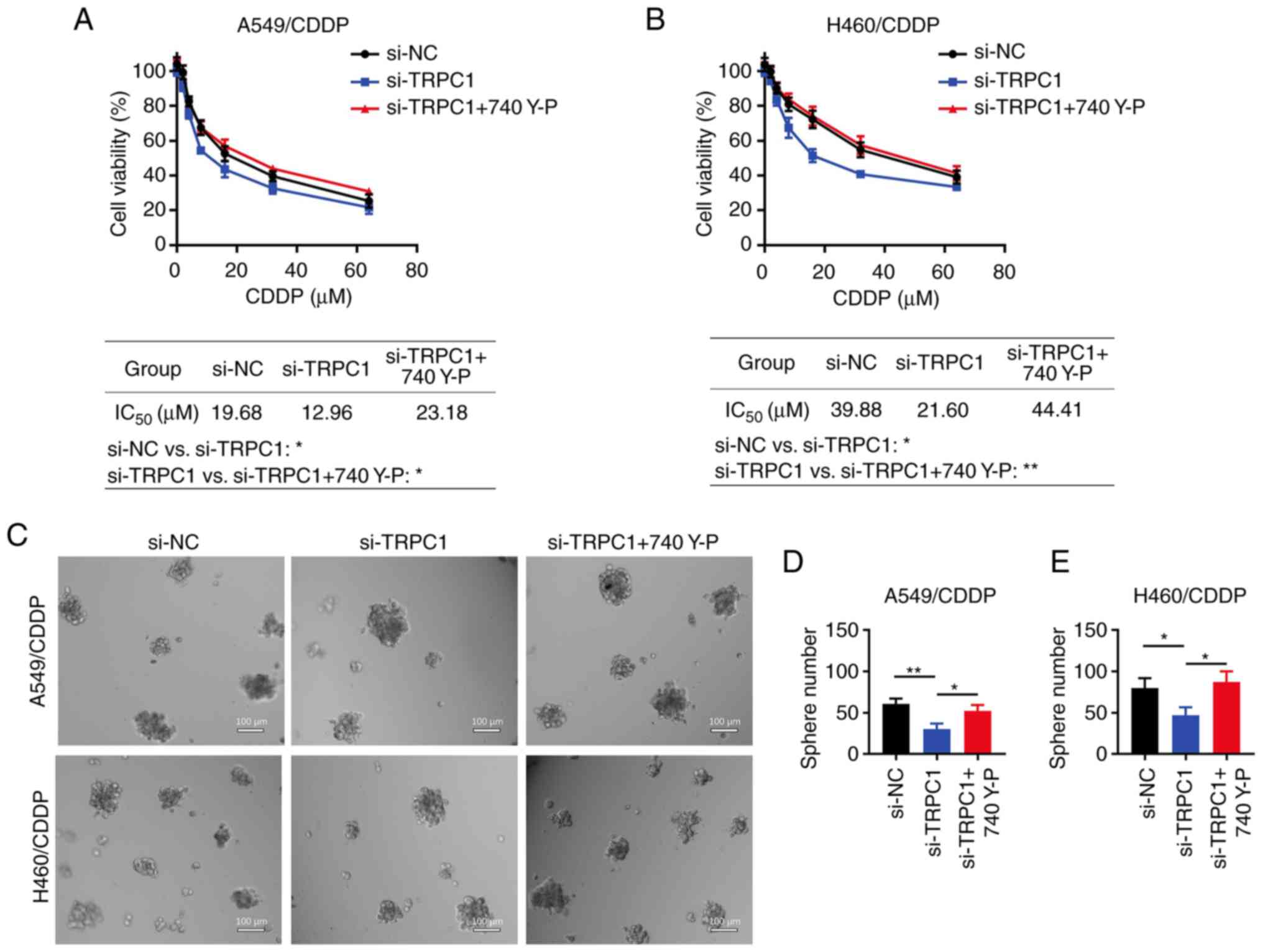
Cell treatment with 740 Y-P alleviates
the effect of TRPC1 knockdown on NSCLC cell chemoresistance and
stemness
A549/CDDP (both P<0.01; Fig. 6A and B) and H460/CDDP (both
P<0.001; Fig. 6A and C) cell
treatment with 740 Y-P increased the p-PI3K/PI3K and p-AKT/AKT
ratios, which were previously decreased by TRPC1 knockdown.
Additionally, treatment with 740 Y-P increased the IC50
values of CDDP in si-TRPC1-transfected A549/CDDP cells (23.18 vs.
12.96 µM; P<0.05; Fig. 7A).
Similar results were observed in the H460/CDDP cells (44.41 vs.
21.60 µM; P<0.01; Fig. 7B).
Finally, the number of spheres formed was increased in the
TRPC1-knockdown A549/CDDP (P<0.05; Fig. 7C and E) and H460/CDDP cells
(P<0.05; Fig. 7D and E) treated
with 740 Y-P.
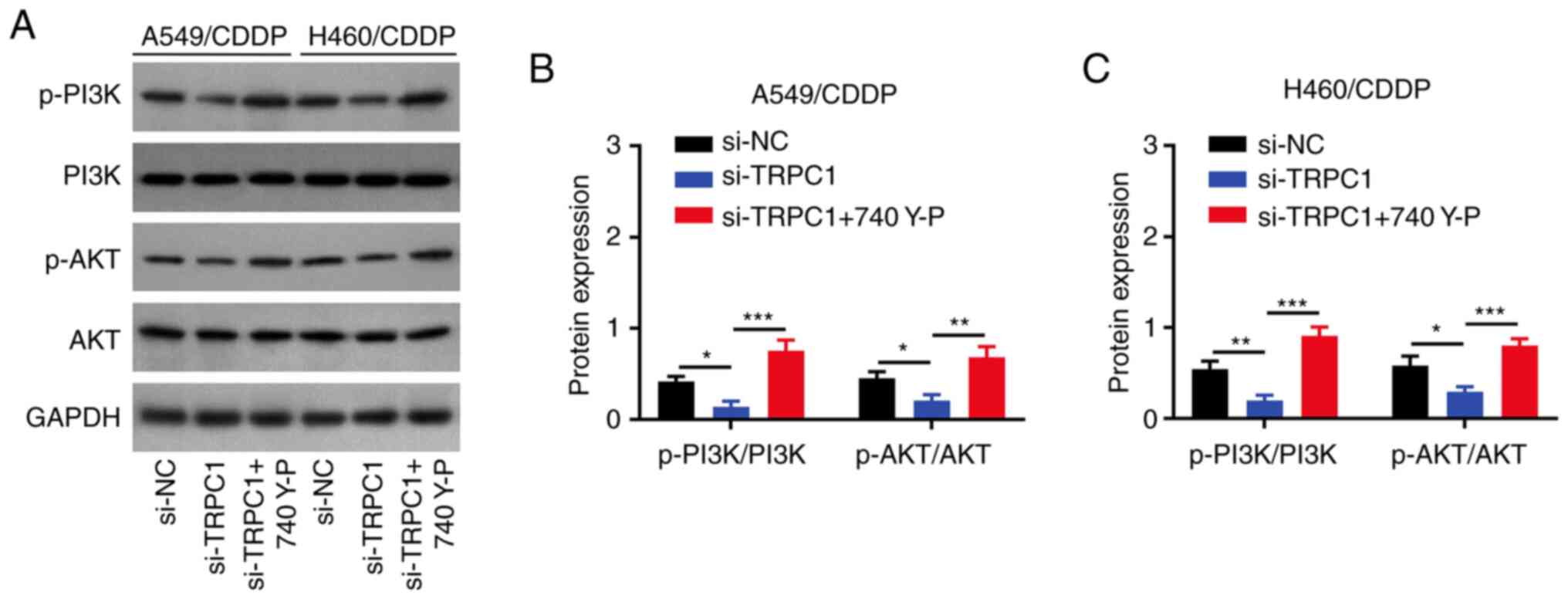 | Figure 6.Effect of 740 Y-P and TRPC1 siRNA on
PI3K/AKT signaling. (A) Representative blots of p-PI3K, PI3K,
p-AKT, AKT, and GAPDH expression in the si-NC transfected NSCLC
cells as well as si-TRPC1 transfected NSCLC cells with and without
740 Y-P treatment. Densitometry analysis of the p-PI3K/PI3K and
p-AKT/AKT ratios in the (B) si-NC and si-TRPC1 transfected
A549/CDDP cells with and without 740 Y-P treatment, and (C) in the
si-NC transfected and si-TRPC1 transfected H460/CDDP cells with and
without 740 Y-P treatment. *P<0.05, **P<0.01, ***P<0.001.
TRPC1, transient receptor potential canonical 1; CDDP,
cis-Diamminedichloroplatinum; si, small interfering; NC, negative
control; p-, phospho; NSCLC, non-small cell lung cancer. |
Discussion
TRPC1, a member of the calcium channel family of
proteins, modulates several cellular functions including cell
proliferation, survival, and migration (18). The role of TRPC1 in cancer has been
gradually uncovered (19–23). More specifically, a study
illustrated that TRPC1 inhibited cell proliferation and invasion in
esophageal squamous cell carcinoma (19). Another study revealed that TRPC1
overexpression promoted the migration of human malignant glioma
cells (20). Additionally, a
previous study suggested that TRPC1 could exacerbate metastasis in
gastric cancer via regulation of a circular RNA-7/microRNA-135a-5p
axis (21). More importantly, the
regulatory role of TRPC1 in NSCLC has also been investigated; TRPC1
was shown to interact with EGFR and correspondingly facilitate the
proliferation of NSCLC cells (22).
In addition, another study indicated that TRPC1 could promote the
proliferation of NSCLC cells (23).
However, the effect of TRPC1 on NSCLC chemoresistance and stemness
has not been previously explored. Consequently, the present study
is the first to explore the regulatory effect of TRPC1 on NSCLC
chemoresistance and stemness, as well as to determine the
underlying mechanism.
Regarding the role of TRPC1 on chemoresistance, it
has been reported that TRPC1 modulates chemoresistance in several
types of cancer (24,25). A previous study demonstrated that
TRPC1 enhanced store-operated Ca2+ entry and was thus
involved in the resistance of breast cancer cells to
chemotherapeutic drugs, such as cisplatin, 5-fluorouracil and
paclitaxel (24). Another study
showed that TRPC1 could interact with stromal interaction molecule
1 and calcium release-activated calcium channel protein 1 to induce
chemoresistance in hepatocellular carcinoma (25). However, its effect on
chemoresistance in NSCLC cells remains unknown. In the current
study, TRPC1 was upregulated in chemoresistant NSCLC cells, while
TRPC1 knockdown restored chemosensitivity in CDDP-resistant NSCLC
cells. The above effects could be due to the fact that TRPC1
knockdown could prevent autophagy, which in turn could inhibit
tumor cell apoptosis mediated by chemotherapeutic drugs. Therefore,
TRPC1 silencing may decrease chemoresistance in NSCLC cells
(26,27). Secondly, TRPC1 knockdown could
inhibit the activity of CDK1 and CyclinB1, which are involved in
the G2 to the M phase transition of the cell cycle (28). Additionally, chemotherapeutic drugs,
such as CDDP, bind with DNA primarily during the G2/M phase
transition to exert a cytotoxic effect (29). Therefore, TRPC1 knockdown may
enhance chemosensitivity in chemoresistant NSCLC cells.
Cancer stemness plays a critical role in the
pathology of chemoresistance. It has been reported that the
microenvironment of cancer stem cells can promote chemoresistance
through several factors (30).
Regarding the effect of TRPC1 on cancer stemness, a previous study
reported that TRPC1 was associated with stemness in dental pulp
stem cells (31). Herein, TRPC1
knockdown decreased sphere formation and reduced the expression
levels of CD133 and CD44 in chemoresistant NSCLC cells. A possible
explanation could be that TRPC1 silencing could attenuate EMT via
inhibiting the PI3K/AKT signaling pathway. It has been reported
that EMT is associated with cancer stemness in several types of
cancer (32,33), suggesting that TRPC1 knockdown could
reduce cancer stemness in NSCLC cells. In addition, another
interesting finding of the present study was that there was no
difference in CD44/GAPDH expression between the si-NC transfected
H460/CDDP cells and si-TRPC1 transfected H460/CDDP cells, whereas
CD44/GAPDH was lower in the si-TRPC1 transfected A549/CDDP cells
compared with si-NC transfected A549/CDDP cells; a possible
explanation for this could be that: TRPC1 has a limited effect on
CD44 expression in H460 cells compared with A549/CDDP cells. Hence,
TRPC1 has a limited effect on CD44 expression in H460/CDDP cells
compared with that in A549/CDDP cells; however, this effect
requires further validation.
The effect of TRPC1 on PI3K/AKT signaling has been
previously investigated. TRPC1 promotes hypoxia-associated EMT via
activation of the PI3K/AKT signaling pathway in breast cancer cells
(11). Another study showed that
TRPC1 could enhance the resistance of colon cancer cells to drugs
by regulating PI3K/AKT signaling (29). However, whether these mechanisms
occur in NSCLC cells has not been determined. Herein, it was shown
that TRPC1 knockdown inhibited PI3K/AKT signaling. However, cell
treatment with 740 Y-P promoted PI3K/AKT signaling,
chemoresistance, and stemness in TRPC1-depleted chemoresistant
NSCLC cells. The above finding could be due to an increase in the
influx of Ca2+ from the extracellular environment, which
in turn may reverse the TRPC1 knockdown-mediated inhibition of
downstream AKT phosphorylation. That is, TRPC1 knockdown attenuated
the PI3K/AKT signaling pathway, further enhancing chemosensitivity
and attenuating the stemness of chemoresistant NSCLC cells
(34).
The current study has some limitations: i) There are
no data to show whether this mechanism is observed in vivo;
ii) Since TRPC1 expression was relatively high in CDDP-resistant
NSCLC cells, a TRPC1 overexpression plasmid may not exert any
notable effects on CDDP-resistant NSCLC cells, thus overexpression
plasmids were not used in the current study. However, experiments
where TRPC1 expression is overexpressed following knockdown may
have value to show the necessity and sufficiency of TRPC1
expression; iii) although some previous studies have already
investigated the regulatory role of TRPC1 on the proliferation and
differentiation of NSCLC cells (22,23),
the absence of these experiments to evaluate the effect of TRPC1 on
these phenotypes in NSCLC cells is a limitation of the present
study; iv) the absence of non-cancerous cell lines as a negative
control is a major limitation of the present study.
Collectively, the results of the current study
suggested that targeting TRPC1 could attenuate NSCLC stemness and
chemoresistance via inactivation of PI3K/AKT signaling; however,
further studies are required to determine if this effect is
observed in vivo.
Acknowledgments
Not applicable.
Funding
This study was supported by the Beijing Medical Award Foundation
of China (grant no. YXJL-2020-0785-0178).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG contributed to the conception and design of the
study. JJ and XY performed the experiment. JH was responsible for
the interpretation of data for the work. YZ, HZ, KZ and YW
contributed to the data acquisition, data analysis and data
interpretation. All authors contributed to the drafting/revising of
article. JH and JG confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodak O, Peris-Diaz MD, Olbromski M,
Podhorska-Okołów M and Dzięgiel P: Current landscape of non-small
cell lung cancer: Epidemiology, histological classification,
targeted therapies, and immunotherapy. Cancers (Basel).
13:47052021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah P, Sands J and Normanno N: The
expanding capability and clinical relevance of molecular diagnostic
technology to identify and evaluate EGFR mutations in
advanced/metastatic NSCLC. Lung Cancer. 160:118–126. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaft JE, Rimner A, Weder W, Azzoli CG,
Kris MG and Cascone T: Evolution of systemic therapy for stages
I–III non-metastatic non-small-cell lung cancer. Nat Rev Clin
Oncol. 18:547–557. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fadejeva I, Olschewski H and Hrzenjak A:
MicroRNAs as regulators of cisplatin-resistance in non-small cell
lung carcinomas. Oncotarget. 8:115754–115773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Graeff A, Slebos RJ and Rodenhuis S:
Resistance to cisplatin and analogues: Mechanisms and potential
clinical implications. Cancer Chemother Pharmacol. 22:325–332.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jing N, Gao WQ and Fang YX: Regulation of
formation, stemness and therapeutic resistance of cancer stem
cells. Front Cell Dev Biol. 9:6414982021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elzamzamy OM, Penner R and Hazlehurst LA:
The role of TRPC1 in modulating cancer progression. Cells.
9:3882020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baudel MASM, Shi J, Large WA and Albert
AP: Insights into activation mechanisms of store-operated TRPC1
channels in vascular smooth muscle. Cells. 9:1792020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van den Eynde C, De Clercq K, Van Bree R,
Luyten K, Annibali D, Amant F, Han S, Van Nieuwenhuysen E, Baert T,
Peeraer K, et al: TRP channel expression correlates with the
epithelial-mesenchymal transition and high-risk endometrial
carcinoma. Cell Mol Life Sci. 79:262021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tai YK, Chan KKW, Fong CHH, Ramanan S, Yap
JLY, Yin JN, Yip YS, Tan WR, Koh APF, Tan NS, et al: Modulated
TRPC1 expression predicts sensitivity of breast cancer to
doxorubicin and magnetic field therapy: Segue towards a precision
medicine approach. Front Oncol. 11:7838032022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Las Rivas J, Brozovic A, Izraely S,
Casas-Pais A, Witz IP and Figueroa A: Cancer drug resistance
induced by EMT: Novel therapeutic strategies. Arch Toxicol.
95:2279–2297. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan C, Jin L, Wang X, Li Y, Chun J, Boese
AC, Li D, Kang HB, Zhang G, Zhou L, et al: Inositol-triphosphate
3-kinase B confers cisplatin resistance by regulating
NOX4-dependent redox balance. J Clin Invest. 129:2431–2445. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian S, Lou L, Tian M, Lu G, Tian J and
Chen X: MAPK4 deletion enhances radiation effects and triggers
synergistic lethality with simultaneous PARP1 inhibition in
cervical cancer. J Exp Clin Cancer Res. 39:1432020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Liang J, Li H, Han J, Jiang J, Li
Y, Feng Z, Zhao R and Tian H: Oncogenic role of abnormal
spindle-like microcephaly-associated protein in lung
adenocarcinoma. Int J Oncol. 58:232021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nesin V and Tsiokas L: TRPC1. Handb Exp
Pharmacol. 222:15–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng YZ, Zhang YQ, Chen JY, Zhang LY, Gao
WL, Lin XQ, Huang SM, Zhang F and Wei XL: TRPC1 inhibits cell
proliferation/invasion and is predictive of a better prognosis of
esophageal squamous cell carcinoma. Front Oncol. 11:6277132021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bomben VC, Turner KL, Barclay TT and
Sontheimer H: Transient receptor potential canonical channels are
essential for chemotactic migration of human malignant gliomas. J
Cell Physiol. 226:1879–1888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Ren L, Zhao Q, Lu G, Ren M, Lu X,
Yin Y, He S and Zhu C: TRPC1 exacerbate metastasis in gastric
cancer via ciRS-7/miR-135a-5p/TRPC1 axis. Biochem Biophys Res
Commun. 529:85–90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tajeddine N and Gailly P: TRPC1 protein
channel is major regulator of epidermal growth factor receptor
signaling. J Biol Chem. 287:16146–16157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang HN, Zeng B, Zhang Y, Daskoulidou N,
Fan H, Qu JM and Xu SZ: Involvement of TRPC channels in lung cancer
cell differentiation and the correlation analysis in human
non-small cell lung cancer. PLoS One. 8:e676372013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hasna J, Hague F, Rodat-Despoix L, Geerts
D, Leroy C, Tulasne D, Ouadid-Ahidouch H and Kischel P: Orai3
calcium channel and resistance to chemotherapy in breast cancer
cells: The p53 connection. Cell Death Differ. 25:693–707. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang BD, Xia X, Lv XF, Yu BX, Yuan JN, Mai
XY, Shang JY, Zhou JG, Liang SJ and Pang RP: Inhibition of
Orai1-mediated Ca2+ entry enhances chemosensitivity of
HepG2 hepatocarcinoma cells to 5-fluorouracil. J Cell Mol Med.
21:904–915. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sukumaran P, Sun Y, Antonson N and Singh
BB: Dopaminergic neurotoxins induce cell death by attenuating
NF-κB-mediated regulation of TRPC1 expression and autophagy. FASEB
J. 32:1640–1652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Usman RM, Razzaq F, Akbar A, Farooqui AA,
Iftikhar A, Latif A, Hassan H, Zhao J, Carew JS, Nawrocki ST and
Anwer F: Role and mechanism of autophagy-regulating factors in
tumorigenesis and drug resistance. Asia Pac J Clin Oncol.
17:193–208. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma S, Kong D, Fu X, Liu L, Liu Y, Xue C,
Tian Z, Li L and Liu X: p53-induced autophagy regulates
chemotherapy and radiotherapy resistance in multidrug resistance
cancer cells. Dose Response. 19:155932582110480462021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barbato L, Bocchetti M, Di Biase A and
Regad T: Cancer stem cells and targeting strategies. Cells.
8:9262019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao W, Wang L, Huang H, Li X, Wang P, Mi
K, Cheng J, Liu H, Gu C, Huang L and Huang J: All-trans retinoic
acid reduces cancer stem cell-like cell-mediated resistance to
gefitinib in NSCLC adenocarcinoma cells. BMC Cancer. 20:3152020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan G, Liu Y, Shang L, Zhou F and Yang S:
EMT-associated microRNAs and their roles in cancer stemness and
drug resistance. Cancer Commun (Lond). 41:199–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu F, Liu XC, Li L, Ma CN and Zhang YJ:
Effects of TRPC1 on epithelial mesenchymal transition in human
airway in chronic obstructive pulmonary disease. Medicine
(Baltimore). 96:e81662017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zanou N, Schakman O, Louis P, Ruegg UT,
Dietrich A, Birnbaumer L and Gailly P: Trpc1 ion channel modulates
phosphatidylinositol 3-kinase/Akt pathway during myoblast
differentiation and muscle regeneration. J Biol Chem.
287:14524–14534. 2012. View Article : Google Scholar : PubMed/NCBI
|