Introduction
Cancer in the oral cavity is the sixth most common
type of malignancy worldwide, with approximately 275,000 cases
diagnosed annually (1). In Japan,
buccal mucosa squamous cell carcinoma (BMSCC) accounts for
approximately 10% of oral cancers (2). The buccal mucosa is a large component
of the oral cavity extending from the line of attachment between
the upper and lower alveolar ridges to the pterygomandibular raphe.
It is divided into the buccal mucosa, retromolar area,
buccal-alveolar sulcus, and lip mucosa (3).
Cervical lymph node metastasis (CLNM), which
significantly impacts the prognosis of patients with head and neck
cancers, is encountered in >20% of squamous cell carcinomas
(SCCs) (4). Tumor thickness has
been reported to be significantly associated with the presence or
absence of lymph node metastasis in BMSCC (5). Appropriate neck management in patients
with head and neck SCC is important because CLNM is the most
significant independent indicator for survival (6).
A new parameter, depth of invasion (DOI), was
introduced in the 8th edition of the Union for International Cancer
Control (UICC) guidelines, which is strongly correlated with CLNM
(7,8). DOI has been reported to be an
independent prognosticator of occult cervical metastasis,
recurrence, and disease-specific survival (DSS) with the literature
supporting an optimal cut-off depth of 4 mm for elective neck
dissection (END) (9,10). There is general agreement that END
is indicated when there is a high likelihood of occult, clinically
undetectable lymph node metastases, when the neck needs to be
entered for surgical treatment of the primary tumor, or when the
patient will be unavailable for regular follow-up (11). This study aimed to investigate the
risk factors for developing CLNM and the indications for END in
BMSCC.
Materials and methods
Patients
This retrospective study included patients with
BMSCC who underwent surgery at the Department of Oral and
Maxillofacial Surgical Oncology of Tokyo Medical and Dental
University between January 2008 and December 2017. Patients who
were previously treated at other hospitals or who underwent
radiotherapy were excluded. The Institutional Review Board of the
Faculty of Dental Hospital of Tokyo Medical and Dental University
approved this clinicopathological study, and written informed
consent was obtained from all of the patients (approval no.
D2015-600). The authors confirm that all experiments were conducted
in accordance with the relevant guidelines and regulations.
Data collection
The following data were collected and analyzed: sex,
age, primary lesion subsite, tumor/node/metastasis stage (UICC 8th
edition), clinical growth patterns, tumor differentiation,
lymphovascular invasion, perineural invasion, pathological DOI
(p-DOI), extent of tumor invasion, clinical outcomes, and
Yamamoto-Kohama mode of invasion (YK classification) (12). Tumor differentiation, lymphovascular
invasion, perineural invasion, mode of invasion, p-DOI, and extent
of tumor invasion were determined using tissue blocks with the
maximum cross-sectional area by two pathologists with more than 15
years of experience.
Treatment strategy
Resection of the primary tumor was performed with a
margin of at least 1 cm. If bone invasion was observed, we
performed marginal mandibulectomy or segmental mandibulectomy
together. END was performed in cN0 patients who underwent
reconstruction using a vascularized free flap. We performed
supraomohyoid neck dissection (SOHND) as END. Postoperative
treatment was performed in patients with positive margins,
pathological CLNM in at least four nodes, or the presence of
pathological extranodal extension (ENE), which may cause
dissemination to the surrounding tissues outside the neck
dissection area (13).
Postoperative radiotherapy was performed at a dose of 50 Gy in all
patients (13). If the renal
function was within the normal limits, platinum-based anticancer
agents (cisplatin 80–100 mg/m2, two courses) were
co-administered in combination with radiotherapy.
Statistical analysis
The primary endpoint was DSS. The follow-up period
was 8–135 months (mean, 58.3±34.2 months). The end of the follow-up
period was December 2019, and the median follow-up time was 58
months. Survival rates were calculated from the time of diagnosis
until the end of the follow-up period, and Kaplan-Meier curves were
plotted. Log-rank tests were used to determine statistical
differences between patients with and without CLNM. Receiver
operating characteristic (ROC) curve analysis was used to analyze
the optimal cut-off value of p-DOI in predicting CLNM. Univariate
analyses were performed using the χ2 test or Fisher's
exact test and the Mann-Whitney U non-parametric test, as
appropriate. Multivariate analysis was performed using multiple
logistic regression to determine significant independent predictive
risk factors for CLNM in BMSCC. All statistical analyses were
performed using PASW Statistics 18 software for Windows (SPSS
Japan, Tokyo, Japan). Statistical significance was set at
P<0.05.
Results
Subject for study
A total of 778 patients with primary oral SCC were
examined at our department during the study period, of whom 86
(11.1%) were diagnosed with BMSCC. Of these, 75 underwent surgery
for the primary lesion. Eleven patients who underwent radiotherapy
were excluded from the study.
Patient characteristics
Table I shows the
clinical and pathological characteristics of 75 patients who
underwent surgery. There were 43 males and 32 females, with a mean
age of 68.8 years (range, 26–89 years). Furthermore, 48 patients
presented with lesions in the buccal mucosa, 14 in the retromolar
area, 12 in the buccal-alveolar sulcus, and one in the lower lip
mucosa. In the clinical T stage (cT), cT2 was the most common
classification (48.0%). In the clinical N stage (cN), cN0 was the
most in 50 patients (66.7%), and cN (+) was 25 patients (33.3%).
Distant metastasis was not found in any patient (M0).
 | Table I.Clinical and pathological
characteristics of patients. |
Table I.
Clinical and pathological
characteristics of patients.
| Classification | Value (%) |
|---|
| Sex |
|
| Male | 43 (57.3) |
|
Female | 32 (42.7) |
| Age, years |
|
| Mean | 68.8 |
|
Range | 26-89 |
| Subsite |
|
| Buccal
mucosa | 48 (64.0) |
|
Retromolar area | 14 (18.7) |
|
Buccal-alveolar sulcus | 12 (16.0) |
| Lower lip
mucosa | 1 (1.3) |
| cT stage |
|
| T1 | 11 (14.7) |
| T2 | 36 (48.0) |
| T3 | 10 (13.3) |
|
T4a | 14 (18.7) |
|
T4b | 4 (5.3) |
| pT stage |
|
| T1 | 20 (26.7) |
| T2 | 27 (36.0) |
| T3 | 14 (18.7) |
|
T4a | 11 (14.6) |
|
T4b | 3 (4.0) |
| cN stage |
|
| N0 | 50 (66.7) |
| N1 | 10 (13.3) |
|
N2b | 12 (16.0) |
|
N2c | 1 (1.3) |
|
N3b | 2 (2.7) |
| pN stage |
|
| N0 | 24 (32.0) |
| N1 | 2 (2.7) |
|
N2a | 3 (4.0) |
|
N2b | 3 (4.0) |
|
N3b | 22 (29.3) |
| NX | 21 (28.0) |
| Clinical growth
patterns |
|
|
Superficial type | 18 (24.0) |
|
Exophytic type | 14 (18.7) |
|
Endophytic type | 43 (57.3) |
| Tumor
differentiation |
|
|
Well | 42 (56.0) |
|
Moderate | 27 (36.0) |
|
Poor | 6 (8.0) |
| Lymphovascular
invasion |
|
| No | 49 (65.3) |
|
Yes | 26 (34.7) |
| Perineural
invasion |
|
| No | 68 (90.7) |
|
Yes | 7 (9.3) |
| YK
classification |
|
| Grade
1 | 5 (6.7) |
| Grade
2 | 16 (21.3) |
| Grade
3 | 35 (46.7) |
| Grade
4C | 13 (17.3) |
| Grade
4D | 6 (8.0) |
| Adjuvant
therapy |
|
| No
adjuvant therapy | 54 (72.0) |
|
Chemoradiotherapy | 11 (14.7) |
|
Chemotherapy | 5 (6.7) |
|
Radiotherapy | 5 (6.7) |
Clinicopathological findings
In the pathological T stage (pT), pT1 increased
(26.7%). In the pathological N stage (pN), pT3b increased
significantly (29.3%). Of the clinical growth patterns, the
endophytic type was the most common in 43 patients (57.3%). There
were six poorly differentiated tumors (8.0%). Lymphovascular and
perineural invasion were found in 26 (34.7%) and seven (9.3%)
patients, respectively. Mode of tumor invasion was classified as
grade 4C in 13 patients (17.3%) and grade 4D in six patients (8.0%)
(8). Postoperative treatments were
performed in 21 patients (28%); 11 patients underwent
chemoradiotherapy, 5 received chemotherapy, and 5 underwent
radiotherapy.
Mode of CLNM
Neck dissection was performed at 51 sites among 49
patients (65.3%) as the initial treatment. Of the 49 patients, 25
with cN(+) disease underwent therapeutic neck dissection (level
I–V). The remaining 24 patients with cN0 disease underwent both
excision of the primary tumor and reconstruction using a
vascularized free flap, with SOHND performed as END. CLNM developed
subsequently in five patients (10%) with cN0 disease, who then
underwent neck dissection.
Histological CLNM was confirmed at 31 sites in 30
patients. The incidence of CLNM in BMSCC was 40% (30/75 patients).
The number of metastatic lymph nodes ranged from 1 to 10 (mean 3.2,
median 3), and ENE was found in 25 patients (83.3%). All but four
patients had lymph node metastasis at ipsilateral levels I–III.
Metastasis at level IB was noted in 29 patients (96.7%). The
remaining four patients had lymph node metastasis at the mandibular
node, buccinator node, lateral retropharyngeal lymph node, and
contralateral level IB and III nodes (Table II).
 | Table II.Mode of cervical lymph node
metastasis. |
Table II.
Mode of cervical lymph node
metastasis.
| Number and level of
metastatic lymph nodes | Patients with pN(+)
(n=30) |
|---|
| Number |
|
| 1 | 6 |
| 2 | 6 |
| 3 | 6 |
| ≥4 | 12 |
| Levela |
|
| Level
IA | 3 |
| Level
IB | 29 |
| Level
II | 17 |
| Level
III | 4 |
|
Othersb | 4 |
Risk factors for CLNM
The p-DOI ranged from 0–23.0 mm, with a mean of 5.3
mm and a median of 2.7 mm. Histopathologically, 31 patients (41.3%)
had tumor invasion to the submucosal tissue, 33 (44.0%) had
invasion to the buccinator muscle, and 11 (14.7%) had invasion to
the mandible. Extent of tumor invasion, p-DOI, and CLNM values are
shown in Table III. Among the 33
patients with buccinator muscle involvement of the tumor, 24
(72.7%) developed CLNM, which was significantly different from
those with submucosal tissue involvement and mandibular involvement
(P<0.001 and P<0.05, respectively). No significant difference
was found in the p-DOI of each group with different regions of
extent of tumor invasion upon comparison of their CLNM status
(P>0.05 for each group). The ROC curve indicated that the best
cut-off value for p-DOI in predicting CLNM was 1.9 mm (sensitivity
80%, specificity 55.5%, area under the curve 0.711). Univariate
analysis was used to evaluate the tumor classification, tumor
differentiation, clinical growth patterns, subsite, extent of tumor
invasion, p-DOI, lymphovascular invasion, perineural invasion, and
mode of invasion to investigate the risk factors for developing
CLNM. Of these, the significant predictors of CLNM were clinical
growth patterns, extent of tumor invasion, p-DOI, lymphovascular
invasion, perineural invasion, and the mode of invasion. Multiple
logistic regression analysis revealed that the extent of tumor
invasion and lymphovascular invasion were significant predictors of
CLNM (P<0.001 and P=0.011, respectively; Table IV), of which the extent of tumor
invasion was confirmed as the most predictive risk factor for CLNM
in patients with BMSCC.
 | Table III.Extent of tumor invasion, p-DOI, and
CLNM. |
Table III.
Extent of tumor invasion, p-DOI, and
CLNM.
|
|
|
| Mean p-DOI, mm |
|
|---|
|
|
|
|
|
|
|---|
| Extent of tumor
invasion | Incidence of CLNM,
% (n) | P-value | CLNM(+) (n=30) | CLNM(−) (n=45) | P-value |
|---|
| Submucosa
(n=31) | 9.7 (3) |
<0.001a | 1.2 | 1.1 | >0.05 |
| Buccinator muscle
(n=33) | 72.7 (24) |
| 8.5 | 8.8 | >0.05 |
| Mandible
(n=11) | 27.3 (3) |
<0.05b | 8.1 | 7.1 | >0.05 |
 | Table IV.Multiple logistic regression analysis
of CLNM. |
Table IV.
Multiple logistic regression analysis
of CLNM.
|
|
|
Multivariateb |
|---|
|
|
|
|
|---|
| Variables |
Univariatea P-value | P-value | OR (95% CI) |
|---|
| T classification
(T1-T2 vs. T3-T4) | 0.071 |
|
|
| Tumor
differentiation (well vs. moderate, poor) | 0.097 |
|
|
| Growth pattern
(superficial, exophytic vs. endophytic) | 0.002c | 0.992 | 1.0 (1.0-1.0) |
| Subsite (buccal
mucosa vs. others) | 0.222 |
|
|
| Extent of tumor
invasion (buccinator muscle vs. others) |
<0.001c |
<0.001a | 12.3
(2.8-53.2) |
| p-DOI (≥1.9 mm vs.
<1.9 mm) | 0.004c | 0.753 | 0.8 (0.2-4.0) |
| Lymphovascular
invasion (positive vs. negative) |
<0.001c | 0.011c | 6.3 (1.5-25.7) |
| Perineural invasion
(positive vs. negative) | 0.015c | 0.325 | 4.4 (0.2-85.3) |
| YK classification
(Grade 1, 2 vs. Grade 3, 4C, 4D) | 0.001c | 0.248 | 3.2 (0.5-22.8) |
Clinical outcomes
Local, regional, and locoregional recurrences were
observed in four, three, and two patients, respectively. The
recurrence rate of BMSCC was 12%. In local recurrences, three
patients underwent additional surgical treatment, and two were
salvaged. In regional recurrences, two patients underwent
additional surgical treatment and chemoradiotherapy, and both were
salvaged. Two patients with locoregional recurrences had a policy
of best supportive care. Furthermore, distant metastasis was
confirmed in five patients (6.7%), and all patients were pN(+) with
buccinator muscle invasion. Thus, total ten patients (13.3%) died
due to primary disease (local-related death in four,
cervical-related death in one and distal metastases-related death
in five), six patients (8.0%) died due to other diseases, and 59
patients (78.7%) achieved no-evidence-of-disease status.
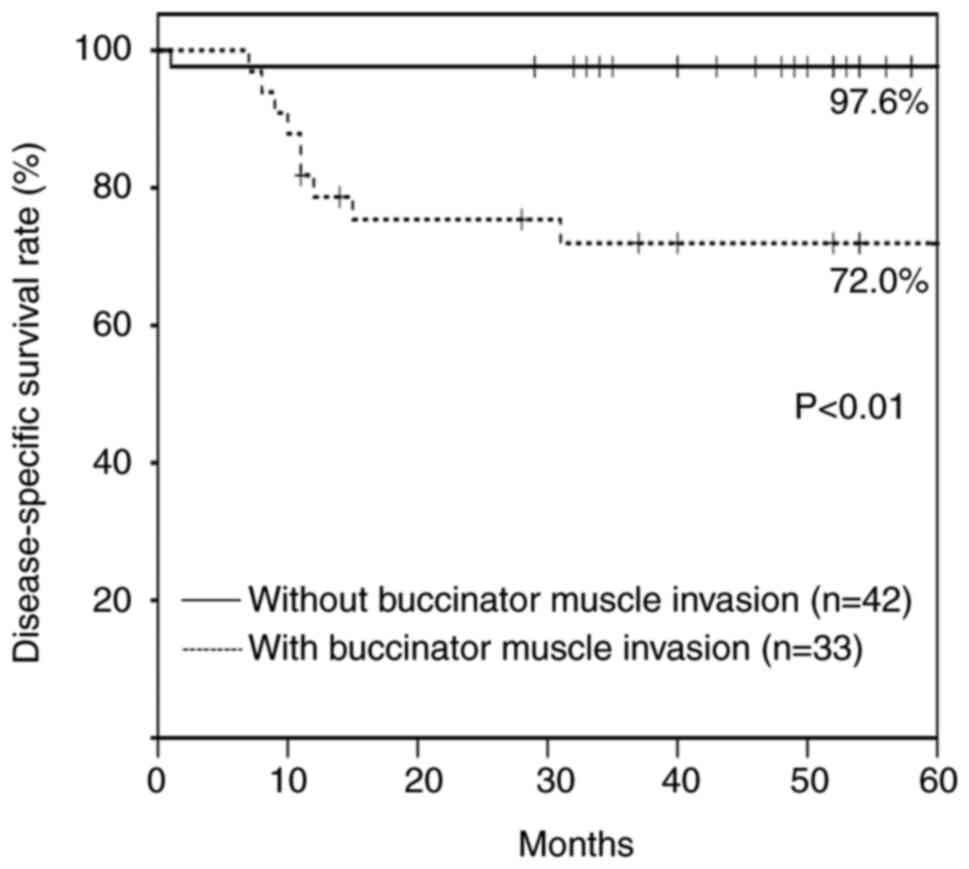
The cumulative overall and 5-year DSS were 78.8 and
86.5%, respectively. The cumulative 5-year DSS was significantly
different between patients without buccinator muscle invasion
(97.6%; n=42) and with buccinator muscle invasion (72.0%; n=33)
(P<0.01) (Fig. 1).
Discussion
The treatment of choice for BMSCC differs based on
the extent and location of the disease and geographic location
(14). In some areas of Southeast
Asia, radiotherapy is the treatment of choice. However, surgery is
the treatment of choice in Western countries (14,15).
In this study, 87% of patients (75/86 patients) underwent surgery,
and the remaining underwent radiotherapy.
Oral SCC has a strong tendency for developing CLNM,
which is well-known to be its most significant prognostic factor
(16). Therefore, CLNM has a
significant impact on treatment strategy and prognosis in patients
with BMSCC.
In this study, the incidence of CLNM was 40% (30/75
patients), which is consistent with previous reports (5,17). A
previous study reported that metastatic lymph nodes were most often
found at levels I–III (18). The
lymphatic system of the buccal mucosa drains primarily into the
submandibular space through collectors that pierce through the
buccinator muscle toward the facial artery and vein (19–21).
In this study, metastasis to level IB was confirmed in 96.7% of
patients with pN(+) (29/30 patients). Hence, the level IB node
should be carefully monitored in patients with BMSCC. The
buccinator and mandibular nodes were recognized as the other
metastatic sites by Tomioka et al (22), who reported that 0.4% of patients
with oral SCC had metastases at these sites. Additionally,
buccinator and mandibular node metastases were found in 1.9 and
5.8% of patients who had BMSCC without and with CLNM, respectively
(22). BMSCC is characterized by
being more likely to metastasize to these lymph nodes than other
oral SCCs. Therefore, treatment of these lymph nodes is important
for improving survival. These lymph nodes are usually outside the
dissection area in a typical neck dissection. When we performed
neck dissection to excise the primary tumor of the buccal mucosa,
we performed an en-bloc resection of the primary lesion and
the dissected tissue, including the adipose tissue surrounding the
facial artery and vein. Thus, we were able to dissect the lymphatic
tissue from the cheek to the neck, including the buccinator and
mandibular nodes. Lateral retropharyngeal lymph node metastasis was
identified in one patient. Oikawa et al (23) reported that the incidence of
retropharyngeal lymph node metastasis in patients with oral cancer
was 1.2%, and the prognosis was significantly poor. In this study,
no adhesions in the internal carotid artery were found in the
patient with retropharyngeal lymph node metastasis; hence, surgical
resection was performed. However, tumor recurrence occurred in the
neck.
Previous studies have reported that tumor thickness
is a more reliable predictor of CLNM (5,6). Ahmed
et al (5) reported that the
risk of CLNM in BMSCC increased in tumors with a thickness ≥2 mm.
Soni et al (24) also
reported that a DOI in SCC of the buccal-alveolar sulcus increased
the incidence of CLNM, but the trend was not statistically
significant. In this study, CLNM was found in three out of 31
patients (9.7%) with submucosal tissue invasion, three out of 11
patients (27.3%) with mandibular invasion, and 24 out of 33
patients (72.7%) with buccinator muscle invasion. These values were
significantly different from each other. This time, we focused on
the fact that the anatomical structure of the buccal mucosa differs
depending on the subsite. The submucosal group is superficial and
does not involve the buccinator muscle. The retromolar area
carcinoma can easily invade the mandible. As shown in Table III, the mandible group had a p-DOI
comparable to that of the buccinator muscle group, but there was a
clear and significant difference in CLNM. This indicates that the
extent of tumor invasion (i.e., buccinator muscle invasion), not
p-DOI, affects CLNM in BMSCC. Previous studies have reported that
lymphovascular invasion is a risk factor for CLNM in oral SCC
(25,26). In the multivariate analysis of this
study, lymphovascular and buccinator muscle invasion showed
significant differences, but buccinator muscle invasion was found
to be a more significant predictive risk factor for CLNM in BMSCC.
In addition, buccinator muscle invasion can be evaluated
preoperatively using computed tomography, magnetic resonance
imaging, and ultrasound, and it is a useful factor in determining
treatment strategy.
D'Cruz et al (27) reported the significance of
performing END for clinical stage I/II disease (T1-2N0). However,
it may be unnecessary in approximately 70% of patients without
metastasis (28). Okura et
al (29) reported that END was
recommended if the probability of occult metastasis was >44.4%.
In the present study, 24 out of 33 patients (72.7%) with buccinator
muscle involvement had CLNM. Hence, END should be performed if
buccinator muscle invasion is clinically identified.
In the previous studies, the 5-year survival rates
for BMSCC have been reported to range from 54.1-74.5% (30–32).
In this study, the cumulative 5-year DSS was 86.5%, which is more
favorable than that reported in previous studies. Among 10 deaths
from primary disease, CLNM was identified in nine patients.
Metastases in multiple regions were also found in seven patients.
The poor prognosis for patients with CLNM is consistent with that
reported in previous studies (33,34).
Since buccinator muscle invasion is an independent risk factor for
CLNM, in this study, the cumulative 5-year DSS rates were 97.6 and
72% for patients without and with buccinator muscle invasion,
respectively. Furthermore, five patients were found to have died
due to distant metastases. All five patients who died were in the
group with buccinator muscle invasion and CLNM. Hence, adjuvant
chemotherapy should be considered for patients with CLNM in
multiple regions.
The most significant limitation of this study was
the relatively small sample size and its retrospective nature.
Moreover, since the surrounding tissues in BMSCC differ according
to the subsite, it is difficult to measure the p-DOI of the
mandible and buccinator muscles in a standardized manner. For
prospective studies, it is necessary to classify tumors by subsite
and to include more cases to overcome these limitations.
We evaluated 75 patients with BMSCC who underwent
surgery. We found that tumor invasion of the buccinator muscle was
the most significant predictive risk factor for CLNM in BMSCC. The
survival rate of patients with BMSCC may be improved by performing
END in patients with buccinator muscle invasion and adjuvant
chemotherapy for patients with CLNM in multiple regions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HHi and HHa conceptualized this study. HHi, NN, TO,
TKug, TKur and YM curated and investigated the data. HHi, HT and YO
developed the statistical analysis plan and conducted statistical
analysis. KK and TI performed the pathological investigation. HT
and HHa supervised and organized this study. HHi wrote the first
draft of the manuscript. HHi and HHa reviewed and edited the
manuscript. HHi and HHa confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of the Faculty of
Dental Hospital of Tokyo Medical and Dental University approved
this clinicopathological study, and written informed consent was
obtained from all of the patients (approval no. D2015-600).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Japan Society for Head and Neck Cancer
Registry Committee, . Report of head and neck cancer registry of
Japan. Clinical statistics of registered patients. Jpn J Head Neck
Cancer. 32:15–34. 2016.
|
|
3
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
John Wiley & Sons; New York, NY: 2016
|
|
4
|
Pimenta Amaral TM, Da Silva Freire AR,
Carvalho AL, Pinto CA and Kowalski LP: Predictive factors of occult
metastasis and prognosis of clinical stages I and II squamous cell
carcinoma of the tongue and floor of the mouth. Oral Oncol.
40:780–786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmed SQ, Junaid M, Awan S, Choudhary MM,
Kazi M, Masoom A and Khan HU: Relationship of tumor thickness with
neck node metastasis in buccal squamous cell carcinoma: An
experience at a tertiary care hospital. Int Arch Otorhinolaryngol.
21:265–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Essig H, Warraich R, Zulfiqar G and Rana
M, Eckardt AM, Gellrich NC and Rana M: Assessment of cervical lymph
node metastasis for therapeutic decision-making in squamous cell
carcinoma of buccal mucosa: A prospective clinical analysis. World
J Surg Oncol. 10:2532012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tam S, Amit M, Zafereo M, Bell D and Weber
RS: Depth of invasion as a predictor of nodal disease and survival
in patients with oral tongue squamous cell carcinoma. Head Neck.
41:177–184. 2019.PubMed/NCBI
|
|
8
|
Faisal M, Abu Bakar M, Sarwar A, Adeel M,
Batool F, Malik KI, Jamshed A and Hussain R: Depth of invasion
(DOI) as a predictor of cervical nodal metastasis and local
recurrence in early stage squamous cell carcinoma of oral tongue
(ESSCOT). PLoS One. 13:e02026322018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larson AR, Kemmer J, Formeister E,
EI-Sayed I, Ha P, George J, Ryan W, Chan E and Heaton C: Beyond
depth of invasion: adverse pathologic tumor features in early oral
tongue squamous cell carcinoma. Laryngoscope. 130:1715–1720. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kallarakkal TG, Siriwardena BSMS,
Samaranayaka A, De Silva R and Tilakaratne WM: A validated
predictive model for risk of nodal metastasis in node negative oral
squamous cell carcinoma of the buccal mucosa and tongue. J Oral
Pathol Med. 51:436–443. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Bree R, Takes RP, Shah JP, Hamoir M,
Kowalski LP, Robbins KT, Rodrigo JP, Sanabria A, Medina JE, Rinaldo
A, et al: Elective neck dissection in oral squamous cell carcinoma:
Past, present and future. Oral Oncol. 90:87–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto E, Kohama G, Sunakawa H, Iwai M
and Hiratsuka H: Mode of invasion, bleomycin sensitivity, and
clinical course in squamous cell carcinoma of the oral cavity.
Cancer. 51:2175–2180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirai H, Ohsako T, Kugimoto T, Tomioka H,
Michi Y, Kayamori K, Yoda T, Miura M, Yoshimura R and Harada H:
Comparison of 50- and 66-Gy total irradiation doses for
postoperative cervical treatment of patients with oral squamous
cell carcinoma. Oral Oncol. 107:1047082020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bachar G, Goldstein DP, Barker E, Lea J,
O'Sullivan B, Brown DH, Gullane PJ, Gilbert RW, Xu W, Su J and
Irish JC: Squamous cell carcinoma of the buccal mucosa: Outcomes of
treatment in the modern era. Laryngoscope. 122:1552–1557. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin D, Bucci MK, Eisele DW and Wang SJ:
Squamous cell carcinoma of the buccal mucosa: A retrospective
analysis of 22 cases. Ear Nose Throat J. 87:582–586. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng CY, Sun FJ and Liu CJ: The influence
of cervical lymph node number of neck dissection on the prognosis
of the early oral cancer patients. J Dent Sci. 15:519–525. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moratin J, Metzgar K, Kansy K, Ristow O,
Engel M, Hoffmann J, Flechtenmacher C, Freier K, Freudlsperger C
and Horn D: The prognostic significance of the lymph node ratio in
oral cancer differs for anatomical subsites. Int J Oral Maxillofac
Surg. 49:558–563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoda N, Bc R, Ghosh S, Ks S, B VD and
Nathani J: Cervical lymph node metastasis in squamous cell
carcinoma of the buccal mucosa: A retrospective study on pattern of
involvement and clinical analysis. Med Oral Patol Oral Cir Bucal.
26:e84–e89. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Werner JA, Dünne AA and Myers JN:
Functional anatomy of the lymphatic drainage system of the upper
aerodigestive tract and its role in metastasis of squamous cell
carcinoma. Head Neck. 25:322–332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haagensen CD: The Lymphatics in Cancer.
1st edition. Saunders; Philadelphia, PA: 1972
|
|
21
|
Pan WR, Le Roux CM and Briggs CA:
Variations in the lymphatic drainage pattern of the head and neck:
Further anatomic studies and clinical implications. Plast Reconstr
Surg. 127:611–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomioka H, Mochizuki Y, Ohsako T, Hirai H,
Shimamoto H and Harada H: Buccinator and mandibular node metastases
in oral squamous cell carcinoma. J Oral Maxillofac Surg.
77:867–873. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oikawa Y, Michi Y, Tsushima F, Tomioka H,
Mochizuki Y, Kugimoto T, Osako T, Nojima H, Yokokawa M, Kashima Y
and Harada H: Management of retropharyngeal lymph node metastasis
in oral cancer. Oral Oncol. 99:1044712019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soni S, Soni TP and Patni N: Association
between nodal metastasis and histopathological factors in
postoperative gingivo-buccal complex squamous cell carcinoma: A
retrospective study. Gulf J Oncolog. 1:66–71. 2019.PubMed/NCBI
|
|
25
|
Bae MR, Roh JL, Kim JS, Choi SH, Nam SY
and Kim SY: Prediction of cervical metastasis and survival in cN0
oral cavity cancer using tumour 18F-FDG PET/CT
functional parameters. J Cancer Res Clin Oncol. 146:3341–3348.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spoerl S, Gerken M, Fischer R, Mamilos A,
Spoerl S, Wolf S, Pohl F, Klingelhöffer C, Ettl T, Reichert TE and
Spanier G: Lymphatic and vascular invasion in oral squamous cell
carcinoma: Implications for recurrence and survival in a
population-based cohort study. Oral Oncol. 111:1050092020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
D'Cruz AK, Vaish R, Kapre N, Dandekar M,
Gupta S, Hawaldar R, Agarwal JP, Pantvaidya G, Chaukar D, Deshmukh
A, et al: Elective versus therapeutic neck dissection in
node-negative oral cancer. N Engl J Med. 373:521–529. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanai N, Asakage T, Kiyota N, Homma A and
Hayashi R: Controversies in relation to neck management in N0 early
oral tongue cancer. Jpn J Clin Oncol. 49:297–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okura M, Aikawa T, Sawai NY, Iida S and
Kogo M: Decision analysis and treatment threshold in a management
for the N0 neck of the oral cavity carcinoma. Oral Oncol.
45:908–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pandey M, Bindu R and Soumithran CS:
Results of primary versus salvage surgery in carcinoma of the
buccal mucosa. Eur J Surg Oncol. 35:362–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singhania V, Jayade BV, Anehosur V,
Gopalkrishnan K and Kumar N: Carcinoma of buccal mucosa: A site
specific clinical audit. Indian J Cancer. 52:605–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bobdey S, Sathwara J, Jain A, Saoba S and
Balasubramaniam G: Squamous cell carcinoma of buccal mucosa: An
analysis of prognostic factors. South Asian J Cancer. 7:49–54.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wan XC, Egloff AM and Johnson J:
Histological assessment of cervical lymph node identifies patients
with head and neck squamous cell carcinoma (HNSCC): Who would
benefit from chemoradiation after surgery? Laryngoscope.
122:2712–2722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lo WL, Kao SY, Chi LY, Wong YK and Chang
RCS: Outcomes of oral squamous cell carcinoma in Taiwan after
surgical therapy: Factors affecting survival. J Oral Maxillofac
Surg. 61:751–758. 2003. View Article : Google Scholar : PubMed/NCBI
|