Introduction
Lung cancer is one of the most common malignancies
worldwide and its incidence and mortality rate are both the highest
among cancers in China (1).
Approximately half of all patients with lung cancer suffer from
metastatic disease at the time of diagnosis, with the most common
metastatic regions being lymph nodes, liver, adrenal gland, bone
and brain (2). However, gastric
metastasis is uncommon with a prevalence of 0.1-6.8% according to
clinical and autoptic results (3–5). In
most cases, gastric metastases are discovered at an advanced stage
when patients experience symptoms, such as abdominal pain, anemia
or vomiting, due to perforation, bleeding or obstruction caused by
the metastatic mass (6,7), which usually predict poor prognosis;
the average time between discovery of gastrointestinal metastasis
and death ranges from 96.5 to 130.3 days, as reported in certain
case series (8,9). Asymptomatic and diminutive gastric
metastases are rarely found in the clinic and the manifestations of
gastric metastases from lung cancer under chromoendoscopy and
magnifying endoscopy have not been reported in the literature, to
the best of our knowledge; furthermore, although there are certain
reports regarding the systemic treatment outcome of primary lung
cancer with gastric metastasis, which mainly focus on the
therapeutic effect of primary tumor treatment (10,11),
the effect of systemic treatment on gastric metastatic lesions is
not well documented. In the two cases presented in the current
study, the gastric metastases from lung adenocarcinoma manifested
as diminutive nodules or erosion endoscopically and had certain
common characteristics under magnifying endoscopy with blue laser
imaging (BLI-ME). After systemic anticancer therapy, the gastric
metastases had effectively regressed to scars in both cases.
Case reports
Case 1
A 66-year-old female patient complained of shortness
of breath for >10 days. The chest computed tomography (CT) scan
at the local hospital indicated a pulmonary nodular lesion
measuring 2.9×2.7 cm in the right lower lobe, with mediastinal and
bilateral hilar enlarged lymph nodes. The patient was admitted to
the Cancer Hospital and Shenzhen Hospital, Chinese Academy of
Medical Sciences (Shenzhen, China) in May 2021. The common
bronchoscopy was normal, but endobronchial ultrasound-guided
transbronchial needle aspiration from lymph nodes of the 11R and 7
regions confirmed pulmonary adenocarcinoma. In addition to the
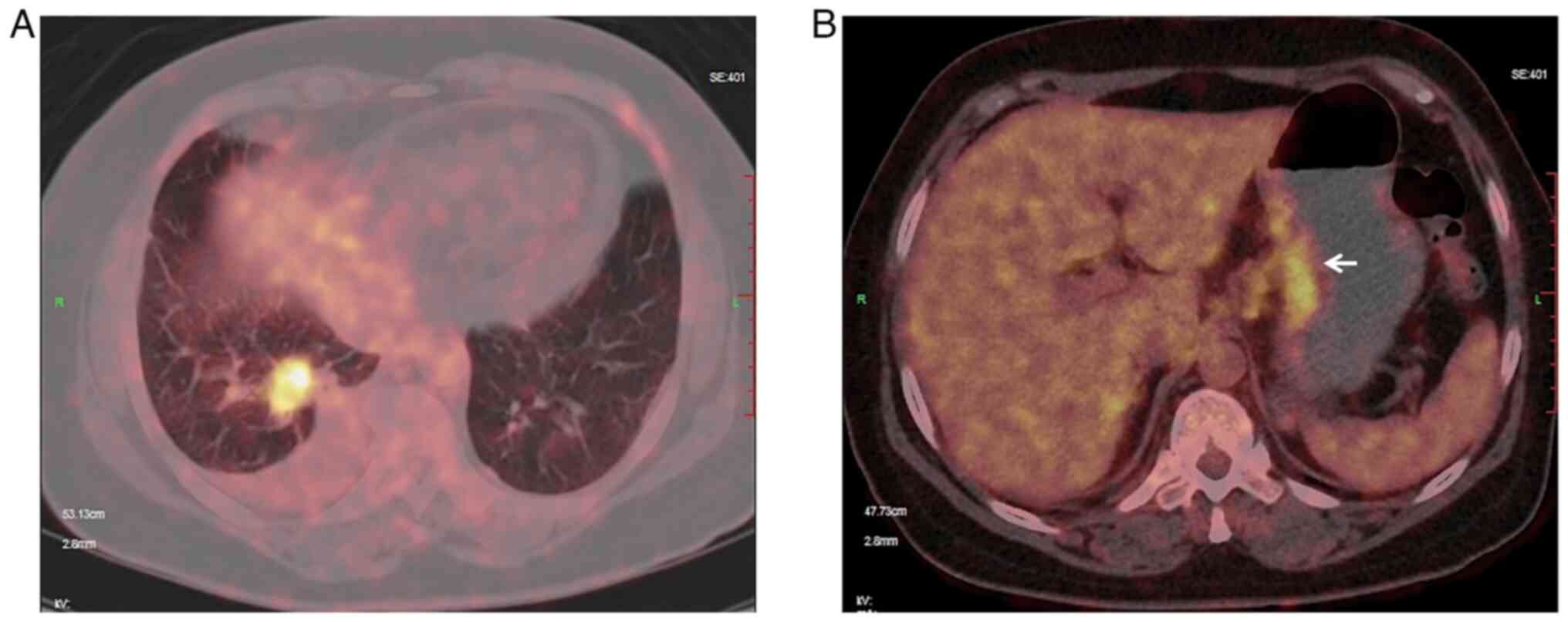
pulmonary tumor, positron emission tomography (PET)/CT revealed a
highly fluorodeoxyglucose (FDG)-up-taking lesion in the gastric
lesser curvature (Fig. 1).
Furthermore, lymph node and bone metastases were considered due to
high FDG uptake. Although the patient denied any abdominal
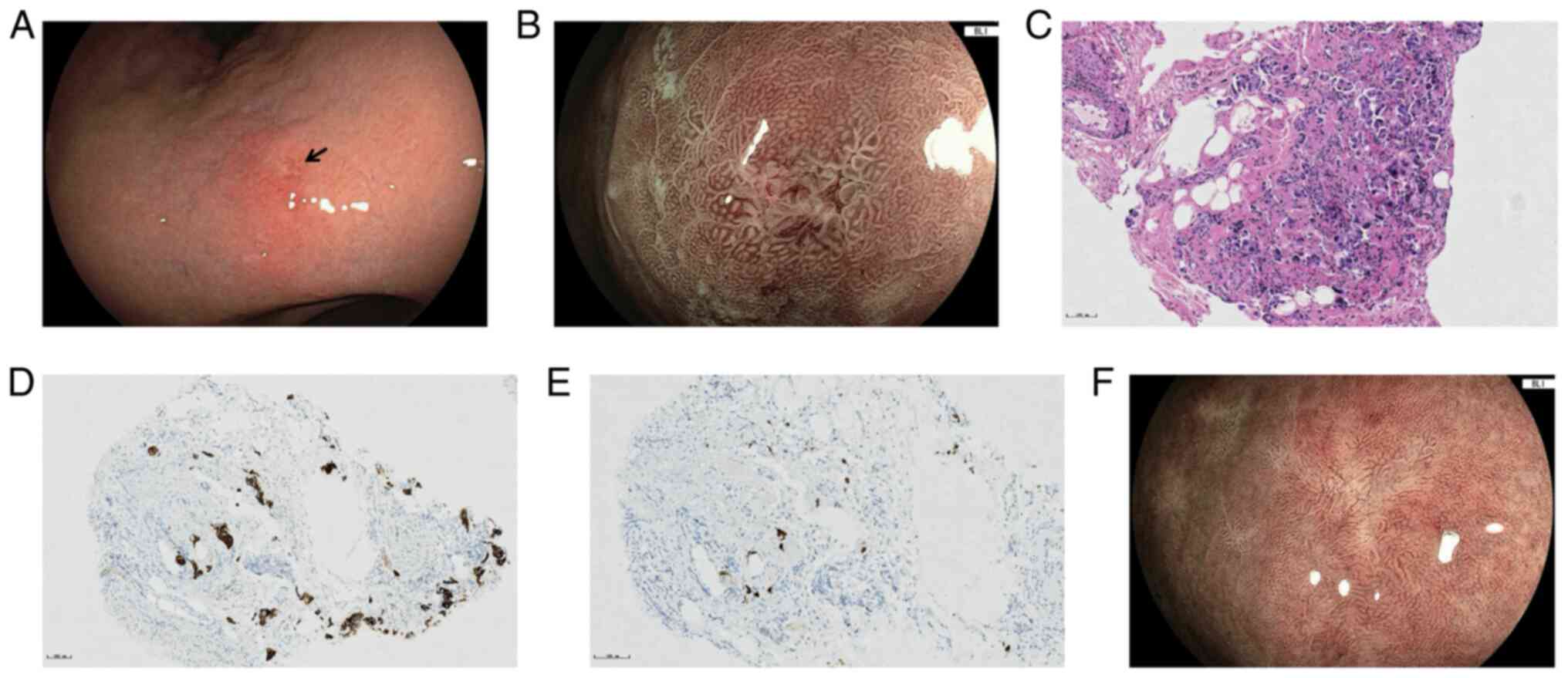
complaints, esophagogastroduodenoscopy (EGD) was recommended and a
diminutive erosion sized 5×4 mm was identified in the gastric
lesser curvature with slight swelling and redness of the
surrounding mucosa (Fig. 2A).
BLI-ME revealed heterogeneous morphology of the marginal crypt
epithelium and a widened intervening part (IP), the subepithelial
capillary network (SECN) was extended and a demarcation line was
recognized between the erosion and background mucosa (Fig. 2B). Biopsies were taken on the
erosion and hematoxylin-and-eosin (H&E) staining of the tissues
(according to standard procedures, the biopsy specimens were fixed
in 10% neutral-buffered formalin solution, embedded in paraffin,
serially cut into 3–4 mm-thick sections and stained with H&E)
revealed poorly differentiated carcinoma infiltration (Fig. 2C). Further immunohistochemical
staining performed with the Benchmark XT Staining Instrument
(Ventana Medical Systems, Inc.) indicated positivity for
cytokeratin 7 (CK7; rabbit monoclonal antibody; cat. no. 790-4462)
and thyroid transcriptional factor-1 (TTF-1; rabbit monoclonal;
cat. no. 790-4756) (Fig. 2D and E),
and negativity for cytokeratin 7 (CK20; rabbit monoclonal; cat. no.
790-4431) and caudal-related homeobox 2 (CDX-2; rabbit monoclonal;
cat. no. 760-4380; all from Ventana Medical Systems, Inc.), which
confirmed the pulmonary origin. Before the genetic test results
came out, the patient started the AP chemotherapy regimen
[Pemetrexed 0.8 g day (d)1, cisplatin 40 mg d1-3, every 3 weeks].
After 4 cycles of chemotherapy, the follow-up CT revealed shrinkage
of the primary lung tumor and lymph nodes, and EGD also found that
the gastric lesion had regressed to a white scar (Fig. 2F). However, MRI revealed newly
emerged brain metastases at the same time, and on the account of a
V600E mutation in the BRAF gene and PD-L1 TC-positive (80%) results
of genetic testing, the therapeutic strategy was switched to
targeted therapy and immunotherapy, and whole-brain radiotherapy
was also performed due to enlarged brain metastases in January
2022. At the time of writing, the patient was still under targeted
therapy (bevacizumab 400 mg, every 3 weeks) with regular follow-up
every month.
Case 2
A 76-year-old female experienced weight loss of 10
kg within half a year, falling below the normal Body Mass Index
range, accompanied by chest tightness and cough, without any
abdominal complaints. PET/CT at the local hospital indicated a
pulmonary mass in the right upper lobe with high FDG uptake,
together with multiple enlarged hilar and mediastinal lymph nodes,
and ilium and sigmoid colon metastases were also suspected. The
patient was admitted to the Cancer Hospital & Shenzhen
Hospital, Chinese Academy of Medical Sciences (Shenzhen, China) in
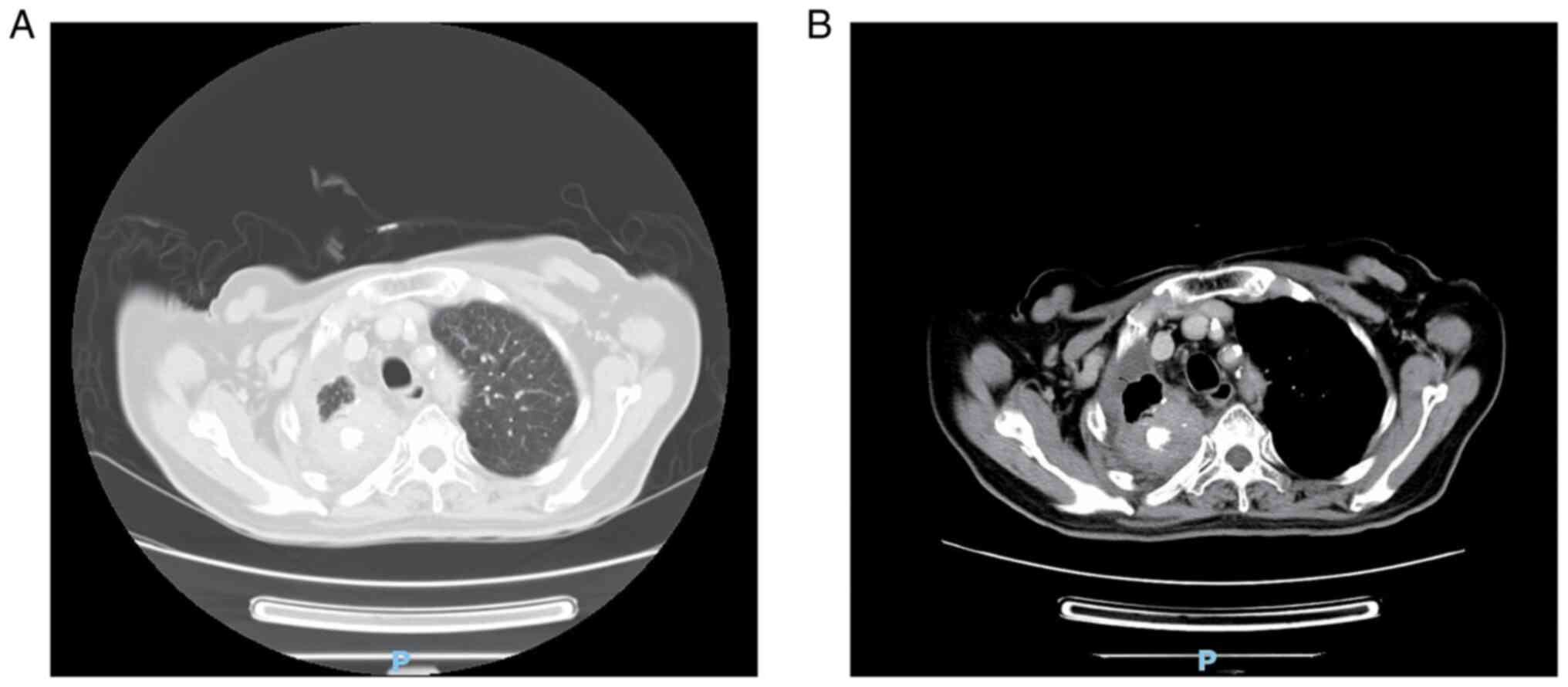
July 2021. CT indicated a pulmonary irregular mass with
calcification in the right upper lobe (maximum diameter, 5.0 cm)
(Fig. 3) and adenocarcinoma was
confirmed by CT-guided percutaneous lung biopsy. For colonic
lesions suspected by PET/CT, the patient received upper and lower
gastrointestinal endoscopy. The colonoscopy was unremarkable,
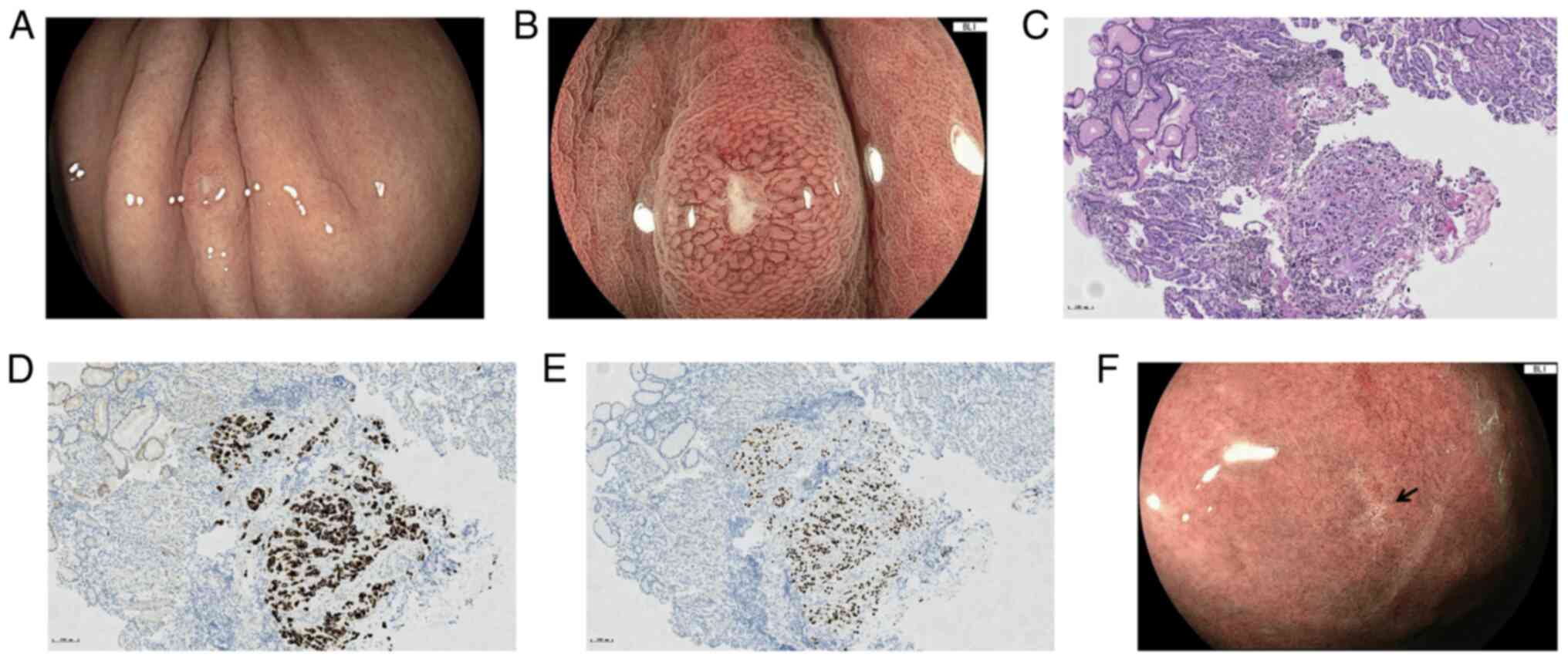
whereas EGD found a diminutive nodule with central depression sized
4×3 mm in the upper greater curvature of the stomach (Fig. 4A). BLI-ME revealed a homogeneously
extended SECN and widened IP around the depression, and within the
depression, the microvascular and microsurface structures were
absent, and the demarcation line between the nodule and background
was unclear (Fig. 4B). Biopsy of
the nodule revealed poorly differentiated carcinoma cells (Fig. 4C), and immunohistochemical staining
was positive for CK7 and TTF-1 (Fig. 4D
and E) but negative for CK20 and CDX-2, which confirmed the
pulmonary origin. Since gene tests of the lung lesion indicated a
positive L858R mutation in exon 21, the patient received targeted
therapy (gefitinib 250 mg, every day, bevacizumab 600 mg, every 3
weeks). The follow-up EGD indicated that the gastric lesion had
regressed to a scar after 3 months of treatment (Fig. 4F), and the pulmonary tumor remained
stable. Due to drug-induced liver injury and a rash, the patient
stopped taking medicine for 5 months on her own account, and
repeated CT indicated that the lung tumor had grown to a size
larger than that at the first presentation. The targeted agent was
then switched to Almonertinib (110 mg, qd) in May 2022 and the
patient tolerated it well, the last chest CT showed no enlargement
of the pulmonary tumor in October 2022.
Discussion
Gastric metastasis is uncommon for lung cancer in
the clinic. Metastatic lesions may develop at any site of the
stomach, mainly locating in the middle and upper thirds (11,12),
and solitary gastric metastases are more common than multiple
metastases (13). The endoscopic
manifestations of gastric metastases may be diverse and the most
common endoscopic appearance is submucosal mass with or without
ulceration, and the ulceration at the apex of the submucosal mass
usually be described as volcano-like or umbilicated (7,11).
Apart from that, multiple nodules, polypoid lesions or an
infiltrating ‘linitis plastica’ pattern have also been reported
(14,15).
The two cases reported in the present study are
gastric metastasis from lung adenocarcinoma without any related
symptoms. They presented as diminutive nodules or erosion under
conventional white-light endoscopy, which is different from the
common endoscopic appearance of gastric metastasis (6,10). In
addition, most of the literature only describes the manifestations
of gastric metastasis from lung cancer under white-light endoscopy
(6,14). The present study further reported
the characteristics of small gastric metastases under BLI-ME; the
microvascular and microsurface patterns of the covering mucosa may
be regular or irregular, and the demarcation line between lesion
and normal mucosa may exist or not correspond to the development
stage of metastases; however, in the present study, certain common
characteristics between the two cases were found under BLI-ME, such
as an obviously widened IP and extended SECN, indicating that
lesions developed beneath the superficial epithelium. These above
endoscopic characteristics may be explained by the growth pattern
of metastasis from a distant primary cancer; cancerous cells
traveling through the blood or lymph system usually disseminate
into the gastric submucosal layer first and then develop as a
submucosal lesion (12,16), with further growth it may break
through the covered epithelium and present as a central defection
of the mucosa, or even erosion and ulcer. Endoscopic
ultrasonography (EUS) has been widely used in the diagnosis and
management of gastrointestinal neoplasms, particularly in gastric
subepithelial lesions due to its excellent capability in evaluating
the originating layer, echo level and internal echo pattern
(17). As for gastric metastases,
EUS may identify the hypoechoic and demarcated mass originating
from the muscular or submucosal layer (12,18),
EUS-guided tissue acquisition and the following immunohistochemical
examination may provide a reliable method to identify gastric
metastases (19,20).
Since the endoscopic manifestations of gastric
metastasis may be diverse, it is at times difficult to
differentiate it from other gastric neoplasms; when the metastatic
lesions manifest as linitis plastica-like tumor or submucosal
tumor, it is easily confused with primary gastric cancer or gastric
gastrointestinal stromal tumor (GIST) (15,18).
In the two cases of the present study, the metastases were small
and less obvious, and they required to be differentiated from early
gastric cancer (EGC) and small submucosal tumors. EGC usually
demonstrates slight changes in color and surface morphology under
white-light endoscopy in the very early stage. Magnifying endoscopy
may contribute to improving diagnostic accuracy; according to the
‘VS classification system’ proposed by Yao et al (21), an irregular microvascular pattern
and/or an irregular microsurface pattern together with a clear
demarcation line under magnifying endoscopy are the hallmarks of
EGC. When considering a different origin, the ‘VS classification
system’ may not be applied to the diagnosis of gastric metastasis.
Gastric GIST is the common submucosal tumor of the stomach, which
usually has a smooth overlying mucosal surface under white-light
endoscopy without any microvascular or microsurface changes under
magnifying endoscopy; on EUS, it typically appears as hypoechoic
solid lesions arising from the muscle layer with a well-demarcated
border (22). Another kind of
gastric submucosal tumor is neuroendocrine tumors (NETs), which
generally present as multifocal polypoid protrusions or sporadic
nodular subepithelial lesions endoscopically, and on EUS, gastric
NETs appear as mucosal and/or submucosal well-edged isoecho or
hypoecho lesions (23). Further
differentiation may require histologic examination by biopsy or
EUS-guided tissue acquisition.
Target biopsy and further immunohistochemical
staining is the gold standard for distinguishing primary from
metastatic gastric tumor. The coordinated expression of CK7 and
CK20 in tumor cells is useful for distinguishing the origin of
carcinomas, and 90% of lung adenocarcinomas demonstrate a
CK7+/CK20-immunoprofile. On the other hand, intestinal carcinomas
generally have an CK7-/CK20+ immunophenotype (24). Other specific markers may further
aid the diagnosis: TTF-1 is positive in lung and thyroid cancer but
negative in gastric cancer, and it is accepted as a specific marker
for primary lung cancer (25,26).
CDX-2 is commonly expressed in intestinal-type adenocarcinomas from
the gastrointestinal tract and negative staining for CDX-2 further
supports the presence of metastasis from an extra-intestinal tumor
(27).
The correct diagnosis of distant gastric metastasis
may facilitate accurate staging of the primary tumor and earlier
detection may affect the treatment strategy. With the development
of targeted therapy and immunotherapy, the 5-year survival rate of
lung cancer has increased (28);
furthermore, with the rising popularization of gastrointestinal
endoscopy, more asymptomatic stomach metastases may be detected
early and prior to the appearance of related symptoms, which may
help physicians to comprehensively treat this condition and avoid
life-threatening events, such as severe bleeding or perforation due
to metastasis. According to the literature, certain patients with
solitary gastric metastasis may get a survival benefit from surgery
(4). However, when gastric
metastasis is identified, most patients may exhibit metastatic
lesions in other organs, and systemic treatments, including
chemotherapy, targeted therapy and immunotherapy, are major
strategies to manage them. In the two cases presented in the
current study, EGD revealed that the gastric metastatic lesions had
regressed to scars after systemic anticancer therapy. The systemic
treatment outcomes of primary lung cancer with gastric metastasis
have been previously reported, but the focus was on the therapeutic
effect on the primary tumor rather than the gastric metastatic
lesions (10). To the best of our
knowledge, only one previous study reported the systemic treatment
outcomes of gastric metastasis from lung cancer; the gastric
metastasis exhibited complete regression after 3 months of oral
treatment with Osimertinib (12).
This is in accordance with the cases of the present study and it
may suggest that gastric metastases respond well to systemic
treatment.
In conclusion, clinicians should direct their
attention to small nodules or erosion in gastric mucosa of patients
with confirmed primary cancer. Careful observation under
white-light endoscopy, chromoendoscopy combined with magnifying
endoscopy, may identify certain characteristics indicating a lesion
originates in the subepithelial layer. Target biopsy or EUS-guided
tissue acquisition in conjunction with further immunohistochemical
staining is the gold standard for distinguishing primary lesions
from metastases. Comprehensive anticancer treatment may be
effective for eliminating gastric metastatic lesions.
Acknowledgements
Not applicable.
Funding
The present study was supported by Sanming Project of Medicine
in Shenzhen (grant no. SZSM201911008) and China Cancer Foundation
Beijing Hope Marathon Special funds (grant no. LC2018A10).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MW conceived the study and drafted the manuscript.
WZ, CF and JG analyzed and interpreted the data. XN and FY
interpreted the data and critically revised the article. MW and FY
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for the publication of any
associated data and accompanying images was obtained from both
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao M and Chen W: Epidemiology of lung
cancer in China. Thorac Cancer. 10:3–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McNeill PM, Wagman LD and Neifeld JP:
Small bowel metastases from primary carcinoma of the lung. Cancer.
59:1486–1489. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taira N, Kawabata T, Gabe A, Furugen T,
Ichi T, Kushi K, Yohena T, Kawasaki H, Higuchi D, Chibana K, et al:
Analysis of gastrointestinal metastasis of primary lung cancer:
Clinical characteristics and prognosis. Oncol Lett. 14:2399–2404.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee PC, Lo C, Lin MT, Liang JT and Lin BR:
Role of surgical intervention in managing gastrointestinal
metastases from lung cancer. World J Gastroenterol. 17:4314–4320.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oda Kondo H, Yamao T, Saito D, Ono H,
Gotoda T, Yamaguchi H, Yoshida S and Shimoda T: Metastatic tumors
to the stomach: Analysis of 54 patients diagnosed at endoscopy and
347 autopsy cases. Endoscopy. 33:507–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altintas E, Sezgin O, Uyar B and Polat A:
Acute upper gastrointestinal bleeding due to metastatic lung
cancer: An unusual case. Yonsei Med J. 47:276–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green LK: Hematogenous metastases to the
stomach: A review of 67 cases. Cancer. 65:1596–1600. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH,
Han MS, Kim CH and Lee JC: Gastrointestinal metastasis of lung
cancer with special emphasis on a long-term survivor after
operation. J Cancer Res Clin Oncol. 135:297–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang
TH, Sheu CC, Tsai JR and Huang MS: Gastro-intestinal metastasis of
primary lung carcinoma: Clinical presentations and outcome. Lung
Cancer. 54:319–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan X, Zhao X and Wang S: An ALK-positive
lung adenocarcinoma with gastric and skin metastasis: A case report
and literature review. Ann Palliat Med. 10:5797–5807. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nitipir C, Ginghina O, Popa L, Andrei F,
Tudor N, Radu I, Iaciu C, Orlov C, Vasilescu F, Balalau C, et al: A
rare case of advanced lung cancer presenting as a symptomatic
gastric tumor. Mol Clin Oncol. 8:595–599. 2018.PubMed/NCBI
|
|
12
|
Tang D, Lv J, Liu Z, Zhan S and Gao Y:
Gastric metastasis of primary lung cancer: Case report and
systematic review with pooled analysis. Front Oncol. 12:9220162022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Palma GD, Masone S, Rega M, Simeoli I,
Donisi M, Addeo P, Iannone L, Pilone V and Persico G: Metastatic
tumors to the stomach: Clinical and endoscopic features. World J
Gastroenterol. 12:7326–7328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qasrawi A, Abu Ghanimeh M, Albadarin S and
Yousef O: Gastric metastases from lung adenocarcinoma causing
gastrointestinal bleeding. ACG Case Rep J. 4:e252017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okazaki R, Ohtani H, Takeda K, Sumikawa T,
Yamasaki A, Matsumoto S and Shimizu E: Gastric metastasis by
primary lung adenocarcinoma. World J Gastrointest Oncol. 2:395–398.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeda J, Miyake M, Tokita K, Iwahashi N,
Nakano T, Tamura S, Hada T and Higashino K: Small cell lung cancer
with extensive cutaneous and gastric metastases. Intern Med.
31:1325–1328. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Standards of Practice Committee, . Faulx
AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V,
Eloubeidi MA, Fanelli RD, Gurudu SR, et al: The role of endoscopy
in subepithelial lesions of the GI tract. Gastrointest Endosc.
85:1117–1132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sileri P, D'Ugo S, Del Vecchio Blanco G,
Lolli E, Franceschilli L, Formica V, Anemona L, De Luca C and
Gaspari AL: Solitary metachronous gastric metastasis from pulmonary
adenocarcinoma: Report of a case. Int J Surg Case Rep. 3:385–388.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamao K, Kitano M, Kudo M and Maenishi O:
Synchronous pancreatic and gastric metastasis from an ovarian
adenocarcinoma diagnosed by endoscopic ultrasound-guided
fine-needle aspiration. Endoscopy. 47 Suppl 1:UCTN. E596–E597.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antonini F, Laterza L, Fuccio L,
Marcellini M, Angelelli L, Calcina S, Rubini C and Macarri G:
Gastric metastasis from ovarian adenocarcinoma presenting as a
subepithelial tumor and diagnosed by endoscopic ultrasound-guided
tissue acquisition. World J Gastrointest Oncol. 9:452–456. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao K, Anagnostopoulos GK and Ragunath K:
Magnifying endoscopy for diagnosing and delineating early gastric
cancer. Endoscopy. 41:462–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rajravelu RK and Ginsberg GG: Management
of gastric GI stromal tumors: Getting the GIST of it. Gastrointest
Endosc. 91:823–825. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Toole D and Palazzo L: Endoscopy and
endoscopic ultrasound in assessing and managing neuroendocrine
neoplasms. Front Horm Res. 44:88–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chu PG and Weiss LM: Keratin expression in
human tissues and neoplasms. Histopathology. 40:403–439. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Merchant SH, Amin MB, Tamboli P, Ro J,
Ordóñez NG, Ayala AG, Czerniak BA and Ro JY: Primary signet-ring
cell carcinoma of lung: Immunohistochemical study and comparison
with non-pulmonary signet-ring cell carcinomas. Am J Surg Pathol.
25:1515–1519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheppard MN: Specific markers for
pulmonary tumours. Histopathology. 36:273–276. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rossi G, Marchioni A, Romagnani E,
Bertolini F, Longo L, Cavazza A and Barbieri F: Primary lung cancer
presenting with gastrointestinal tract involvement:
Clinicopathologic and immunohistochemical features in a series of
18 consecutive cases. J Thorac Oncol. 2:115–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thandra KC and Barsouk A, Saginala K,
Aluru JS and Barsouk A: Epidemiology of lung cancer. Contemp Oncol
(Pozn). 25:45–52. 2021.PubMed/NCBI
|