Introduction
Small cell carcinoma (SCC) is a high grade malignant
neuroendocrine tumor and is most commonly of pulmonary origin
(1). The existence of ‘real’ small
cell carcinoma of the thyroid gland is still debated, and in the
4th edition of the World Health Organization thyroid tumor
classification (2), it was not
listed as a distinct tumor type. However, intermittent reports of
primary thyroid small cell carcinoma with clinical findings
supporting thyroid origin and immunohistochemical results valid for
SCC have been reported (3–5).
Combined small-cell carcinoma is defined as SCC
combined with additional components that consist of any
histological type of non-small cell carcinoma. Combined SCC is
mostly reported in the lung, and head and neck, few cases of
combined SCCs have been previously reported in the larynx (6,7). To
the best of our knowledge, there has been no previous report of
combined SCC in the thyroid. Herein, a case of primary SCC combined
with PDTC in thyroid is reported.
Case report
A 34 year old male was admitted to Jeonbuk National
University Hospital (Jeonju, Republic of Korea) in September 2019,
because of a thyroid mass. The patient had undergone chemotherapy
and bone marrow transplantation 10 years previously as treatment
for T-cell lymphoblastic leukemia. Neck computed tomography showed
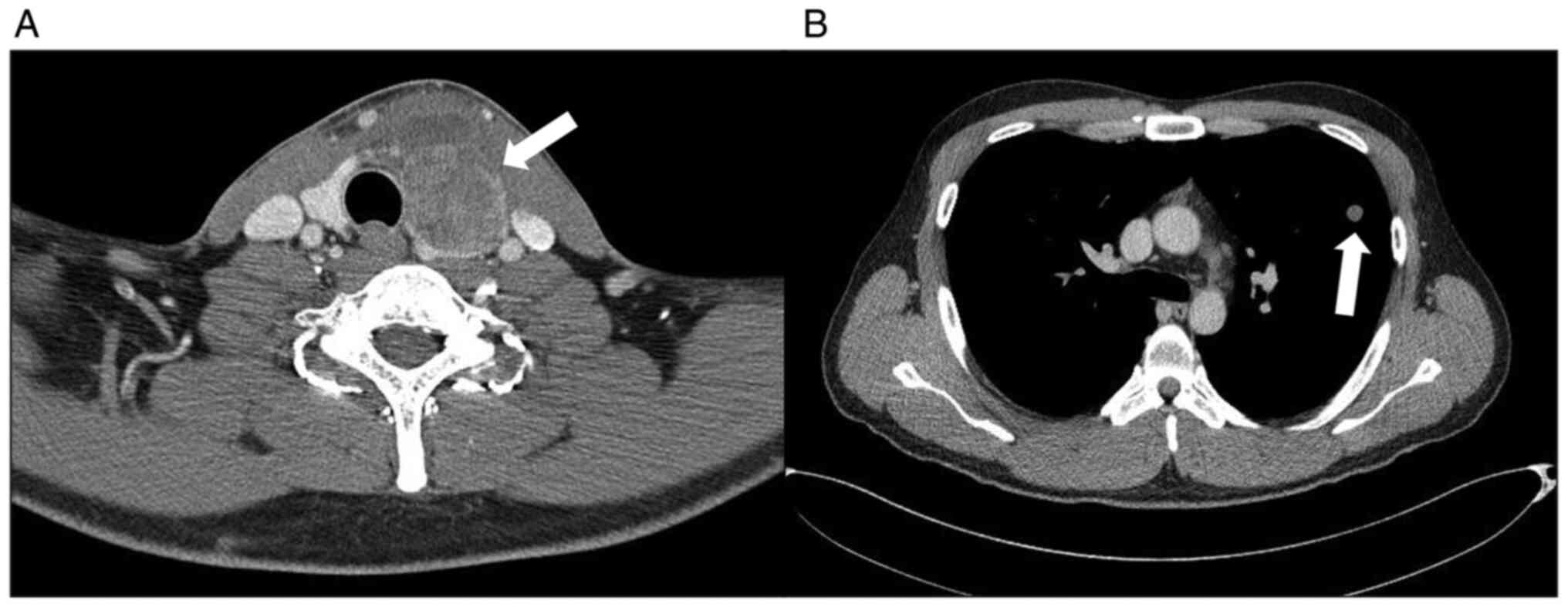
a 4.0 cm sized mass in the left lobe of the thyroid gland (Fig. 1A). Positron emission tomography and
computed tomography were performed, and metastatic lesions were not
identified. A total thyroidectomy with neck lymph node dissection
was performed. Macroscopically, a soft, yellowish tumor of 3.2×1.7
cm was detected. The surgical specimen was fixed in 10% neutral
formaldehyde at room temperature for 24 h and the representative
part of the tumor was routinely embedded in paraffin, sliced to 4–5
um thickness sections and stained with hematoxylin and eosin
(H&E) for ~45 min at room temperature using a Roche VENTANA
HE600 fully automated system (Roche Tissue Diagnostics; Roche
Diagnostics, Ltd.) and histological characteristics were assessed
using a light microscope. Microscopically, the tumor was composed
of two components and had infiltrated into the extra-thyroid
tissue. The main component constituted ~80% of the tumor and
demonstrated typical PDTC morphology. The tumor had a mainly solid
growth pattern and certain parts showed follicular growth with
frequent mitoses (Fig. 2A and B).
The remaining 20% of the tumor demonstrated SCC morphology. The
tumor cells demonstrated hyperchromatic oval to spindle-shaped
nuclei without prominent nucleoli, had scant cytoplasm, showed
occasional nuclear molding and mitoses were frequent (Fig. 2C and D). Tissue sections were
processed according to the aforementioned method for hematoxylin
and eosin staining and used for immunohistochemistry. The sections
were stained on a Benchmark ULTRA automated immunohistochemistry
stainer (Roche Tissue Diagnostics; Roche Diagnostics, Ltd.) using
an OptiView DAB IHC Detection Kit (Roche Tissue Diagnostics; Roche
Diagnostics, Ltd.) according to the following procedure. Heat
induced epitope retrieval was performed using ULTRA cell
conditioning solution (ULTRA CC1; Roche Tissue Diagnostics; Roche
Diagnostics, Ltd.) for 32 min at 100°C. Sections were incubated
with Optiview Peroxidase Inhibitor (3% hydrogen peroxide solution)
for 4 min at room temperature and with primary antibodies for
calcitonin (1:100; cat. no. 760-2611; Roche Diagnostics), ready to
use CD56 (cat. no. 760-4596; Roche Diagnostics), ready to use CEA
(cat. no. 760-4594; Roche Diagnostics), ready to use chromogranin
(cat. no. 780-4422; Roche Diagnostics), ready to use cytokeratin
(cat. no. 790-4555; Roche Diagnostics) and ready to use
synaptophysin (cat. no. 760-4595; Roche Diagnostics) for 12 min at
37°C, followed by sequential incubation with the OptiView DAB IHC
Detection Kit (Optiview HQ Universal Linker for 8 min, Optiview HRP
Multimer for 8 min, Optiview DAB and Optiview
H2O2 for 8 min, Optiview Copper for 4 min),
at room temperature. The OptiView HQ Universal Linker kit (cat. no.
760-770; Roche Diagnostics) contained a cocktail of HQ-labeled
secondary antibodies (goat anti-mouse IgG, goat anti-mouse IgM and
goat anti-rabbit) at an unspecific concentration <50 µg/ml in
buffer and OptiView HRP Multimer contained a mouse monoclonal
anti-HQ labeled HRP antibody at an unspecific concentration <40
µg/ml in buffer. Ten slides were removed from the stainer and
counterstained using Mayer's hematoxylin (ScyTek Laboratories,
Inc.) for 2 min at room temperature manually. Immunohistochemical
staining was evaluated using a light microscope. The PDTC component
was positive for cytokeratin and thyroid transcription factor-1
(TTF-1), and negative for calcitonin, chromogranin and
synaptophysin (8). The SCC
component was positive for synaptophysin and CD56, and negative for
calcitonin, chromogranin, CEA and TTF-1 (Fig. 2E and F) (9). Conventional nuclear features of
papillary thyroid carcinoma, such as nuclear clearing, intranuclear
groove and nuclear pseudo-inclusions, were not identified (10). Seven months after thyroid surgery,
two lung nodules were detected (Fig.
1B). A wedge resection of the left upper lobe was performed.
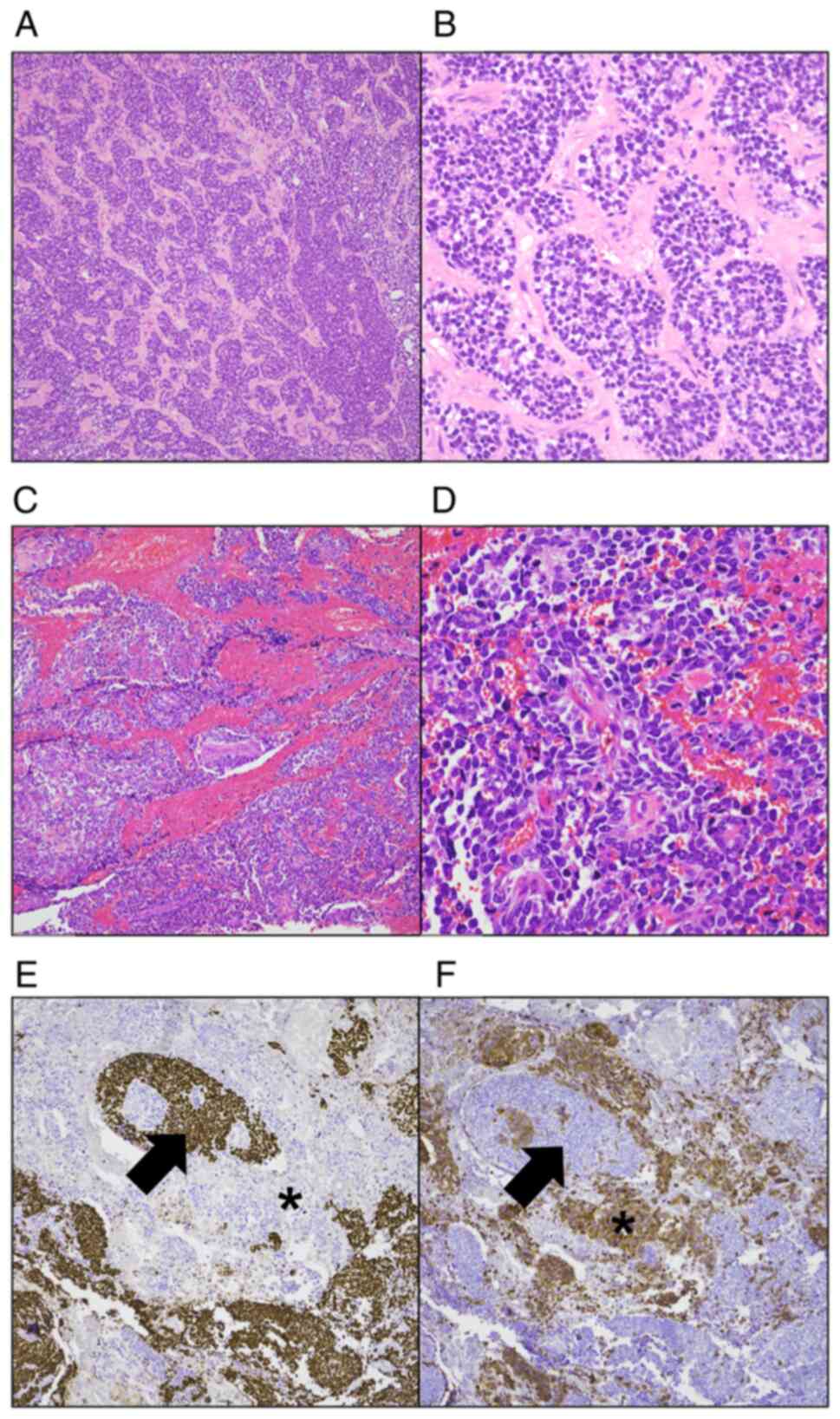
Histologically, the lung tumor was similar to the SCC component of
the thyroid tumor (Fig. 3A and B).
The lung tumor was positive for synaptophysin and CD56, and
negative for TTF-1, chromogranin and calcitonin (Fig. 3C and D). At the time of diagnosis of
the patient's thyroid tumor, no lung lesions suggestive of
metastasis were observed on the chest computed tomography. Next
generation sequencing (NGS) was performed to investigate the
relationship between the small cell component and PDTC of thyroid
cancer, and to evaluate the relationship between the lung cancer
and thyroid cancer. Targeted NGS was performed on both thyroid and
lung lesions. Formalin-fixed paraffin-embedded (FFPE) tumor tissue
was used. Hematoxylin and eosin-stained slides were reviewed, and
tumor areas with sufficient viable tumor cells were marked and used
as a guide for macro-dissection. Areas with >50% tumor volume
were used for examination. Briefly, total nucleic acid was isolated
from tumor tissue using a Recover All Total Nucleic Acid Isolation
Kit for FFPE (Ambion; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. After extracting DNA and RNA from
FFPE specimens, the quality and concentration of DNA and RNA were
assessed using a Qubit 3.0 Fluorometer with the Qubit™ dsDNA HS
Assay Kit (cat. no. Q32854; Thermo Fisher Scientific, Inc) and
Qubit™ RNS HS Assay Kit (cat. no. Q32852; Thermo Fisher Scientific,
Inc). Library preparation for an Oncomine™ Comprehensive Assay v3
(IonTorrent; Thermo Fisher Scientific, Inc.) was performed. For
library preparation, the multiplex PCR-based Ion Torrent AmpliSeq™
technology (Thermo Fisher Scientific, Inc.) with Oncomine™
Comprehensive Assay v3M Kit (cat. no. A36111; Thermo Fisher
Scientific, Inc) was used. The individual libraries were diluted to
a final concentration of 50 pM and samples were pooled and
processed to library amplification on Ion Spheres using an Ion 550™
Kit (cat. no. A34538; Thermo Fisher Scientific, Inc.) and library
enrichment on the Ion Chef (Thermo Fisher Scientific, Inc.). An
IonTorrent S5 XL platform was used for sequencing according to the
manufacturer's protocols. The direction of sequencing was single
end type and 200 bp for length. Alignment to the hg19 human
reference genome and variant calling were performed using Torrent
Suite version 5.12.1 (Thermo Fisher Scientific, Inc.) and Ion
Reporter software version 5.18 (Thermo Fisher Scientific, Inc.).
Only the PDTC area was included in the examination for the thyroid
lesion. Alterations of ATRX (c.6793G>T, p.Glu2265*),
TP53 (c.377A>G, p.Tyr126Cys) and MYCL
(c.332G>T, p.Gly111Val) were demonstrated in both lesions. In
the lung SCC lesions, KIT and PDGFRA amplification
and CDK2 (c.783C>A, p.Ser261Arg) mutations were also
demonstrated. The patient was scheduled to receive 6 cycles of
paclitaxel, cisplatin and etoposide-based chemotherapy, according
to the normal procedures of Jeonbuk National University Hospital.
However, the response was poor and after receiving 4 cycles of
chemotherapy the patient died due to progression of the disease.
The patient's death occurred 12 months after the total
thyroidectomy.
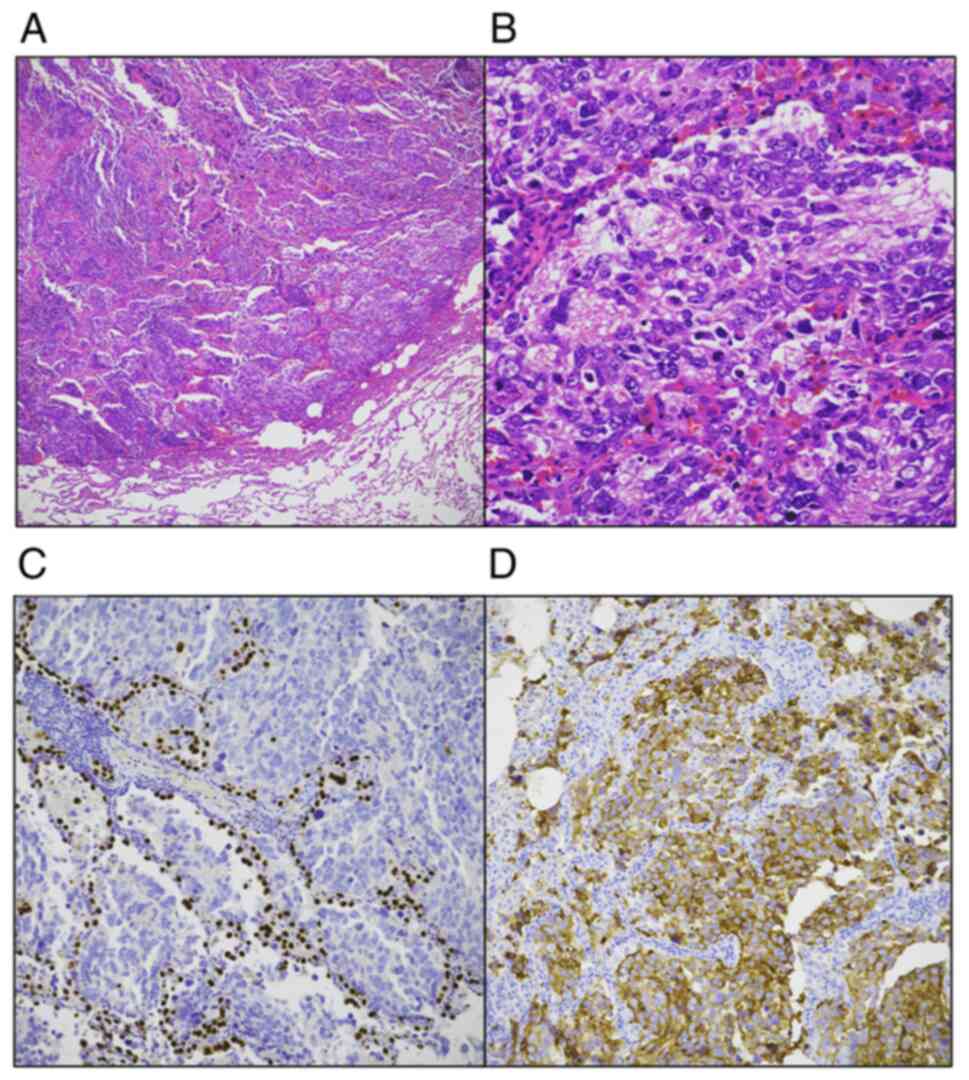
 | Figure 2.Histopathologic features of the
thyroid tumor. The tumor was composed of two components; the main
component was a PDTC and the secondary component was a SCC. (A) The
PDTC component demonstrated a solid growth pattern (H&E
staining; original magnification, ×200). (B) The high-power field
of view the tumor cells of the PDTC component demonstrated scant
cytoplasm with round to oval nuclei and mild pleomorphism (H&E
staining; original magnification, ×400). (C) The SCC component
demonstrated a solid growth pattern (H&E staining; original
magnification, ×200). (D) In the high-power field of view of the
SCC component, the tumor cells demonstrated minimal cytoplasm with
hyperchromatic oval to spindle-shaped pleomorphic nuclei and were
without prominent nucleoli. Numerous mitoses were also seen
(H&E staining; original magnification, ×400). (E)
Immunohistochemical staining for TTF-1 showed positivity in the
PDTC component (arrow). Whereas, the SCC component was negative for
TTF-1 (asterisk) (original magnification, ×200). (F)
Immunohistochemical staining for synaptophysin. The tumor cells of
the PDTC component did not show immunoreactivity to synaptophysin
(arrow). Whereas, the SCC component was positive for synaptophysin
(asterisk) (original magnification, ×200). PDTC, poorly
differentiated thyroid carcinoma; SCC, small cell carcinoma;
H&E, hematoxylin and eosin; TTF-1, thyroid transcription
factor-1. |
Discussion
Primary SCCs have been intermittently reported
previously, but most of them have presented as low-grade lymphoma
or PDTC following immunohistochemical evaluation. Approximately 6–7
cases of primary small cell thyroid carcinomas (SCTCs) meeting the
diagnostic criteria for small cell carcinoma have been reported in
previous years (3,4,5,9,11).
Eusebi et al (5) reported
two cases of SCC that met the morphologic and immunohistochemical
criteria for SCC. Since then, there have been intermittent reports
of SCC of the thyroid gland, but it is very rare and has not yet
been accepted as a distinct disease entity. Much like its pulmonary
counterparts, primary SCTC demonstrated an aggressive clinical
course and poor outcome (5).
The present case report reported a thyroid cancer
composed of two different components. The main component
demonstrated typical morphologic and immunohistochemical features
of PDTC. Whereas the second component demonstrated morphologic
features similar to pulmonary SCC including hyperchromatic oval to
spindle-shaped nucleus, no nucleoli, scant cytoplasm, occasional
nuclear molding, and frequent mitoses. The second component was
positive for synaptophysin and CD56, and negative for calcitonin,
chromogranin, CEA and TTF-1. There were no amyloid depositions in
the tumor. In the 4th edition of WHO classification of thyroid
tumors, high-grade neuroendocrine carcinomas are not listed as a
distinctive type (2) and the fact
that this tumor exhibited neuroendocrine differentiation, meant
that calcitonin-negative medullary thyroid carcinoma (MTC) needed
to be considered in the differential diagnosis. However, the tumor
had no histological characteristics for MTC, and the morphological
characteristics, such as minimal cytoplasm, nuclear molding, high
mitotic figure, and immunohistochemical features such as being
positive for CD56 and synaptophysin were similar to those of lung
SCC (9). Sporadic MTCs are
well-differentiated and locally aggressive tumors (12). However, in this tumor, metastasis to
the lung occurred rapidly and was observed just 7 months
post-surgery. The patient's rapid lung metastasis was also
consistent with the biological behavior of SCC (13). For proper treatment and accurate
prediction of prognosis, it was considered more appropriate to
diagnose the second component of this tumor as SCC rather than as a
variant of medullary carcinoma.
NGS tests were performed on lung lesions and PDTC
regions in the thyroid lesions. Three identical mutations were
observed in the thyroid and lung lesions, which confirmed that the
lung lesions had metastasized from the thyroid gland. Furthermore,
as the same genetic mutations were observed in PDTC and SCC, it was
possible to infer that the origin of the two components were the
same. In the SCC lesion, KIT and PDGFRA amplification were
additionally observed. Previous studies have reported that
TP53 mutation and, KIT and PDGFRA
amplification are rare in well-differentiated thyroid cancer and
frequently observed in anaplastic thyroid carcinomas (14,15).
The genetic alterations demonstrated in both lesions suggested that
both tumors were of the same cell origin and that SCC may arise
through the acquisition of additional genetic alterations. It is
generally accepted that ATC and PDTC usually develop from the
dedifferentiation of differentiated thyroid cancer (16). The concept of combined small cell
lung cancer (CSCLC) is well-established in the lung, and the
prevalence of CSCLC is reported to be 2–28% of all small cell lung
cancer cases (17); however, the
cellular origin of CSCLC remains unclear. Mangum et al
reported that the cellular origin of CSCLC showed that different
components (small cell vs non-small cell) of CSCLC shared nearly
75% common mutations (17). Based
on these results, it was reported that one component of CSCLC arose
separately from the other component at a relatively late time point
in the presence of a different microenvironment (17). These previous reports support the
hypothesis of the present case report that the patient's PDTC and
SCC developed from a common precursor.
Acknowledgements
Not applicable.
Funding
This case report was supported by The Fund of Biomedical
Research Institute, Jeonbuk National University Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YTH performed the surgery and provided treatment for
the patient. ARA, KMK and MJC analyzed the NGS sequencing data. MJC
and KMK evaluated the histopathological images and prepared the
figures. MJC wrote the manuscript. KMK and MJC confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The report of this study was approved by the
Institutional Review Board of the Jeonbuk National University
Hospital with a waiver of informed consent for publication (IRB no.
2022-09-055).
Consent for publication
Written informed consent was obtained from the
patient for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Somnay YR, Schneider D and Mazeh H:
Thyroid: Medullary carcinoma. Atlas Genet Cytogenet Oncol Haematol.
17:291–296. 2013.PubMed/NCBI
|
|
2
|
Lloyd RV, Osamura RY, Klöppel G and Rosai
J: WHO Classification of Tumours of Endocrine Organs. (4th
edition). IARC; Lyon: 2017
|
|
3
|
Beach DF, Klump WJ, Haddad G, Reid LM,
Schwarting R and Hageboutros A: Extrapulmonary small cell: A novel
case of small cell carcinoma of the thyroid gland. Med Oncol.
29:1405–1408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mussazhanova Z, Miura S, Stanojevic B,
Rougounovitch T, Saenko V, Shiraishi T, Kurashige T, Shichijo K,
Kaneko K, Takahashi H, et al: Radiation-associated small cell
neuroendocrine carcinoma of the thyroid: A case report with
molecular analyses. Thyroid. 24:593–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eusebi V, Damiani S, Riva C, Lloyd R and
Capella C: Calcitonin free oat-cell carcinoma of the thyroid gland.
Virchows Archiv A Pathol Anat Histopathol. 417:267–271. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aggarwal G, Jackson L and Sharma S:
Primary combined small cell carcinoma of larynx with lateralized
histologic components and corresponding side-specific neck nodal
metastasis: Report of a unique case and review of literature. Int J
Clin Exp Pathol. 4:111–117. 2011.
|
|
7
|
Ferlito A, Recher G and Caruso G: Primary
combined small cell carcinoma of the larynx. Am J Otolaryngol.
6:302–308. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bongiovanni M and Faquin WC: Poorly
differentiated thyroid carcinoma. The Bethesda System For Reporting
Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes.
Springer; pp. 129–138. 2010, View Article : Google Scholar
|
|
9
|
Prado MCM, Staino IRFL, Silva HDBd, Castro
AFd, França L and Barros PF: Primary small cell carcinoma of the
thyroid: A case report. Brazil J Oncol. 15:1–7. 2019. View Article : Google Scholar
|
|
10
|
Volante M, Collini P, Nikiforov YE,
Sakamoto A, Kakudo K, Katoh R, Lloyd RV, LiVolsi VA, Papotti M,
Sobrinoh-Simoes M, et al: Poorly differentiated thyroid carcinoma:
The Turin proposal for the use of uniform diagnostic criteria and
an algorithmic diagnostic approach. Am J Surg Pathol. 31:1256–1264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chorny JA, Orrego JJ and
Cameselle-Teijeiro JM: Primary high-grade calcitonin-negative
neuroendocrine carcinoma of the thyroid: A very rare cancer.
Endocrinol Diabetes Metab Case Rep. 17:18–36. 2018.PubMed/NCBI
|
|
12
|
Rendl G, Manzl M, Hitzl W, Sungler P and
Pirich C: Long-term prognosis of medullary thyroid carcinoma. Clin
Endocrinol (Oxf). 69:497–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walenkamp AM, Sonke GS and Sleijfer DT:
Clinical and therapeutic aspects of extrapulmonary small cell
carcinoma. Cancer Treat Rev. 35:228–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song YS, Lim JA and Park YJ: Mutation
profile of well-differentiated thyroid cancer in Asians. Endocrinol
Metab (Seoul). 30:252–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasaian K, Wiseman SM, Walker BA, Schein
JE, Zhao Y, Hirst M, Moore RA, Mungall AJ, Marra MA and Jones SJM:
The genomic and transcriptomic landscape of anaplastic thyroid
cancer: Implications for therapy. BMC Cancer. 15:1–11. 2015.
View Article : Google Scholar
|
|
16
|
Ma B, Xu W, Wei W, Wen D, Lu Z, Yang S,
Chen T, Wang Y, Wang Y and Ji Q: Clinicopathological and survival
outcomes of well-differentiated thyroid carcinoma undergoing
dedifferentiation: A retrospective study from FUSCC. Int J
Endocrinol. 21:23837152018.PubMed/NCBI
|
|
17
|
Mangum M, Greco F, Hainsworth J, Hande K
and Johnson D: Combined small-cell and non-small-cell lung cancer.
J Clin Oncol. 7:607–612. 1989. View Article : Google Scholar : PubMed/NCBI
|