Introduction
Anti-CD19 chimeric antigen receptor (CAR)-T cells
have demonstrated notable therapeutic effects on B cell leukemia
and lymphoma in clinical trials following landmark studies
(1,2). Anti-CD19 CAR-T cell therapy is
effective for treating relapsed or refractory aggressive B cell
lymphoma and second-generation anti-CD19 CARs have been approved by
the US Food and Drug Administration and are currently being
evaluated in clinical trials to expand their applications (3–5). In
certain cases, persistent CD4+ CAR-T cells have achieved leukemia
remission lasting a decade (6).
However, CD19-expressing malignant B cells evade CAR-T cell therapy
(7) and T cell exhaustion can occur
due to continued antigen stimulation by cancer cells (8). Furthermore, there is a risk of
cytokine release syndrome (9), and
autologous blood is the only source of CAR-T cells in the clinic to
prevent potential graft-versus-host disease (GVHD). CAR-T cells
produce cytokines and exert cytotoxicity to control tumor cells;
tumor cells can produce cytokines that interact with immune cells
in the tumor microenvironment (10). Infusion of CAR-T cells increases
circulating cell count, resulting in elevated serum inflammatory
cytokine levels, which elicit cytokine release syndrome (9).
To overcome the limitations of CAR-T cell therapy,
natural killer (NK) cells are suitable candidates for cancer
immunotherapy. They are not major histocompatibility complex
(MHC)-restricted and are thus less likely to elicit GVHD while
retaining anti-tumor activity via degranulation of cytolytic
molecules, including granzyme and perforin, and cytokine secretion
(11). In addition, NK cells have
short lifespans, which may prevent cytokine release syndrome.
Infusion of human leukocyte antigen-mismatched anti-CD19 CAR-NK
cells derived from umbilical cord blood does not cause cytokine
release syndrome, neurotoxicity or GVHD in patients with CD19+
relapsed or refractory lymphoma or leukemia (12). Notably, 73% of patients exhibit a
response to treatment with these cells, and there is no increase in
inflammatory cytokine levels (12).
According to phase I clinical trials, infusion of NK-92, a human NK
cell line, is safe in patients with various types of solid tumor,
refractory lymphoma, and multiple myeloma (MM) (13–15).
NK-92 cells are easily cultured and have a higher transfection
efficiency than primary NK cells (16). NK-92 cells express high levels of
molecules involved in the perforin-granzyme apoptosis pathway
(17). To eliminate in vivo
proliferation and tumorigenic potential of CAR-NK-92, irradiation
is often performed before administration in patients, and phase I
clinical trials demonstrating their stability profile have been
performed (18,19). The safety of anti-CD33 CAR-NK-92
cells has also been previously examined in patients with relapsed
and refractory acute myeloid leukemia (20).
CD22 is expressed during the pre-B cell stage and is
maintained in mature B cells, making it a promising target for
immunotherapy against malignant B cells (21,22).
CD19 and CD22 are expressed in normal B cells and associated
malignancies and CD22 expression is sustained in CD19low
or CD19− malignant B cells (4,23). By
targeting both CD19 and CD22, bispecific CARs may broaden the range
of diseases treated by CAR-based immune cells. Anti-CD19 and
anti-CD22 CAR-T cell cocktail therapy and anti-CD19/CD22 bispecific
CAR-T cell therapy have shown promising results in phase I clinical
trials (4,24,25).
To the best of our knowledge, however, the efficacy of
anti-CD19/CD22 bispecific CAR-NK cells has not been reported.
The present study aimed to overcome the limitations
of anti-CD19 CAR-T cell therapy. To generate anti-CD19/CD22
bispecific CAR structures, anti-CD22 monoclonal antibodies (mAbs)
were developed, and their antigen-binding sites were integrated
into CAR backbone structures. The present study also aimed to
evaluate the cytotoxicity of anti-CD19/CD22 bispecific CAR-NK-92
cells against OCI-Ly7 cells, a human diffuse large B cell lymphoma
cell line, in vitro and in vivo and identify the
anti-CD19/CD22 CAR structures that had the highest efficacy in NK
cells.
Materials and methods
Generation of anti-CD22 single-chain
variable fragment (scFv) Ab
Specific-pathogen-free (SPF) White Leghorn chickens
(Gallus gallus) were hatched from SPF eggs (VALO BioMedia GmbH).
All chickens were kept at 24–30°C and 9:15 light-dark cycle in
40–60% humidity, and had free access to food and water until the
end of the experiment. A total of three 4-week-old male chickens
were immunized with 20 µg recombinant human CD22-Fc protein (cat.
no. 1968-SL; R&D Systems, Inc.) subcutaneously four times at
two-week intervals by BIOPOA Co., Ltd. The spleen, bursa of
Fabricius and bone marrow were harvested for total RNA isolation
using TRI Reagent™ (Invitrogen, Thermo Fisher Scientific, Inc.).
Complementary DNA was synthesized using the Superscript IV
First-Strand Synthesis system (Invitrogen, Thermo Fisher
Scientific, Inc.), with oligo(dT) primers, according to the
manufacturer's instructions. The genes encoding Vλ and VH were
amplified from the oligo(dT)-synthesized cDNA using gene-specific
PCR primers. Primer sequences for Vλ (CSCVK_forward:
5′GTGGCCCAGGCGGCCCTGACTCAGCCGTCCTCGGTGTC-3′ and
CKJo-B_reverse:5′-GGAAGATCTAGAGGACTGACCTAGGACGGTCAGG-3′) and VH
(CSCVHo-FL_forward:
5′-GGTCAGTCCTCTAGATCTTCCGGCGGTGGTGGCAGCTCCGGTGGTGGCGGTTCCGCCGTGACGTTGGACGAG-3′
and CSCG-B_reverse:
5′-CTGGCCGGCCTGGCCACTAGTGGAGGAGACGATGACTTCGGTCC-3′) were
synthesized by Integrated DNA Technologies, Inc. (26). scFv-displaying phage libraries were
generated as described previously (27). The libraries were subjected to five
rounds of bio-panning with CD22-conjugated magnetic beads (cat. no.
14302; Invitrogen, Thermo Fisher Scientific, Inc.). To select
candidates able to bind CD22, reactivity of antibody-displaying
phages was tested in phage enzyme immunoassay. The microtiter
plates were coated with 100 ng/well human CD22-His protein (cat.
No. 11958-H08H, Sino Biological, Inc.) and incubated at 4°C
overnight. After blocking with 3% bovine serum albumin (BSA, cat.
no. BSAS 0.1; Bovogen Biologicals Pty Ltd.) dissolved in PBS (w/v),
the plate was then sequentially incubated with scFv-displaying
phages in the culture supernatant, HRP-conjugated anti-M13 antibody
(dilution fold 1:5,000, cat. no. 27-9421-01;Cytiva) and then
finally with 2,2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic
acid]-diammonium salt (ABTS) substrate solutions (cat. no.002024,
Thermo Fisher Scientific, Inc.) with intermittent washing using
0.05% Tween-20 in PBS. The optical density was measured at 405 nm
using a microplate spectrophotometer (Sunrise™, Tecan. Phage clones
showing positive signals were selected (optical density at 405 nm
≥0.6), and their nucleotide sequences were determined by Sanger
sequencing (Cosmo Genetech Co., Ltd., Korea).
Expression and purification of
anti-CD22 scFv-Cκ-hemagglutinin (HA) fusion protein
The gene encoding anti-CD22 scFv was subcloned into
modified pCEP4 mammalian expression vectors to express human
constant κ region (Cκ) and HA-tagged proteins as described
previously (28). The construct was
transfected into FreeStyle™ 293-F cells (cat. no. R790-07, Thermo
Fisher Scientific, Inc.) using 25-kDa linear polyethylenimine (PEI,
cat. no. 23966-1, Polysciences). The mixture of 2 µg plasmid DNA
and 4 µg linear PEI in 100 µl 150 mM NaCl solution was prepared per
ml of cell culture volume. Following 15-min incubation at room
temperature, the mixture was added to HEK293F cells
(2×106 cells ml−1) and the cells were grown
in FreeStyle 293 Expression Medium (cat. no. 12338018, Thermo
Fisher Sciences) for 5 days at 37°C in an atmosphere containing 7%
CO2 on an orbital shaking incubator (cat/no. NB-206CXL,
N-Biotek) at 135 rpm. The scFv-Cκ-HA fusion proteins were purified
from culture supernatant which was collected 48 h after
transfection by affinity chromatography using KappaSelect Resin
(cat. no. 17545801, Cytiva) according to the manufacturer's
instructions.
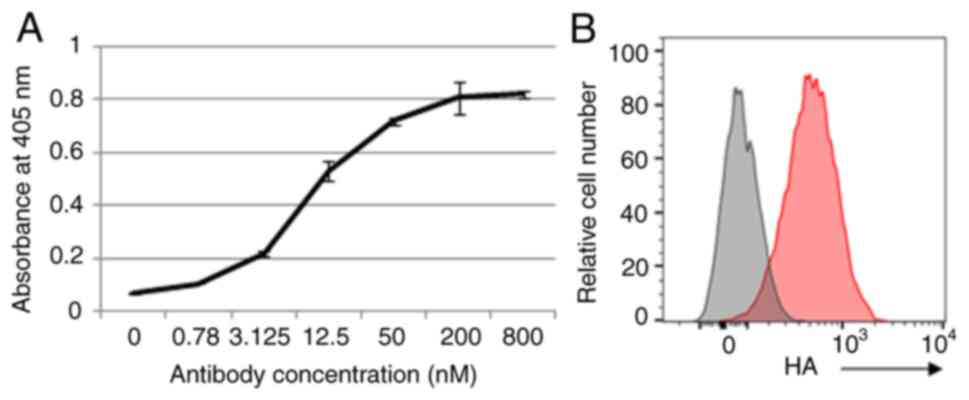
Binding assay using anti-CD22
scFv-Cκ-HA fusion protein
Microtiter plates were coated with 100 ng/well human
CD22-His protein (Sino Biological, Inc.) and incubated at 4°C
overnight. Wells were blocked with 150 ml 3% BSA (Sigma Aldrich;
Merck KGaA) in PBS at 37°C for 1 h. The anti-CD22 scFv-Cκ-HA fusion
protein was diluted serially in 3% BSA in PBS starting from 800 nM
with 4-fold dilutions, resulting in seven dilutions, and added to
each well. After incubation at 37°C for 1.5 h, the plate was washed
three times with 0.05% Tween-20 in PBS and incubated with
HRP-conjugated anti-human Cκ antibody (dilution fold 1:5,000, cat.
no. AP502P, MilliporeSigma) at 37°C for 1 h. The plate was washed
three times with 0.05% Tween-20 in PBS and bound antibody was
determined by adding ABTS solution. Absorbance at 405 nm was
measured using a microplate spectrophotometer.
OCI-Ly7 cells, donated by Professor Sung Ho Jeon
(Hallym University, Chuncheon, Korea) or Raji cells (ATCC) were
seeded into a 1.5 ml tube at a density of 3×105
cells/tube. The anti-CD22 scFv-Cκ-HA fusion protein was added at
0.25 µM to each tube and incubated on ice for 30 min. After washing
twice with MACS buffer (Miltenyi Biotec GmbH), Alexa Fluor
647-labeled mouse anti-HA antibody (Clone 912426; cat. no. IC6875R;
R&D Systems) was added at 1:1,000 dilution and the sample was
incubated on ice for 30 min. The cells were washed twice with MACS
buffer, resuspended in 150 µl 1% paraformaldehyde in MACS buffer,
and incubated at 37°C overnight. The cells were analyzed using flow
cytometry with a FACS Canto II instrument (BD Biosciences) using a
FACSDiva 6.0 software (BD Biosciences). For each sample, 10,000
cells were analyzed.
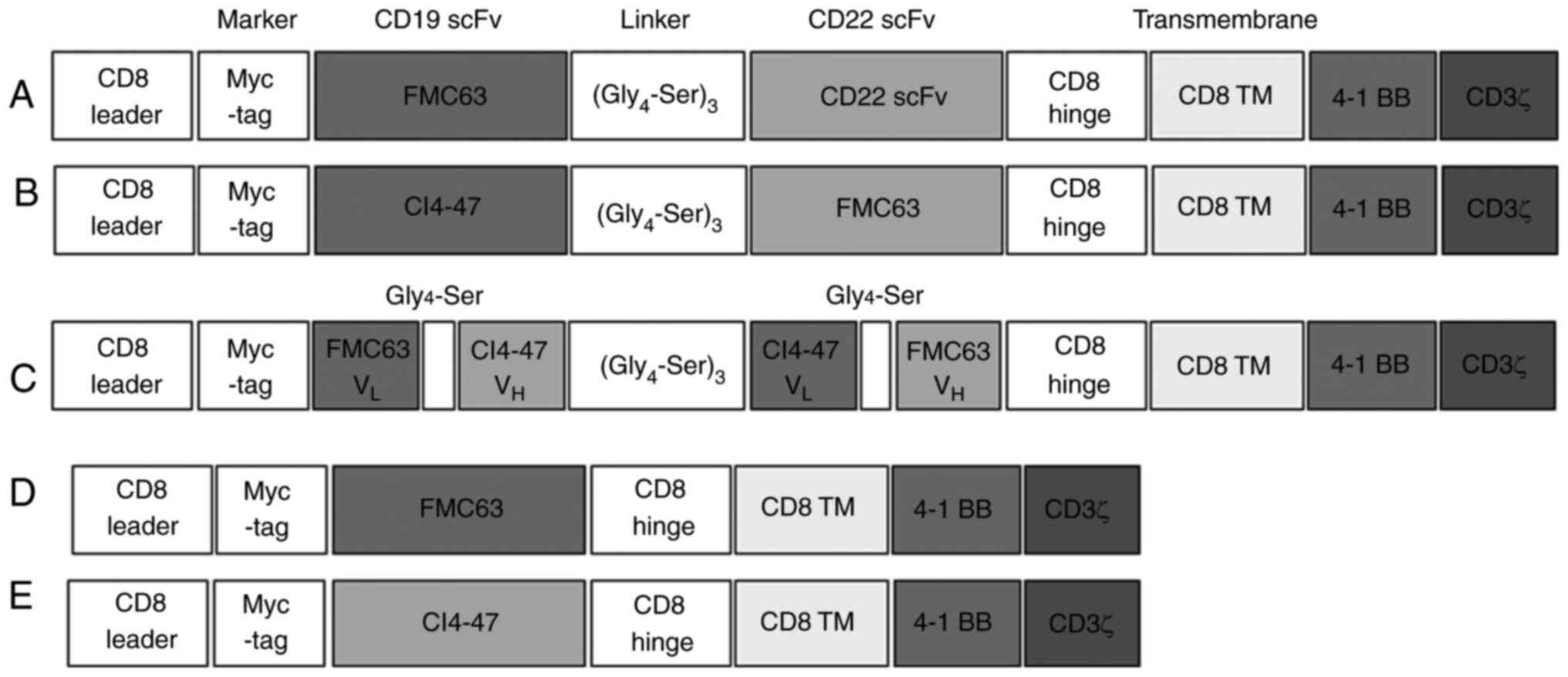
Anti-CD19/CD22 bispecific CAR
construction
CD19 CAR-expressing vector (FMC63-CD8
hinge-BBz-pCL20C-MND) was provided by Dr Byoung Ryu (St Jude
Hospital, Memphis, TN, USA). The pCAG vector backbone was derived
from pCAGGS prepared by Dr Jun-ichi Miyazaki (Kumamoto University,
Kumamoto, Japan) (29). CD19 CAR
construct contained the FMC63 scFv, hinge and transmembrane domain
of CD8, cytoplasmic domain of 4-1BB and CD3ζ signaling domain. The
CD19 CAR vector was tagged with MYC. Representative structures of
CARs are shown in Fig. 1.
Cell culture
NK-92 cells were obtained from American Type Culture
Collection (cat. no. CRL-2407) and maintained in α-Minimum
Essential Medium (Welgene, Inc.) containing 12.5% non-heat
inactivated FBS (Welgene, Inc.), 12.5% heat-inactivated horse serum
(Gibco; Thermo Fisher Scientific, Inc.) 10 U/ml penicillin, 10
µg/ml streptomycin, 20 mM folic acid, 20 mM myo-inositol
(MilliporeSigma), 55 mM 2-mercaptoethanol and 100 U/ml recombinant
human interleukin-2 (rhIL-2; PeproTech, Inc.); this was named NK92
medium. The human B cell lymphoma cell lines, OCI-Ly7 and Raji, and
human embryonic kidney epithelial cell line 293(T) cells (Korean
Cell Line Bank; Korean Cell Line Research Foundation) were
maintained in DMEM (Welgene, Inc.) containing 10% heat-inactivated
FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. All cells were
maintained in a fully humidified incubator at 37°C in 5%
CO2.
Generation of CAR-transduced
cells
Plasmid DNA was transformed by heat shock into
competent Escherichia coli Stbl3 cells in a 0.1 M solution CaCl2,
following standard protocols (30).
The CAR vector was isolated using a Plasmid DNA Midi prep kit
(Qiagen GmbH). To produce lentivirus particles, the CAR-expressing
vector was transfected into 1×105 293(T) cells with
packaging plasmid vectors pCAG-KGP1-1R, pCAG4-RTR2, and pCAG-VSVG
at a ratio of 6:3:1:1 µg using 100 µl of Lipofectamine®
3000 (Invitrogen, Thermo Fisher Scientific, Inc.) for 30 min at
room temperature. The virus-containing medium was harvested at 48
and 72 h after transfection and filtered through a 0.45 µM filter
(MilliporeSigma). The viral supernatant was used to transfect NK92
cells pre-treated with 8 µg/ml polybrene (MilliporeSigma) for 5 min
at room temperature. CAR-NK-92 cells were sorted to 80–99% purity
CAR-expressing Myc+ cells using a BD FACSAria™III cell sorter. Flow
cytometry was performed using allophycocyanin anti-CD56 (clone
NCAM, Cat. No. 17-0567-42; Invitrogen, Thermo Fisher Scientific,
Inc.), AlexaFluor-647-conjugated anti-Myc-Tag mouse monoclonal
antibody (Clone 9B11, Cat. No. 2276; Cell Signaling Technology,
Inc.), and matched AlexaFluor-647-conjugated mouse IgG2a, κ isotype
control (Clone MOPC-173, Cat. No. 400234; BioLegend, Inc.) by
CytoFLEX (Beckman Coulter, Inc.) and FlowJo v10 software (Tree
Star, Inc.) following 30 min incubation at 1:200 dilution on ice.
The selected CAR-NK-92 cells were cryopreserved in heat-inactivated
FBS containing 10% DMSO and stored at −196°C in liquid nitrogen
until use. The purity of the Myc+ CAR-NK-92 cells was over 90%
after thawing (Fig. S1). In in
vitro experiments, naked lentiviral vector was transfected as
NV control.
Apoptosis test
Cytotoxicity was analyzed by performing
7-amino-actinomycin D (7-AAD) and Annexin V assay. Target OCI-Ly7
cells were labeled with carboxyfluorescein diacetate succinimidyl
ester (CFSE) using a Cell Trace Cell Proliferation kit (Thermo
Fisher Scientific, Inc.), as previously described (31). CAR-NK-92 cells were co-cultured with
CFSE-labeled target cells at an effector-to-target ratio of 2:1.
After incubation at 37°C overnight, the cells were washed with
Annexin V binding buffer (BD Biosciences) and incubated at 37°C for
30 min. CAR-NK-92 cells were cultured at 37°C for 4 h, pre-treated
with human FcR blocking reagent at 1:200 dilution (Miltenyi Biotec
GmbH) for 5 min, and stained with 7-AAD Viability Staining Solution
(BioLegend, Inc.) and Pacific Blue™ Annexin V (BioLegend, Inc.) for
15 min on ice. The CFSE+ target cells were evaluated for
early and late apoptosis using flow cytometry using CytoFLEX
(Beckman Coulter, Inc.). The data were analyzed using FlowJo v10
software (Tree Star, Inc.).
In vivo experiments
Female 8–12 week-old NSG
(NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice were purchased (JABio,) and
maintained in SPF facilities at 20–23°C and a 12/12 light/dark
cycle with 40–60% humidity. They were free to access food and
water. On day 0, mice were injected intraperitoneally with
CFSE-labeled OCI-Ly7 cells at a density of 5×106
cells/200 µl in 1X PBS. After 30 min, mice were injected
intraperitoneally with 5×106 CAR-transduced cells. On
day 2, 15 ml 1X PBS was injected into the peritoneal cavity, and
the peritoneal lavage was harvested (32). For euthanasia, 30–70% vol/min
CO2 was administered in a closed box, following
injection of Tribromoethanol 200–400 mg/kg as anesthetics,
intraperitoneally. For harvesting peritoneal lavage, anesthetics
(Zoletil 20–40 mg/kg + Zylazine 5–10 mg/kg) were injected
intraperitoneally. The ratio and number of CFSE+ cells
were measured using flow cytometry, as described above. The data
were analyzed with FlowJo v10 software. All mouse experimental
procedures were approved by the Institutional Animal Care and Use
Committee of Asan Medical Center, Seoul, Korea (approval no.
2022-12-090) and performed ethically and in accordance with Animal
Protection Act by Korean Government (33).
Statistical analysis
Graphical and statistical analysis was performed
using GraphPad Prism v6 software (Dotmatics). P-values were
calculated by one-way ANOVA followed by post hoc Tukey-Kramer and
Bartlett's tests. P<0.05 was considered to indicate a
statistically significant difference. Data are shown as the mean ±
standard error of the mean or SD of at least four independent
experimental repeats.
Results
Production of anti-CD19/CD22
bispecific CAR constructs
Binding of mAb clones to CD22 protein was evaluated
through enzyme immune assays (Figs.
2A and S1A) and flow cytometry
(Figs. 2B and S1B). The antigen-binding domain of
selected mAb clones, CI4-47, CI3-23, 2-3-14, and 2-3-16, was
integrated into a CAR backbone structure, a second-generation
molecule containing 4-1BB and CD3ζ signaling domains. To optimize
the bispecific CAR structures for NK cells, the order of the
anti-CD19 (clone FMC63) and anti-CD22 (clone CI4-47) was changed
(Fig. 1A and B) and the loop
structure was prepared (Fig. 1C).
CI4-47 clone was selected by binding assay using flow cytometry
(Fig. S1B). Anti-CD19- or
anti-CD22 specific CAR-expressing plasmids were also produced
(Fig. 1D and E) in same lentiviral
vector.
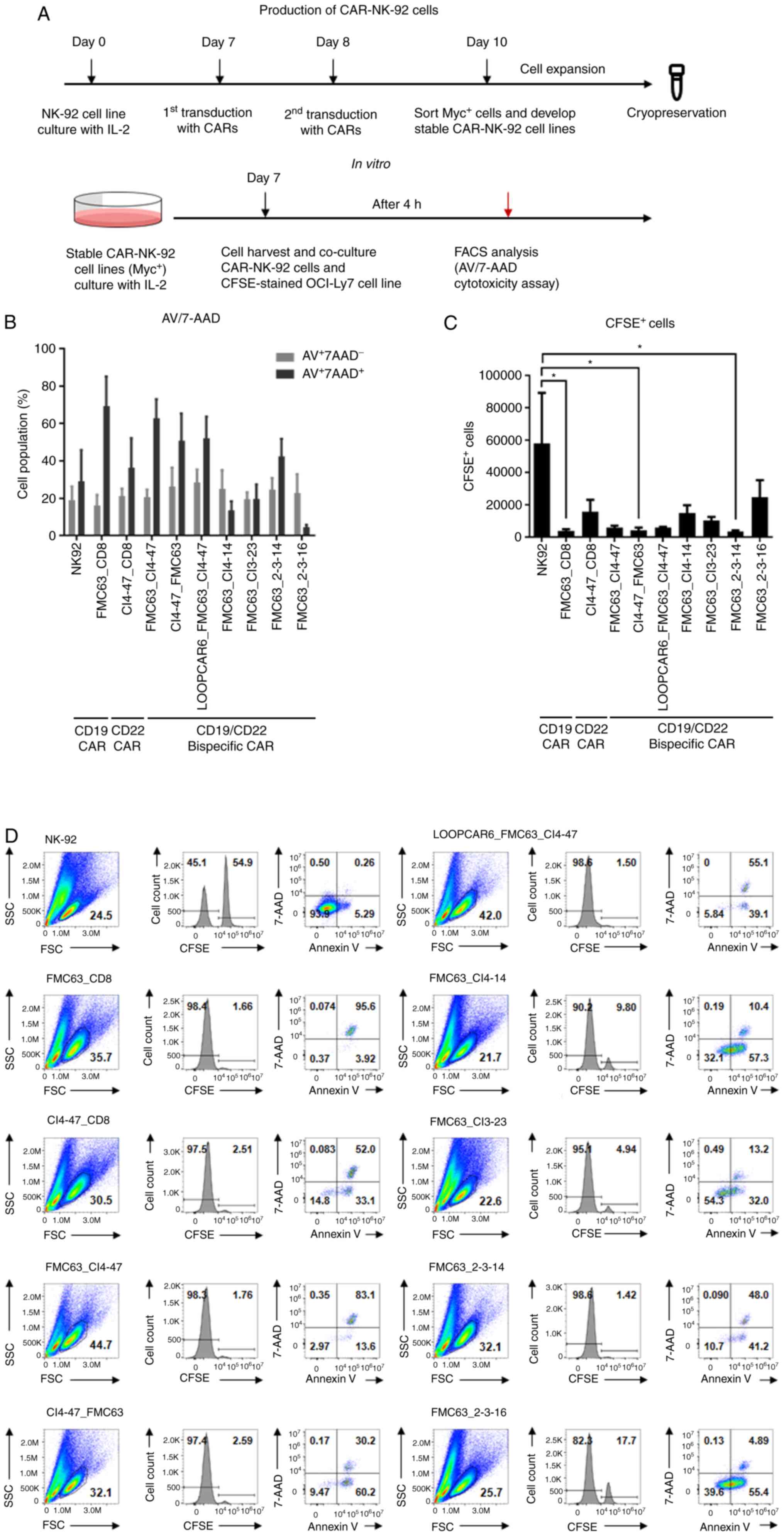
Selection of anti-CD19/CD22 bispecific
CAR-NK-92 cells in vitro
CAR-expressing lentivirus particles were used to
infect NK-92 cells twice. After 48 h, CAR-expressing NK-92 cells
were sorted using flow cytometry (Figs.
3A and S2). The CAR-NK-92
cells were cultured to obtain sufficient cell numbers, and CAR
expression was confirmed before the experiments (Fig. S2). Following co-incubation with
CFSE-labeled OCI-Ly7 cells, a B cell lymphoma cell line used as
target cells, the death of target cells was assessed by calculating
the percentages of CFSE+ cells using Annexin V/7-AAD
staining (Figs. S3 and 3D). OCI-Ly7 cells expressed high levels of
CD19 and CD22 (Fig. S4). The
number of late apoptotic cells that were
AV+7-AAD+ increased following 4-h
co-incubation with anti-CD19/CD22 bispecific CAR-NK-92 cells,
whereas the number of early apoptotic cells that were
AV+7-AAD− did not increase due to the effect
of CAR-NK-92 cells (Fig. 3B).
Counting of CFSE+ target cells revealed that CAR-NK-72
cells exhibited higher cytotoxicity than NK-92 cells (Fig. 3C). Anti-CD19 single CAR, anti-CD22
(CI4-47)/CD19 (FMC63) bispecific CAR and anti-CD19 (FMC63)/CD22
(2-3-14) bispecific CAR significantly decreased target cell numbers
compared with the effects of anti-CD19 specific CAR. The
anti-CD19/CD22 (FMC62-CI4-47) and loop-structured anti-CD19/CD22
(LOOPCAR-FMC63-CI4-47) bispecific CARs decreased target cell
numbers, but to a lesser extent. The NK-92 cells infected with
naked lentiviral vector control did not change the viability of
target cells compared with NK-92 cells without viral vector
(Fig. S5). Therefore, five CAR
structures were selected for further in vivo
investigation.
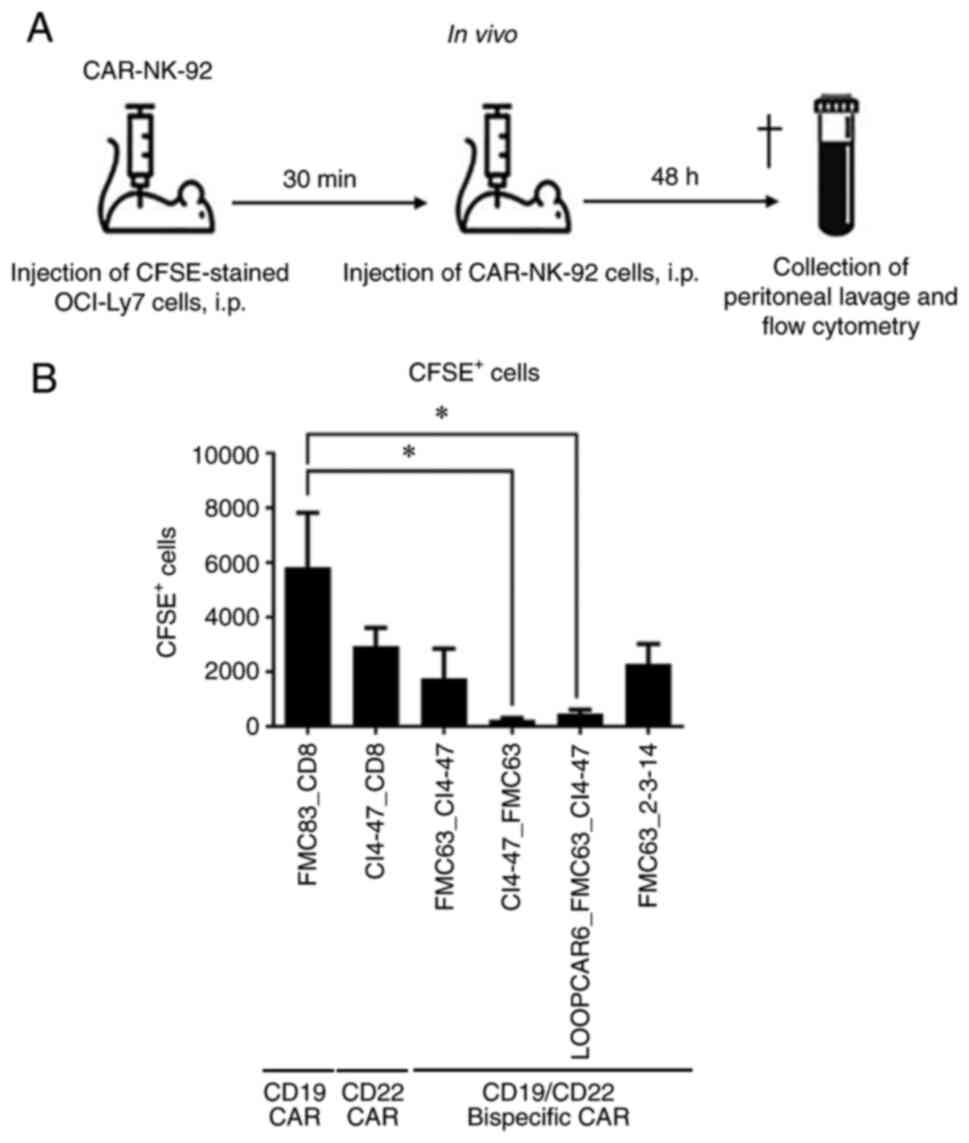
Efficacy of anti-CD22/CD19 and
loop-structured anti-CD19/CD22 CAR-NK-92 cells in vivo
To evaluate whether CAR-NK-92 cells induced cell
death in B cell lymphoma cells in vivo, NSG immune-deficient
mice were inoculated with CFSE-labeled OCI-Ly7 cells and with
CAR-NK-92 cells (Fig. 4A). Among
the CAR structures tested, anti-CD22/CD19 (CI4-47-FMC63) and
loop-structured anti-CD19/CD22 (LOOPCAR-FMC63-CI4-47) bispecific
CAR-NK-92 cells significantly eliminated target cells compared with
anti-CD19 specific CAR-NK-92 cells after 48 h (Fig. 4B). The results suggested that
anti-CD19/CD22 bispecific CARs were more cytotoxic than anti-CD19
CAR against B cell lymphoma cells in vivo. Additionally, the
structures of bispecific CAR may modify the efficacy in NK-92
cells.
Discussion
Anti-CD19/CD22 bispecific CARs were developed and
the efficacy of anti-CD19/CD22 CAR-NK-92 cells in vitro and
in vivo was demonstrated. To the best of our knowledge, the
present study is the first report of anti-CD19/CD22 bispecific
CAR-NK-92 cells from a novel clone of chicken anti-CD22 Ab. The two
distinct backbone structures of the bispecific CARs showed
significant efficacy in a xenograft model of B cell lymphoma.
Notably, anti-CD22/CD19 (CI4-47-FMC63) and loop-structured
anti-CD19/CD22 (LOOPCAR-FMC63-CI4-47) bispecific CAR-NK-92 cells
showed significantly higher cytotoxicity in mice after 48 h
compared with anti-CD19 CAR-NK-92 cells. The CAR structures
evaluated in the present study can be introduced into stem
cell-derived CAR-NK cells. Notably, the two structures appeared to
function more efficiently in vivo than in vitro;
inconsistent results between in vitro and in vivo
experiments may be associated with different experimental times (4
vs. 48 h) and the tumor environment in vivo.
To optimize the design of CARs, modules of CAR
structures should be carefully considered, including the
ligand-binding, spacer, transmembrane and cytoplastic domains
(33). In addition to the affinity
and avidity of antigen binding sites, structural factors such as
antigen epitope proximity and accessibility, scFv aggregation,
length of spacer domain and epitope proximity to the membrane
affect the efficacy of CAR-T cells (33). The protein structures were varied,
including the order of scFv sequences, to optimize the stability
and efficacy of the molecules. Although the present study did not
have the means to predict the actual tertiary structures of the CAR
and antigens, it is hypothesized that the order of the two antigen
binding sites may determine the interaction and proximity between
target cells and NK cells. CI4-47-FMC63 and LOOPCAR6-FMC63-CI4-47
may interact with the membrane proximal epitope of CD22 via CI4-47.
Furthermore, changes in variable region heavy chain (VH)/variable
region light chain (VL) combination, scFv order, length and
flexibility/rigidity of the linkers and extracellular spacer length
in the structures of bispecific CARs modify the expression and the
activity of the CARs (34). The
linker domain in LOOPCAR6-FMC63-CI4-47 is shorter than that of
FMC63-CI4-47. This difference in length may facilitate optimal
space between target cells and NK cells, allowing the formation of
immune synapses. In a previous study, loop-structured, bi-specific
CD19/CD22 CAR-T cells were developed and evaluated in
patient-derived mouse xenograft models (35). Although underlying mechanisms of how
structural changes in CARs improve their efficacy in NK-92 cells
remains unclear, the present results suggested that optimizing CAR
structures in NK and T cells may lead to improved clinical
outcomes.
The anti-CD22 mAb clones used in the present study
were produced in chickens. Due to the phylogenetic and evolutionary
divergence of avians from mammals, raising Abs against mammalian
antigens with better chances due to less homology (36). Furthermore, the use of chicken VH
and VL genes simplifies mining of rearranged genes and preserves
library diversity by avoiding the loss of rare transcripts during
PCR amplification. Chicken scFv is flexible in structure and
tolerate a range of mutations without compromising binding activity
and solubility (37). Therefore,
chicken scFv was used in the present study. ELISA and flow
cytometry suggested that strong binding affinity may not guarantee
improved efficacy to target cells as CAR, as the binding affinity
of CI4-47 was not the best among the clones tested. However, the
anti-CD19/CD22 bispecific CAR with CI4-14 showed improved efficacy
in vivo compared with the 2-3-14 clone. The present results
suggested the importance of optimizing CAR structures in addition
to binding affinity.
NK-92 cells, although immortalized, have been shown
to be safe in phase I clinical trials (13–15)
and irradiation of NK-92 cells can further improve their safety
(18–20). These results support the use of
CAR-NK-92 cells as off-the-shelf therapeutics (38). However, NK cell activity is
decreased in patients with MM, particularly in advanced stages, and
patients with aggressive extramedullary cutaneous plasmacytoma
(39,40). Furthermore, patients with MM who
exhibit higher NK cell activity tend to show improved survival
(40). As irradiated anti-CD33
CAR-NK-92 cells are safe for use in humans, infusion of NK-92 cells
may be beneficial for patients with MM. In one study, the serum
levels of IL-6 and IL-10 in one patient increased 5–6 days
post-infusion, but returned to normal within a further 48 h
(20). The other two patients did
not show increased cytokine levels. However, IL-6 and IL-10
suppress NK cell activity (41), so
it would be beneficial to control inflammatory cytokines to improve
NK cell activity. Although cytokine production was not measured in
mice injected with CAR-NK-92 cells, no mice showed any visible
signs of sickness or weakness during the experiments. Hematopoietic
and induced pluripotent stem cell-derived CAR-NK cells can be used
in the clinic, instead of NK-92 cells (42). In phase I/II clinical trials, human
leukocyte antigen-mismatched anti-CD19 CAR-NK cells are effective
in treating patients with relapsed or refractory CD19+ lymphoma,
with a 73% response rate (12,43).
The infused CAR-NK cells proliferate and persist at low levels for
at least 12 months, without increases in inflammatory cytokine
levels. NK cells are unlikely to cause cytokine release syndrome
and GVHD due to their relatively short lifespan and lack of MHC
restriction, respectively. Irradiated NK-92 cells are safe, have a
short lifespan, and are cost-effective, making repeated
administration feasible in the clinic. The in vivo
experimental model in the present study was a tumor rejection assay
in the peritoneal cavity, rather than orthotropic xenograft model.
This in vivo model was chosen because blood-origin tumors,
which are often circulating or metastatic tumors, as well as
residual tumor cells, are more likely to be targeted by CAR-NK-92
cells than primary solid tumors in the clinic. In addition, CAR
expression remained stable after cells were cultured, frozen, and
thawed.
In conclusion, the present results suggest that a
novel CD19/CD22 bispecific CAR, originating from a novel antibody
clone from chickens, was stably expressed in NK-92 cells and was
effective in eliminating a B cell lymphoma cell line in
vitro and in vivo. The present results supported the
potential use of anti-CD19/CD22 bispecific CAR-NK-92 cells for the
treatment of B cell leukemia/lymphoma as well as the need for
further experiments to improve CAR structures.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Byoung Ryu (St
Jude Hospital, Memphis, Tennessee, USA) for providing CAR backbone
vectors.
Funding
The present study was supported by the Basic Science Research
Program through the National Research Foundation of Korea funded by
the Ministry of Science and ICT (grant. no.
NRF-2018R1C1B6008852).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HyoK, HyeK, HJI, KNK and NK designed the research
and wrote the manuscript. HyoK, MH, MK and NK performed the
research and analyzed the results. HyeK, HJI and KNK provided
samples. KNK obtained the grant. HyoK and NK confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
the Institutional Animal Care and Use Committee of Asan Medical
Center, Seoul, Korea (approval no. 2022-12-090).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
7-AAD
|
7-amino-actinomycin D
|
|
CAR
|
chimeric antigen receptor
|
|
CFSE
|
carboxyfluorescein diacetate
succinimidyl ester
|
|
GVHD
|
graft versus host disease
|
|
NK
|
natural killer
|
|
scFv
|
single chain variable fragment
|
References
|
1
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ying Z, Huang XF, Xiang X, Liu Y, Kang X,
Song Y, Guo X, Liu H, Ding N, Zhang T, et al: A safe and potent
anti-CD19 CAR T cell therapy. Nat Med. 25:947–953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang N, Hu X, Cao W, Li C, Xiao Y, Cao Y,
Gu C, Zhang S, Chen L, Cheng J, et al: Efficacy and safety of
CAR19/22 T-cell cocktail therapy in patients with
refractory/relapsed B-cell malignancies. Blood. 135:17–27. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bouchkouj N, Kasamon YL, de Claro RA,
George B, Lin X, Lee S, Blumenthal GM, Bryan W, McKee AE and Pazdur
R: FDA approval summary: Axicabtagene ciloleucel for relapsed or
refractory large B-cell lymphoma. Clin Cancer Res. 25:1702–1708.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Melenhorst JJ, Chen GM, Wang M, Porter DL,
Chen C, Collins MA, Gao P, Bandyopadhyay S, Sun H, Zhao Z, et al:
Decade-long leukaemia remissions with persistence of CD4+ CAR T
cells. Nature. 602:503–509. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plaks V, Rossi JM, Chou J, Wang L, Poddar
S, Han G, Wang Z, Kuang SQ, Chu F, Davis RE, et al: CD19 target
evasion as a mechanism of relapse in large B-cell lymphoma treated
with axicabtagene ciloleucel. Blood. 138:1081–1085. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wherry EJ: T cell exhaustion. Nat Immunol.
12:492–499. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fajgenbaum DC and June CH: Cytokine storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim N, Lee DH, Choi WS, Yi E, Kim H, Kim
JM, Jin HS and Kim HS: Harnessing NK cells for cancer
immunotherapy: Immune checkpoint receptors and chimeric antigen
receptors. BMB Rep. 54:44–58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu E, Marin D, Banerjee P, Macapinlac HA,
Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan
M, et al: Use of CAR-transduced natural killer cells in
CD19-positive lymphoid tumors. N Engl J Med. 382:545–553. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arai S, Meagher R, Swearingen M, Myint H,
Rich E, Martinson J and Klingemann H: Infusion of the allogeneic
cell line NK-92 in patients with advanced renal cell cancer or
melanoma: A phase I trial. Cytotherapy. 10:625–632. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tonn T, Schwabe D, Klingemann HG, Becker
S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG and Bug G:
Treatment of patients with advanced cancer with the natural killer
cell line NK-92. Cytotherapy. 15:1563–1570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Williams BA, Law AD, Routy B, den
Hollander N, Gupta V, Wang XH, Chaboureau A, Viswanathan S and
Keating A: A phase I trial of NK-92 cells for refractory
hematological malignancies relapsing after autologous hematopoietic
cell transplantation shows safety and evidence of efficacy.
Oncotarget. 8:89256–89268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grund EM and Muise-Helmericks RC: Cost
efficient and effective gene transfer into the human natural killer
cell line, NK92. J Immunol Methods. 296:31–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu H, Zhao X, Li Z, Hu Y and Wang H: From
CAR-T cells to CAR-NK cells: A developing immunotherapy method for
hematological malignancies. Front Oncol. 11:7205012021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klingemann HG, Wong E and Maki G: A
cytotoxic NK-cell line (NK-92) for ex vivo purging of leukemia from
blood. Biol Blood Marrow Transplant. 2:68–75. 1996.PubMed/NCBI
|
|
19
|
Fabian KP and Hodge JW: The emerging role
of off-the-shelf engineered natural killer cells in targeted cancer
immunotherapy. Mol Ther Oncolytics. 23:266–276. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu
T, Yin J, You F, Zhu M, Shen W, et al: First-in-man clinical trial
of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients
with relapsed and refractory acute myeloid leukemia. Am J Cancer
Res. 8:1083–1089. 2018.PubMed/NCBI
|
|
21
|
Lanza F, Maffini E, Rondoni M, Massari E,
Faini AC and Malavasi F: CD22 expression in B-cell acute
lymphoblastic leukemia: Biological significance and implications
for inotuzumab therapy in adults. Cancers (Basel). 12:3032020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haso W, Lee DW, Shah NN, Stetler-Stevenson
M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, FitzGerald DJ,
Barrett DM, et al: Anti-CD22-chimeric antigen receptors targeting
B-cell precursor acute lymphoblastic leukemia. Blood.
121:1165–1174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fry TJ, Shah NN, Orentas RJ,
Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S,
Delbrook C, Yates B, et al: CD22-targeted CAR T cells induce
remission in B-ALL that is naive or resistant to CD19-targeted CAR
immunotherapy. Nat Med. 24:20–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spiegel JY, Patel S, Muffly L, Hossain NM,
Oak J, Baird JH, Frank MJ, Shiraz P, Sahaf B, Craig J, et al: CAR T
cells with dual targeting of CD19 and CD22 in adult patients with
recurrent or refractory B cell malignancies: A phase 1 trial. Nat
Med. 27:1419–1431. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cordoba S, Onuoha S, Thomas S, Pignataro
DS, Hough R, Ghorashian S, Vora A, Bonney D, Veys P, Rao K, et al:
CAR T cells with dual targeting of CD19 and CD22 in pediatric and
young adult patients with relapsed or refractory B cell acute
lymphoblastic leukemia: A phase 1 trial. Nat Med. 27:1797–1805.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andris-Widhopf J, Rader C, Steinberger P,
Fuller R and Barbas CF III: Methods for the generation of chicken
monoclonal antibody fragments by phage display. J Immunol Methods.
242:159–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rader C and Barbas CF III: Phage display
of combinatorial antibody libraries. Curr Opin Biotechnol.
8:503–508. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee Y, Kim H and Chung J: An antibody
reactive to the Gly63-Lys68 epitope of NT-proBNP exhibits
O-glycosylation-independent binding. Exp Mol Med. 46:e1142014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niwa H, Yamamura K and Miyazaki J:
Efficient selection for high-expression transfectants with a novel
eukaryotic vector. Gene. 108:193–199. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF-α induced apoptosis is accompanied
with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr
Biol. 239:115–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sambrook J, FritscheE F and Maniatis T:
Molecular cloning: A laboratory manual. 3. Cold Spring Harbor
Laboratory Press; 1989
|
|
32
|
Saudemont A, Garçon F, Yadi H,
Roche-Molina M, Kim N, Segonds-Pichon A, Martín-Fontecha A,
Okkenhaug K and Colucci F: p110gamma and p110delta isoforms of
phosphoinositide 3-kinase differentially regulate natural killer
cell migration in health and disease. Proc Natl Acad Sci USA.
106:5795–5800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
https://www.law.go.kr
|
|
34
|
Jayaraman J, Mellody MP, Hou AJ, Desai RP,
Fung AW, Pham AHT, Chen YY and Zhao W: CAR-T design: Elements and
their synergistic function. EBioMedicine. 58:1029312020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zanetti SR, Velasco-Hernandez T,
Gutierrez-Agüera F, Díaz VM, Romecín PA, Roca-Ho H,
Sánchez-Martínez D, Tirado N, Baroni ML, Petazzi P, et al: A novel
and efficient tandem CD19- and CD22-directed CAR for B cell ALL.
Mol Ther. 30:550–563. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qin H, Ramakrishna S, Nguyen S, Fountaine
TJ, Ponduri A, Stetler-Stevenson M, Yuan CM, Haso W, Shern JF, Shah
NN and Fry TJ: Preclinical development of bivalent chimeric antigen
receptors targeting both CD19 and CD22. Mol Ther Oncolytics.
11:127–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee W, Syed Atif A, Tan SC and Leow CH:
Insights into the chicken IgY with emphasis on the generation and
applications of chicken recombinant monoclonal antibodies. J
Immunol Methods. 447:71–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoon A, Shin JW, Kim S, Kim H and Chung J:
Chicken scFvs with an artificial cysteine for site-directed
conjugation. PLoS One. 11:e01469072016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang C, Oberoi P, Oelsner S, Waldmann A,
Lindner A, Tonn T and Wels WS: Chimeric antigen receptor-engineered
NK-92 cells: An off-the-shelf cellular therapeutic for targeted
elimination of cancer cells and induction of protective antitumor
immunity. Front Immunol. 8:5332017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jurisic V, Colovic N, Konjevic G, Minic I
and Colovic M: An aggressive extramedullary cutaneous plasmacytoma
associated with extreme alterations in the innate immune system.
Onkologie. 33:113–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jurisic V, Srdic T, Konjevic G, Markovic O
and Colovic M: Clinical stage-depending decrease of NK cell
activity in multiple myeloma patients. Med Oncol. 24:312–317. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Konjević GM, Vuletić AM, Mirjačić
Martinović KM, Larsen AK and Jurišić VB: The role of cytokines in
the regulation of NK cells in the tumor environment. Cytokine.
117:30–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arias J, Yu J, Varshney M, Inzunza J and
Nalvarte I: Hematopoietic stem cell- and induced pluripotent stem
cell-derived CAR-NK cells as reliable cell-based therapy solutions.
Stem Cells Transl Med. 10:987–995. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gambella M, Carlomagno S, Raiola AM,
Giannoni L, Ghiggi C, Setti C, Giordano C, Luchetti S, Serio A, Bo
A, et al: CD19-targeted immunotherapies for diffuse large B-cell
lymphoma. Front Immunol. 13:8374572022. View Article : Google Scholar : PubMed/NCBI
|