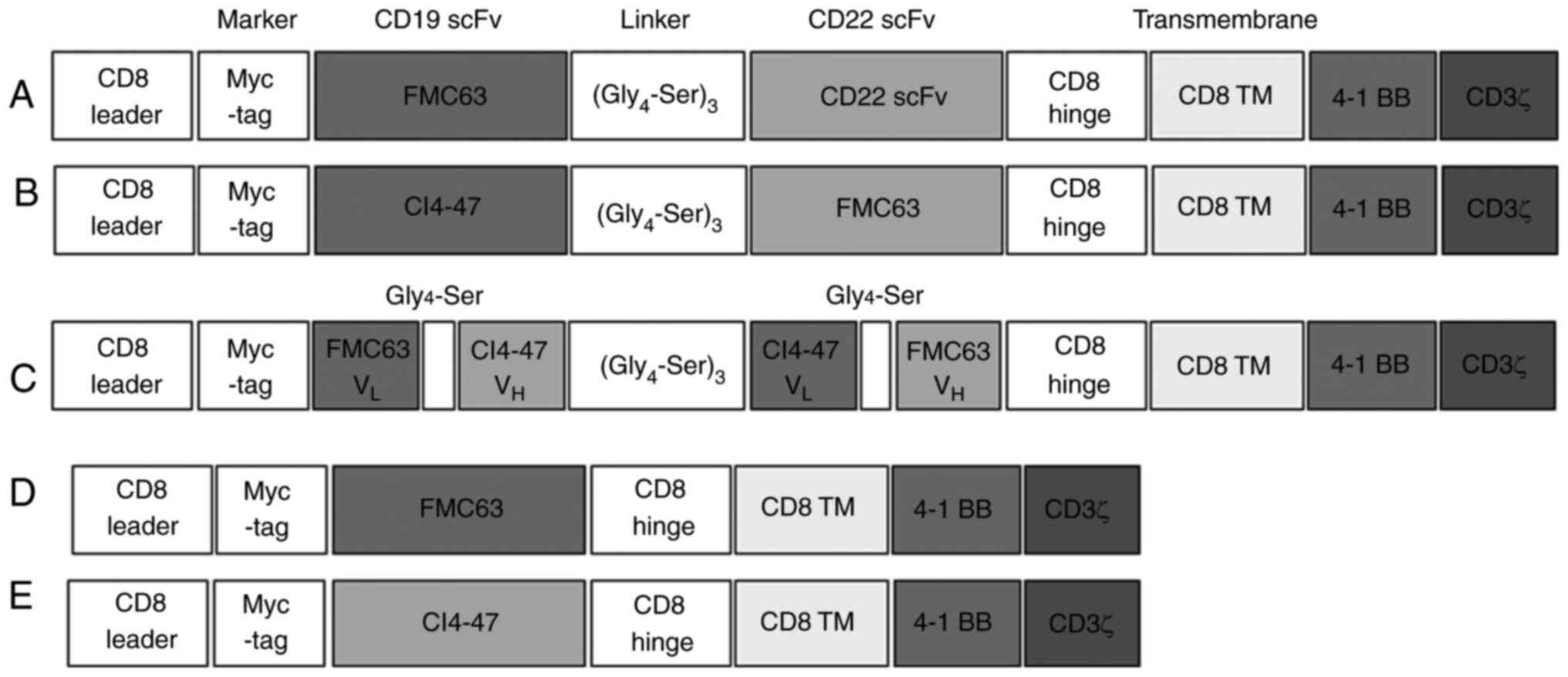
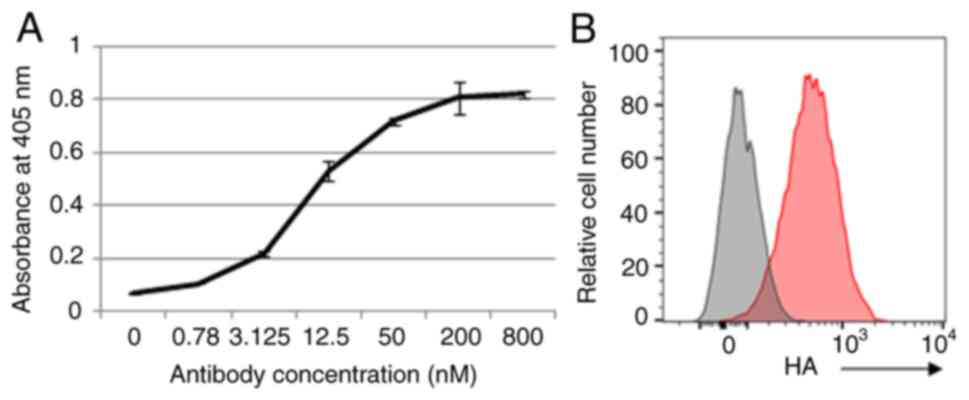
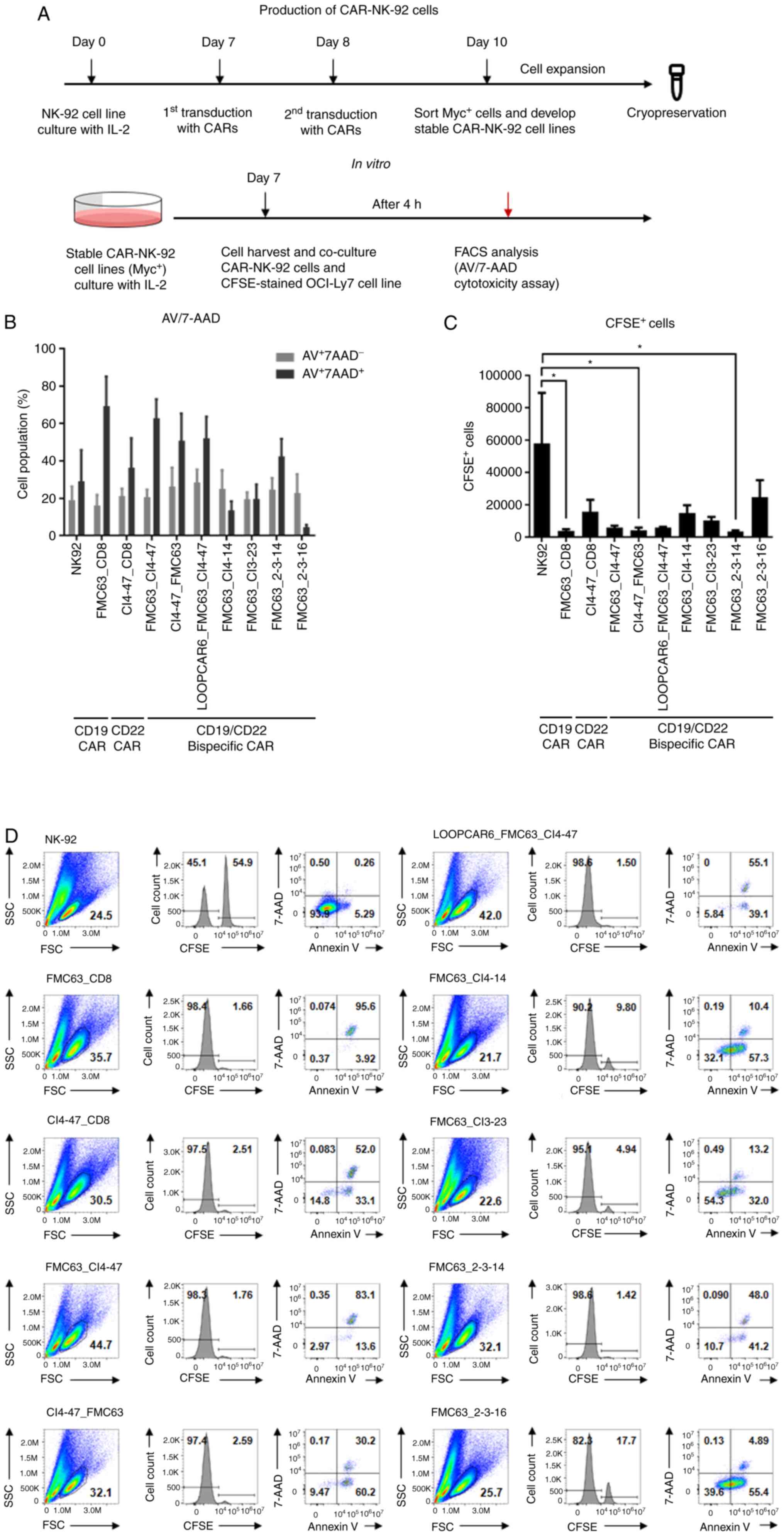
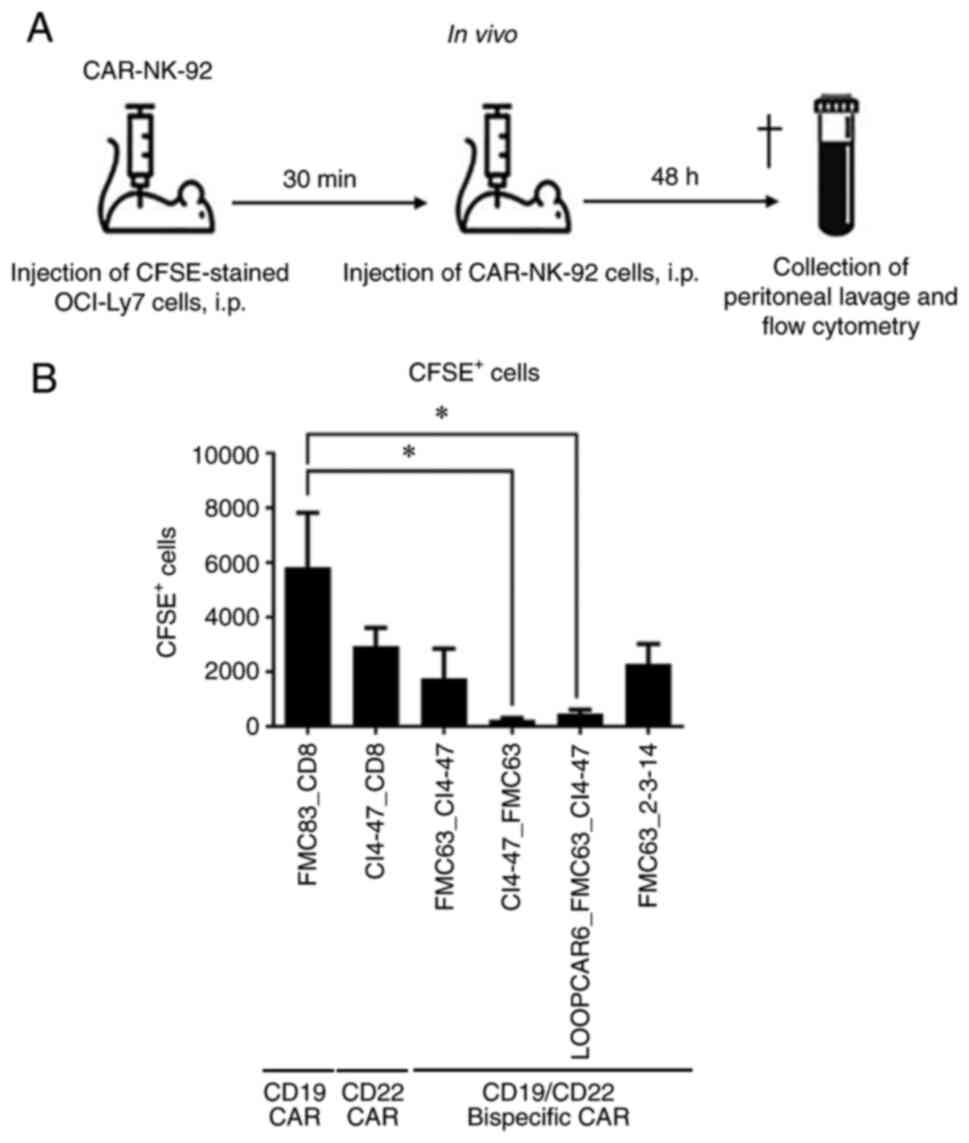
|
1
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ying Z, Huang XF, Xiang X, Liu Y, Kang X,
Song Y, Guo X, Liu H, Ding N, Zhang T, et al: A safe and potent
anti-CD19 CAR T cell therapy. Nat Med. 25:947–953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang N, Hu X, Cao W, Li C, Xiao Y, Cao Y,
Gu C, Zhang S, Chen L, Cheng J, et al: Efficacy and safety of
CAR19/22 T-cell cocktail therapy in patients with
refractory/relapsed B-cell malignancies. Blood. 135:17–27. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bouchkouj N, Kasamon YL, de Claro RA,
George B, Lin X, Lee S, Blumenthal GM, Bryan W, McKee AE and Pazdur
R: FDA approval summary: Axicabtagene ciloleucel for relapsed or
refractory large B-cell lymphoma. Clin Cancer Res. 25:1702–1708.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Melenhorst JJ, Chen GM, Wang M, Porter DL,
Chen C, Collins MA, Gao P, Bandyopadhyay S, Sun H, Zhao Z, et al:
Decade-long leukaemia remissions with persistence of CD4+ CAR T
cells. Nature. 602:503–509. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plaks V, Rossi JM, Chou J, Wang L, Poddar
S, Han G, Wang Z, Kuang SQ, Chu F, Davis RE, et al: CD19 target
evasion as a mechanism of relapse in large B-cell lymphoma treated
with axicabtagene ciloleucel. Blood. 138:1081–1085. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wherry EJ: T cell exhaustion. Nat Immunol.
12:492–499. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fajgenbaum DC and June CH: Cytokine storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim N, Lee DH, Choi WS, Yi E, Kim H, Kim
JM, Jin HS and Kim HS: Harnessing NK cells for cancer
immunotherapy: Immune checkpoint receptors and chimeric antigen
receptors. BMB Rep. 54:44–58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu E, Marin D, Banerjee P, Macapinlac HA,
Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan
M, et al: Use of CAR-transduced natural killer cells in
CD19-positive lymphoid tumors. N Engl J Med. 382:545–553. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arai S, Meagher R, Swearingen M, Myint H,
Rich E, Martinson J and Klingemann H: Infusion of the allogeneic
cell line NK-92 in patients with advanced renal cell cancer or
melanoma: A phase I trial. Cytotherapy. 10:625–632. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tonn T, Schwabe D, Klingemann HG, Becker
S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG and Bug G:
Treatment of patients with advanced cancer with the natural killer
cell line NK-92. Cytotherapy. 15:1563–1570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Williams BA, Law AD, Routy B, den
Hollander N, Gupta V, Wang XH, Chaboureau A, Viswanathan S and
Keating A: A phase I trial of NK-92 cells for refractory
hematological malignancies relapsing after autologous hematopoietic
cell transplantation shows safety and evidence of efficacy.
Oncotarget. 8:89256–89268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grund EM and Muise-Helmericks RC: Cost
efficient and effective gene transfer into the human natural killer
cell line, NK92. J Immunol Methods. 296:31–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu H, Zhao X, Li Z, Hu Y and Wang H: From
CAR-T cells to CAR-NK cells: A developing immunotherapy method for
hematological malignancies. Front Oncol. 11:7205012021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klingemann HG, Wong E and Maki G: A
cytotoxic NK-cell line (NK-92) for ex vivo purging of leukemia from
blood. Biol Blood Marrow Transplant. 2:68–75. 1996.PubMed/NCBI
|
|
19
|
Fabian KP and Hodge JW: The emerging role
of off-the-shelf engineered natural killer cells in targeted cancer
immunotherapy. Mol Ther Oncolytics. 23:266–276. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu
T, Yin J, You F, Zhu M, Shen W, et al: First-in-man clinical trial
of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients
with relapsed and refractory acute myeloid leukemia. Am J Cancer
Res. 8:1083–1089. 2018.PubMed/NCBI
|
|
21
|
Lanza F, Maffini E, Rondoni M, Massari E,
Faini AC and Malavasi F: CD22 expression in B-cell acute
lymphoblastic leukemia: Biological significance and implications
for inotuzumab therapy in adults. Cancers (Basel). 12:3032020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haso W, Lee DW, Shah NN, Stetler-Stevenson
M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, FitzGerald DJ,
Barrett DM, et al: Anti-CD22-chimeric antigen receptors targeting
B-cell precursor acute lymphoblastic leukemia. Blood.
121:1165–1174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fry TJ, Shah NN, Orentas RJ,
Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S,
Delbrook C, Yates B, et al: CD22-targeted CAR T cells induce
remission in B-ALL that is naive or resistant to CD19-targeted CAR
immunotherapy. Nat Med. 24:20–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spiegel JY, Patel S, Muffly L, Hossain NM,
Oak J, Baird JH, Frank MJ, Shiraz P, Sahaf B, Craig J, et al: CAR T
cells with dual targeting of CD19 and CD22 in adult patients with
recurrent or refractory B cell malignancies: A phase 1 trial. Nat
Med. 27:1419–1431. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cordoba S, Onuoha S, Thomas S, Pignataro
DS, Hough R, Ghorashian S, Vora A, Bonney D, Veys P, Rao K, et al:
CAR T cells with dual targeting of CD19 and CD22 in pediatric and
young adult patients with relapsed or refractory B cell acute
lymphoblastic leukemia: A phase 1 trial. Nat Med. 27:1797–1805.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andris-Widhopf J, Rader C, Steinberger P,
Fuller R and Barbas CF III: Methods for the generation of chicken
monoclonal antibody fragments by phage display. J Immunol Methods.
242:159–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rader C and Barbas CF III: Phage display
of combinatorial antibody libraries. Curr Opin Biotechnol.
8:503–508. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee Y, Kim H and Chung J: An antibody
reactive to the Gly63-Lys68 epitope of NT-proBNP exhibits
O-glycosylation-independent binding. Exp Mol Med. 46:e1142014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niwa H, Yamamura K and Miyazaki J:
Efficient selection for high-expression transfectants with a novel
eukaryotic vector. Gene. 108:193–199. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF-α induced apoptosis is accompanied
with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr
Biol. 239:115–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sambrook J, FritscheE F and Maniatis T:
Molecular cloning: A laboratory manual. 3. Cold Spring Harbor
Laboratory Press; 1989
|
|
32
|
Saudemont A, Garçon F, Yadi H,
Roche-Molina M, Kim N, Segonds-Pichon A, Martín-Fontecha A,
Okkenhaug K and Colucci F: p110gamma and p110delta isoforms of
phosphoinositide 3-kinase differentially regulate natural killer
cell migration in health and disease. Proc Natl Acad Sci USA.
106:5795–5800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
https://www.law.go.kr
|
|
34
|
Jayaraman J, Mellody MP, Hou AJ, Desai RP,
Fung AW, Pham AHT, Chen YY and Zhao W: CAR-T design: Elements and
their synergistic function. EBioMedicine. 58:1029312020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zanetti SR, Velasco-Hernandez T,
Gutierrez-Agüera F, Díaz VM, Romecín PA, Roca-Ho H,
Sánchez-Martínez D, Tirado N, Baroni ML, Petazzi P, et al: A novel
and efficient tandem CD19- and CD22-directed CAR for B cell ALL.
Mol Ther. 30:550–563. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qin H, Ramakrishna S, Nguyen S, Fountaine
TJ, Ponduri A, Stetler-Stevenson M, Yuan CM, Haso W, Shern JF, Shah
NN and Fry TJ: Preclinical development of bivalent chimeric antigen
receptors targeting both CD19 and CD22. Mol Ther Oncolytics.
11:127–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee W, Syed Atif A, Tan SC and Leow CH:
Insights into the chicken IgY with emphasis on the generation and
applications of chicken recombinant monoclonal antibodies. J
Immunol Methods. 447:71–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoon A, Shin JW, Kim S, Kim H and Chung J:
Chicken scFvs with an artificial cysteine for site-directed
conjugation. PLoS One. 11:e01469072016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang C, Oberoi P, Oelsner S, Waldmann A,
Lindner A, Tonn T and Wels WS: Chimeric antigen receptor-engineered
NK-92 cells: An off-the-shelf cellular therapeutic for targeted
elimination of cancer cells and induction of protective antitumor
immunity. Front Immunol. 8:5332017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jurisic V, Colovic N, Konjevic G, Minic I
and Colovic M: An aggressive extramedullary cutaneous plasmacytoma
associated with extreme alterations in the innate immune system.
Onkologie. 33:113–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jurisic V, Srdic T, Konjevic G, Markovic O
and Colovic M: Clinical stage-depending decrease of NK cell
activity in multiple myeloma patients. Med Oncol. 24:312–317. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Konjević GM, Vuletić AM, Mirjačić
Martinović KM, Larsen AK and Jurišić VB: The role of cytokines in
the regulation of NK cells in the tumor environment. Cytokine.
117:30–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arias J, Yu J, Varshney M, Inzunza J and
Nalvarte I: Hematopoietic stem cell- and induced pluripotent stem
cell-derived CAR-NK cells as reliable cell-based therapy solutions.
Stem Cells Transl Med. 10:987–995. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gambella M, Carlomagno S, Raiola AM,
Giannoni L, Ghiggi C, Setti C, Giordano C, Luchetti S, Serio A, Bo
A, et al: CD19-targeted immunotherapies for diffuse large B-cell
lymphoma. Front Immunol. 13:8374572022. View Article : Google Scholar : PubMed/NCBI
|