Introduction
Gastric cancer is a digestive tract tumor that
seriously threatens human health and life and is the third leading
cause of cancer-associated death worldwide (1). In recent years, although the incidence
of gastric cancer has decreased worldwide, the incidence and
mortality rate of gastric cancer in China have both risen (2). Therefore, improving the diagnostic
rate of early gastric cancer and finding new therapeutic targets
and prognostic indicators are important directions of current
gastric cancer research.
SPARC, also known as osteoadhesive protein, is a
calcium-binding glycoprotein whose structure and function are
regulated by calcium ions. It belongs to the stromal cell protein
family, which inhibits cell adhesion and promotes tumor cell
proliferation, invasion and metastasis (3). The SPARC protein has three functional
domains: Amino acid terminal Ca-binding region, Cu-binding region
and extracellular Ca-binding region. Because of the three
functional regions of SPARC, it may participate in cell
proliferation and angiogenesis; however, to the best of our
knowledge, no previous study has determined whether serum calcium
and copper levels affect the growth of cancer (4). SPARC is mainly expressed in
mesenchymal cells and its expression may inhibit the synthesis of
mechanistic proteins and alter the composition and structure of
mesenchymal stroma (5). It may also
increase the synthesis of mechanistic degradation enzymes, thus
promoting tumor cells to break through the basement membrane of
blood vessels and lymphatic vessels for distant metastasis
(6). In addition, SPARC can induce
gastric tumor cells to become round in shape and have an
anti-adhesion role, thereby improving the invasive ability of
gastric tumor cells (7).
In most studies on cancer published to date, SPARC
was indicated to be abnormally expressed and closely related to the
biological behavior of malignant tumors, such as cancer cell
invasion and metastasis. SPARC inhibits the mitogenic effect of
VEGF in human microvascular endothelium and reduces the tyrosine
phosphorylation of the protein kinase activated by VEGF, further
attenuating the activity of VEGF (8). High expression of SPARC in gastric
cancer mesenchyme inhibits the progression of gastric cancer, while
VEGF promotes the development of this type of cancer. SPARC
inhibits tumor angiogenesis by regulating VEGF expression. As the
expression of SPARC decreases, the inhibitory effect gradually
decreases and tumor microvessels become more and more numerous,
causing cancer cells to metastasize to distant sites (9). SPARC regulates extracellular matrix
components and has a role in promoting the secretion of TGF-β1
protein, which reduces the adherence of gastric tumor cells,
enhances their concentration dependence, and inhibits their growth
and replication (10).
Previous studies have indicated that SPARC
expression is elevated in colorectal, renal and prostate cancers
(11–13). High SPARC expression is closely
associated with tumor development and has an important role in
tumor invasion and metastasis, leading to poor prognosis. In the
present study, a meta-analysis and bioinformatics analysis were
performed to analyze the relationship between SPARC expression and
the clinicopathological characteristics and prognosis of gastric
cancer.
Materials and methods
Literature search and data
extraction
The published literature was obtained by searching
the Pubmed and CNKI databases (May 2022) using the following key
words: ‘SPARC’ AND ‘gastric’ (OR ‘stomach’) AND ‘cancer’ (OR
‘carcinoma’ OR ‘tumor’). The inclusion criteria were as follows: i)
Patients with gastric cancer; ii) immunohistochemical staining for
SPARC expression; iii) article containing SPARC protein expression
and clinicopathological features; iv) none of the patients received
any medical treatment prior to surgery. The exclusion criteria were
as follows: i) Patients received chemotherapy, radiation therapy or
other treatment prior to surgery; ii) the article type was
abstract, case report, review or meeting; iii) SPARC expression was
detected by western blot or reverse transcription (RT)-PCR; iv)
repeated publications.
Data extraction and quality
assessment
The main information of the articles was extracted
by two authors (JS and ZGF). The information extracted included the
following: First author, year of publication, country, antibody
company, number of cases and controls, expression changes and
quality score assessment. The quality scores of the included
articles were independently completed by two authors based on the
Newcastle Ottawa Oncomine Scale (14).
Bioinformatics analysis
Using the Human Protein Atlas (HPA) database
(www.proteinatlas.org), the protein
expression of SPARC in normal gastric tissues and gastric cancer
tissues was analyzed and the survival curves of patients with
gastric cancer were drawn. The prognostic significance of SPARC
mRNA expression was analyzed in gastric cancer using the
Kaplan-Meier (KM)-plotter database (https://kmplot.com/analysis). The Oncomine database
(www.oncomine.org) was used to analyze the SPARC
mRNA expression levels in gastric cancer tissues and normal
tissues. According to The Cancer Genome Atlas (TCGA) database
(www.cancer.gov), the raw data were integrated,
the expression of SPARC mRNA in gastric cancer was analyzed and its
association with the clinicopathological and prognostic data of
patients was determined. SPARC expression in gastric cancer was
also analyzed using the Gene Expression Profiling Interactive
Analysis (GEPIA; http://gepia.cancer-pku.cn/) and University of ALabama
at Birmingham CANcer (UALCAN; http://ualcan.path.uab.edu/index.html) databases and
the relationship between SPARC expression and patient prognosis was
examined. The TIMER database (https://cistrome.shinyapps.io/timer/) was used to
investigate the relationship between SPARC gene expression and
clinical outcomes and immune-cell infiltration.
Statistical analysis
Revman version 5.3 (The Cochrane Institute) was used
for the present analysis. The expression of SPARC was estimated
using odds ratios (ORs) and 95% CIs. The heterogeneity of included
articles was tested with the χ2 test and if there was no
statistically significant heterogeneity among studies (P>0.1,
I2≤50%), a fixed-effects model was used. If there was
significant heterogeneity among the studies (P<0.1,
I2≥50%), a random-effects model was used. Funnel plots
were drawn to assess publication bias and its asymmetry was
quantified using Begg's test and Egger's test. Cox risk regression
models were used for the univariate and multivariate analyses. This
model analyzed the effect of risk factors, the hazard ratio and 95%
CI. P<0.05 was considered to indicate a statistically
significant difference. All data analyses were performed with SPSS
17.0 software (SPSS, Inc.).
Results
Characteristics of included
studies
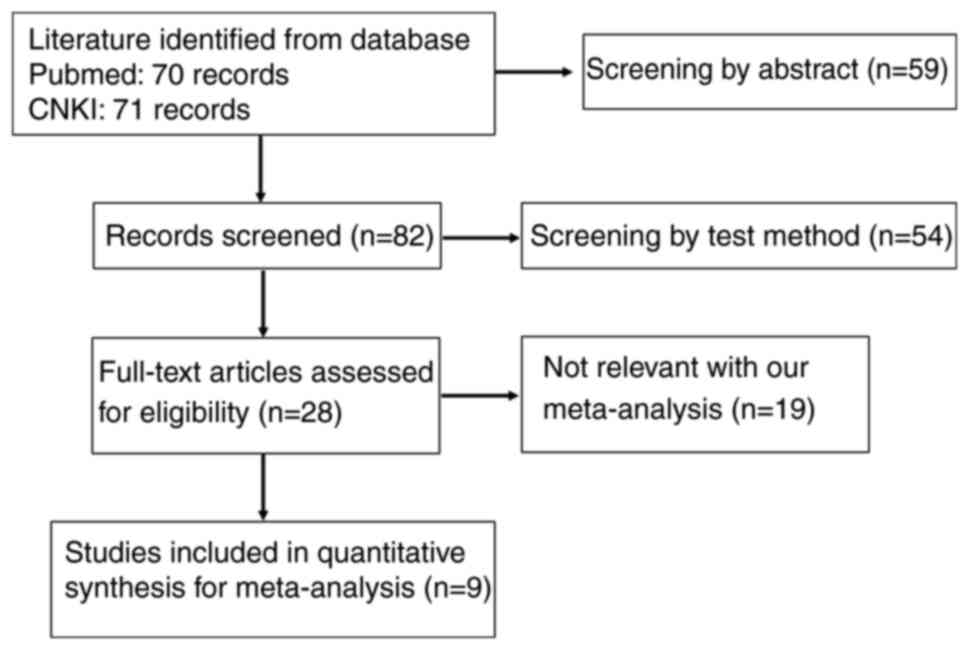
As illustrated in Fig.
1, a total of 9 eligible studies were chosen for inclusion in
the present analysis (9,10,15–21).
The main characteristics of the included studies are presented in
Table I. Information on the
expression of SPARC in gastric cancer tissues, as well as
clinicopathological characteristics of patients, including
histological type, tumor location, TNM stage, degree of
differentiation, distant metastasis, lymph node metastasis, gender
and age, was extracted.
 | Table I.Main characteristics of studies
included in the present meta-analysis. |
Table I.
Main characteristics of studies
included in the present meta-analysis.
| First author | Year | Country | Antibody
supplier | Cases | Controls | Risk of cancer
associated with high SPARC expression | Quality (NOS) | (Refs.) |
|---|
| Li | 2014 | China | BIOSS | 65 | 90 | Increased | 7 | (10) |
| Li | 2014 | China | NS | 46 | 61 | Increased | 8 | (15) |
| Yang | 2012 | China | ZSGB-BIO | 61 | 80 | Increased | 8 | (9) |
| Dong | 2011 | China | BIOSS | 95 | 123 | Increased | 8 | (17) |
| Li | 2015 | China | ZYMED | 95 | 108 | Increased | 8 | (16) |
| Ma | 2019 | China | CST | 91 | 192 | Increased | 7 | (18) |
| Franke | 2009 | China | Germany | 38 | 40 | Increased | 8 | (19) |
| Wang | 2004 | China | ZYMED | 25 | 40 | Increased | 8 | (20) |
| Zhao | 2009 | China | Santa Cruz | 144 | 436 | Increased | 8 | (21) |
|
|
|
| Biotechnology,
Inc. |
|
|
|
|
|
Association between SPARC expression
and clinicopathological characteristics of patients with gastric
cancer
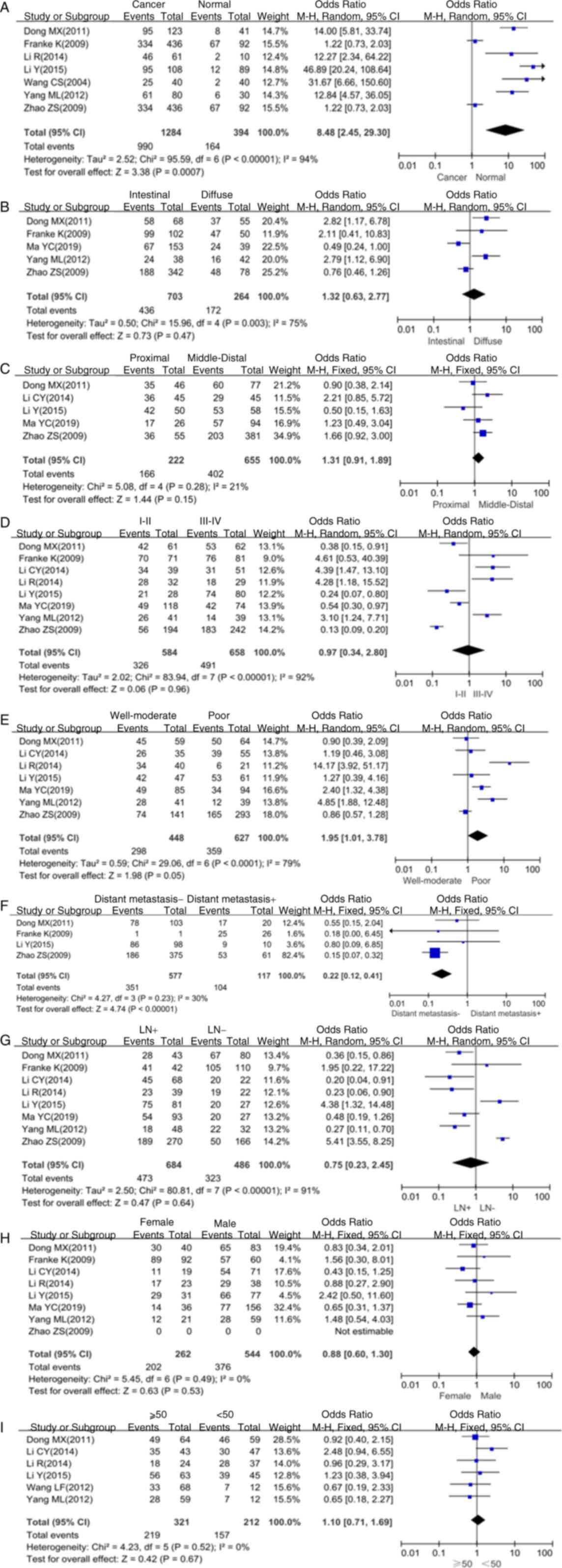
Forest plots for the association of SPARC with
various parameters of patients with gastric cancer are provided in
Fig. 2. The expression of SPARC in
gastric cancer tissue was higher than that in normal gastric
mucosal tissue (P<0.05; Fig.
2A). The meta-analysis further indicated that SPARC expression
was not associated with the histological type (P>0.05; Fig. 2B), tumor location (P>0.05;
Fig. 2C), TNM stage (P>0.05;
Fig. 2D), lymph node metastasis
(P>0.05; Fig. 2G), gender
(P>0.05; Fig. 2H) and age
(P>0.05; Fig. 2I). However,
SPARC was associated with the degree of differentiation (P<0.05;
Fig. 2E) and distant metastasis
(P<0.05; Fig. 2F).
Publication bias
As presented in Fig.
3, funnel plots were used to detect heterogeneity among
studies. Sensitivity analysis is used to assess the impact of a
single study on summary results, deleting one study at a time from
the summary analysis. Based on Egger's test, there was no
significant publication bias in the present meta-analysis.
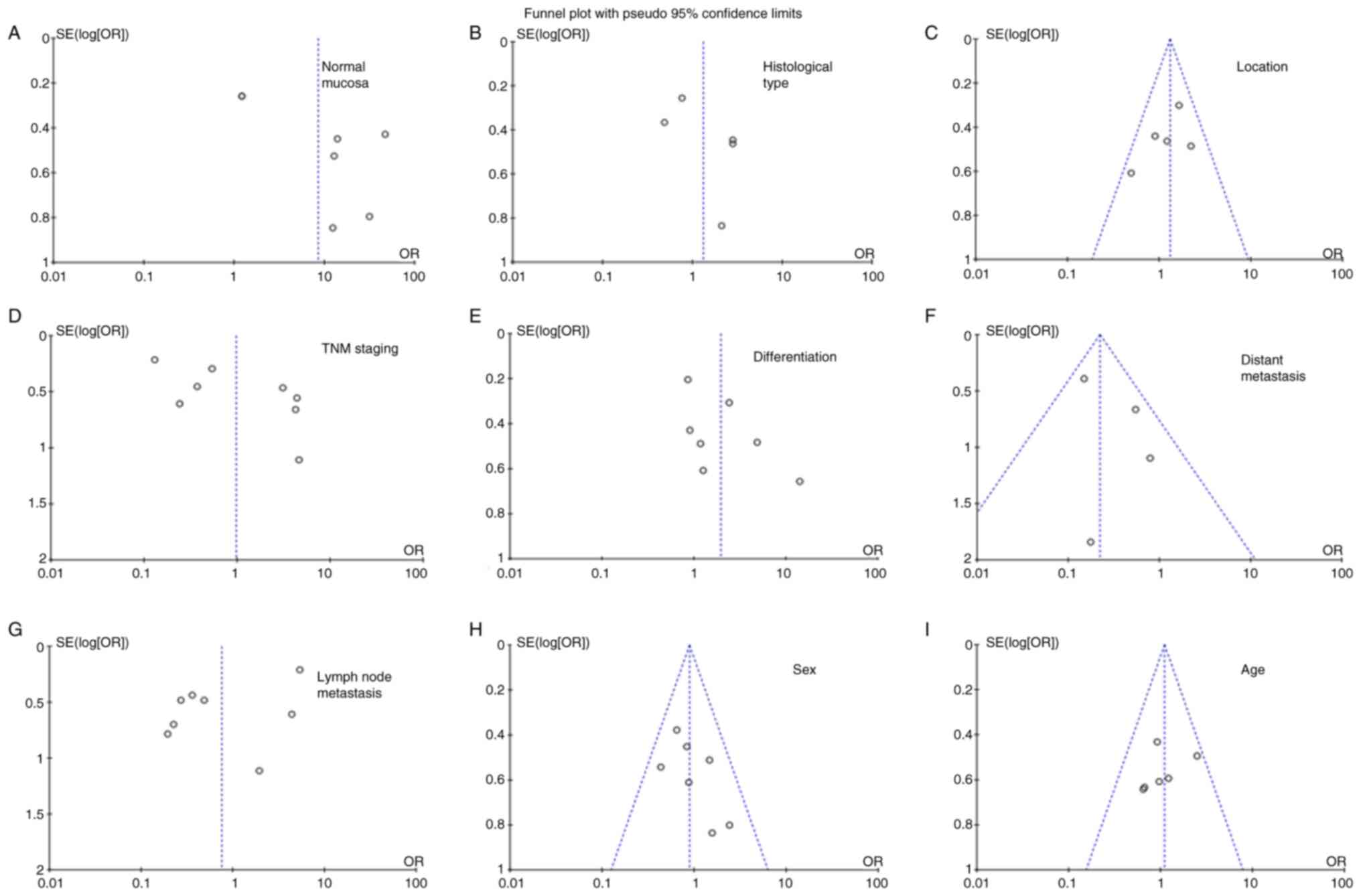 | Figure 3.Funnel plot for testing publication
bias for the association of SPARC with gastric cancer. Publication
bias was also tested for the association between SPARC expression
and clinicopathological features of gastric cancer, including (A)
gastric mucosa, (B) histological type, (C) location, (D) TNM
staging, (E) differentiation, (F) distant metastasis, (G) lymph
node metastasis, (H) sex and (I) age. SPARC, secreted protein
acidic and rich in cysteine; OR, odds ratio; SE, standard
error. |
Clinicopathological and prognostic
significance of SPARC expression in gastric cancers
According to KM-plotter, it was indicated that
higher SPARC expression was negatively associated with overall
survival (OS) rates (P<0.05; Fig.
4A), even after the stratification of the patients by TNM
stage, distant metastasis, perforation, treatment, Lauren's
classification and Her2 status (P<0.05; Table II). These findings were the same
for female patients, N1-N3 stage and well-differentiated tumors
(P<0.05; Table II). In
addition, higher SPARC expression was negatively associated with
post-progression survival rates (P<0.05; Fig. 4A), even after the stratification of
the patients by lymph node metastasis, distant metastasis and
Lauren's classification (P<0.05; Table II). This was also the same for
female patients with gastric cancer, those with TNM stage II–IV,
treated with surgery alone or adjuvant therapy other than 5-FU, as
well as Her2-negative tumors (P<0.05; Table II). The first progression survival
rate during the entire treatment period of the patients with high
expression of SPARC was significantly lower than that in the low
expression group, and this was also the case in the subgroups
stratified by tumor size and treatment (P<0.05; Fig. 4A and Table II).
 | Table II.Prognostic significance of secreted
protein acidic and rich in cysteine mRNA (high vs. low) in gastric
cancer. |
Table II.
Prognostic significance of secreted
protein acidic and rich in cysteine mRNA (high vs. low) in gastric
cancer.
|
| Overall
survival | Post-progression
survival | First
progression |
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Hazard ratio | P-value | Hazard ratio | P-value | Hazard ratio | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Female | 1.80
(1.26-2.57) | 0.0011 | 2.05
(1.33-3.17) | 0.00093 | 1.84
(1.25-2.73) | 0.0019 |
|
Male | 1.22
(0.99-1.51) | 0.065 | 1.26
(0.97-1.63) | 0.087 | 1.17
(0.91-1.51) | 0.23 |
| TNM staging |
|
|
|
|
|
|
| 1 | 0.21
(0.05-0.95) | 0.026 | 0.30
(0.04-2.55) | 0.25 | 0.27
(0.06-1.22) | 0.068 |
| 2 | 2.08
(1.03-4.22) | 0.037 | 2.29
(1.10-4.77) | 0.023 | 1.77
(0.91-3.46) | 0.088 |
| 3 | 1.50
(1.12-2.01) | 0.0066 | 2.40
(1.55-3.70) |
4.7×10−5 | 1.80
(1.24-2.60) | 0.0017 |
| 4 | 1.62
(1.10-2.38) | 0.013 | 1.77
(1.10-2.84) | 0.017 | 1.47
(0.97-2.22) | 0.066 |
| Tumor stage |
|
|
|
|
|
|
| 2 | 1.90
(1.24-2.92) | 0.0028 | 2.11
(1.34-3.32) | 0.00094 | 1.74
(1.14-2.64) | 0.0089 |
| 3 | 1.55
(1.09-2.22) | 0.015 | 1.67
(1.14-2.45) | 0.0081 | 1.46
(1.03-2.07) | 0.034 |
| 4 | 3.72
(1.23-11.26) | 0.013 | 1.56
(0.62-3.94) | 0.34 | 3.22
(1.43-7.25) | 0.0032 |
| Nodal stage |
|
|
|
|
|
|
| 0 | 1.67
(0.71-3.96) | 0.24 | 3.28
(1.00-10.68) | 0.038 | 1.66
(0.70-3.93) | 0.24 |
|
1-3 | 2.08
(1.60-2.72) |
3.5×10−8 | 2.16
(1.61-2.90) |
1.4×10−7 | 1.97
(1.53-2.55) |
1.2×10−7 |
| 1 | 2.44
(1.61-3.71) |
1.4×10−5 | 2.50
(1.58-3.95) |
5.1×10−5 | 2.30
(1.55-3.42) |
2.1×10−5 |
| 2 | 1.89
(1.20-2.99) | 0.0057 | 1.81
(1.11-2.94) | 0.015 | 2.07
(1.31-3.27) | 0.0015 |
| 3 | 1.89
(1.09-3.71) | 0.021 | 1.94
(1.08-3.47) | 0.023 | 1.44
(0.85-2.43) | 0.17 |
| Metastasis
stage |
|
|
|
|
|
|
| 0 | 1.80
(1.36-2.39) |
3.7×10−5 | 2.07
(1.52-2.82) |
2.0×10−6 | 1.76
(1.34-2.31) |
3.3×10−5 |
| 1 | 1.95
(1.08-3.54) | 0.025 | 3.18
(1.41-7.18) | 0.0036 | 1.38
(0.76-2.51) | 0.29 |
| Perforation |
|
|
|
|
|
|
| - | 1.66
(1.12-2.48) | 0.012 | 1.37
(0.74-2.52) | 0.31 | 1.63
(1.11-2.40) | 0.011 |
| Treatment |
|
|
|
|
|
|
| Surgery
alone | 1.71
(1.23-2.38) | 0.0014 | 1.85
(1.35-2.53) |
8.8×10−5 | 1.56
(1.17-2.09) | 0.0022 |
|
5-FU-based adjuvant | 0.58
(0.39-0.87) | 0.0069 | 0.72
(0.48-1.08) | 0.11 | 0.62
(0.42-0.92) | 0.016 |
| Other
adjuvant | 4.21
(1.74-10.19) | 0.00053 | 4.11
(1.69-10.02) | 0.00075 | 3.75
(1.71-8.23) |
4.0×10−4 |
| Degree of
differentiation |
|
|
|
|
|
|
|
Well-differentiated | 2.97
(1.24-7.11) | 0.01 | - | - | - | - |
|
Moderately differentiated | 1.59
(0.79-3.21) | 0.19 | 0.61
(0.24-1.58) | 0.31 | 1.76
(1.33-3.45) | 0.096 |
| Poorly
differentiated | 1.38
(0.91-2.11) | 0.13 | 0.69
(0.37-1.30) | 0.25 | 1.53
(0.94-2.51) | 0.087 |
| Lauren's
classification |
|
|
|
|
|
|
|
Intestinal type | 1.69
(1.16-2.45) | 0.0054 | 1.57
(1.04-2.38) | 0.03 | 1.56
(1.09-2.22) | 0.014 |
| Diffuse
type | 2.17
(1.53-3.06) |
7.8×10−6 | 2.33
(1.58-3.42) |
1.0×10−5 | 2.07
(1.46-2.94) |
2.9×10−5 |
| Mixed
type | 2.77
(0.97-7.93) | 0.049 | - | - | 2.21
(0.79-6.20) | 0.12 |
| Her2 status |
|
|
|
|
|
|
| - | 1.43
(1.14-1.81) | 0.0023 | 1.87
(1.39-2.52) |
3.1×10−5 | 1.42
(1.09-1.85) | 0.0085 |
| + | 1.45
(1.08-1.96) | 0.013 | 1.36
(0.93-1.98) | 0.11 | 1.29
(0.91-1.82) | 0.16 |
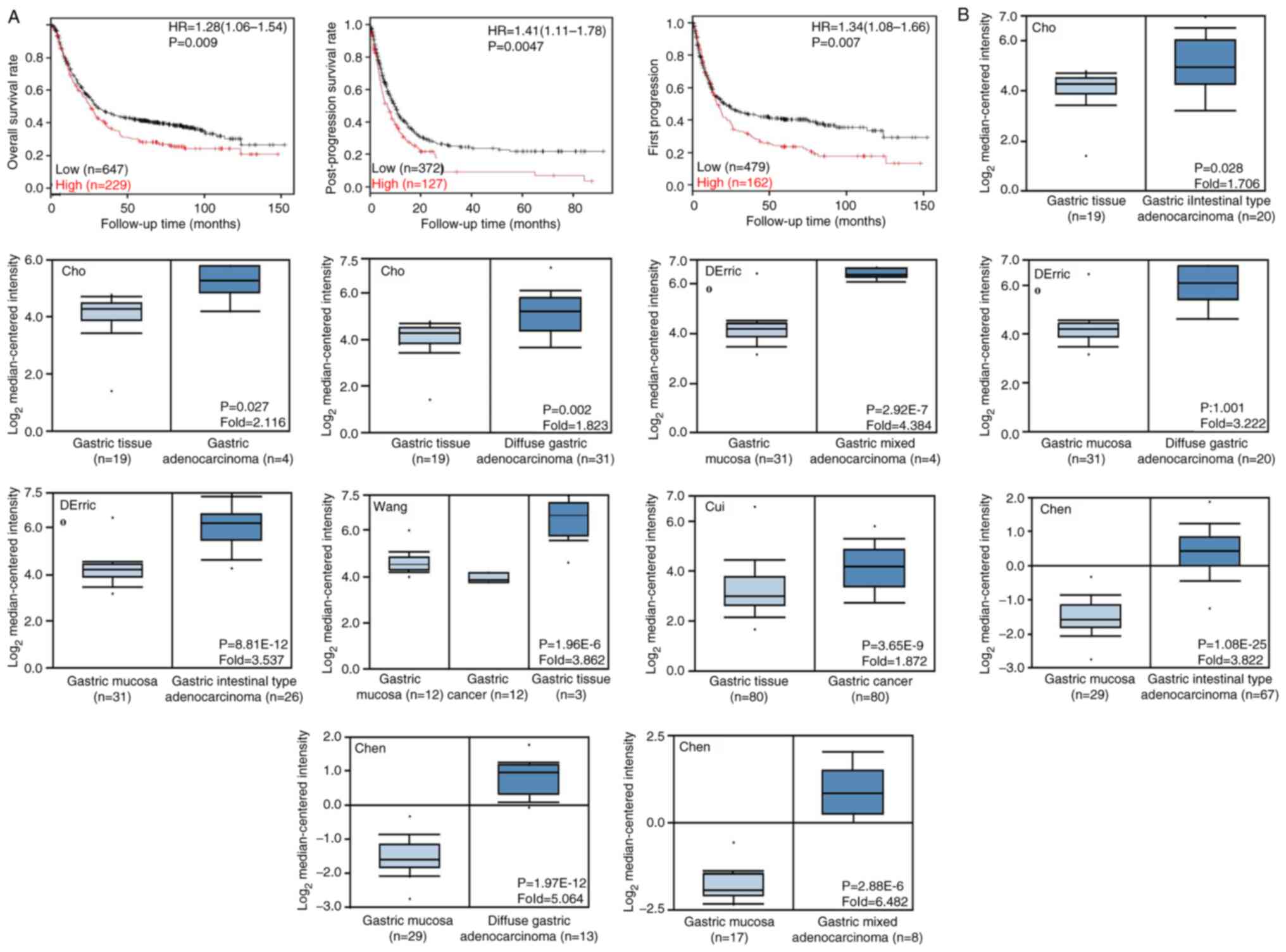
According to the Oncomine database, SPARC mRNA
expression was higher in gastric cancer tissue compared with that
in normal tissues (P<0.05; Fig.
4B), even when cases were stratified as diffuse, intestinal and
mixed-type carcinoma. Higher SPARC expression was negatively
associated with OS rates based on the UALCAN database (P<0.05;
Fig. 5A). Based on the UALCAN
database, SPARC was also indicated to be highly expressed in
gastric cancer tissues and high SPARC expression was strongly
associated with grade, stage, lymph node metastasis, TP53 status
and Helicobacter pylori infection status (Fig. 5B-G), but there was no significant
difference between sexes or among different age groups (Fig. 5H and I).
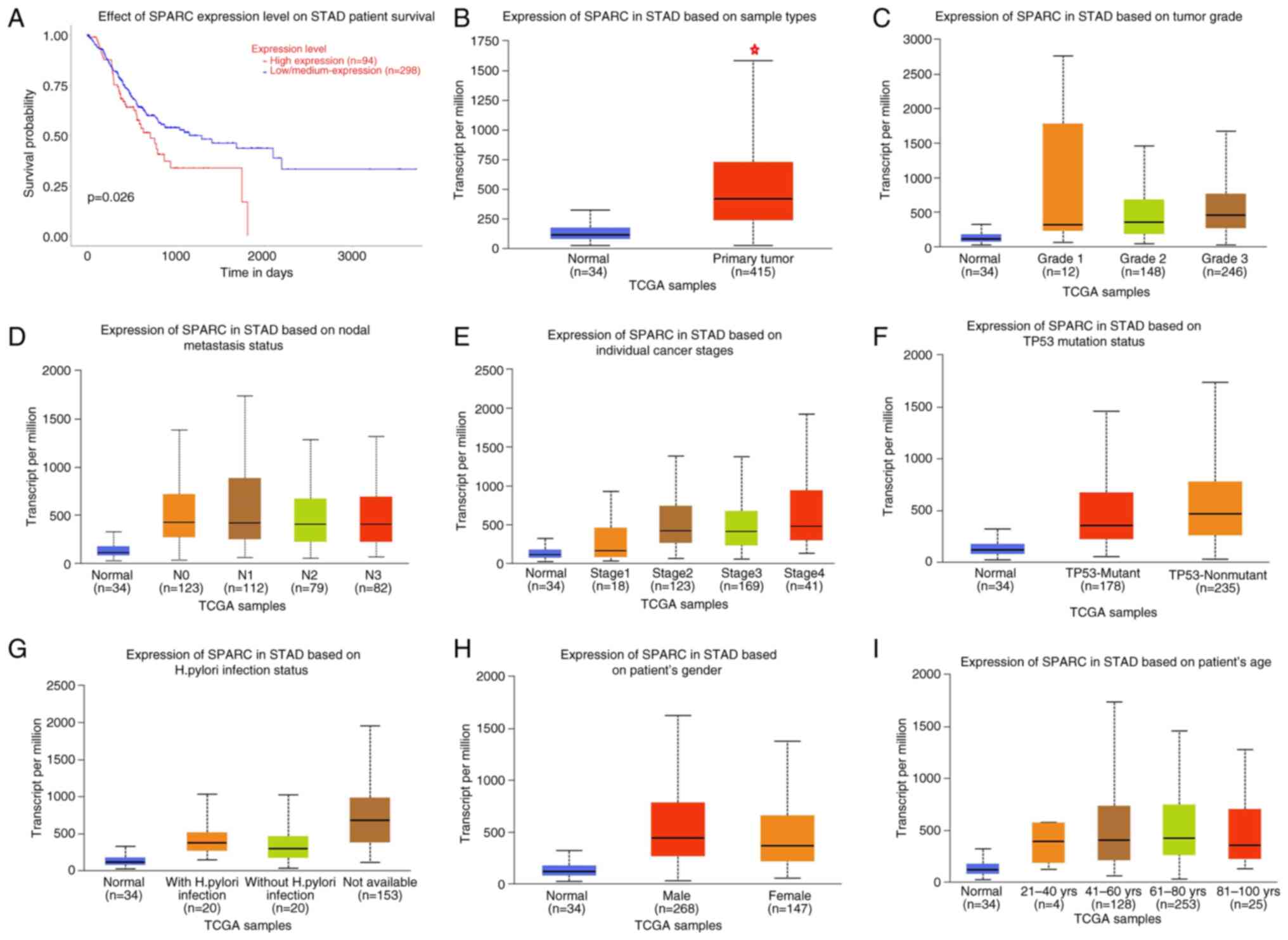 | Figure 5.Prognostic value of SPARC mRNA
expression in patients with gastric cancer according to the UALCAN
database. (A) Overall survival, (B) SPARC was higher expressed in
gastric cancer than normal tissues, (C) tumor grade, (D) node
metastasis status, (E) individual cancer stages, (F) TP53 mutation
status, (G) Helicobacter pylori infection status, (H) gender
and (I) age. *P<0.05. SPARC, secreted protein acidic and rich in
cysteine; TCGA, The Cancer Genome Atlas; STAD, stomach
adenocarcinoma. |
The GEPIA database indicated that SPARC expression
was higher in gastric cancer than in normal tissue and was
positively correlated with the TNM stage (P<0.05; Fig. 6A-C).
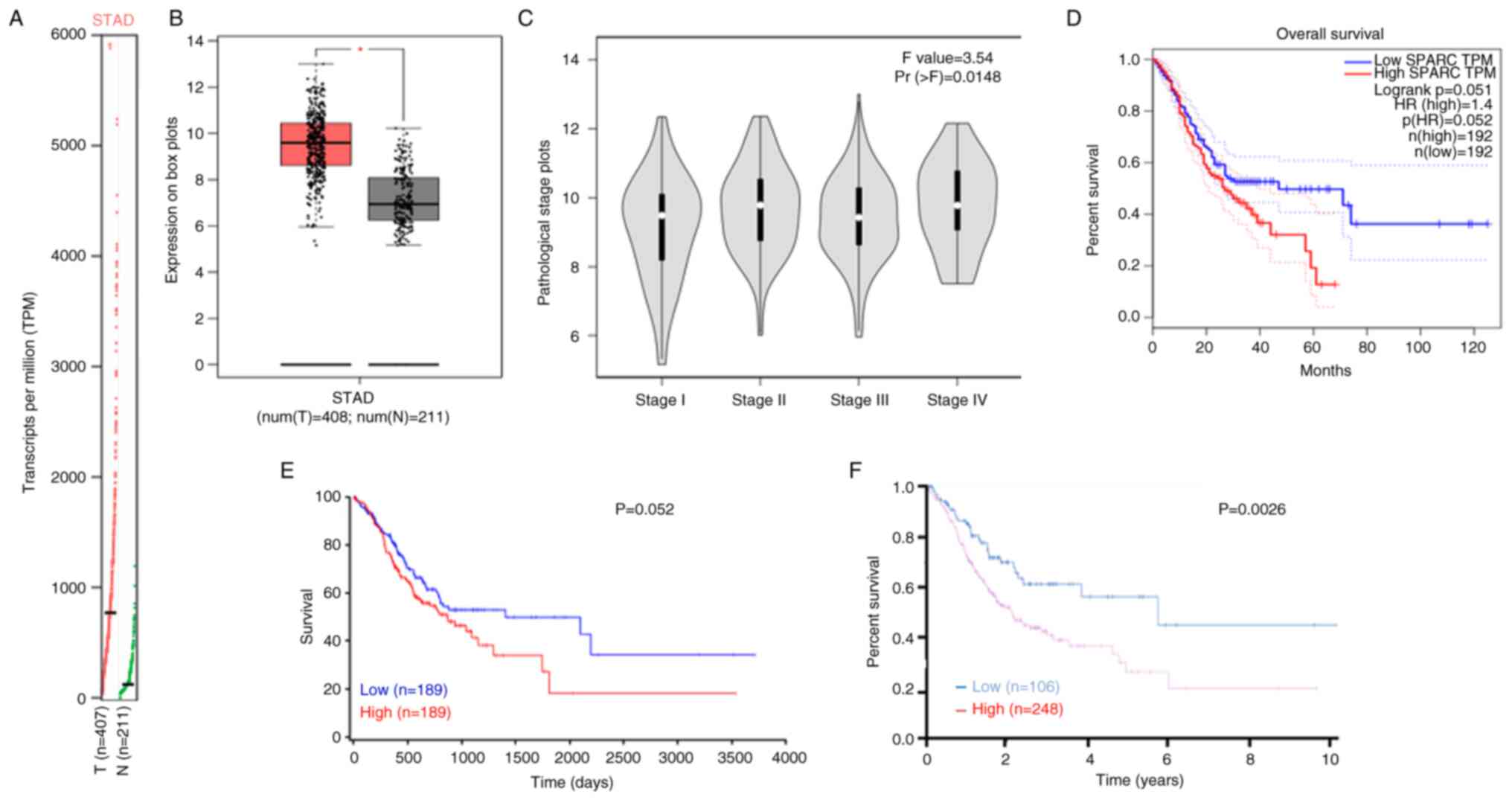 | Figure 6.Prognostic value of SPARC mRNA
expression in patients with gastric cancer according to the GEPIA,
HPA and ONCOLNC databases. Based on the gene expression Profile (A)
and box plots (B) in the GEPIA database, we found that SPARC
expression was higher in gastric cancer than in normal tissue and
(C) was negatively correlated with the TNM stage. The relationship
between SPARC expression in gastric cancer and overall survival of
patients based on the (D) GEPIA, (E) ONCOLNC and (F) HPA databases.
*P<0.05. T, tumor; N, normal; SPARC, secreted protein acidic and
rich in cysteine; HR, hazard ratio; GEPIA, Gene Expression
Profiling Interactive Analysis; HPA, Human Protein Atlas; STAD,
stomach adenocarcinoma. |
SPARC expression was not significantly associated
with OS according to the GEPIA and ONCOLNC databases (P>0.05;
Fig. 6D and E); however, higher
SPARC expression was negatively associated with OS rates based on
the HPA (P<0.05; Fig. 6F). A
univariate survival analysis performed in the TCGA database
indicated that distant metastasis and lymph node metastasis were
positively associated with the prognosis of patients with gastric
cancer (P<0.05; Table III).
Furthermore, multivariate analysis suggested that high SPARC
expression, age and distant metastasis were important factors
affecting patients' survival (P<0.05; Table IV). An analysis in the Timer
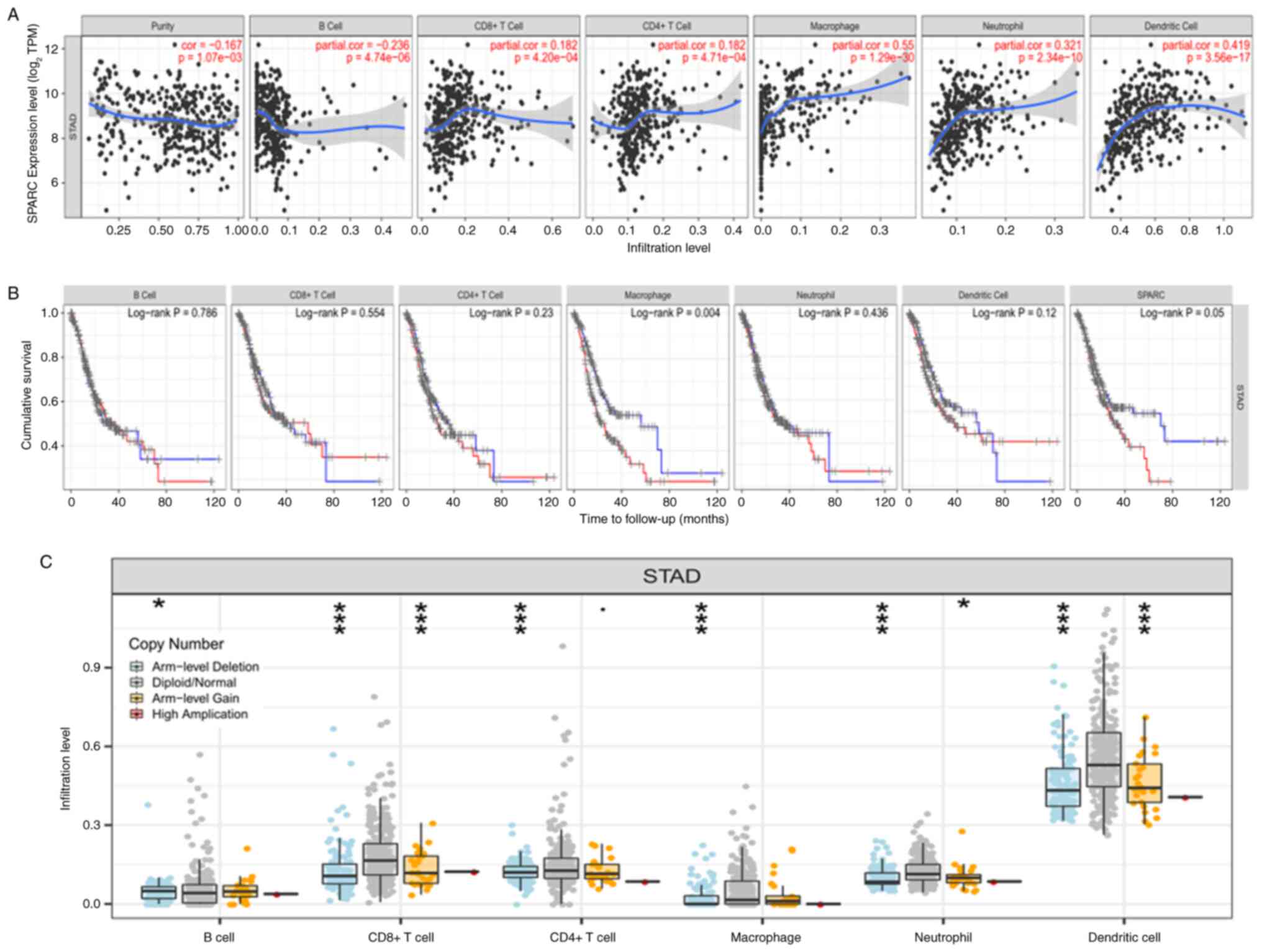
database indicated that SPARC expression was closely associated
with the proportion of 7 immune-cell infiltrates in gastric cancer
(B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils and
dendritic cells; P<0.05; Fig.
7A). When the degree of macrophage infiltration decreases, the
survival time of cancer patients increases. However, survival and
prognosis in cancer patients were negatively correlated with SPARC
expression (P<0.05; Fig. 7B). As
presented in Fig. 7C, based on DNA
copy variation data, it was found that diploid/normal is more
common than deep deletion in CD8+, CD4+T cells, macrophages,
neutrophils and dendritic cells, which is related to the deletion
and gain at arm level.
 | Table III.Univariate analysis of prognostic
risk factors in patients with gastric cancer. |
Table III.
Univariate analysis of prognostic
risk factors in patients with gastric cancer.
| Characteristic | Patients, n
(%) | Relative risk (95%
CI) | P-value |
|---|
| Sex |
|
|
|
|
Female | 127 (37.1) | Ref. |
|
|
Male | 215 (62.9) | 1.030
(0.696-1.524) | 0.883 |
| Age, years |
|
|
|
|
<60 | 114 (33.3) | Ref. |
|
|
≥60 | 228 (66.7) | 1.342
(0.880-2.049) | 0.173 |
| TNM stage |
|
|
|
|
I/II | 46 (14.1) | Ref. |
|
|
III/IV | 280 (85.9) | 1.605
(0.857-3.006) | 0.139 |
| Invasion |
|
|
|
| - | 17 (5.0) | Ref. |
|
| + | 322 (95.0) | 6.58
(0.915-47.62) | 0.061 |
| Lymph node
metastasis |
|
|
|
| - | 107 (32.4) | Ref. |
|
| + | 223 (67.6) | 2.066
(1.279-3.344) | 0.003 |
| Distant
metastasis |
|
|
|
| - | 303 (92.9) | Ref. |
|
| + | 23 (7.1) | 3.040
(1.686-5.464) | <0.001 |
| Degree of
differentiation |
|
|
|
|
Well-differentiated | 10 (2.4) | Ref. |
|
|
Moderately-poorly
differentiated | 400 (97.6) | 1.193
(0.946-1.684) | 0.314 |
 | Table IV.Multivariate analysis of
clinicopathological variables influencing the survival of patients
with gastric cancer. |
Table IV.
Multivariate analysis of
clinicopathological variables influencing the survival of patients
with gastric cancer.
| Clinicopathological
parameters | Relative risk (95%
CI) | P-value |
|---|
| SPARC expression
(+) | 0.014
(0.001-0.190) | 0.001 |
| Age (≥60
years) | 1.857
(1.105-2.999) | 0.011 |
| Sex (female) | 0.898
(0.581-1.390) | 0.631 |
| Depth of invasion
(T2-4) | 5.451
(0.687-43.275) | 0.109 |
| Lymph node
metastasis (+) | 1.892
(0.982-3.645) | 0.057 |
| Distant metastasis
(+) | 3.056
(1.555-6.006) | 0.001 |
| TNM staging
(III/IV) | 0.649
(0.257-1.636) | 0.359 |
Discussion
SPARC is a calcium-binding glycoprotein with
multiple functions, promoting the following effects: Tumor-cell
detachment, invasion and metastasis, matrix composition changes,
basement membrane degradation and endothelial cell migration, and
angiogenesis and cell growth stimulation (22). As a potential cell cycle inhibitor,
SPARC may act as a cyclin to cause cell cycle arrest in G1 phase.
The carboxyl terminal of region IV in the extracellular calcium
binding region of SPARC contains a calcium binding site with high
affinity, which may release active peptides after binding with
calcium to act on endothelial cells and inhibit the proliferation
of endothelial cells, thus affecting the biological activity of
SPARC (23). It may bind to the
dimer of platelet-derived growth factor (PDGF), thereby changing
the structure of the dimer, which hinders the binding of PDGF to
cell surface receptors, leading to the regulation of cell growth
(24). In ovarian cancer, SPARC may
inhibit the activation of ERK mediated by β-fibroblast growth
factor and VEGF in endothelial cells and may also inhibit the
phosphorylation levels of MAPK and ERK (25).
The strongly positive expression rate and score of
SPARC in liver cancer tissues were significantly higher than those
in normal liver tissues, which may promote tumor invasion and
metastasis, demonstrating that it may act as a diagnostic tool for
liver cancer. In addition, SPARC expression was different in liver
cancer tissues with different Tumor, Nodes, American Joint
Committee on Cancer stages and tumor differentiation. Univariate
and multivariate survival analyses indicated that strongly positive
expression of SPARC was a prognostic factor affecting OS and
disease-free survival of patients with liver cancer, providing
evidence that the SPARC expression pattern may aid the
prognostication of liver cancer (26–28).
A previous study indicated that the expression of
SPARC was significantly higher in colorectal cancer tissues, and
was positively associated with tumor differentiation and
metastasis, suggesting that SPARC has a role in tumor invasion and
metastasis (29). Studies on colon
cancer suggested that SPARC has the effect of inhibiting tumor
angiogenesis, which is related to the expression of VEGF. The
function of SPARC in tumors is the same: SPARC has the effect of
inhibiting tumor angiogenesis, but it has a significant negative
correlation with the expression of VEGF. It is well-known that VEGF
is able to promote tumor blood angiogenesis; therefore, SPARC is
mainly expressed in tumor stroma, and the anti-angiogenesis effect
of SPARC may be achieved by regulating the expression of VEGF
(29,30). SPARC is associated with distant
metastasis and lymph node metastasis in malignant melanoma and may
be used as a predictor of melanoma prognosis.
Another study, which used a transgenic mouse model,
demonstrated that high expression of SPARC in prostate stromal
adenocarcinoma inhibits the growth of cancer cells by affecting the
cell cycle, thereby limiting the progression of prostate cancer
(31,32). The expression of SPARC in breast
cancer tissues is higher than that in normal tissues and its
expression in tumor stromal cells is even higher than that in tumor
cells. Studies have indicated that SPARC expression is positively
associated with histological grade and TNM stage: Univariate
analysis and Cox multivariate analysis suggested that lymph node
metastasis, distant metastasis, age and TNM stage were negatively
associated with the prognosis of patients with breast cancer.
However, analysis with KM-plotter indicated that low expression of
SPARC was negatively associated with OS, post-progression survival
and distant metastasis. Metastasis and TNM stage are important
factors affecting the survival time of patients with breast cancer
and SPARC expression may be a good indicator of prognosis in
patients with breast cancer (33).
At the same time, SPARC expression was found in the MDA-MB-231
breast cancer cell line and to promote breast cancer metastasis to
the lung (34).
Previous studies used small interfering RNA to
inhibit SPARC in high-expressing cell lines and found that the
proliferation and survival of gastric cancer cells in the
SPARC-knockdown group were significantly reduced, which was
confirmed in a tumor formation experiment in nude mice (35,36).
The detection of 10 commonly used gastric cancer cell lines
indicated that 8 cell lines had SPARC promoter methylation and 7 of
them had loss of SPARC expression. After pyrimidine nucleoside
treatment, SPARC expression was restored to normal levels in all
cell lines. The phenomenon of SPARC promoter methylation provides a
new direction for gastric cancer prognostication and selection of
therapeutic targets (37). Liao
et al (38) and Li et
al (39) found SPARC, one of
the genes closely related to the occurrence of gastric cancer,
through database screening of differentially expressed genes, and
determined that the SPARC gene is closely related to the prognosis
of patients with gastric cancer through KM-plotter and TCGA
database analyses. However, in the present study, the expression of
SPARC in gastric cancer tissue was examined through a meta-analysis
and the relationship between its expression and clinical
characteristics of patients was analyzed. The TCGA and KM-plotter
databases were also used to analyze the prognosis of patients with
SPARC gene expression, but the relationship between SPARC mRNA
expression and clinicopathological characteristics of patients with
gastric cancer was assessed in more detail. Furthermore, the
expression of SPARC protein and mRNA in gastric cancer was examined
in three additional databases, GEPIA, HPA and UALCAN, making the
results more convincing. The influence of SPARC on immune-cell
infiltration in gastric cancer was also analyzed through the Time
database. The present results comprehensively explain the
significance of the SPARC gene for patients with gastric cancer and
lay a foundation for future research.
The expression of SPARC mRNA in gastric cancer
tissue as determined by RT-PCR was significantly higher than that
in normal tissue and immunohistochemical analysis demonstrated that
SPARC was highly expressed in interstitial cells, while its
expression was lower in normal mucosal cells in tumor nuclei. High
SPARC expression was negatively associated with TNM stage and lymph
node metastasis (40,41). This is consistent with the present
analysis, which indicated that SPARC expression was higher in
gastric cancer tissues than in normal tissues. High SPARC
expression was negatively associated with the degree of
differentiation and distant metastasis. Furthermore, the KM-plotter
analysis indicated that high SPARC mRNA expression was negatively
associated with OS, post-progression and first progression survival
rates of patients. Univariate analysis suggested that lymph node
metastasis and distant metastasis were associated with the
prognosis of patients with gastric cancer. Cox multifactorial
analysis indicated that high SPARC expression, age and distant
metastasis were independent factors affecting the survival time of
patients with gastric cancer. In the TCGA database, the number of
stratified samples of indicators included is not completely
consistent, which may have led to inconsistent results regarding
the TNM stage, lymph node metastasis and distant metastasis in the
univariate analysis. Univariate analysis is the effect of each
clinicopathological feature on prognosis. Multivariate analysis is
a statistical analysis method that takes prognosis as the dependent
variable and other pathological characteristics as independent
variables, establishes a linear or nonlinear mathematical model to
estimate the quantitative relationship between multiple variables,
and uses sample data for analysis. Therefore, the results of the
single-factor and multi-factor analysis may be different.
In conclusion, SPARC protein and mRNA expression is
upregulated in gastric cancer. SPARC is positively associated with
lymph node metastasis and distant metastasis of gastric cancer.
High expression of SPARC may be a potential marker of tumorigenesis
and metastasis in patients with gastric cancer.
Acknowledgements
Not appliable.
Funding
Funding: No funding was received.
Availability data and materials
All data generated or analyzed during this study are
included in this published article. The expression level of SPARC
was analyzed using Oncomine. In addition, the prognostic
significance of SPARC mRNA was analyzed using the KM-plotter,
GEPIA, HPA, UALCAN, Timer, ONCOLNC and TCGA databases. The data for
the meta-analysis were obtained from the studies listed in Table I.
Authors' contributions
JS and ZGF performed the meta-analysis and wrote the
manuscript. JS and YKB analyzed the data from databases. The
authenticity of the raw data was confirmed by JS and YKB. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuo TT, Zheng RS, Zeng HM, Zhang SW and
Chen WQ: Epidemiology of stomach cancer in China. Chin J Clin
Oncol. 44:52–59. 2017.(In Chinese).
|
|
3
|
Han W, Xu XX, He DW, Cao F and Ding HZ:
Effect of SPARCL1 gene on the proliferation and apoptosis of
hepatocellular carcinoma SMMC-7721 cells. J Jiangsu Univ.
26:52016.(In Chinese).
|
|
4
|
Chun Y, Yamakoshi Y, Kim JW, Iwata T, Hu
JCC and Simmer JP: Porcine SPARC: Isolation from dentin, cDNA
sequence, and computer model. Eur J Oral Sci. 114:78–85. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yiu GK, Chan WY, Ng SW, Chan PS, Cheung
KK, Berkowitz RS and Mok SC: SPARC (secreted protein acidic and
rich in cysteine) induces apoptosis in ovarian cancer cells. Am J
Pathol. 159:609–622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rotllant J, Liu D, Yan YL, Postlethwait
JH, Westerfield M and Du SJ: Sparc (Osteonectin) functions in
morphogenesis of the pharyngeal skeleton and inner ear. Matrix
Biol. 27:561–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji R, Zhou YN, Ren TW, Zhang ZY and Li Q:
Expression and clinical significance of MMP-9 in gastric cancer and
metastatic lymphnodes. Chin J Clin Oncol. 37:377–380. 2010.(In
Chinese).
|
|
8
|
De S, Chen J, Narizhneva NV, Heston W,
Brainard J, Sage EH and Byzova TV: Molecular pathway for cancer
metastasis to bone. J Biol Chem. 278:39044–39050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang ML, Zhai LL, Ma LH, et al: Expression
of SPARC and VEGF in Gastric Carcinoma and Their Relationship with
Angiogenesis. J Diag Pathol. 19:52–55. 2012.(In Chinese).
|
|
10
|
Li CY and Gu KS: Expression of CD133,
SPARC and ARID1A in gastric cancer and its clinical significance.
Chin Clin Oncol. 19:411–416. 2014.(In Chinese). PubMed/NCBI
|
|
11
|
Wang GM, Yuan YP, Zhao CX and Song XL:
Expression and significance of MMP-2, MMP-9 and VEGF in colon
carcinoma tissues. Chin J Cancer Prevent Treat. 18:1267–1269.
2011.(In Chinese).
|
|
12
|
Nishie A, Masuda K, Otsubo M, Migita T,
Tsuneyoshi M, Kohno K, Shuin T, Naito S, Ono M and Kuwano M: High
expression of the Cap43 gene in infiltrating macrophages of human
renal cell carcinomas. Clin Cancer Res. 7:2145–2151.
2001.PubMed/NCBI
|
|
13
|
Thomas R, True LD, Bassuk JA, Lange PH and
Vessella RL: Differential expression of osteonectin/SPARC during
human prostate cancer progression. Clin Cancer Res. 6:1140–1149.
2000.PubMed/NCBI
|
|
14
|
Wells GA, She B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of non-randomised studies in
meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.aspMay
13–2022
|
|
15
|
Li R: Expression of protein Smad3, HER2,
SPARC in gastric cancer tissues and its relation with long-term
efficacy. Shanghai Med Pharm J. 35:32014.(In Chinese).
|
|
16
|
Li Y, Wu JF, Zhang H, Qin R and Wang DB:
Expression of SPARC in gastric cancer tissues. Acta Universitatis
Medicinalis Anhui. 41:626–628. 2006.(In Chinese).
|
|
17
|
Dong MX, Yang YH and Yu JX: Expression of
SPARC in gastric carcinoma and clinical significance. Med J Qilu.
26:105–108. 2011.(In Chinese).
|
|
18
|
Ma Y, Zhu J, Chen S, Ma J, Zhang X, Huang
S, Hu J, Yue T, Zhang J, Wang P, et al: Low expression of SPARC in
gastric cancer-associated fibroblasts leads to stemness
transformation and 5-fluorouracil resistance in gastric cancer.
Cancer Cell Int. 19:1372019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franke K, Carl-Mcgrath S, Rhl FW, Röhl FW,
Lendeckel U, Ebert MP, Tänzer M, Pross M and Röcken C: Differential
expression of SPARC in intestinal-type gastric cancer correlates
with tumor progression and nodal spread. Transl Oncol. 2:310–320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CS, Lin KH, Chen SL, Chan YF and
Hsueh S: Overexpression of SPARC gene in human gastric carcinoma
and its clinic-pathologic significance. Br J Cancer. 91:1924–1930.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao ZS, Wang YY, Chu YQ, Ye ZY and Tao
HQ: SPARC is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 16:260–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang HB, Zhao Y and Yang SJ: Research
progress of SPARC in esophageal cancer. J Int Oncol. 41:42014.
|
|
23
|
Brekken RA and Sage EH: SPARC, a
matricellular protein: At the crossroads of cell-matrix
communication. Matrix Biol. 19:816–827. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kupprion C, Motamed K and Sage EH: SPARC
(BM-40, osteonectin) inhibits the mitogenic effect of vascular
endothelial growth factor on microvascular endothelial cells. J
Biol Chem. 273:29635–29640. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Socha MJ, Said N, Dai Y, Kwong J,
Ramalingam P, Trieu V, Desai N, Mok SC and Motamed K: Aberrant
promoter methylation of sparc in ovarian cancer. Neoplasia.
11:126–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng Y, Yang X and Wang XB: Research
advances in secreted protein acidic and rich in cysteine in
hepatocellular carcinoma. J Clin Hepatol. 34:419–423. 2018.
|
|
27
|
Ouyang HY, Le Y, Zhang YF, Guo RP and Shi
M: Expression of SPARCL1 in primary hepatocellular carcinoma and
its clinical significance. Pract J Cancer. 31:32016.
|
|
28
|
Zheng XW, Li WX and Liu JF: Relationship
of bcl-2 expression with SPARC expression in hepatocellular
carcinoma and their correlation with recurrence, metastasis and
prognosis. Chin J Modern Med. 23:42013.(In Chinese).
|
|
29
|
Jiang DL, Cao SQ and Jiang YL: Expression
and clinical significance of secreted protein acidic and rich in
cysteine and matrix metalloproteinase-2 in human colorectal
carcinoma and colorectal adenoma. J Clin Int Med. 34:42017.(In
Chinese).
|
|
30
|
Liang JF, Wang HK, Hong X, Li N, Cheng CX,
Zhao YZ, Ma YB, Gao JZ, Bai RB and Zheng HX: Relationship and
prognostic significance of SPARC and VEGF protein expression in
colon cancer. J Exp Clin Cancer Res. 29:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nischt R, Wallich M, Reibetanz M, Baumann
P, Krieg T and Mauch C: BM-40 and MMP-2 expression are not
coregulated in human melanoma cell lines. Cancer Lett. 162:223–230.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Said N, Frierson HF Jr, Chernauskas D,
Conaway M, Motamed K and Theodorescu D: The role of SPARC in the
TRAMP model of prostate carcinogenesis and progression. Oncogene.
28:3487–3498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi S, Ma HY, Han XY, Sang YZ, Yang MY and
Zhang ZG: Prognostic significance of SPARC expression in breast
cancer: A meta-analysis and bioinformatics analysis. Bio Res Int.
15:86004192022.PubMed/NCBI
|
|
34
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin J, Chen G, Liu Y, Liu S, Wang P, Wan
Y, Wang X, Zhu J and Gao H: Downregulation of SPARC expression
decreases gastric cancer cellular invasion and survival. J Exp Clin
Cancer Res. 29:592010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jie Y, Chen GW, Si L and Liu YC: Effects
of SPARC on the invasion ability and MMP-2 expression of gastric
cancer cell line HGC27 in vivo. Chin Med Guide. 8:1387–1388.
2010.(In Chinese).
|
|
37
|
Chen ZY, Zhang JL, Yao HX, Wang PY, Zhu J,
Wang W, Wang X, Wan YL, Chen SW, Chen GW and Liu YC: Aberrant
methylation of the SPARC gene promoter and its clinical implication
in gastric cancer. Sci Rep. 4:70352014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liao P, Li W, Liu R, Teer JK, Xu B, Zhang
W, Li X, Mcleod HL and He Y: Genome-scale analysis identifies
SERPINE1 and SPARC as diagnostic and prognostic biomarkers in
gastric cancer. Onco Targets Ther. 11:6969–6980. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li L, Zhu Z, Zhao Y, Zhang Q, Wu X, Miao
B, Cao J and Fei S: FN1, SPARC, and SERPINE1 are highly expressed
and significantly related to a poor prognosis of gastric
adenocarcinoma revealed by microarray and bioinformatics. Sci Rep.
9:78272019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sato T, Oshima T, Yamamoto N, Yamada T,
Hasegawa S, Yukawa N, Numata K, Kunisaki C, Tanaka K, Shiozawa M,
et al: Clinical significance of SPARC gene expression in patients
with gastric cancer. J Surg Oncol. 108:364–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang L, Yang M, Shan L, Qi L, Chai C, Zhou
Q, Yao K, Wu H and Sun W: The role of SPARC protein expression in
the progress of gastric cancer. Pathol Oncol Res. 18:697–702. 2012.
View Article : Google Scholar : PubMed/NCBI
|