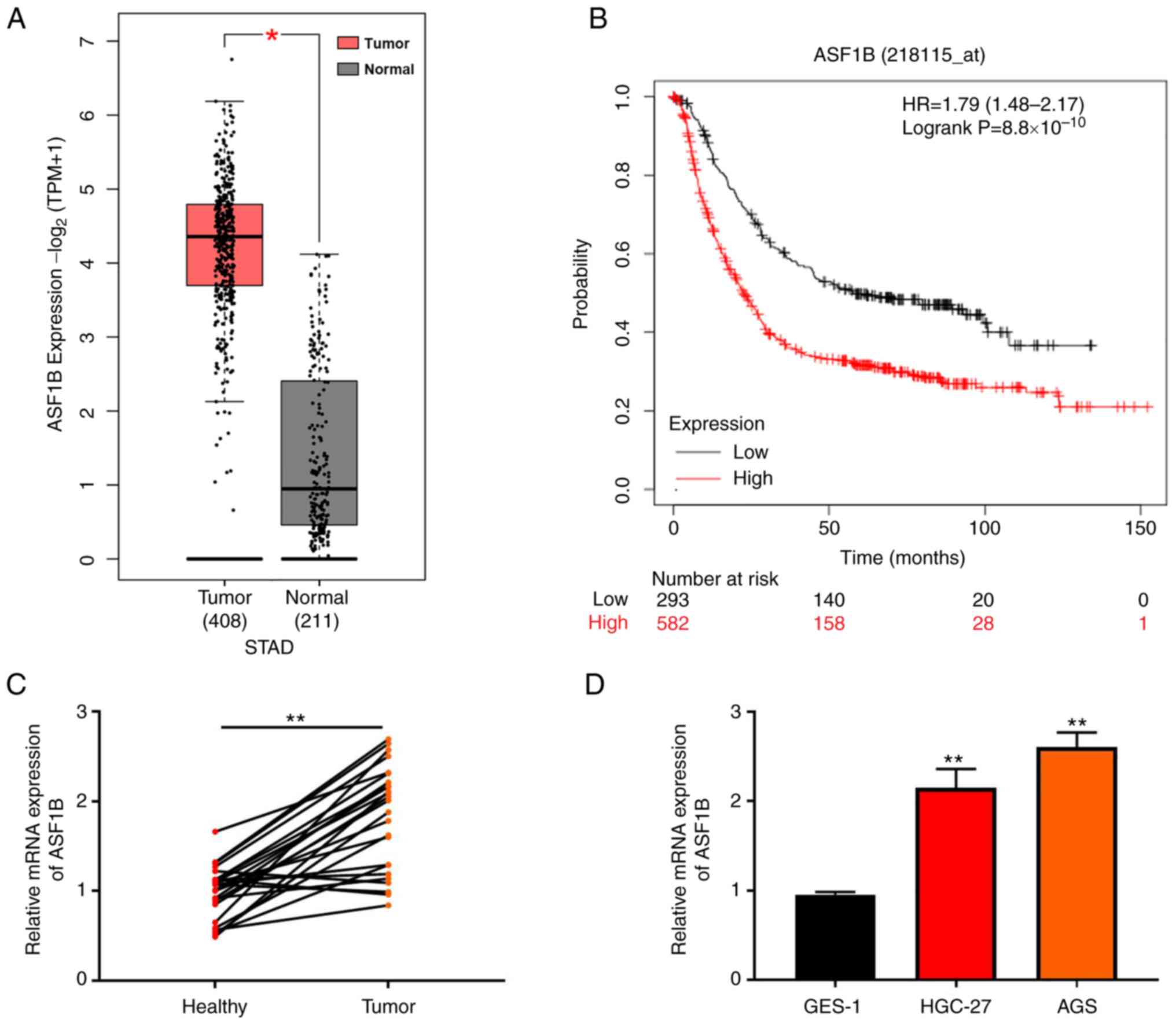
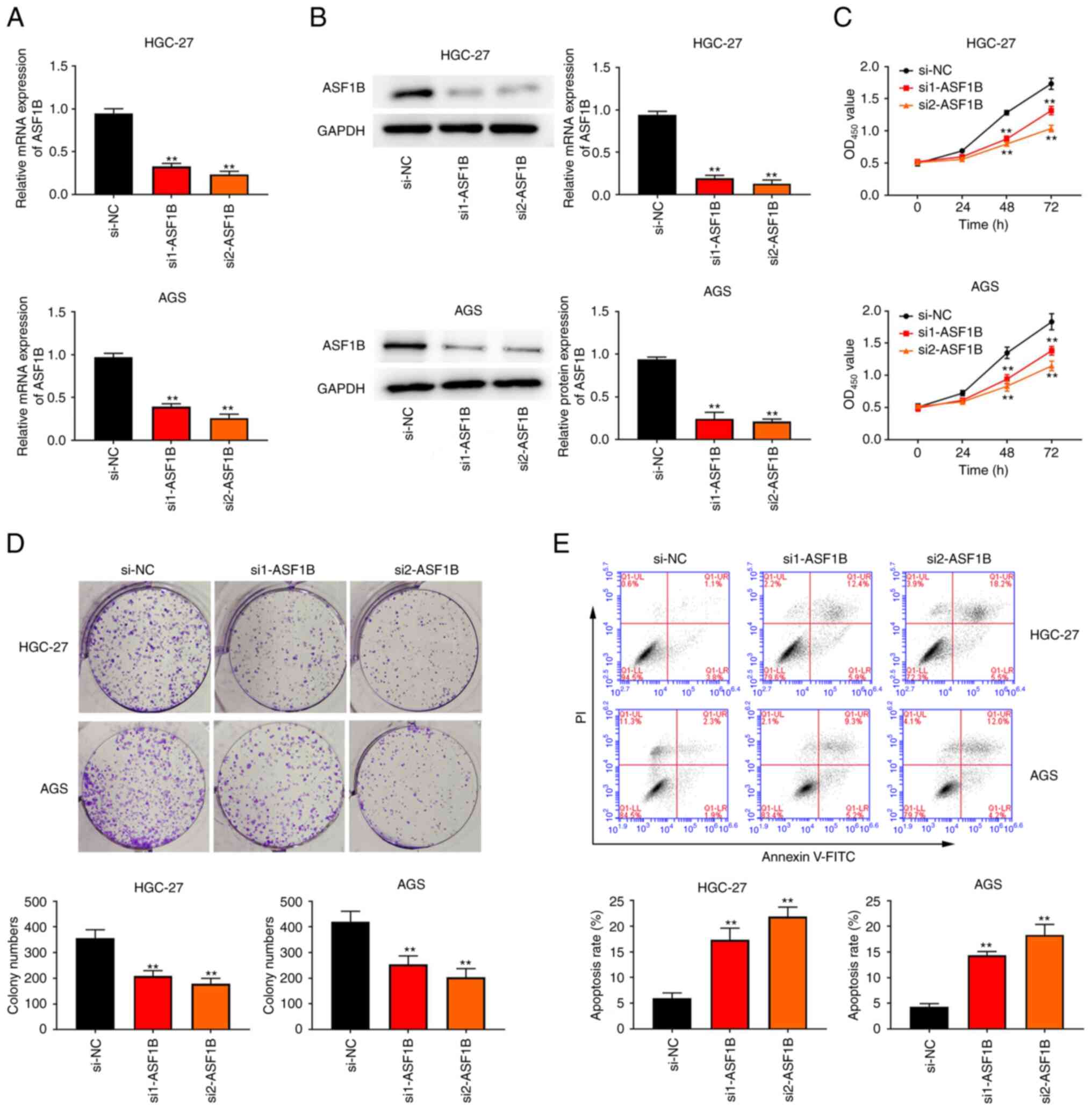
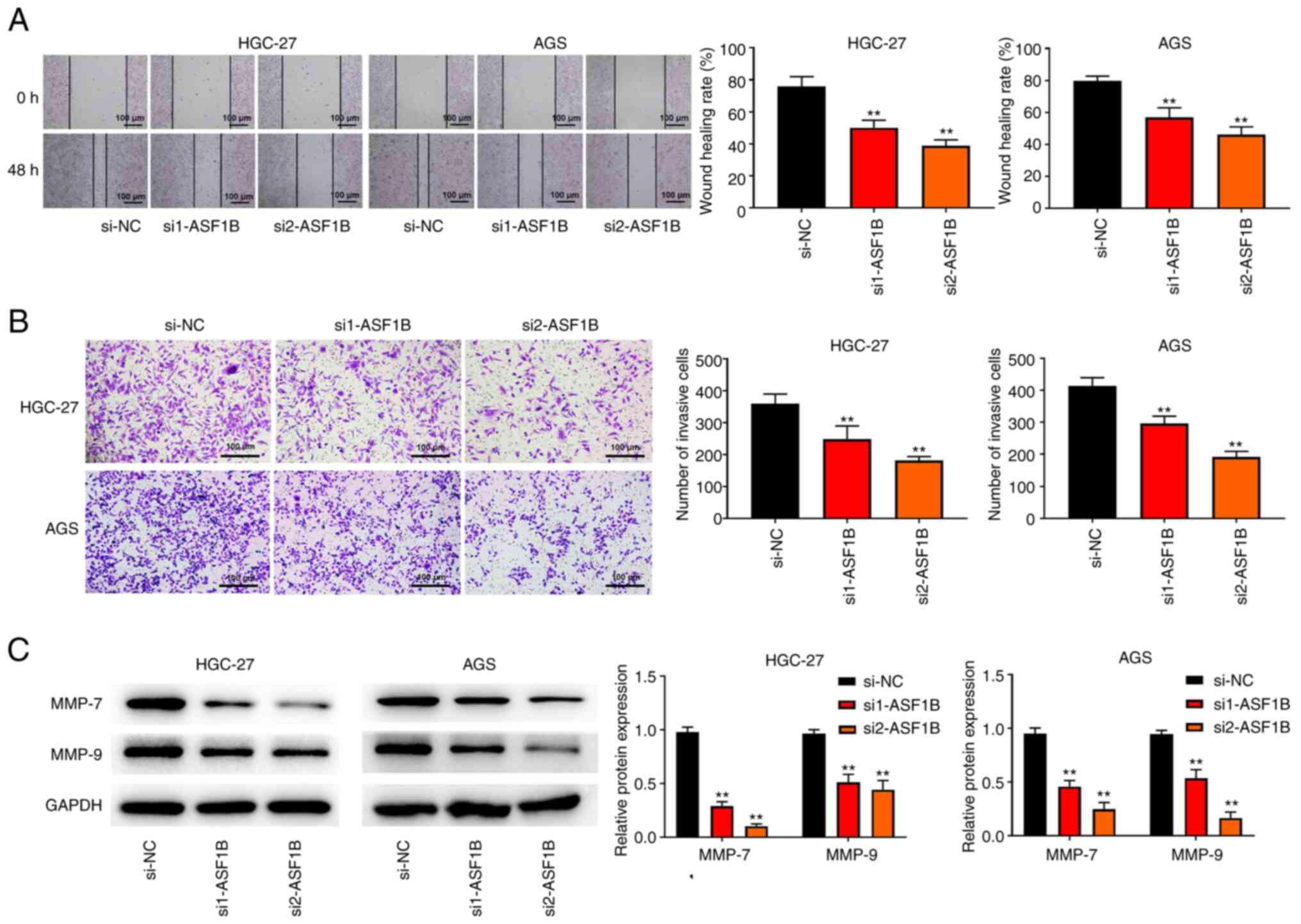
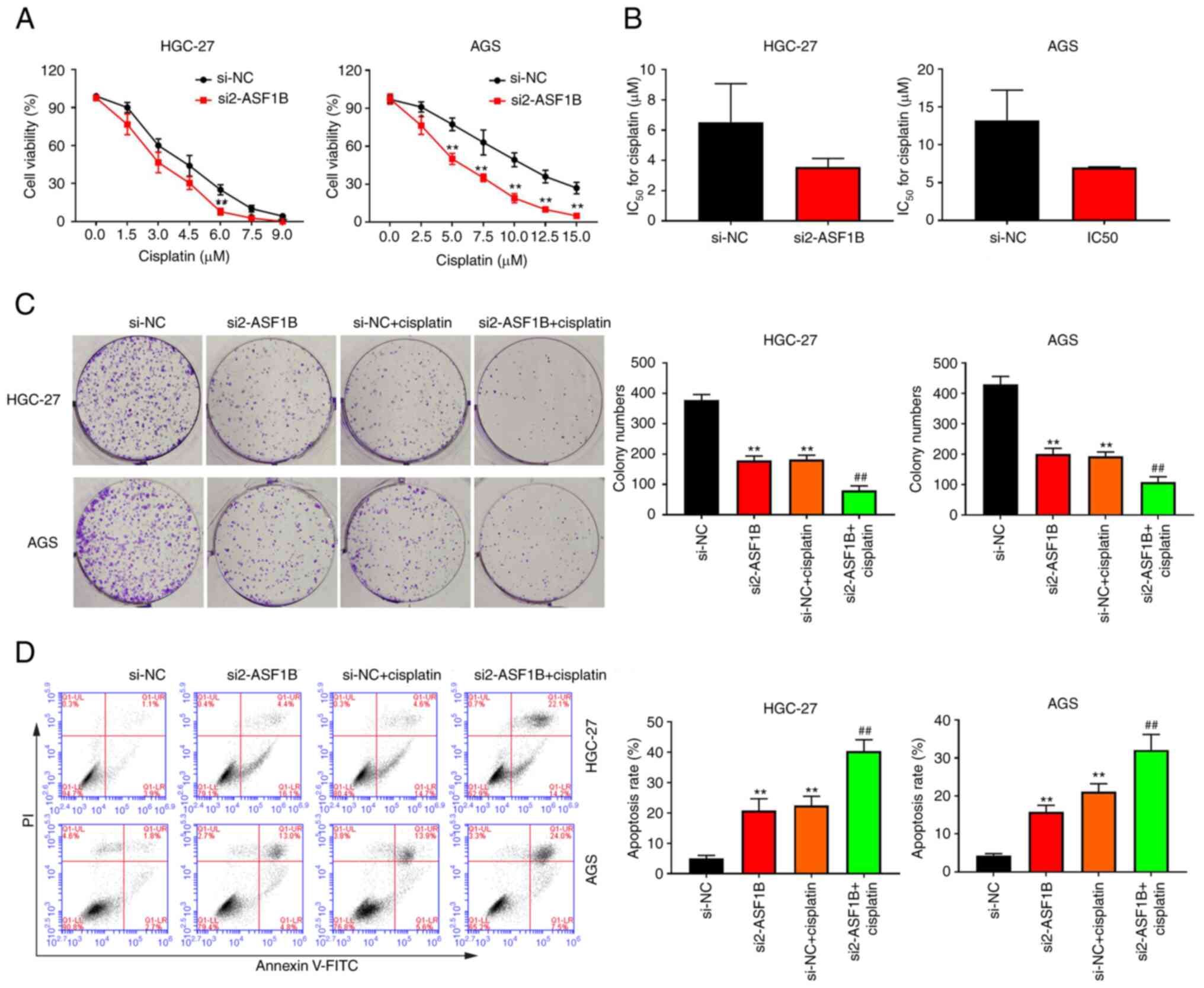
|
1
|
Sexton RE, Al Hallak MN, Diab M and Azmi
AS: Gastric cancer: A comprehensive review of current and future
treatment strategies. Cancer Metastasis Rev. 39:1179–1203. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brodsky AS, Khurana J, Guo KS, Wu EY, Yang
D, Siddique AS, Wong IY, Gamsiz Uzun ED and Resnick MB: Somatic
mutations in collagens are associated with a distinct tumor
environment and overall survival in gastric cancer. BMC Cancer.
22:1392022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan Z: Recent advances in the surgical
treatment of advanced gastric cancer: A Review. Med Sci Monit.
25:3537–3541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joshi SS and Badgwell BD: Current
treatment and recent progress in gastric cancer. CA Cancer J Clin.
71:264–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kenmotsu H, Yamamoto N, Yamanaka T,
Yoshiya K, Takahashi T, Ueno T, Goto K, Daga H, Ikeda N, Sugio K,
et al: Randomized phase III study of pemetrexed plus cisplatin
versus vinorelbine plus cisplatin for completely resected stage II
to IIIA nonsquamous non-small-cell lung cancer. J Clin Oncol.
38:2187–2196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang DM, Gupta S, Kitchlu A, Meraz-Munoz
A, North SA, Alimohamed NS, Blais N and Sridhar SS: Defining
cisplatin eligibility in patients with muscle-invasive bladder
cancer. Nat Rev Urol. 18:104–114. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao L, Wang JM, Liu BQ, Yan J, Li C, Jiang
JY, Zhao FY, Qiao HY and Wang HQ: m6A-YTHDF1-mediated TRIM29
upregulation facilitates the stem cell-like phenotype of
cisplatin-resistant ovarian cancer cells. Biochim Biophys Acta Mol
Cell Res. 1868:1188782021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Safi A, Bastami M, Delghir S, Ilkhani K,
Seif F and Alivand MR: miRNAs modulate the dichotomy of cisplatin
resistance or sensitivity in breast cancer: An update of
therapeutic implications. Anticancer Agents Med Chem. 21:1069–1081.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Fan J, Ai G, Liu J, Luo N, Li C and
Cheng Z: Berberine in combination with cisplatin induces
necroptosis and apoptosis in ovarian cancer cells. Biol Res.
52:372019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu L, Yuan Y, Yuan L, Li L, Liu F, Liu J,
Chen Y, Lu Y and Cheng J: Activation of TFEB-mediated autophagy by
trehalose attenuates mitochondrial dysfunction in cisplatin-induced
acute kidney injury. Theranostics. 10:5829–5844. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raudenska M, Balvan J, Fojtu M, Gumulec J
and Masarik M: Unexpected therapeutic effects of cisplatin.
Metallomics. 11:1182–1199. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cocetta V, Ragazzi E and Montopoli M:
Links between cancer metabolism and cisplatin resistance. Int Rev
Cell Mol Biol. 354:107–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ouyang X, Lv L, Zhao Y, Zhang F, Hu Q, Li
Z, Zhu D and Li L: ASF1B serves as a potential therapeutic target
by influencing cell cycle and proliferation in hepatocellular
carcinoma. Front Oncol. 11:8015062021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Song J, Zhang Y, Wang H, Sun H,
Feng X, Hou M, Chen G, Tang Q and Ji M: ASF1B promotes cervical
cancer progression through stabilization of CDK9. Cell Death Dis.
11:7052020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu L and Jie B: Protumor effects of
histone H3-H4 chaperone antisilencing Feature 1B gene on lung
adenocarcinoma: In silico and in vitro analyses. Comput Math
Methods Med. 16:50054592021.PubMed/NCBI
|
|
17
|
Ma J, Han W and Lu K: Comprehensive
pan-cancer analysis and the regulatory mechanism of ASF1B, a gene
associated with thyroid cancer prognosis in the tumor
micro-environment. Front Oncol. 11:7117562021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan T, Gao X, Wang G, Li F, Shen J, Lu C,
Xu L, Li Y and Zhang J: Construction of novel lncRNA-miRNA-mRNA
network associated with recurrence and identification of
immune-related potential regulatory axis in hepatocellular
carcinoma. Front Oncol. 11:6266632021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiangqiao Z, Tao Q, Zhongbao C, Xiaoxiong
M, Long Z, Jilin Z and Tianyu W: Anti-silencing function 1B histone
chaperone promotes cell proliferation and migration via activation
of the AKT pathway in clear cell renal cell carcinoma. Biochem
Biophys Res Commun. 511:165–172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Lv W, Liu Y, Fu W, Chen B, Ma Q,
Gao X and Cui X: Knockdown of serum- and glucocorticoid-regulated
Kinase 1 enhances cisplatin sensitivity of gastric cancer through
suppressing the nuclear factor kappa-b signaling pathway. Balkan
Med J. 38:331–340. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Wang X and Han M: Loss of DAB2IP
contributes to cell proliferation and cisplatin resistance in
gastric cancer. Onco Targets Ther. 14:979–988. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song W, Cui Z, Liu H, Xue L and Ju H: The
expression and prognostic value of miR-195-5p in patients with
advanced gastric cancer after chemotherapy. J BUON. 25:2332–2340.
2020.PubMed/NCBI
|
|
26
|
Qiao XL, Zhong ZL, Dong Y and Gao F:
LncRNA HMGA1P4 promotes cisplatin-resistance in gastric cancer. Eur
Rev Med Pharmacol Sci. 24:8830–8836. 2020.PubMed/NCBI
|
|
27
|
Ge L, Hou L, Yang Q, Wu Y, Shi X, Li J and
Yang K: A systematic review and network meta-analysis protocol of
adjuvant chemotherapy regimens for resected gastric cancer.
Medicine (Baltimore). 98:e144782019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo Z, Rong Z and Huang C: Surgery
strategies for gastric cancer with liver metastasis. Front Oncol.
9:13532019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu X, Zhu H, Zhang X, He X and Xu X:
Comprehensive analysis of pan-cancer reveals potential of ASF1B as
a prognostic and immunological biomarker. Cancer Med. 10:6897–6916.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang W, Xiao L, Pan D and Hu L: ASF1B
enhances migration and invasion of lung cancers cell via regulating
the P53-mediated epithelial-mesenchymal transformation (EMT)
signaling pathway. Neoplasma. 69:3692022. View Article : Google Scholar
|
|
31
|
Qi L, Luo Q and Zhang Y: Advances in
toxicological research of the anticancer drug cisplatin. Chem Res
Toxicol. 32:1469–1486. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Wu X, Yang H, Li L, Ye Z and Rao Y:
A nuclear targeted Dox-aptamer loaded liposome delivery platform
for the circumvention of drug resistance in breast cancer. Biomed
Pharmacother. 117:122019. View Article : Google Scholar
|
|
33
|
Liang W, Zheng Y, Zhang J and Sun X:
Multiscale modeling reveals angiogenesis-induced drug resistance in
brain tumors and predicts a synergistic drug combination targeting
EGFR and VEGFR pathways. BMC Bioinformatics. 20 (Suppl 7):S2032019.
View Article : Google Scholar
|
|
34
|
Li Y, Zu X, Hu X, Wang L and He W:
Forkhead Box R2 knockdown decreases chemoresistance to cisplatin
via MYC pathway in bladder cancer. Med Sci Monit. 25:8928–8939.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mei Y, Liu YB, Hu DL and Zhou HH: Effect
of RIF1 on response of non-small-cell lung cancer patients to
platinum-based chemotherapy by regulating MYC signaling pathway.
Int J Biol Sci. 14:1859–1872. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pyndiah S, Tanida S, Ahmed KM, Cassimere
EK, Choe C and Sakamuro D: c-MYC suppresses BIN1 to release
poly(ADP-ribose) polymerase 1: A mechanism by which cancer cells
acquire cisplatin resistance. Sci Signal. 4:20015562011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muvarak N, Kelley S, Robert C, Baer MR,
Perrotti D, Gambacorti-Passerini C, Civin C, Scheibner K and
Rassool FV: c-MYC Generates repair errors via increased
transcription of alternative-NHEJ factors, LIG3 and PARP1, in
tyrosine kinase-activated leukemias. Mol Cancer Res. 13:699–712.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia P, Zhang H, Xu K, Jiang X, Gao M, Wang
G, Liu Y, Yao Y, Chen X, Ma W, et al: MYC-targeted WDR4 promotes
proliferation, metastasis, and sorafenib resistance by inducing
CCNB1 translation in hepatocellular carcinoma. Cell Death Dis.
12:6912021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li M, Fang L, Kwantwi LB, He G, Luo W,
Yang L, Huang Y, Yin S, Cai Y, Ma W, et al: N-Myc promotes
angiogenesis and therapeutic resistance of prostate cancer by TEM8.
Med Oncol. 38:1272021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun W, Li J, Zhou L, Han J, Liu R, Zhang
H, Ning T, Gao Z, Liu B, Chen X and Ba Y: The
c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in
colorectal cancer. Theranostics. 10:1981–1996. 2020. View Article : Google Scholar : PubMed/NCBI
|