Introduction
Chronic myeloid leukemia (CML) is a neoplasm of
myeloid origin that has an annual incidence of 1 to 2 cases per
100,000 individuals worldwide and a mean age at time of diagnosis
of 65 years (1,2). Multiple myeloma (MM) is a
hematological malignancy that has characteristic abnormal clonal
plasma cells present in the bone marrow. MM is diagnosed in an
estimated 588,161 individuals worldwide each year and a median age
at time of diagnosis of 70 years (3,4). To
the best of our knowledge, these 2 uncommon diseases in the same
patient is extremely rare, and, since 1972, there have been 15
cases reported in the world literature. MM accompanied by CML is
rare, and few related studies have been reported worldwide since
1972 (5–8). A literature review shows that new
hematopathological neoplasms occur following the initial treatment
of a hematological malignancy (7,9–11).
This can include a single or up to three new additional neoplasms
subsequent to treatment. An example of this is the occurrence of MM
following treatment of CML with tyrosine kinase inhibitor (TKI)
(7). The origin of these two
malignancies in such patients remains unknown, and further research
is required to enhance our understanding. The present study
describes the unusual case of a patient treated with TKI who
developed MM 6 years after the diagnosis of CML.
Case report
A 79-year-old male was admitted to The First
Affiliated Hospital of Jishou University (Jishou, China) in
February 2016 due to a 2-month history of an unexplained fever
combined with a cough and expectoration. Prior to hospitalization,
the patient experienced an intermittent mild fever (~38.0°C),
mainly in the afternoon. Intravenous cephalosporin antibiotics (2 g
ceftazidim twice a day for 3 days; 0.5 g moxifloxacin sodium
chloride every day for 1 week) and fluid replacement were used to
control the fever, but the efficacy was limited. The patient
developed an oral ulcer during this time, but the symptoms improved
after the aforementioned intravenous anti-inflammatory treatment.
An initial hematological examination showed an elevated white blood
cell (WBC) count of 43.86×109/l (normal range,
4.0-10.0×109/l) and a neutrophil count of
36.47×109/l (normal range, 1.8-6.3×109/l).
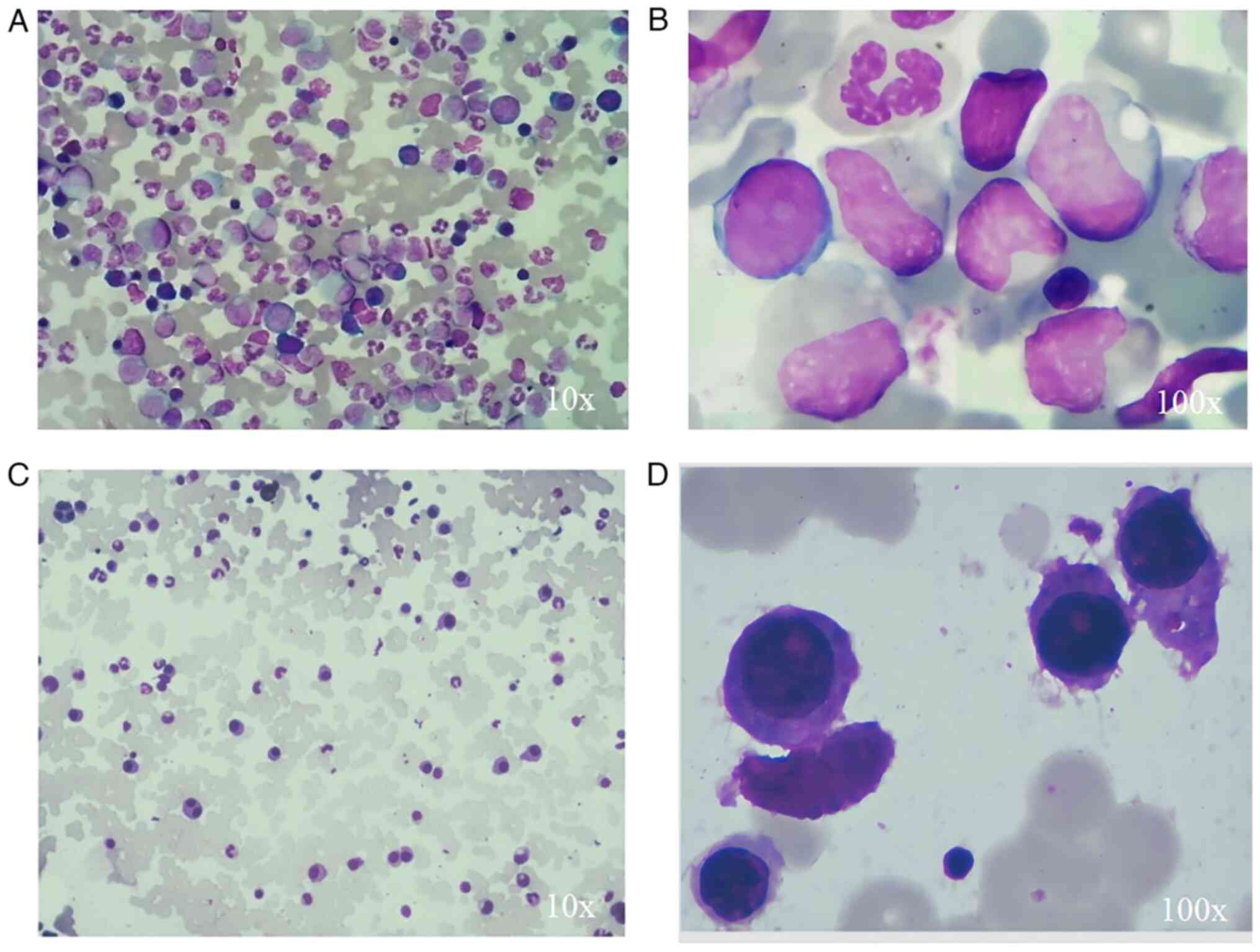
Bone marrow aspiration revealed 85% granulocytes, mainly with
hyperplasia of the mesocytic granulocyte and rod-shaped nuclear
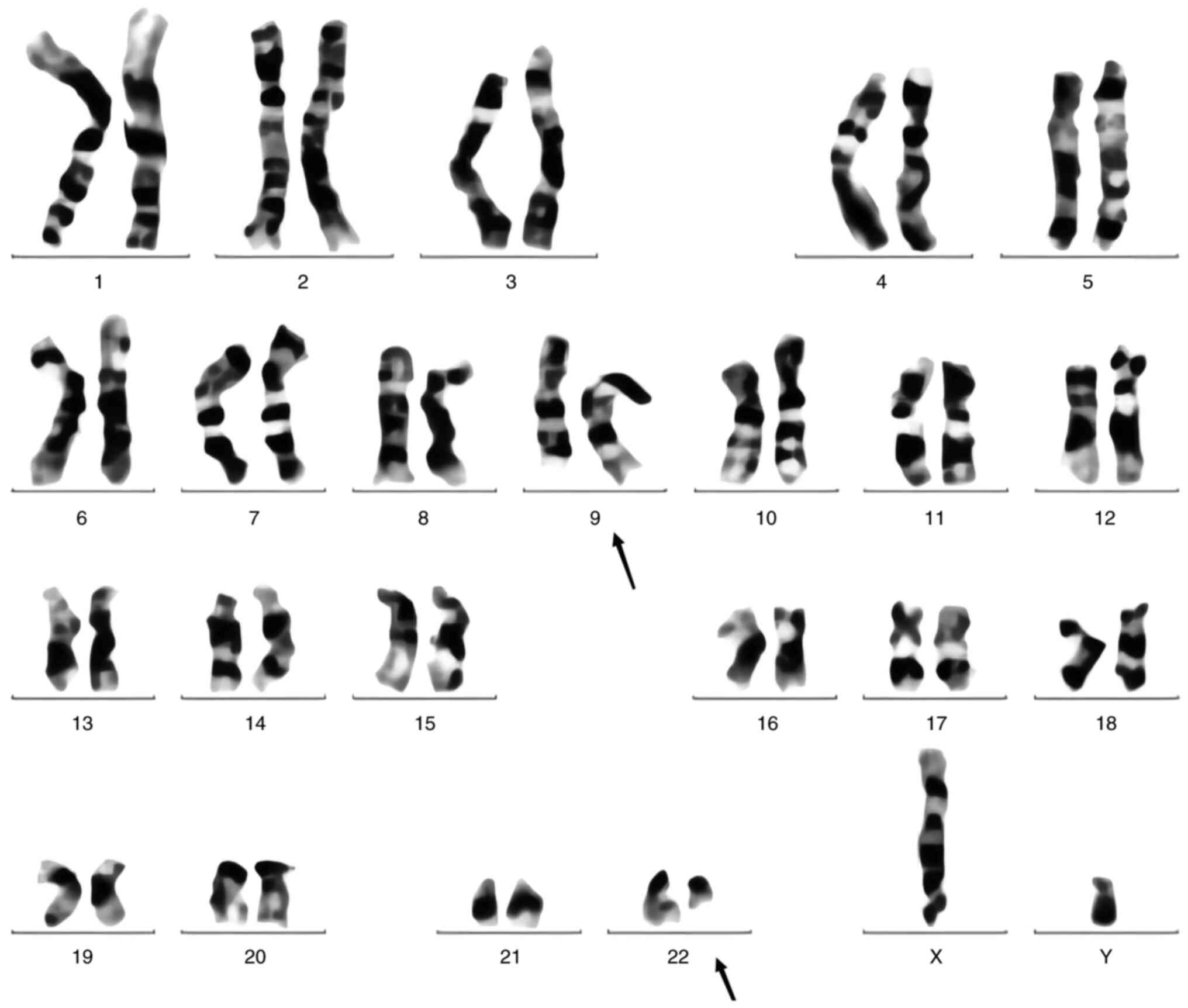
granulocytes (Fig. 1). Karyotype
analysis detected Ph [t(9;22)(q34;q11)] in 8 out of 20 metaphases
analyzed (Fig. 2). BCR-ABL t(9;22)
translocation was also confirmed by fluorescence in situ
hybridization (FISH) assays (Data
S1), which showed fusion signals for [BCR-ABL1 International
Standard (IS), 71.833%]. A peripheral smear showed only rare blasts
(<10%) and the patient appeared to be in the chronic phase of
CML. The patient was diagnosed with CML (Sokal score 1.1) (12) in February 2016 and treated with
imatinib (400 mg daily), with intermittent follow-up in the
Outpatient Department. During this period, the patient was advised
to change to a second-generation TKI owing to the poor efficacy of
the initial treatment, but this was refused due to economic
factors. In addition, due to these economic factors and the poor
compliance of the patient, no patient-related data was obtained
between February 2016 and September 2020, which is a deficiency of
this case report. However, from the clinical data of the patient at
the initial diagnosis of CML, there was no basis for active MM. The
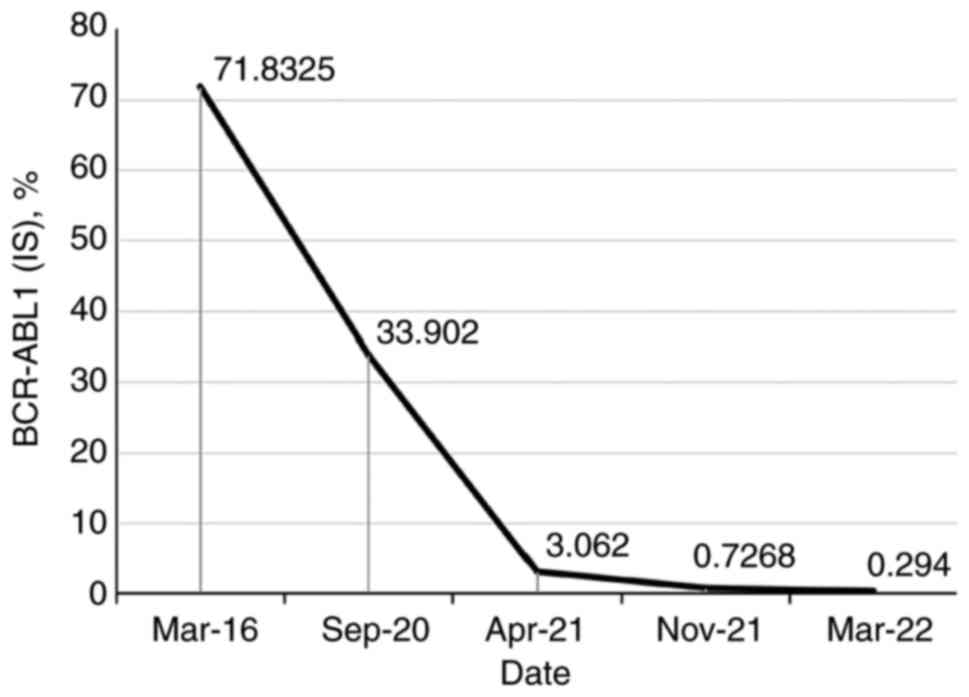
curative effect in the patient was still not satisfactory after
treatment with imatinib for ~4 years [BCR-ABL1(IS), 33.902%]. Thus,
the treatment was switched to flumatinib (600 mg daily) in
September 2020. With regular follow-up, the BCR-ABL1(IS) count had
decreased to 3.062% after 6 months (Fig. 3).
In April 2022, the patient complained of bone pain
and was found to exhibit a high level of immunoglobulin (IgG)
(30.40 g/l; normal range, 7.51-15.60 g/l). Further work-up revealed
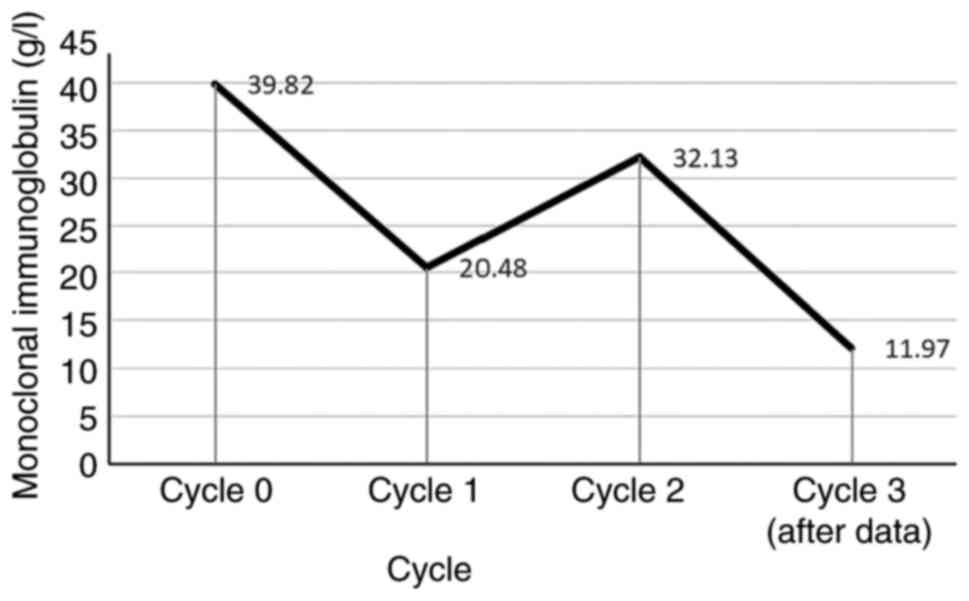
IgG-κ monoclonality, with an M-spike of 39.82 g/l. Moreover, a
skeletal survey revealed multiple bony lesions in the skull, but
magnetic resonance imaging of the spine showed no obvious damage to
the sclerotin. The β-2 microglobulin level reached 5.52 mg/l
(normal range, 1.0-3.0 mg/l), and the serum calcium and renal
function were normal. The serum κ free light chain level was
elevated at 131.97 mg/l, with an increase in the κ:λ chain ratio at
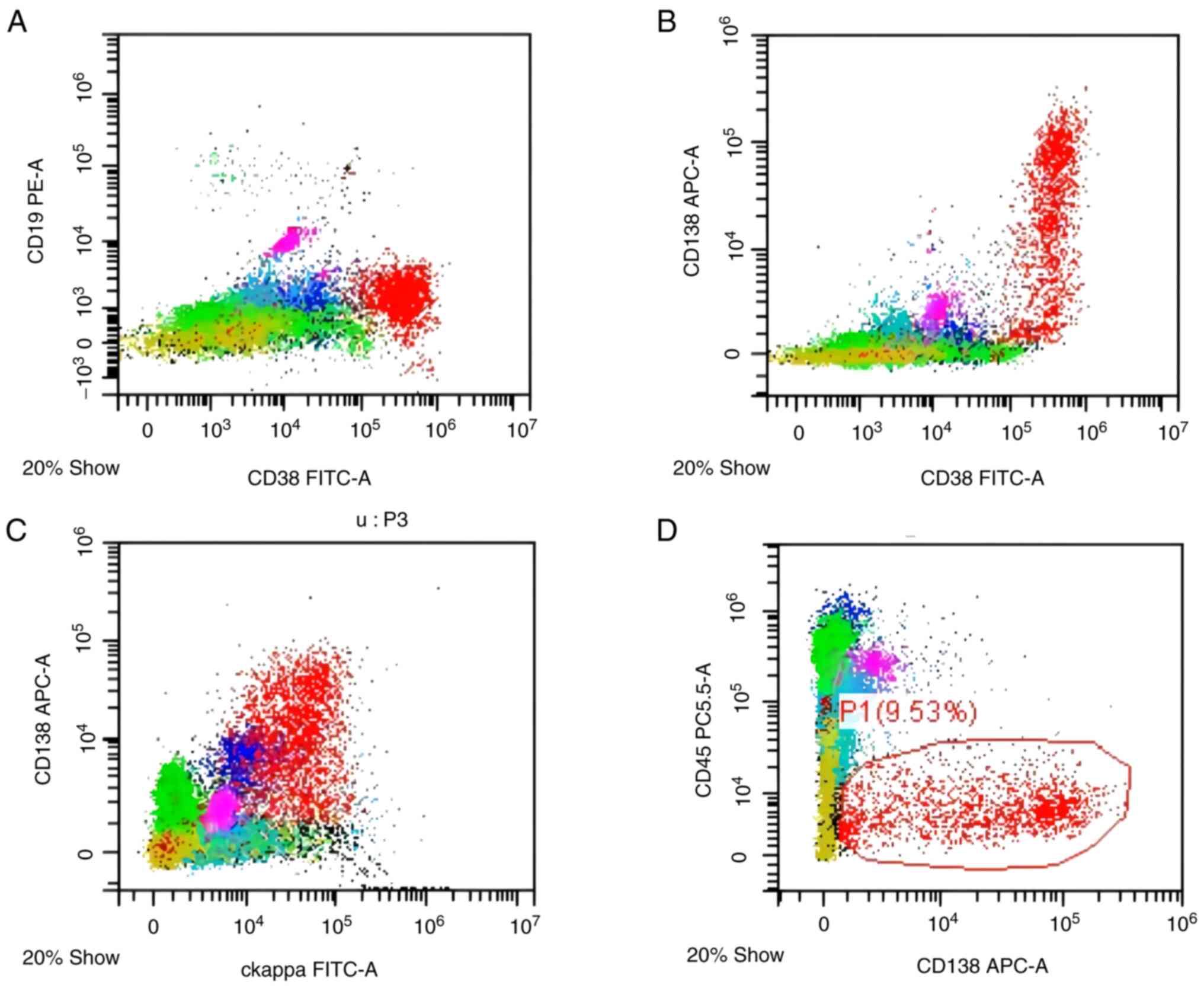
13.8. A bone marrow smear showed 21% primitive plasma cells, and
flow cytometry further demonstrated plasma cells positive for κ
chain, CD38 and CD138 (Fig. 4).
Flow cytometry was performed on a DxFLEX flow cytometer (Beckman
Coulter, Inc.). Cytogenetic analysis of bone marrow cells revealed
a normal male karyotype of 46, while the XY-FISH analysis (Fig. 5) detected a complex chromosome
rearrangement
[52,XY,+3,add(4)(q31),+7,add(7)(q32),+8,+11,-13,+15,+19,+mar[4]/46,XY[16]].
Therefore, the patient was diagnosed with IgG-κ MM, Durie-Salmon
stage IIIA (13), International
Staging System (ISS) stage III (14) and revised ISS stage II (15), and treated with combination
chemotherapy using an VRd regimen [2 mg bortezomib on days 1, 4, 8
and 11; 25 mg lenalidomide on days 1–14; and 20 mg dexamethasone
(DXM) on days 1 and 2, 4 and 5, 8 and 9, and 11 and 12; 28-day
cycles]. After two cycles of chemotherapy, the curative effect did
not reach a partial response status, so the scheme was adjusted to
a DVD regimen (800 mg daratumumab on days 0, 7, 14 and 21; 2 mg
bortezomib on days 1, 4, 8 and 11; and 20 mg DXM on days 1 and 2, 4
and 5, 8 and 9, and 11 and 12; 28-day cycles). After one cycle, the
monoclonal Ig level decreased markedly to 11.97 g/l (Fig. 6). The patient continued to receive
600 mg flumatinib when at home, and the general condition of the
patient was reported to be good, although no further laboratory
results were obtained.
 | Figure 5.Karyotype from a bone marrow specimen
at the time of the multiple myeloma diagnosis showing
52,XY,+3,add(4)(q31),+7,add(7)(q32),+8,+11,-13,+15,+19,+mar[4]/46,XY[16]. |
Discussion
CML is a myeloproliferative disorder caused by
pluripotent hematopoietic stem cells. MM is a monoclonal disorder
of plasma cells that have differentiated from lymphoid B cells. The
abnormal cell types of CML and MM are therefore distinctly
different. The mechanism of these two concomitant malignant tumors
is not fully understood. Several factors have been postulated to be
related to the presence of accompanying diseases, including
host-specific characteristics, prior chemotherapy and radiation,
exposure to environmental carcinogens or radiation, and epigenetic
upregulation/downregulation. The present study discusses some
assumptions about the mechanism of MM accompanying CML.
First, an intuitive hypothesis is that MM arises as
a consequence of the therapy for CML (16). Several studies reviewed the
association between chemotherapy and the development of MM in the
months following treatment, particularly in imatinib-treated CML
(7,17). During the treatment of CML, imatinib
inhibits the proliferation of WBCs by competing with ATP to bind to
BCR-ABL1 tyrosine kinase. In addition, imatinib mesylate is active
in different genes involved in cell transformation, and can inhibit
BCR-ABL1, c-KIT, PGFRα/β, Jak2 and Erk1/2 (5). In a study from 2010, the use of
imatinib mesylate in the treatment of CML was shown to increase the
risk of MM. It was suggested that all patients treated with
imatinib should be regularly monitored by serum protein
electrophoresis (18). This study
provided support for the plasmacyte-specific effects of imatinib.
By contrast, another study revealed that imatinib inhibits the
proliferation of MM cells by arresting cell cycle progression
(19). As a result, no conclusion
can be made at this point due to the conflicting results of
published studies. The long-term effects of TKIs must continue to
be closely monitored due to the lack experience in their use and
the few cases reported.
Another potential hypothesis is that plasma cell
myeloma and CML share the presence of malignant pluripotent
progenitor stem cells (16,20,21).
This reveals the capability of CML to differentiate into either
myeloid lineage or lymphoid lineage. A previous study analyzed
whether overlapping genetic susceptibility loci were present in
myeloproliferative neoplasm (MPN) and MM (21); 23 known MM risk sites were assessed
in individuals from MPN case-control studies, and the most
significant result was noted for PS0RS1C1-rs2285803 in patients
with CML. The polygenic risk score showed that PS0RS1C1-rs2285803
is related to CML risk, suggesting that the combination of multiple
MM risk loci may affect CML risk, and vice versa. This implies a
potential common genetic background between CML and MM (21), which requires further investigation.
Meanwhile, the constitutively active BCR-ABL tyrosine kinase is
known to promote cell survival and proliferation, and is
responsible for the malignant transformation of the disease
(11). Furthermore, Ph has been
associated with an increase in cell proliferation and survival, as
well as malignant transformation, and has been detected in all
hematopoietic cell lineages (16,21–23).
Although the etiology of CML and MM in the present patient could
not be determined, the hypothesis that the diseases evolved from
common malignant pluripotent hematopoietic stem cells has
potential.
Thirdly, the presence of host-specific factors is
also an explanation for the dual presence of the diseases in the
same patient; preexisting CML may create a more sustainable
environment for the formation of secondary malignancies. Plasma
cell myeloma (PCL) is a slow growing malignancy that develops over
a number of years. This in return may reduce the effectiveness of
the immune system, thus impeding its ability to destroy newly
formed malignant cells. The patients may present with seemingly
simultaneous malignant tumors. Notably, given the large number of
chromosomal abnormalities existing in the present patient, the
genetic instability of the host may have produced an atypical
progenitor cell, placing this patient in a more vulnerable position
to acquire multiple disorders. Furthermore, during the treatment,
the expression of NF-κB signaling pathway is downregulated, and
these malignant cells gain anti-apoptotic abilities and mechanisms,
and evade immune surveillance in the context of immune response
loss or decline (5,6,23,24).
The progression or treatment of malignant tumors enhances the
development of malignant cells (gaining anti-apoptotic abilities)
and the mechanisms of evading immune surveillance, chronic antigen
stimulation and genetic polymorphism (23,25).
In addition, the variability of enzymes and repair pathways encoded
by genes may make a person more likely to develop malignant tumors
(5). These properties of MM suggest
that these changes to the microenvironment of the bone marrow may
create sustainability and opportunity for secondary
hematopathological malignancies.
In conclusion, MM in a patient with CML is extremely
rare. The reasons for the occurrence of both diseases in one
patient may be multifactorial, so the exact mechanism of the
present extremely rare case remains unknown. Therefore, further
investigation and monitoring of potential associated cases is
needed to determine the exact cause of concomitant multiple
malignant tumors.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation Platform and
Talent Program of Hunan Province (grant no. 2021SK4050) and the
Research Project of Jishou University (grant no. Jdzd21002).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XLL and KS conceived and designed the study. XLL
collected all relevant data of patients from the database and
drafted the manuscript, while MLi was responsible for medication
guidance. ZWS and LZW analyzed the data. KS revised the manuscript.
KS, JT and MLia participated in making the pathological diagnosis.
All authors confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Jishou
University.
Patient consent for publication
Written consent for publication of the case report
and any accompanying images, without any potentially identifying
information, was provided by the patient's family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hehlmann R, Hochhaus A and Baccarani M;
European LeukemiaNet, : Chronic myeloid leukaemia. Lancet.
370:342–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deininger MW, Shah NP, Altman JK, Berman
E, Bhatia R, Bhatnagar B, DeAngelo DJ, Gotlib J, Hobbs G, Maness L,
et al: Chronic myeloid leukemia, version 2.2021, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
18:1385–1415. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cowan AJ, Green DJ, Kwok M, Lee S, Coffey
DG, Holmberg LA, Tuazon S, Gopal AK and Libby EN: Diagnosis and
management of multiple myeloma: A review. JAMA. 327:464–477. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maerki J, Katava G, Siegel D, Silberberg J
and Bhattacharyya PK: Unusual case of simultaneous presentation of
plasma cell myeloma, chronic myelogenous leukemia, and a jak2
positive myeloproliferative disorder. Case Rep Hematol.
2014:7384282014.PubMed/NCBI
|
|
6
|
Ali N, Pickens PV and Auerbach HE:
Immunoglobulin D multiple myeloma, plasma cell leukemia and chronic
myelogenous leukemia in a single patient treated simultaneously
with lenalidomide, bortezomib, dexamethasone and imatinib. Hematol
Rep. 8:62952016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michael M, Antoniades M, Lemesiou E,
Papaminas N and Melanthiou F: Development of multiple myeloma in a
patient with chronic myeloid leukemia while on treatment with
imatinib mesylate for 65 months. Oncologist. 14:1198–1200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacSween JM and Langley GR: Light-chain
disease (hypogammaglobulinemia and Bence Jones proteinuria) and
sideroblastic anemia-preleukemic chronic granulocytic leukemia. Can
Med Assoc J. 106:995–998. 1972.PubMed/NCBI
|
|
9
|
Garipidou V, Vakalopoulou S and Tziomalos
K: Development of multiple myeloma in a patient with chronic
myeloid leukemia after treatment with imatinib mesylate.
Oncologist. 10:457–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Romanenko NA, Bessmel'tsev SS, Udal'eva
VIu, Zenina MN, Martynkevich IS, Rugal' VI and Abdulkadyrov KM: The
combination of chronic myeloid leukemia and multiple myeloma in one
patient. Vopr Onkol. 59:103–110. 2013.(In Russian). PubMed/NCBI
|
|
11
|
Offiah C, Quinn JP, Thornton P and Murphy
PT: Co-existing chronic myeloid leukaemia and multiple myeloma:
Rapid response to lenalidomide during imatinib treatment. Int J
Hematol. 95:451–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sokal JE, Cox EB, Baccarani M, Tura S,
Gomez GA, Robertson JE, Tso CY, Braun TJ, Clarkson BD, Cervantes F,
et al: Prognostic discrimination in ‘good-risk’ chronic
granulocytic leukemia. Blood. 63:789–799. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Durie BG and Salmon SE: A clinical staging
system for multiple myeloma. Correlation of measured myeloma cell
mass with presenting clinical features, response to treatment, and
survival. Cancer. 36:842–854. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar S, Paiva B, Anderson KC, Durie B,
Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, et
al: International Myeloma Working Group consensus criteria for
response and minimal residual disease assessment in multiple
myeloma. Lancet Oncol. 17:e328–e346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised International staging system
for multiple myeloma: A report from International Myeloma Working
Group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ragupathi L, Najfeld V, Chari A, Petersen
B, Jagannath S and Mascarenhas J: A case report of chronic
myelogenous leukemia in a patient with multiple myeloma and a
review of the literature. Clin Lymphoma Myeloma Leuk. 13:175–179.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Melo JV, Hughes TP and Apperley JF:
Chronic myeloid leukemia. Hematology Am Soc Hematol Educ Program.
2003:132–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carulli G, Cannizzo E, Ottaviano V,
Cervetti G, Buda G, Galimberti S, Baratè C, Marini A and Petrini M:
Abnormal phenotype of bone marrow plasma cells in patients with
chronic myeloid leukemia undergoing therapy with Imatinib. Leuk
Res. 34:1336–1339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katzel JA, Lee-Ma A and Vesole DH: Safety
of a second-generation tyrosine kinase inhibitor and novel targeted
therapy for the treatment of a patient with chronic myeloid
leukemia and multiple myeloma. Anticancer Drugs. 26:907–909. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ide M, Kuwahara N, Matsuishi E, Kimura S
and Gondo H: Uncommon case of chronic myeloid leukemia with
multiple myeloma. Int J Hematol. 91:699–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macauda A, Giaccherini M, Sainz J,
Gemignani F, Sgherza N, Sánchez-Maldonado JM, Gora-Tybor J,
Martinez-Lopez J, Carreño-Tarragona G, Jerez A, et al: Do
myeloproliferative neoplasms and multiple myeloma share the same
genetic susceptibility loci? Int J Cancer. 148:1616–1624. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Swaminathan N, Gupta S and Dourado C: Case
Report: IgG multiple myeloma and chronic myeloid leukemia in a
single patient. F1000Res. 9:4882020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hideshima T, Mitsiades C, Tonon G,
Richardson PG and Anderson KC: Understanding multiple myeloma
pathogenesis in the bone marrow to identify new therapeutic
targets. Nat Rev Cancer. 7:585–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Avet-Loiseau H, Attal M, Moreau P,
Charbonnel C, Garban F, Hulin C, Leyvraz S, Michallet M,
Yakoub-Agha I, Garderet L, et al: Genetic abnormalities and
survival in multiple myeloma: The experience of the Intergroupe
Francophone du Myélome. Blood. 109:3489–3495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas A, Mailankody S, Korde N,
Kristinsson SY, Turesson I and Landgren O: Second malignancies
after multiple myeloma: From 1960s to 2010s. Blood. 119:2731–2737.
2012. View Article : Google Scholar : PubMed/NCBI
|