Introduction
With the increasing incidence and mortality rates,
cancer remains a primary public health problem. According to the
GLOBOCAN 2020 global cancer analysis, the incidence of lung cancer
ranks second in the world, with >2.2 million cases (11.4%) and
>1.79 million deaths (18%) every year (1). Non-small cell lung cancer (NSCLC)
accounts for 80–85% of cases of lung cancer (2,3). In
recent years, EGFR-tyrosine kinase inhibitors (TKIs) have played a
role in molecular targeted therapy of NSCLC, improving the
prognosis of patients with NSCLC. Treatment with TKIs has
revolutionized the overall survival time and quality of life in
patients with NSCLC with EGFR mutations (4). The use of TKIs in NSCLC depends on the
presence of EGFR mutations (5).
Although EGFR plays an important role in the occurrence and
development of lung cancer, patients with lung cancer with EGFR
gene mutations account for 25% of NSCLC cases and are prone to drug
resistance and high cytotoxicity (6). It is an urgent problem to find an
effective anti-lung cancer drug with low toxicity; however, the
elaboration of new mechanisms of action of drugs already in
clinical application, or the study of new derivatives of drugs with
low toxicity, can save the time and cost of drug development
(7).
The discovery of the therapeutic potential of
nitrofuran derivatives dates back to 1948 when it was discovered
how they induced antimicrobial activity (8). Nifuroxazide (NFZ), a gastrointestinal
antibiotic, was first patented in 1961 (England), 1966 (France) and
1966 (USA) by Robert & Carrie Laboratories (9). In previous years, it has been mainly
used to prevent bacillary dysentery and enteritis (10). Previous studies have also shown that
NFZ has anti-inflammatory, anti-infection, anti-diabetes,
anti-renal fibrosis, anti-pulmonary fibrosis and antiproliferative
activity, and ameliorates the lipid and glucose metabolism of HepG2
liver cancer cells (11–15).
The role of NFZS in small cell lung cancer is
unclear. The purpose of the present study was to investigate the
relationship between NFZ and H299 cell apoptosis, reactive oxygen
species (ROS) and ER stress (ERS), and to explore whether NFZ is a
PERK mechanism through one of the classical pathways of ERS.
Materials and methods
Materials and instruments
Human NSCLC cells, NCI-H1299 (National
Infrastructure of Cell Line Resource), were cultured in DMEM high
glucose medium (Hyclone; Cytiva) containing 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) in a cell incubator at 37°C
and 5% CO2.
NFZ and GSK2606414 were purchased from Selleck
Chemicals. With dimethyl sulfoxide (DMSO) as the solvent, they were
prepared into 50 mM storage solutions and stored at −80°C. The
following reagents were also used in the present study: Antibodies
against protein kinase R-like ER kinase (PERK; 1:1,000; #5683; CST
Biological Reagents Co., Ltd.), P-PERK (1:1,000; #3179; CST
Biological Reagents Co., Ltd.), activating transcription factor 4
(ATF4; 1:1,000; #11815; CST Biological Reagents Co., Ltd.), DNA
damage inducible transcript 3 (CHOP; 1:1,000; #2895; CST Biological
Reagents Co., Ltd.), Janus kinase 2 (JAK2; 1:1,000; #3230; CST
Biological Reagents Co., Ltd.), P-JAK2 (1:1,000; #3771; CST
Biological Reagents Co., Ltd.), signal transducer and activator of
transcription 3 (STAT3; 1:1,000; #9139; CST Biological Reagents
Co., Ltd.), P-STAT3 (1:1,000; #9145; CST Biological Reagents Co.,
Ltd.), HRP-linked Anti-Rabbit IgG (1:5,000; #7074; CST Biological
Reagents Co., Ltd.), HRP-linked Anti-Mouse IgG (1:5,000; #7076; CST
Biological Reagents Co., Ltd.), HRP-conjugated β-actin (1:5,000;
#4967; CST Biological Reagents Co., Ltd.) and HRP-conjugated GAPDH
(1:5,000; #3683; CST Biological Reagents Co., Ltd.); Annexin V/PI
kit and BCA kit (both from Yeasen Biotechnology Co., Ltd.); Brite
670 (AmyJet Scientific, Inc.) and Fura Red AM (A&D Technology);
DAPI (Sigma-Aldrich; Merck KgaA).
The following instruments were used in the present
study: BB15 CO2 incubator (Thermo Fisher Scientific,
Inc.), desktop low-temperature 5810R centrifuge, BD Accuri™ C6 flow
cytometer (BD Biosciences), Olympus IX-B53 inverted fluorescence
microscope (Olympus Corporation), Bio-Rad 550 enzyme reader and
electrophoresis/membrane transfer apparatus (both from Bio-Rad
Laboratories, Inc.), and BioSpectrum 810 Imaging System (Analytik
Jena AG).
Cell Counting Kit-8 (CCK-8) cell
viability assay
H1299 cells in the logarithmic growth stage were
divided into two groups, the DMSO and NFZ groups. The NFZ group was
divided into seven sub-groups depending on the concentration of NFZ
and there were three replicates in each group. The single cell
suspension was aliquoted into 96-well plates, with 1×104
cells/well in 100 µl DMEM, the plate was then placed in a
CO2 cell incubator at 37°C. When the cell adhesion
density reached 75%, DMEM high glucose medium containing 1% DMSO
was added to the DMSO group, and DMEM high glucose medium
containing 0.25, 0.5, 1, 2, 4, 8, and 16 µM NFZ was added to the
NFZ sub-groups. At 24, 48 and 72 h time points, 10 µl CCK-8
solution (Yeasen Biotechnology Co., Ltd.) was added to each well
and incubated in the cell incubator for 1 h, and an empty well was
also filled with 10 µl CCK-8 solution as a blank group. The plate
was inserted into the Bio-Rad 550 enzyme microplate reader and each
group was measured at an OD of 450 nm. The experiment was repeated
three times. Relative cell viability rate=[(cell experimental group
A450-blank group A450)/(cell control
A450-blank group A450)] ×100%. The
IC50 of NFZ was calculated using GraphPad Prism 8.0
software (Dotmatics).
H1299 cells were washed with PBS and digested with
25% trypsin-EDTA to form a single cell suspension that was added to
96-well plates. The plates were randomly divided into a DMSO, NFZ,
GSK2606414 and NFZ + GSK2606414 group, and each group had three
wells. At 24 h, cells in each group were treated, except the cells
in the DMSO group. Cells in the NFZ group were treated with 20 µM
NFZ medium, and cells in the GSK2606414 group were treated with 1
µM GSK2606414 medium. Cells in the NFZ + GSK2606414 group were
treated with 1 µM GSK2606414 and 1 h later, 20 µM NFZ and 1 µM
GSK2606414 were added in the form of liquid exchange. All plates
were then placed in the incubator for incubation at 37°C. At 24 h
time points, 10 µl CCK-8 solution was added to each well and
incubated in the cell incubator for 1 h. The plates were inserted
into the Bio-Rad 550 enzyme microplate reader and each group was
measured at an OD of 450 nm.
Optical microscopy
After digestion in 0.25% trypsin, H1299 cells were
seeded into 24-well plates at 3×105 cells/ml in each
well. The cells were placed in the incubator, the morphological
changes were observed and images were captured under an optical
microscope after 24 h.
Inverted fluorescence microscopy
The grouping and dosing methods aforementioned were
repeated and, 24 h later, 10 µl Brite 670 was added to the cells
and the cells were incubated at 37°C for 30 min in the dark. The
cells were then washed twice with 1X PBS and images were captured
using an inverted IX-B53 fluorescence microscope at ×200
magnification. ImageJ (v1.53c) was used for data analysis.
Flow cytometry
H1299 cells were washed with PBS and digested with
25% trypsin-EDTA to form a single cell suspension that was added to
10-cm dishes for culturing. The plates were randomly divided into a
DMSO, NFZ, GSK2606414 and NFZ + GSK2606414 group. At 24 h, cells in
each group were treated, except the cells in the DMSO group. Cells
in the NFZ group were treated with 20 µM NFZ medium, and cells in
the GSK2606414 group were treated with 1 µM GSK2606414 medium.
Cells in the NFZ + GSK2606414 group were treated with 1 µM
GSK2606414 and 1 h later, 20 µM NFZ and 1 µM GSK2606414 were added
in the form of liquid exchange. All plates were then placed in the
incubator for incubation at 37°C for 24 h time. After treatment,
the cells were digested with 0.25% trypsin and collected by
centrifugation (300 × g, 4°C, 5 min). After centrifugation, the
cells were resuspended in PBS, the cell density was adjusted to
1×105 cells/ml and the cells were washed with PBS a
further three times. For detection, 100 µl 1X binding buffer
containing 5 µl Annexin V-FITC and 10 µl PI were added into each
tube and incubated at 37°C for 60 min. Subsequently, 400 µl 1X
binding buffer was added to each tube for cell resuspension, and
cell apoptosis was detected by BD Accuri C6 flow cytometer and the
data were analyzed using FlowJo 10.0 (FlowJo LLC).
To stain intracellular ROS, 10 µl 100 µM Brite 670
was added to the treated cells and ROS was detected by flow
cytometry. Brite 670 was first incubated for 30 min, and then DAPI
was added for 15 min. Finally, the double-stained cells were
cleaned twice with PBS, which led to observation and photography
under fluorescence microscope. In addition, 200 µl Fura Red AM was
added to the treated cells for intracellular Ca2+
staining, and the emission wavelength was detected at 550 nm. The
BD Accuri C6 flow cytometer was used for detection and the data
were analyzed using FlowJo 10.0 (FlowJo LLC).
Western blot analysis
After the aforementioned treatments, the cells were
washed with PBS and centrifuged (300 × g, 4°C, 5 min) to collect
the suspended cells. The cells were lysed with RIPA buffer
(Beyotime Institute of Biotechnology) containing 1:100 protease
inhibitor and 1:100 phosphatase inhibitor, and a BCA kit was used
to detect protein concentration.
Proteins (20 µg) were separated by SDS-PAGE on 10 or
12% gels. Electrophoresis and PVDF membrane transfer were performed
using Bio-Rad electrophoresis and membrane transfer apparatus. For
blocking, the membranes were incubated with 5% BSA (Biological
Industries) on a 4°C with agitation for 2 h. After blocking, the
membranes were washed three times for 10 min intervals. Primary
antibodies were added at a dilution of 1:1,000 and incubated at 4°C
overnight. Subsequently, the membranes were washed three times for
10 min intervals. After the addition of the secondary antibody at a
ratio of 1:5,000, the membranes were incubated with agitation for 2
h at 4°C and then washed three times for 10 min intervals. Finally,
ECL chemiluminescence developer (Yeasen Biotechnology Co., Ltd.)
was added to the membranes and, after full reaction, the membranes
were placed in a Biospectrum 810 Imaging system for exposure
development. The protein expression bands were observed and
recorded, and the gray values of each band were analyzed by ImageJ
software (v1.53c).
Statistical analysis
GraphPad Prism 8.0 software was used for statistical
analysis. The data are presented as the mean ± SD. Student's t-test
was used for data comparison between two unpaired groups. One-way
ANOVA was applied for multiple group comparisons and Dunnett's post
hoc test was performed to compare the means of multiple groups with
those of a single group. All experiments were repeated three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
NFZ inhibits the viability of H1299
cells
After H1299 cells were treated with 0.25, 0.5, 1, 2,
4, 8 and 16 µM NFZ for 24, 48 and 72 h, the viability of the H1299
cells decreased significantly compared with that in the DMSO group
and showed significant cytotoxicity, which was proportional to time
and dose (Fig. 1). The
IC50 of NFZ in H1299 cells at 24 h was calculated by
GraphPad Prism 8.0. The IC50 in H1299 cells at 24 h was
19.78 µM; therefore, 20 µM was chosen as the experimental
concentration.
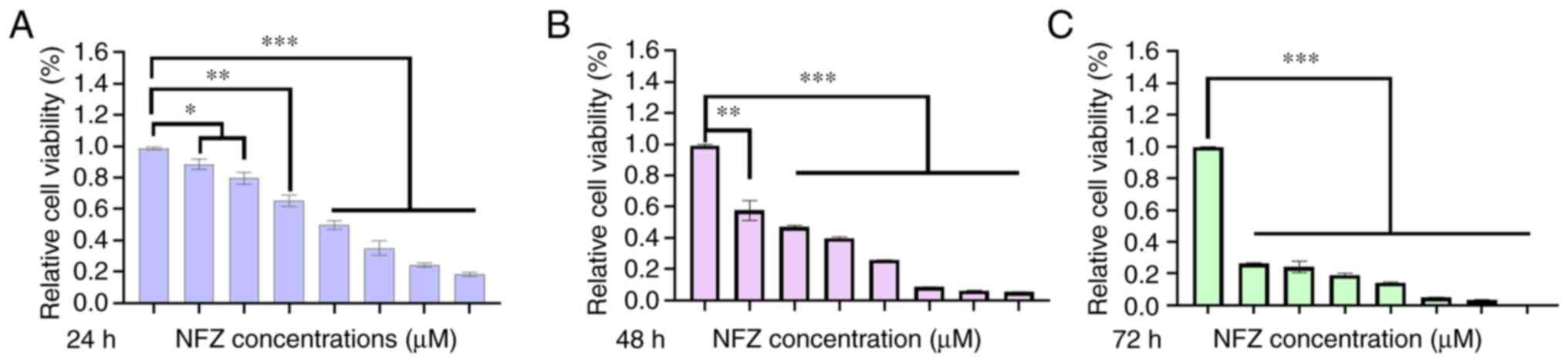 | Figure 1.Effects of different concentrations
of NFZ (0.25, 0.5, 1, 2, 4, 8 and 16 µM) on H1299 cell viability at
(A) 24, (B) 48 and (C) 72 h. Relative cell viability was determined
by Cell Counting Kit-8 assay. Compared with in the DMSO group,
H1299 cell viability of even the low dose (0.25 µM) group was
significantly decreased after 24 h of treatment. *P<0.05,
**P<0.01, ***P<0.001. DMSO, dimethyl sulfoxide; NFZ,
nifuroxazide. |
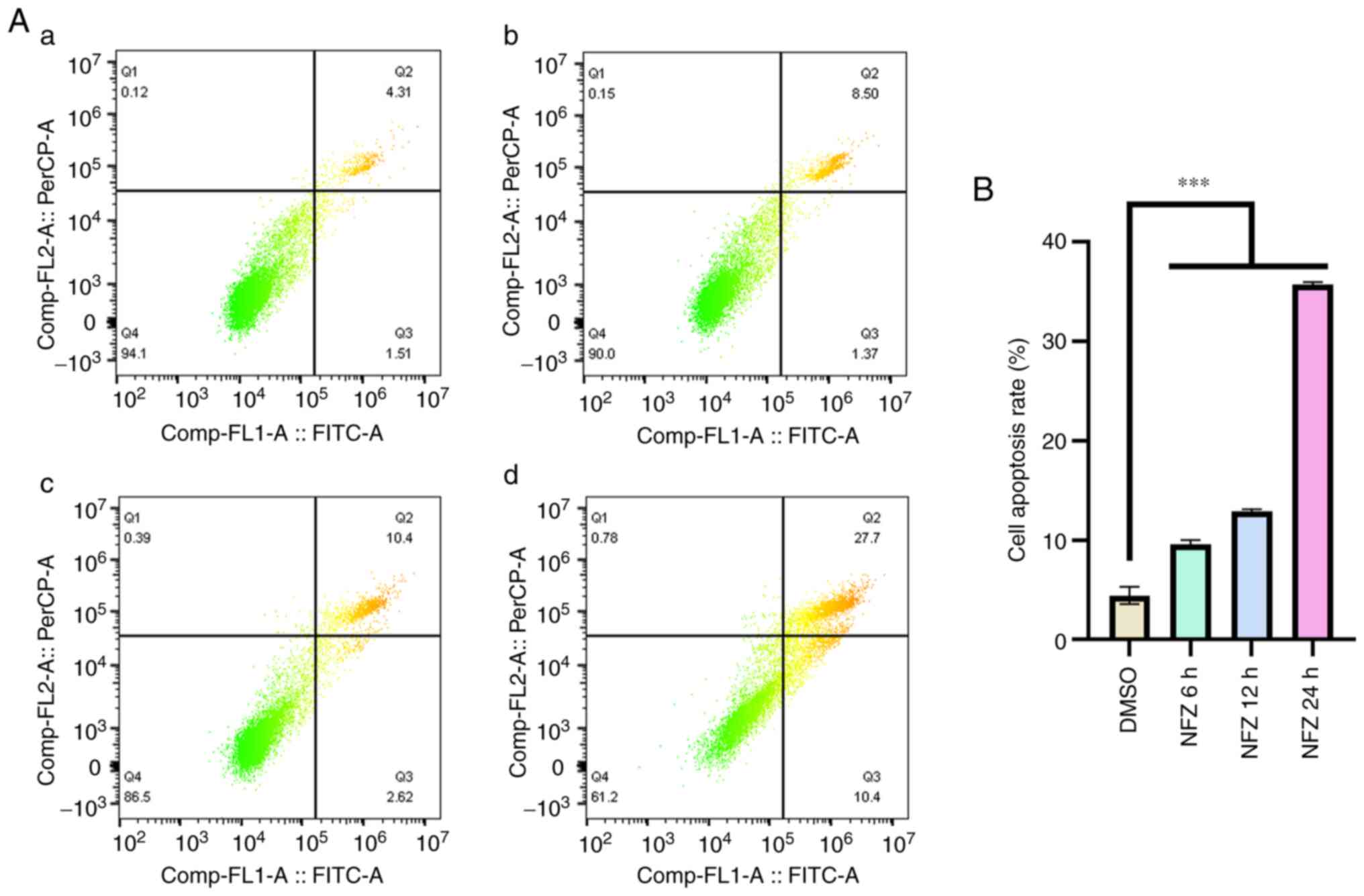
NFZ induces the apoptosis of H1299
cells
H1299 cells were treated with 20 µm NFZ for 6, 12
and 24 h, and the degree of apoptosis of H1299 cells increased in a
time-dependent manner. After 6 h, apoptosis began to increase
significantly (Fig. 2).
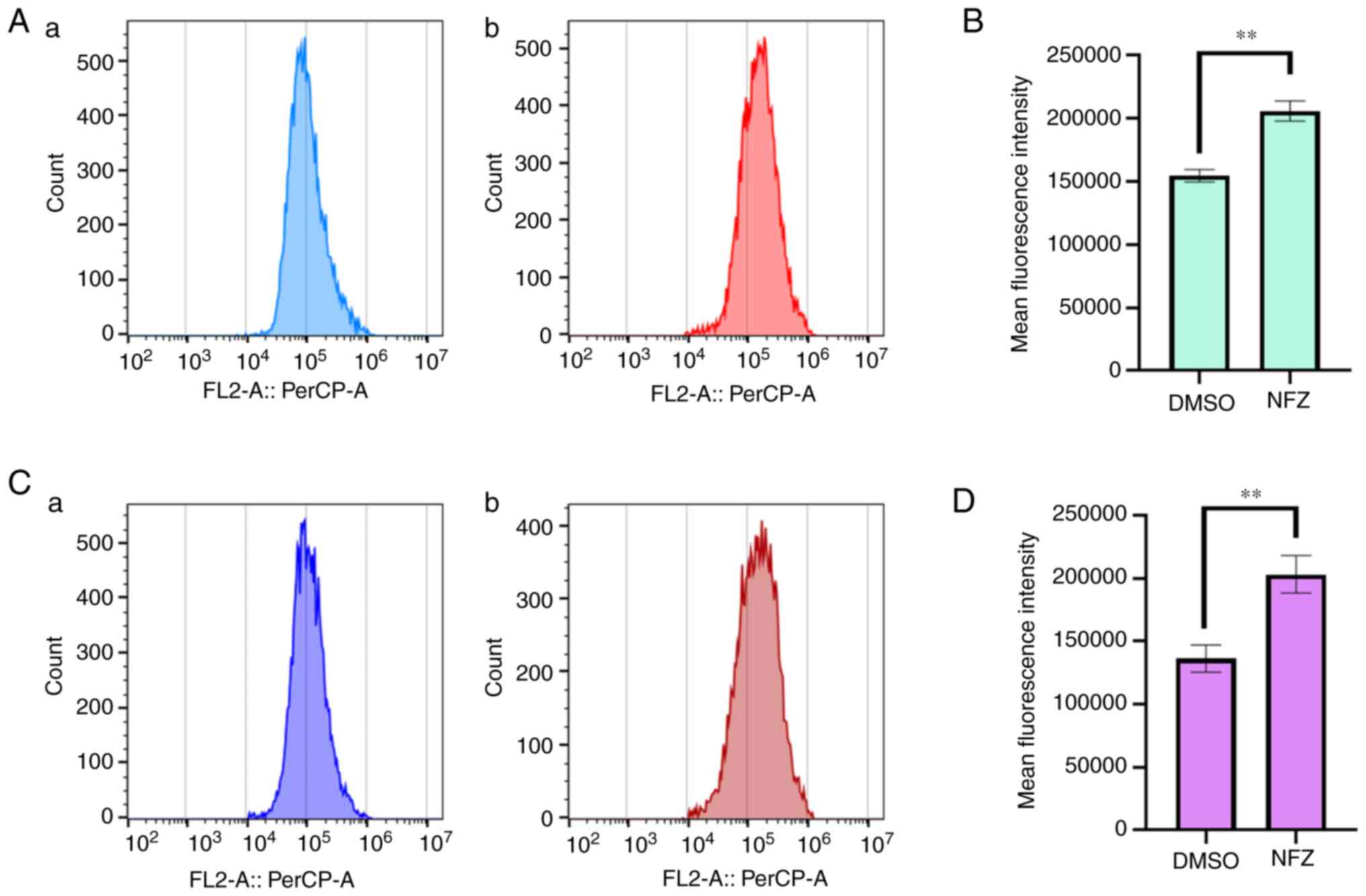
NFZ increases ROS and Ca2+
levels in H1299 cells
After exposure to 20 µM NFZ for 24 h, compared with
those in the DMSO control group, an increase in the intracellular
ROS (Fig. 3A and B) and
Ca2+ levels (Fig. 3C and
D) was observed.
NFZ induces activation of the
ERS-related PERK pathway
NFZ significantly increased the expression levels of
P-PERK, ATF4 and CHOP, key proteins in the PERK pathway of ERS, and
unphosphorylated PERK decreased with treatment of the drug,
compared with that in the DMSO control group (Fig. 4). These results were observed even
after 6 h of treatment.
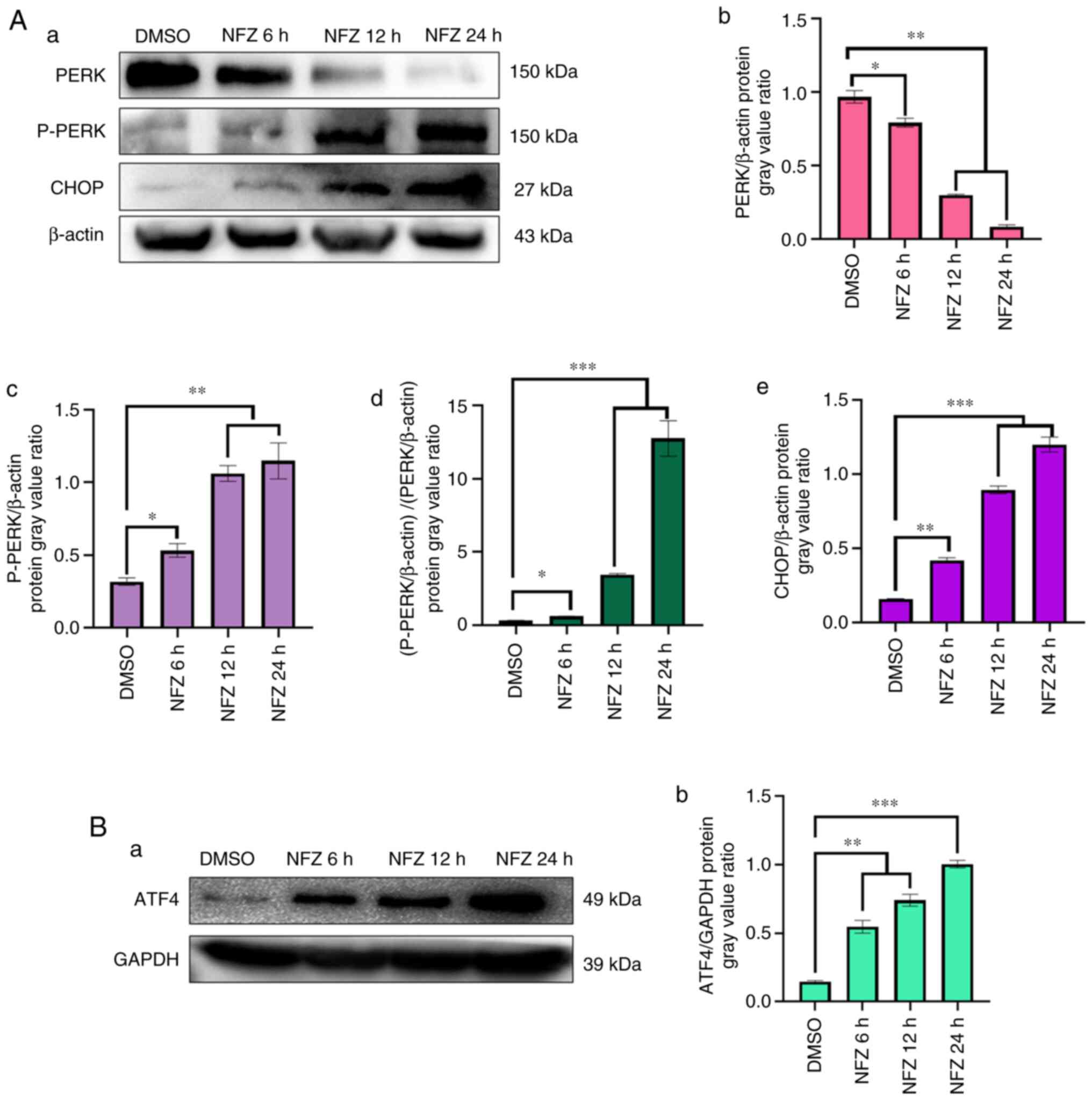 | Figure 4.Changes in PERK, P-PERK, ATF4 and
CHOP expression. (Aa) Expression of PERK, P-PERK and CHOP in H1299
cells exposed to NFZ for 6, 12 and 24 h. (Ab) The ratio of PERK
expression relative to β-actin expression in each group. (Ac) The
ratio of P-PERK expression relative to β-actin expression in each
group. (Ad) The ratio of p-PERK/β-actin to PERK/β-actin. (Ae) The
ratio of CHOP expression relative to β-actin expression in each
group. (Ba) Expression of ATF4 in H1299 cells exposed to NFZ for 6,
12 and 24 h. (Bb) The ratio of ATF4 expression relative to GAPDH
expression in each group. Data are presented as the mean ± SD.
*P<0.05, **P<0.01, ***P<0.001. AFT4, activating
transcription factor 4; CHOP, DNA damage inducible transcript 3;
DMSO, dimethyl sulfoxide; NFZ, nifuroxazide; PERK, protein kinase
R-like ER kinase. |
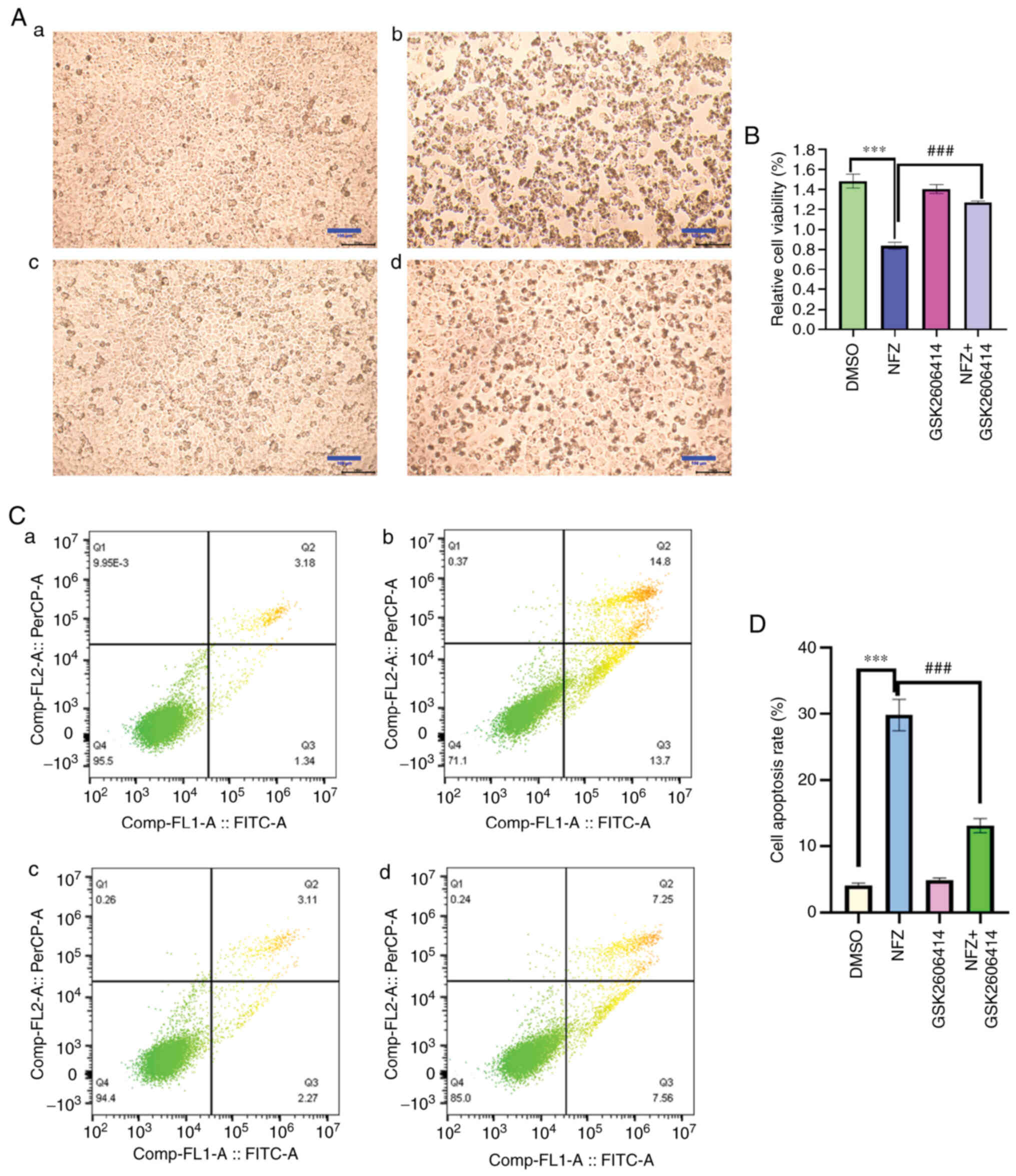
GSK2606414 reduces the morphological
changes and apoptosis induced by NFZ
Compared with in the DMSO group, the cell body of
H1299 cells treated with NFZ decreased and became round, and the
cells did not form colonies. A small amount of granular material
appeared in the cells, there were more cell fragments in the
culture medium and the cell viability was significantly decreased
(Fig. 5A and B). The addition of
GSK2606414 reduced the morphological changes induced by NFZ. The
addition of 1 µM GSK2606414 also reduced the NFZ-induced apoptosis
and cytotoxicity in H1299 cells (Fig.
5B-D).
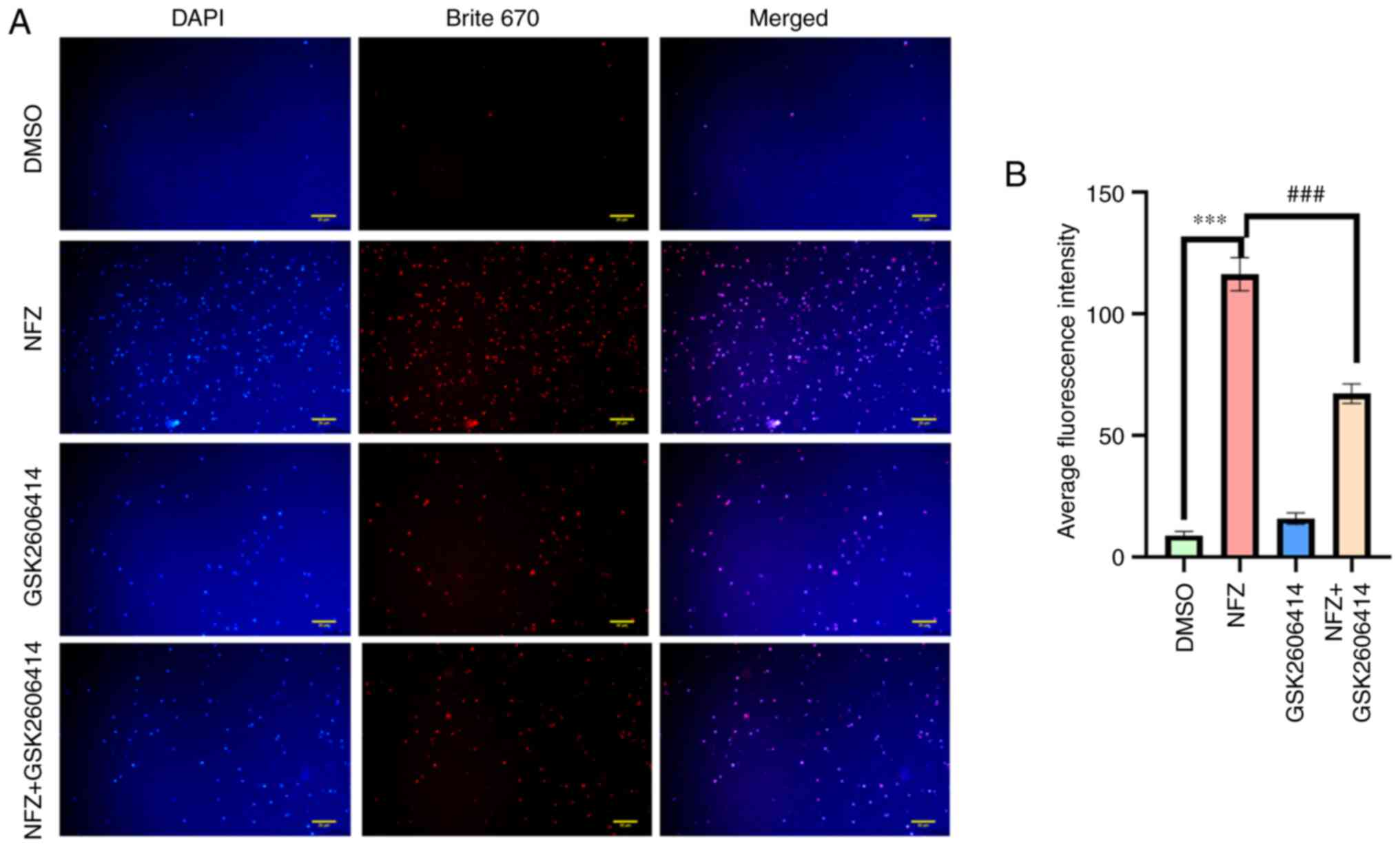
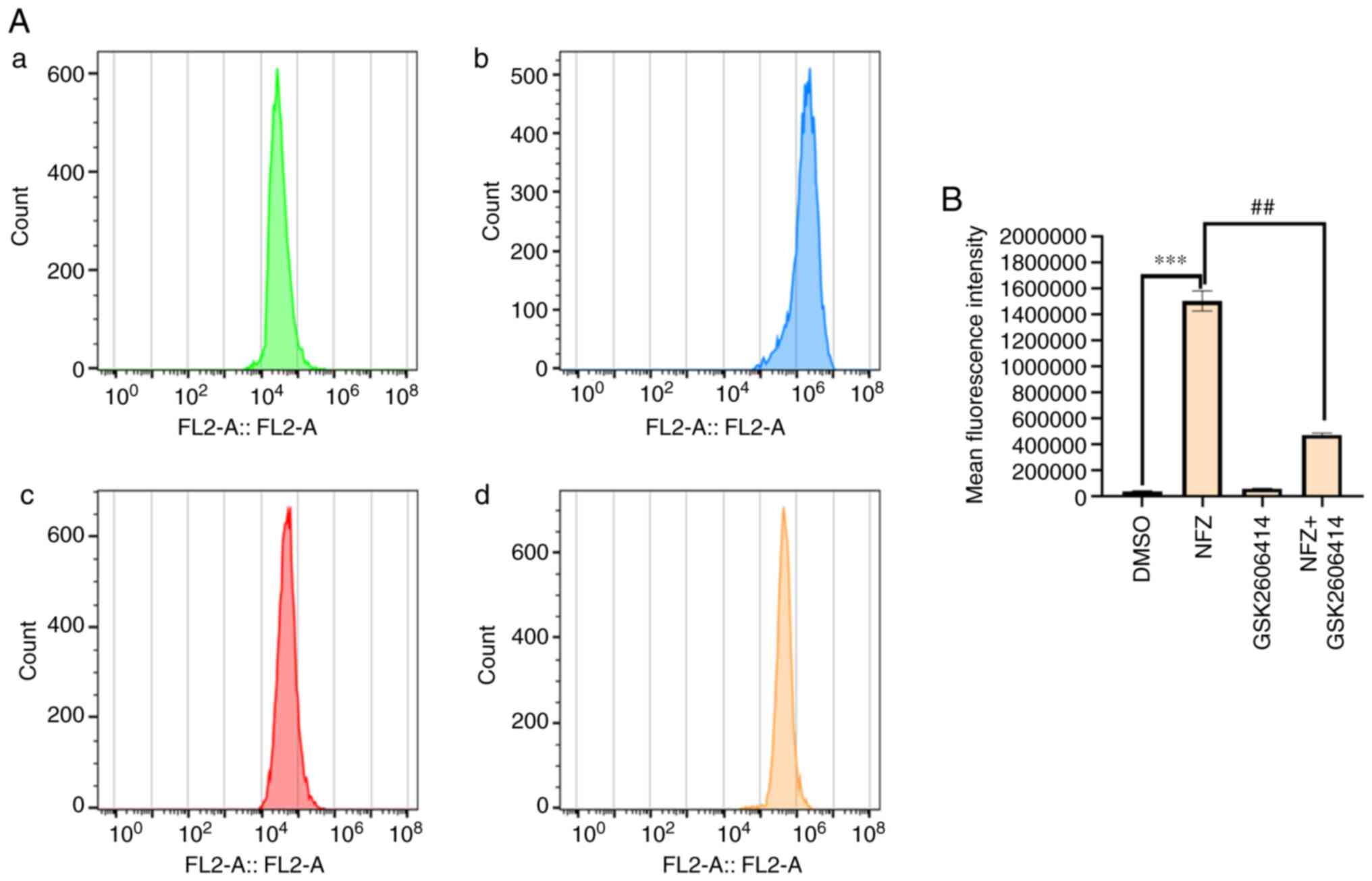
GSK2606414 can inhibit ERS-induced by
NFZ
GSK2606414 significantly reduced the increase of
intracellular ROS induced by NFZ (Fig.
6). ROS is closely related to oxidative stress, and PERK is one
of the classical pathways of ERS (16,17).
The results of the present study indicated that the increase of
intracellular ROS level induced by NFZ can lead to ERS. After ERS
occurs, Ca2+ homeostasis in the ER is unbalanced and
Ca2+ in the ER is released into the cytoplasm, which
increases the level of ROS in the cytoplasm and further aggravates
oxidative stress (16,17). GSK2606414 inhibited the increase in
intracellular Ca2+ induced by NFZ (Fig. 7), indicating that Ca2+
release from the ER is an important mechanism of NFZ-induced H1299
cell apoptosis. GSK2606414 also inhibited the increase in P-PERK,
ATF4 and CHOP protein expression levels induced by NFZ (Fig. 8).
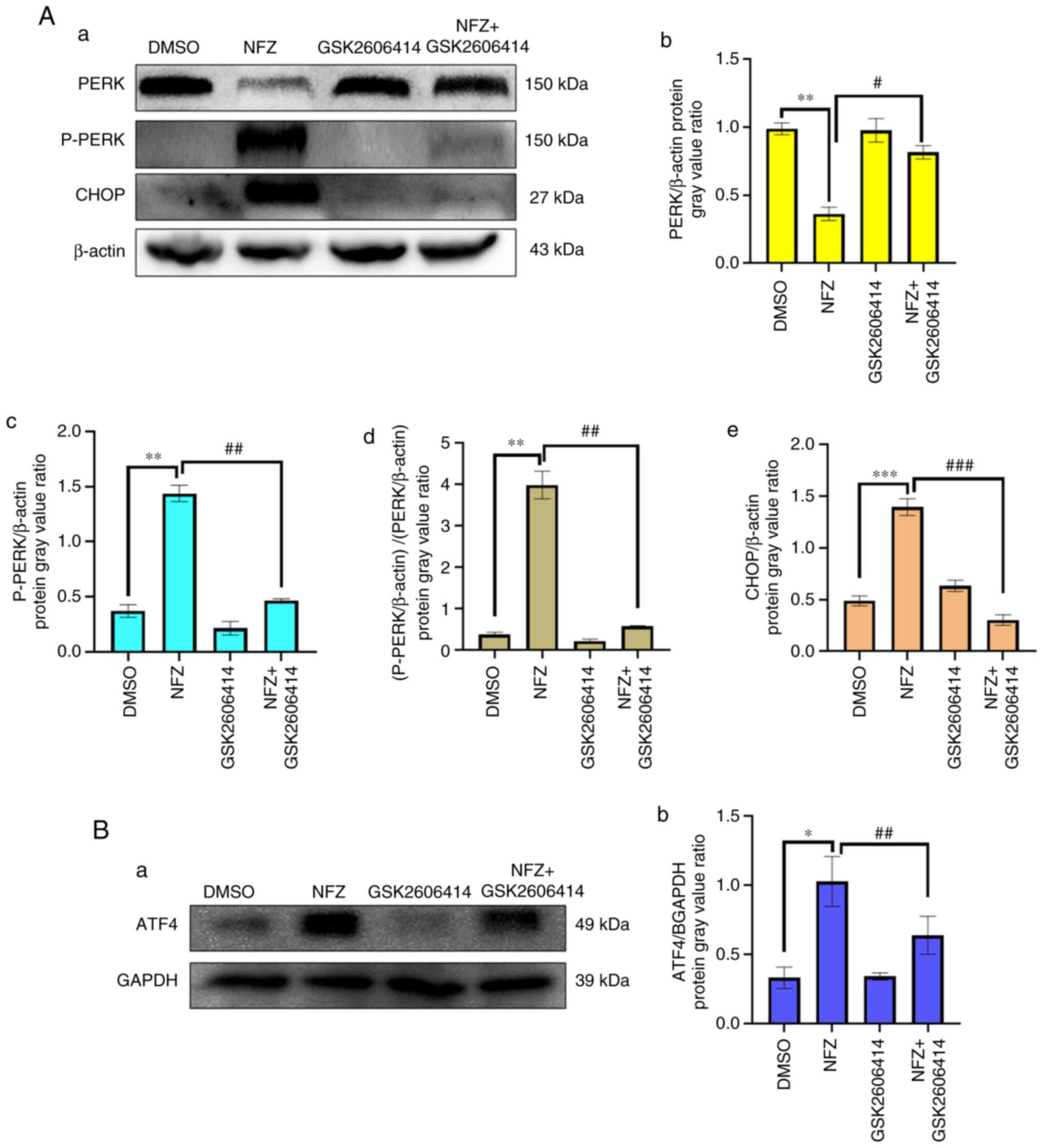 | Figure 8.GSK2606414 inhibits the increases in
P-PERK, ATF4 and CHOP protein expression induced by NFZ. (Aa)
Changes in PERK, P-PERK, ATF4 and CHOP in DMSO, NFZ, GSK2606414 and
NFZ + GSK2606414 groups. (Ab) The ratio of PERK expression relative
to β-actin expression in each group. (Ac) The ratio of P-PERK
expression relative to β-actin expression in each group. (Ad) The
ratio of P-PERK/β-actin to PERK/β-actin. (Ae) The ratio of CHOP
expression relative to β-actin expression in each group; (Ba)
Expression of ATF4 in DMSO, NFZ, GSK2606414 and NFZ + GSK2606414
groups. (Bb) The ratio of ATF4 expression relative to GAPDH
expression in each group. *P<0.05, **P<0.01, ***P<0.001,
#P<0.05, ##P<0.01,
###P<0.001. AFT4, activating transcription factor 4;
CHOP, DNA damage inducible transcript 3; DMSO, dimethyl sulfoxide;
NFZ, nifuroxazide; PERK, protein kinase R-like ER kinase. |
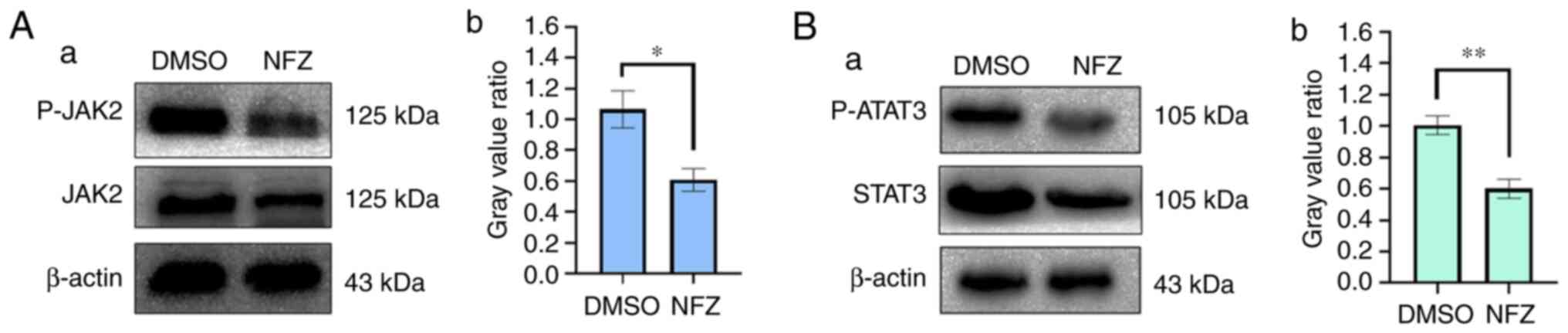
NFZ reduces the phosphorylation of
JAK2 and STAT3
The western blotting results showed that the
phosphorylation levels of STAT3 and JAK2 in the NFZ group were
significantly lower than those in the DMSO group. These findings
indicated that NFZ may be an inhibitor of JAK2 and STAT3 (Fig. 9).
Discussion
NFZ has become a research focus in recent decades
due to its antitumor and JAK2 inhibitor activity (18). NFZ, an oral nitrofuran antibiotic,
reduces the viability of a number of cancer cells (such as
osteosarcoma, multiple myeloma, breast cancer, colorectal carcinoma
and solid Ehrlich carcinoma cells) by inhibiting STAT3
phosphorylation (19–23). NFZ can significantly inhibit the
migration and invasion of osteosarcoma cells through P-STAT3, MMP-2
and MMP-9 mediated signaling pathways (19). In addition, studies have shown that
NFZ has antitumor cell proliferation and metastasis effects on
liver cancer, breast cancer, multiple myeloma and melanoma
(12,20–22,24).
The occurrence of malignant tumors is due to inhibition of the
apoptosis mechanism, leaving cells unable to carry out cell death
and cell elimination. The induction of tumor cell apoptosis is the
main way to eliminate cancer cells. Both the ER and mitochondrial
pathways are typical apoptotic pathways. A previous study reported
that NFZ can induce the apoptosis of osteosarcoma cells through the
mitochondrial pathway (19).
Oxidative stress refers to imbalance of the redox
system and the significant increase of free radicals, which exceeds
the scavenging capacity of the endogenous antioxidant system
(25). However, when the production
of free radicals exceeds the scavenging capacity of the body,
excessive ROS will accumulate in the cells and attack biological
macromolecules and organelles, resulting in different degrees of
oxidative stress response to DNA, proteins, lipids and
carbohydrates (26). Certain
studies have also demonstrated that ROS in tumor cells exceed the
tolerance capacity of cells, resulting in changes in tumor cell
apoptosis (27,28). The present study demonstrated that
intracellular ROS were significantly increased in H1299 lung cancer
cells treated with NFZ for 24 h. CCK-8 and flow cytometry
experiments also demonstrated that NFZ may induce the apoptosis of
H1299 cells via oxidative stress. ERS is closely related to
oxidative stress and early ERS plays a cytoprotective role, but
sustained ERS activates the apoptosis signaling pathway to protect
the functional stability of cells (29). PERK, a member of the elF2a protein
kinase family, is also a type I transmembrane protein kinase
located in the ER membrane, which mainly transduces ERS signals via
three typical ERS-induced apoptotic pathways involving
inositol-requiring enzyme 1, PERK and ATF6 (30,31).
PERK is also activated during tumor progression (32).
ERS can promote apoptosis mainly by promoting the
expression of transcription factors, such as ATF4 and CHOP. ATF4
plays a key role in the transcriptional regulation of pro-survival
genes mainly related to oxidative stress, autophagy, protein
folding, amino acid synthesis and cell differentiation (33). The PERK signaling pathway not only
reduces the folding pressure of newly synthesized proteins from the
ER, but also specifically enhances the transcription level of
certain genes through the fine regulatory mechanism. The
upregulation of these genes is mediated by the transcription
factor, ATF4 (32,34). After synthesis, ATF4 is translocated
to the nucleus, and acts as a transcription factor to upregulate
the transcriptional expression of molecular chaperones in the ER
and amino acid transport proteins. However, sustained
overexpression of ATF4 promotes the upregulation of the gene
encoding CHOP (35,36). It has been confirmed that ERS in
tumor cells leads to the activation of CHOP (32,34).
Apoptosis is primarily inhibited by the presence of the
anti-apoptotic molecule, Bcl-2, which has previously been observed
in various tumor types (including primary cutaneous B-cell
non-Hodgkin's lymphoma cells and oral squamous cells) and high
expression of Bcl-2 is associated with survival and therapeutic
response (37,38). A previous study reported that NFZ
induces apoptosis in breast cancer through the activation of
cleaved caspase-3 and Bax and the downregulation of Bcl-2 (21). Similarly, in another study, NFZ
upregulated CHOP protein levels and induced the caspase molecular
cascade reaction by reducing Bcl-2 levels, thus leading to
apoptosis of tumor cells (39). In
the present study, the experimental results demonstrated that NFZ
significantly increased the expression levels of P-PERK, ATF4 and
CHOP. These results indicated that NFZ may induce H1299 cell
apoptosis through the PERK ERS pathway. ERS is an important pathway
for cell apoptosis, and its process requires the participation of a
number of molecular chaperones and specific proteins, including the
transcriptional activation of CHOP and the Ca2+ pathway
(35,40). Under ERS, Ca2+ is
released from the ER into the cytoplasm, which causes internal
Ca2+ overload and further aggravates ROS production,
thus enhancing oxidative stress. The present study demonstrated
that intracellular Ca2+ increased after NFZ treatment of
H1299 cells for 24 h, and the PERK inhibitor, GSK2606414,
significantly reduced the intracellular ROS, Ca2+ levels
and the protein expression levels of P-PERK, ATF4 and CHOP, thus
inhibiting the level of apoptosis.
As depicted in Fig.
10, NFZ may induce apoptosis of human NSCLC H1299 cells by
activating the PERK-ATF4-CHOP pathway of ERS. The present study
demonstrated the molecular mechanism of NFZ cytotoxicity from the
perspective of ERS-mediated apoptosis, which may provide a new
basis for revealing the cytotoxicity of NFZ to tumor cells. The
present study also provided some toxicological data to support the
scientific and rational use of NFZ and provides a new option and
molecular biological basis for the treatment of NSCLC. However, the
in vitro culture cannot completely simulate the in
vivo internal environment. Therefore, in vivo
experiments will be conducted in a future study.
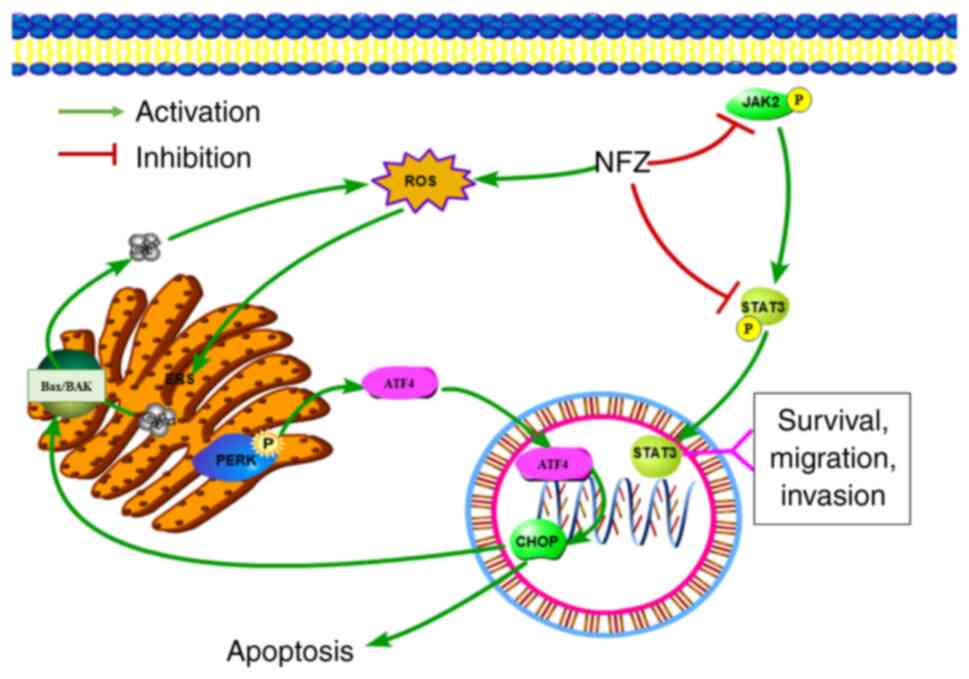 | Figure 10.As a classical inhibitor of
JAK2/STAT3, NFZ can induce ERS by increasing the intracellular ROS
levels, thereby releasing Ca2+ from the ER into the
cytoplasm, increasing the cytoplasmic Ca2+ levels and
further increasing the intracellular ROS levels. In addition, the
PERK pathway, the classical pathway of ERS, is activated, and the
level of CHOP in the nucleus is increased, thus inducing the
apoptosis of H1299 human non-small cell lung cancer cells. AFT4,
activating transcription factor 4; CHOP, DNA damage inducible
transcript 3; ERS, ER stress; JAK2, Janus kinase 2; NFZ,
nifuroxazide; PERK, protein kinase R-like ER kinase; ROS, reactive
oxygen species; STAT3, signal transducer and activator of
transcription 3. |
Acknowledgements
Not applicable.
Funding
Funding was provided by The Qingdao Applied Basic Research
Program (grant no. 19-6-2-31-cg).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available as the subject has not
been fully concluded and the data cannot be disclosed for the time
being, but are available from the corresponding author on
reasonable request.
Authors' contributions
DL and LL co-wrote the manuscript. In addition, DL
and LL jointly performed experiments, analyzed the experimental
data and drew the pathway map. DL, LL and FL searched the
literature together. FL corrected the language for some important
content and helped to design some of the experiments. KG and CM
designed the study together and revised the article. FL also helped
to design the study. KG gave the funding support. All authors read
and approved the final manuscript. All authors confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NFZ
|
nifuroxazide
|
|
ERS
|
endoplasmic reticulum stress
|
|
PERK
|
protein kinase R-like ER kinase
|
|
ATF4
|
activating transcription factor 4
|
|
CHOP
|
DNA damage inducible transcript 3
|
|
DMSO
|
dimethyl sulfoxide
|
|
ROS
|
reactive oxygen species
|
|
JAK2
|
Janus kinase 2
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Basumallik N and Agarwal M: Small cell
lung cancer. StatPearls [Internet]. StatPearls Publishing; Treasure
Island, FL: 2022
|
|
4
|
Jurisic V, Vukovic V, Obradovic J,
Gulyaeva LF, Kushlinskii NE and Djordjević N: EGFR polymorphism and
survival of NSCLC patients treated with TKIs: A systematic review
and meta-analysis. J Oncol. 2020:19732412020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jurišić V, Obradovic J, Pavlović S and
Djordjevic N: Epidermal growth factor receptor gene in
non-small-cell lung cancer: The importance of promoter polymorphism
investigation. Anal Cell Pathol (Amst). 2018:61921872018.PubMed/NCBI
|
|
6
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pushpakom S, Iorio F, Eyers PA, Escott KJ,
Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, et
al: Drug repurposing: Progress, challenges and recommendations. Nat
Rev Drug Discov. 18:41–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ward WC and Dodd MC: A comparative study
of the in vitro bacteriostatic action of some simple derivatives of
furan, thiophene, and pyrrole. J Bacteriol. 56:649–652. 1948.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
CarronMCE, . Antibacterial
Nitrofurfuryldene Derivatives and Methods of Using Same. US Patent
US3290213A, Filed July 9 1975; issued. December 6–1966.
|
|
10
|
Masunari A and Tavares LC: A new class of
nifuroxazide analogues: Synthesis of 5-nitrothiophene derivatives
with antimicrobial activity against multidrug-resistant
Staphylococcus aureus. Bioorg Med Chem. 15:4229–4236. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Said E, Zaitone SA, Eldosoky M and
Elsherbiny NM: Nifuroxazide, a STAT3 inhibitor, mitigates
inflammatory burden and protects against diabetes-induced
nephropathy in rats. Chem Biol Interact. 281:111–120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao T, Jia H, Cheng Q, Xiao Y, Li M, Ren
W, Li C, Feng Y, Feng Z, Wang H and Zheng J: Nifuroxazide prompts
antitumor immune response of TCL-loaded DC in mice with
orthotopically-implanted hepatocarcinoma. Oncol Rep. 37:3405–3414.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gan C, Zhang Q, Liu H, Wang G, Wang L, Li
Y, Tan Z, Yin W, Yao Y, Xie Y, et al: Nifuroxazide ameliorates
pulmonary fibrosis by blocking myofibroblast genesis: A drug
repurposing study. Respir Res. 23:322022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saber S, Nasr M, Kaddah MMY,
Mostafa-Hedeab G, Cavalu S, Mourad AAE, Gaafar AGA, Zaghlool SS,
Saleh S, Hafez MM, et al: Nifuroxazide-loaded cubosomes exhibit an
advancement in pulmonary delivery and attenuate bleomycin-induced
lung fibrosis by regulating the STAT3 and NF-κB signaling: A new
challenge for unmet therapeutic needs. Biomed Pharmacother.
148:1127312022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JY, Zhang YC, Song LN, Zhang L, Yang
FY, Zhu XR, Cheng ZQ, Cao X and Yang JK: Nifuroxazide ameliorates
lipid and glucose metabolism in palmitate-induced HepG2 cells. RSC
Adv. 9:39394–39404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caballano-Infantes E, Terron-Bautista J,
Beltrán-Povea A, Cahuana GM, Soria B, Nabil H, Bedoya FJ and Tejedo
JR: Regulation of mitochondrial function and endoplasmic reticulum
stress by nitric oxide in pluripotent stem cells. World J Stem
Cells. 9:26–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao S, Tang J, Huang Y, Li G, Li Z, Cai W,
Yuan Y, Liu J, Huang X and Zhang H: The road of solid tumor
survival: From drug-induced endoplasmic reticulum stress to drug
resistance. Front Mol Biosci. 8:6205142021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
da Costa MOL, Pavani TFA, Lima AN, Scott
AL, Ramos DFV, Lazarini M and Rando DGG: Nifuroxazide as JAK2
inhibitor: A binding mode proposal and Hel cell proliferation
assay. Eur J Pharm Sci. 162:1058222021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo Y, Zeng A, Fang A, Song L, Fan C, Zeng
C, Ye T, Chen H, Tu C and Xie Y: Nifuroxazide induces apoptosis,
inhibits cell migration and invasion in osteosarcoma. Invest New
Drugs. 37:1006–1013. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nelson EA, Walker SR, Kepich A, Gashin LB,
Hideshima T, Ikeda H, Chauhan D, Anderson KC and Frank DA:
Nifuroxazide inhibits survival of multiple myeloma cells by
directly inhibiting STAT3. Blood. 112:5095–5102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X,
Li Y, Jie H, Liu C, Xiong Y, et al: Nifuroxazide induces apoptosis
and impairs pulmonary metastasis in breast cancer model. Cell Death
Dis. 6:e17012015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye TH, Yang FF, Zhu YX, Li YL, Lei Q, Song
XJ, Xia Y, Xiong Y, Zhang LD, Wang NY, et al: Inhibition of Stat3
signaling pathway by nifuroxazide improves antitumor immunity and
impairs colorectal carcinoma metastasis. Cell Death Dis.
8:e25342017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Sherbiny M, El-Sayed RM, Helal MA,
Ibrahiem AT, Elmahdi HS, Eladl MA, Bilay SE, Alshahrani AM, Tawfik
MK, Hamed ZE, et al: Nifuroxazide mitigates angiogenesis in
Ehlrich's solid carcinoma: Molecular docking, bioinformatic and
experimental studies on inhibition of Il-6/Jak2/Stat3 signaling.
Molecules. 26:68582021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu Y, Ye T, Yu X, Lei Q, Yang F, Xia Y,
Song X, Liu L, Deng H, Gao T, et al: Nifuroxazide exerts potent
anti-tumor and anti-metastasis activity in melanoma. Sci Rep.
6:202532016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sies H: Oxidative stress: Oxidants and
antioxidants. Exp Physiol. 82:291–295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Sun X, Guo Y and Ge K: Evodiamine
induces ROS-Dependent cytotoxicity in human gastric cancer cells
via TRPV1/Ca2+ pathway. Chem Biol Interact.
351:1097562022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, St Clair DK, Xu Y, Crooks PA and St
Clair WH: A NADPH oxidase-dependent redox signaling pathway
mediates the selective radiosensitization effect of parthenolide in
prostate cancer cells. Cancer Res. 70:2880–2890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao B, Liu C, Liu BT, Zhang X, Liu RR and
Zhang XW: TTF1-NPs Induce ERS-Mediated apoptosis and inhibit human
hepatoma cell growth in vitro and in vivo. Oncol Res. 23:311–320.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Limia CM, Sauzay C, Urra H, Hetz C, Chevet
E and Avril T: Emerging roles of the endoplasmic reticulum
associated unfolded protein response in cancer cell migration and
invasion. Cancers (Basel). 11:6312019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oakes SA: Endoplasmic reticulum stress
signaling in cancer cells. Am J Pathol. 190:934–946. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rozpedek W, Pytel D, Mucha B, Leszczynska
H, Diehl JA and Majsterek I: The role of the PERK/eIF2α/ATF4/CHOP
signaling pathway in tumor progression during endoplasmic reticulum
stress. Curr Mol Med. 16:533–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen D, Fan Z, Rauh M, Buchfelder M,
Eyupoglu IY and Savaskan N: ATF4 promotes angiogenesis and neuronal
cell death and confers ferroptosis in a xCT-dependent manner.
Oncogene. 36:5593–5608. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kania E, Pająk B and Orzechowski A:
Calcium homeostasis and ER stress in control of autophagy in cancer
cells. Biomed Res Int. 2015:3527942015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oyadomri S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo FJ, Liu Y, Zhou J, Luo S, Zhao W, Li X
and Liu C: XBP1S protects cells from ER stress-induced apoptosis
through Erk1/2 signaling pathway involving CHOP. Histochem Cell
Biol. 138:447–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colovic N, Jurisic V, Terzic T, Atkinson
HD and Colovic M: Immunochemotherapy for Bcl-2 and MUM-negative
aggressive primary cutaneous B-cell non-Hodgkin's lymphoma. Arch
Dermatol Res. 301:689–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Popović B, Jekić B, Novaković I, Luković
LJ, Tepavcević Z, Jurisić V, Vukadinović M and Milasin J: Bcl-2
expression in oral squamous cell carcinoma. Ann N Y Acad Sci.
1095:19–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karan-Djurasevic T, Palibrk V, Zukic B,
Spasovski V, Glumac I, Colovic M, Colovic N, Jurisic V, Scorilas A,
Pavlovic S and Tosic N: Expression of Bcl2L12 in chronic
lymphocytic leukemia patients: Association with clinical and
molecular prognostic markers. Med Oncol. 30:4052013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao B, Lin D and Zhang X, Zhang M and
Zhang X: TTF1, in the form of nanoparticles, inhibits angiogenesis,
cell migration and cell invasion in vitro and in vivo in human
hepatoma through STAT3 regulation. Molecules. 21:15072016.
View Article : Google Scholar : PubMed/NCBI
|