Introduction
A solitary fibrous tumor (SFT) is a rare soft-tissue
tumor of mesenchymal origin (1).
The reported incidence rate is <0.1/100,000 individuals/year
(2). First recognized in the pleura
(1), with gross and histological
features that overlap with numerous other soft-tissue tumors, SFT
was previously referred to by several other names, including
pleural fibroma, benign mesothelioma, hemangiopericytoma, localized
fibrous tumor and subserosal fibroma (3). SFT is now recognized to occur at all
anatomical sites and comprises a histological spectrum, ranging
from hypocellular fibrous SFT, to hypercellular tumors previously
recognized as hemangiopericytoma, to anaplastic SFT with frank
sarcomatous transformation (3).
Historically, SFTs are divided into three categories: i) Benign
(local disease); ii) not otherwise specified (rarely metastasize);
and iii) malignant (4). The
traditional criteria for malignant SFTs include a mitotic rate of
≥4 per 10 high-power fields (HPFs), pleomorphism, necrosis and a
large tumor size (5). The diagnosis
of an SFT is established by the conjunction of clinical,
pathological, immunohistochemical and molecular features.
The treatment strategy of SFTs depends on the
historical type (3). For the benign
and not otherwise specified SFTs, the best treatment method is
complete surgical excision. Complete en bloc surgical resection
with negative margins (R0) is the gold-standard treatment for
malignant SFT. If the surgery could not achieve an R0 resection due
to anatomical site, then adjuvant radiotherapy and chemotherapy
could give a favorable long-term outcome.
To date, only 33 cases of breast SFTs, including 5
malignant tumors, have been reported (6). In the present report, a rare case of a
48-year-old female patient with a large tumor confirmed to be a
malignant SFT is reported. A brief literature review is also
presented.
Case report
A 48-year-old female patient visited Weifang
People's Hospital (Weifang, China) in September 2021 with the
complaint of a mass in the right breast that had expanded rapidly
in the preceding 2 years. The patient had no family history of
breast and ovarian cancer, and denied any chronic diseases,
including malignancies of other tissues. The patient accidentally
found a small nodule in the right upper outer quadrant measuring
2×2 cm2 ~2 years ago. The nodule grew rapidly and
occupied almost the entire left breast when the patient visited the
breast clinic of Weifang People's Hospital.
Physical examination revealed a giant hard mass
(~25×25×10 cm). The lesion occupied the entire right breast without
skin retraction or other skin alterations. Nipple discharge and
palpable axillary or supraclavicular lymph nodes were not observed.
The liver [glutamic-pyruvic transaminase, 16 U/l (normal range,
0–40 U/l); glutamic oxalacetic transaminase, 15 U/l (normal range,
0–40 U/l)] and kidney [creatinine, 56 µmol/l (normal range, 41–81
µmol/l)] functions were normal, and the serum tumor marker levels
were within the normal range [carcinoembryonic antigen, 0.70 ng/ml
(normal range, 0–5 ng/ml); carbohydrate antigen 153, 6.25 U/ml
(normal range, 0–19 U/ml]. Breast ultrasonography revealed a giant,
well-circumscribed, heterogeneous and hypoechoic lesion with
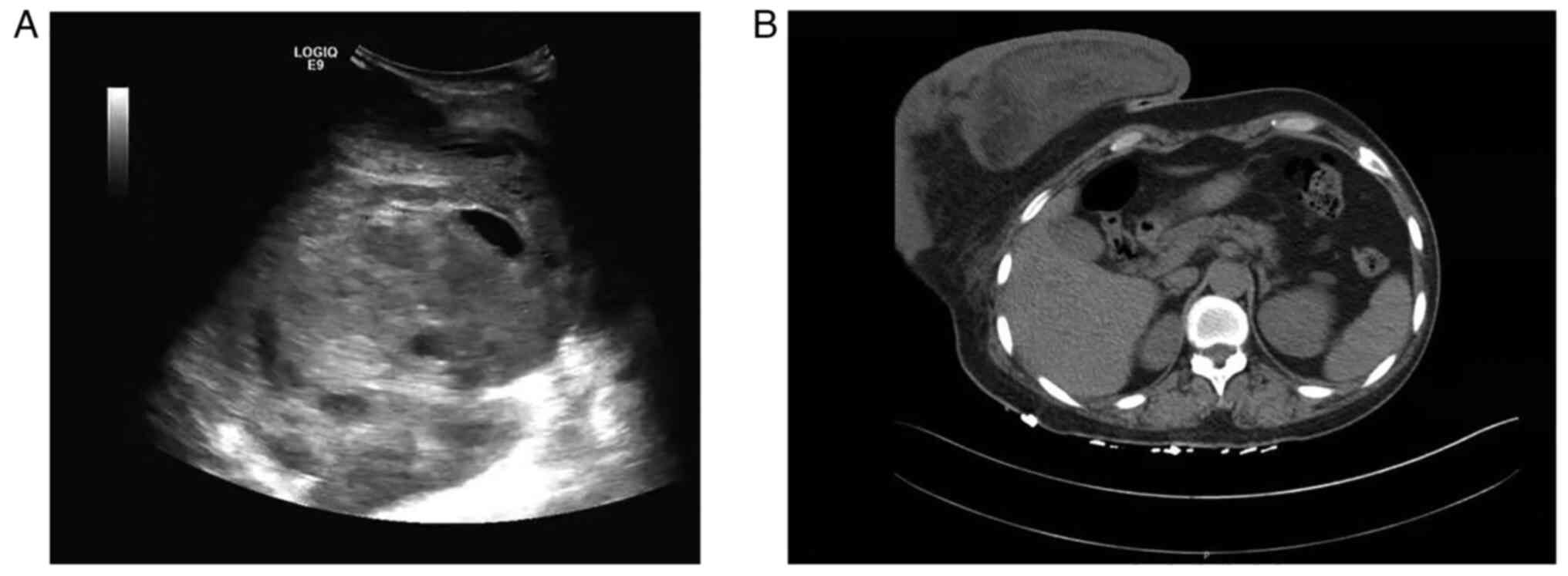
central and peripheral blood flow (Fig.
1A). The diameter of the lesion was not measured using breast
ultrasonography due to its size. Computed tomography revealed a
large heterogeneous tumor of ~25×25×10 cm in the right breast
(Fig. 1B). Mammography and magnetic
resonance imaging were not performed, as the lesion was too
large.
To prepare a surgical plan, a core needle biopsy was
performed. The pathological results indicated a malignant spindle
cell tumor. The patient underwent mastectomy and intraoperative
sentinel lymph node biopsy. The results of the intraoperative
frozen section examination for sentinel lymph nodes showed no
metastasis. Therefore, axillary lymph node dissection,
epidermization or autologous breast reconstruction was not
performed on the patient.
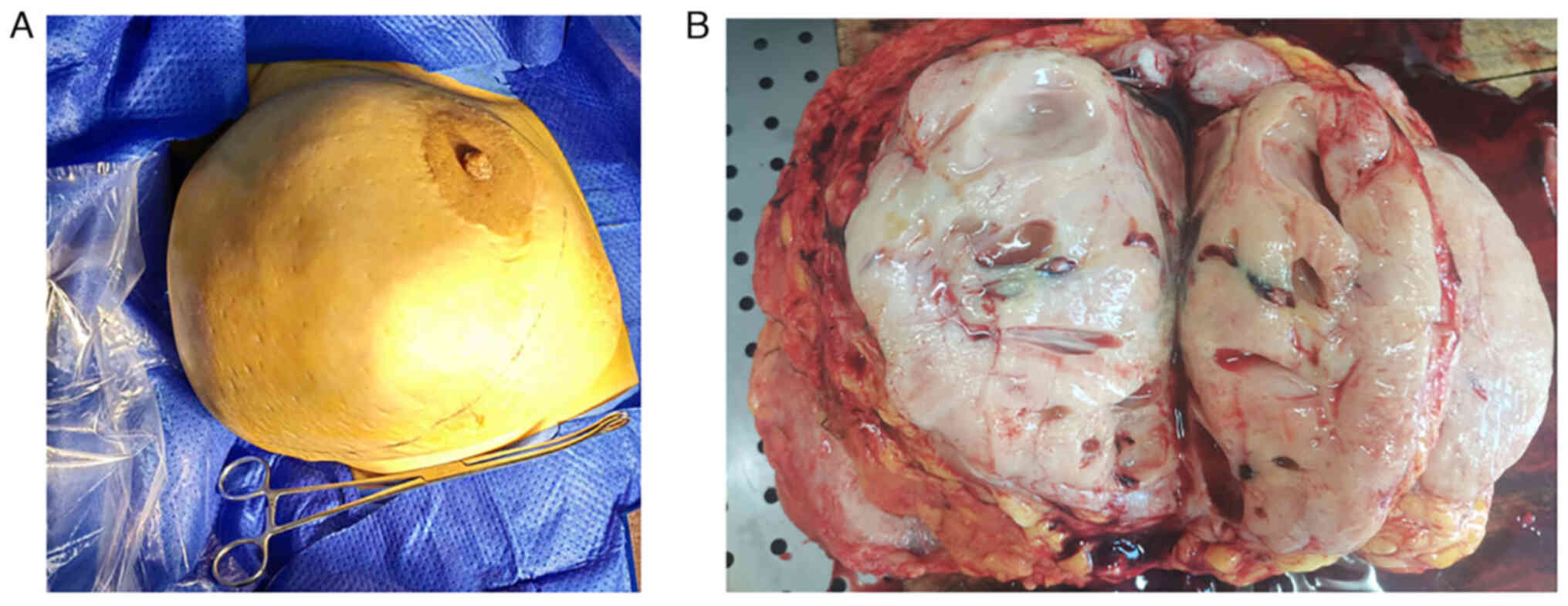
In brief, the resected tumor measured 25×20×8 cm and
occupied almost the entire right breast (Fig. 2A). The cut surface of the tumor was
white-gray in color and exhibited cystic degeneration (Fig. 2B). Specimens were fixed with 4%
formalin at room temperature for 12 h, embedded in paraffin, cut
into 4-µm sections, stained for 5 min at room temperature with
hematoxylin and eosin, and observed under a light microscope (Nikon
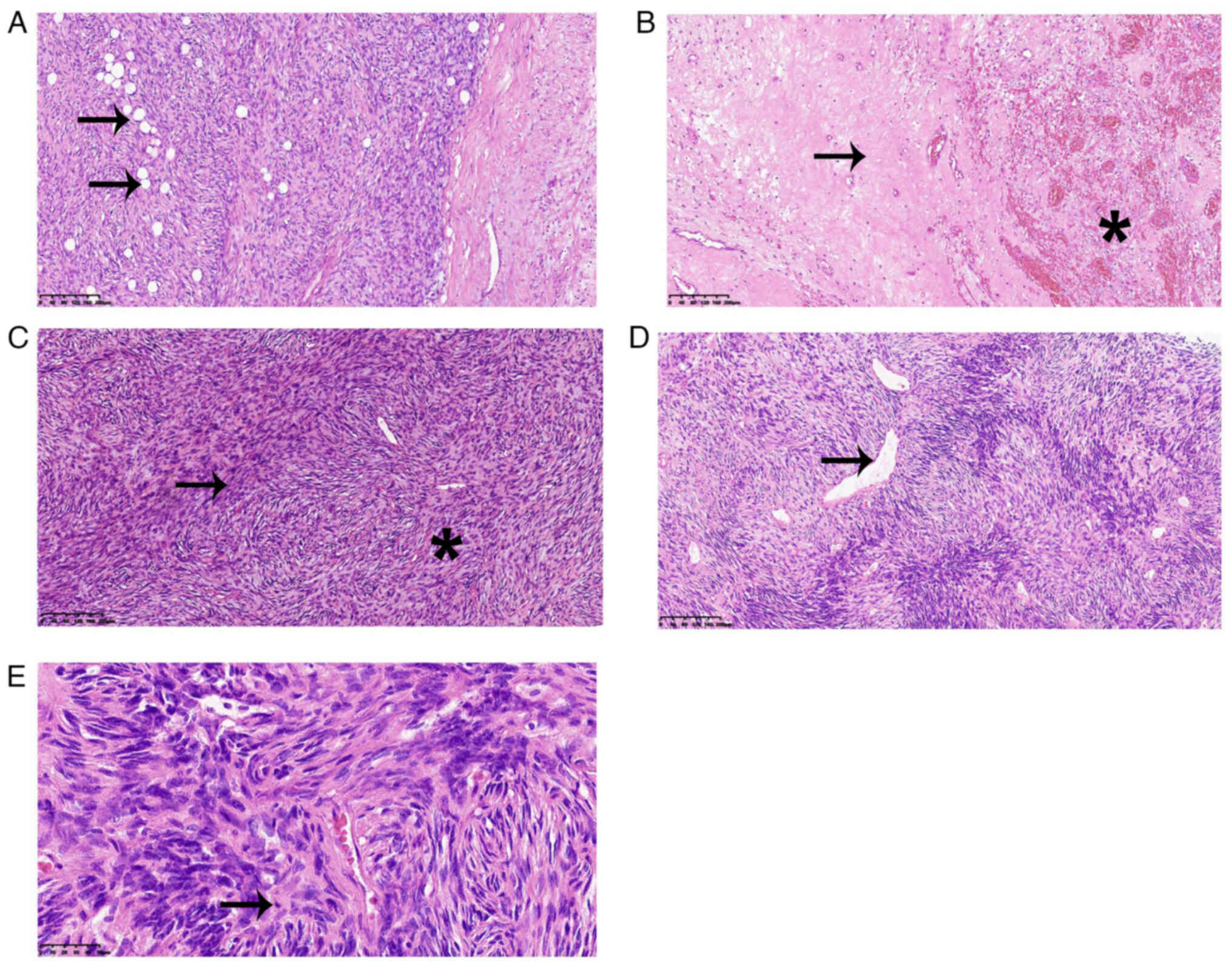
Corporation). As observed under the light microscope with ×200
magnification, the lesion had a well-defined, fibrous capsule and
focally infiltrated the surrounding adipose tissue (Fig. 3A). Necrotic tissue and hemorrhage
were identified in some areas (Fig.
3B). The tumor showed a patternless architecture characterized
by alternating hypercellular and hypocellular areas (Fig. 3C). In the hypercellular area, ovoid
spindle-shaped tumor cells were surrounded by branching and
staghorn vasculature (Fig. 3D).
Under ×400 magnification, relatively greater mitotic activity (6
mitoses in every 10 HPFs) was observed (Fig. 3E).
For immunohistochemical (IHC) staining, the tissue
was fixed with 4% neutral formalin at room temperature for 12 h,
then embedded in paraffin, cut into 4-µm sections and sealed with
3% hydrogen peroxide at room temperature for 10 min. Antigen
retrieval was performed with EDTA at 100°C for 2.5 min, followed by
washing with PBS. Primary antibody incubation was performed at 37°C
for 60 min and secondary antibody incubation at 37°C for 20 min.
The primary antibodies were purchased from Beyotime Institute of
Biotechnology. The following primary antibodies were used: CD34
(cat. no. AG1463; 1:100), Bcl-2 (cat. no. AG1222; 1:100), β-catenin
(cat. no. AF0069; 1:100), STAT6 (cat. no. AF1534; 1:100), desmin
(cat. no. AF1414; 1:100), S-100 (cat. no. AF1945; 1:100), p63 (cat.
no. AF1993; 1:100), smooth muscle actin (cat. no, AF1507; 1:100)
and Ki-67 (cat. no. AF1738; 1:100). Biotinylated Goat anti-Mouse
and -Rabbit secondary antibodies were obtained from OriGene
Technologies, Inc. (cat. no. PV-6000; 1:500). Finally, sections
were stained with DAB at room temperature for 5 min, counterstained
with hematoxylin at room temperature for 5 min and observed under a
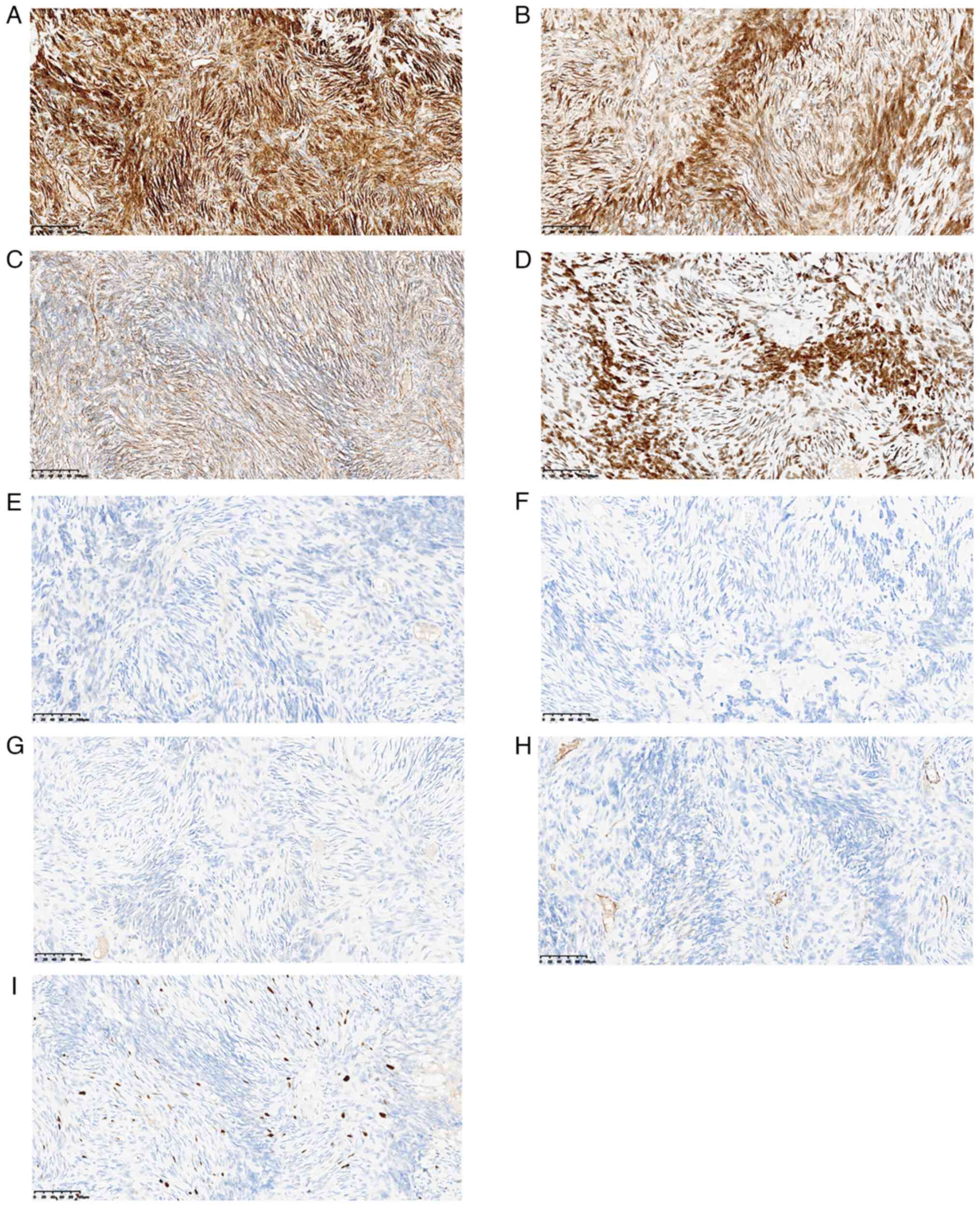
Nikon light microscope (Nikon Corporation). IHC staining revealed
that the tumor cells were CD34+ (Fig. 4A), Bcl-2+ (Fig. 4B), β-catenin+ (Fig. 4C) and STAT6+ (Fig. 4D), but desmin− (Fig. 4E), S-100− (Fig. 4F), p63− (Fig. 4G), and smooth muscle
actin− (Fig. 4H). The
Ki67 labeling index was 10% (Fig.
4I).
Based on the findings of a hypercellular lesion with
a high mitotic index, infiltrating margins and tissue necrosis, and
the IHC results, the final histological diagnosis of the tumor was
malignant SFT.
Owing to the scarcity of evidence on the benefits of
chemotherapy and radiotherapy for malignant breast SFTs, further
treatments were not performed. That patient underwent regular
medical follow-up every 3 months; however, no evidence of local
recurrence or metastasis was observed 1 year after the
resection.
Discussion
Due to the low morbidity of SFTs, data on breast
SFTs are primarily available from case reports. In the present
study, the advances in the understanding of SFTs, including SFT
location in the breast, epidemiology, histology, molecular
techniques used for diagnosis, presentation and therapeutic
strategy are discussed.
An SFT is a rare mesenchymal tumor, and the reported
incidence rate is <0.1/100,000 individuals/year (2). The precise incidence rate of breast
SFTs is difficult to illustrate. Breast SFTs appear to be more
common in female (24 cases) than male (9 cases) patients, whereas
in the pleura and central nervous system SFTs have similar
distribution between male and female patients (6). This difference may be attributed to
the specific epicenter of SFTs. Breast SFTs have been diagnosed in
adults ranging between 38–88 years old, and are most commonly
diagnosed in patients in their 50 to 70s (7). Neither environmental nor hereditary
factors have been shown to increase the incidence rate of SFTs.
SFTs are often slow-growing tumors, and are
frequently diagnosed based on incidental radiological or accidental
findings of painless palpable masses of varying sizes (range,
0.6–10 cm) (6). Pain, nipple
discharge or enlarged regional lymph nodes are usually not observed
in patients with SFTs (7).
Ultrasound examination and mammography are the most common
auxiliary examinations. On ultrasound, SFTs appear to be oval or
lobulated, well-defined hypoechoic lesions with central and
peripheral vascularity. Mammography usually reveals a high-density
round or oval mass with well-defined borders without calcification.
The categorization according to the Breast Imaging-Reporting and
Data System (8) was primarily
category 2–4A using ultrasound and category 3–4A using mammography,
which both indicated a <10% chance of malignancy. Magnetic
resonance imaging generally shows a well-circumscribed oval tumor
with geographic enhancement, and the time-intensity curve usually
shows a rapid-plateau pattern (9).
In addition, few patients with SFTs (<5%) have Doege-Potter
syndrome, and exhibit refractory hypoglycemic syndrome owing to the
overproduction of insulin-like growth factor-2 by large peritoneal
or pleural SFTs (3). Paraneoplastic
syndrome has not been observed in SFTs of the breast likely due to
the rarity of the condition.
Based on its gross appearance, a breast SFT is a
well-circumscribed, lobulated solid mass with or without hemorrhage
and necrosis (3). Histological
characteristics include the following: i) A mass composed of
spindle cells arranged in a disorderly pattern accompanying the
alteration of hypocellular and hypercellular areas; ii) a low
mitotic count and the typical absence of tumor necrosis and nuclear
pleomorphism; and iii) presence of characteristic branching
staghorn vasculature, but mostly inconspicuous or altered by
stromal hyalinization (3). The
aforementioned histopathological patterns are not specific to SFTs,
and the patterns cannot be used to distinguish SFTs from other
mesenchymal tumors, including fibromatosis, inflammatory
myofibroblastic tumors, reactive spindle cell nodules, nodular
fasciitis, fibrosarcoma, spindle cell metaplastic carcinoma and
myoepithelioma. Therefore, IHC analysis is necessary for a
definitive diagnosis.
Immunohistochemically, CD34, nuclear β-catenin and
Bcl-2 are the most consistent markers in breast SFTs. The
sensitivity range of nuclear β-catenin expression is 33–40%
(10,11). Although the sensitivities of CD34
and Bcl-2 in breast SFTs are >95%, these markers lack
specificity, and positive staining results are limited in
mesenchymal tumors, including myofibroblastomas and spindle cell
lipomas (3,4). In addition, actin, desmin and smooth
muscle actin are not expressed in breast SFTs (3,4).
Along with the development of molecular techniques,
detection of NAB2-STAT6 fusion, the defining driver mutation, is
possible (12–14). However, the sensitivity of the
NAB2-STAT6 fusion varies in different studies (range, 55–100%)
depending on the molecular techniques used, including
next-generation sequencing, fluorescence in situ
hybridization probing and reverse transcription PCR (15,16).
The varying sensitivity may be attributed to the small size of the
chromosomal inversion, which is not detected by most break-apart
clinical fluorescence in situ hybridization probes or
multiple breakpoints of NAB2-STAT6, with multiplex PCR being
required for ensuring accuracy (3).
Therefore, NAB2-STAT6 fusion is not required for the diagnosis of
an SFT.
STAT6 is another important marker of an SFT.
Previous studies have shown that a nuclear C-terminus of STAT6 (as
observed by IHC staining) can lead to good diagnostic performance,
with sensitivity and specificity of 98 and >85%, respectively,
for SFTs (17,18). In addition, positive nuclear STAT6
immunoreactivities were observed in all cases (3/3) of breast SFT,
whereas in other spindle cell lesions, such as myofibroblastomas,
desmoid-type fibromatosis, spindle cell metaplastic carcinomas,
benign fibroblastic spindle cell tumors and pseudoangiomatous
stromal hyperplasias, the expression of the C-terminal region of
STAT6 was only focal, and weak cytoplasmic staining was observed
(19). Therefore, in a clinical
study, STAT6 IHC staining may be a reliable surrogate for the
detection of the NAB2-STA6 fusion gene (20).
SFTs are divided into 3 categories: i) Benign (local
disease); ii) not otherwise specified (rarely metastasis); and iii)
malignant (4). The traditional
criteria for malignant SFT include a mitotic rate of ≥4 per 10
HPFs, pleomorphism, necrosis and large tumor size (5). However, other risk factors, such as
age and history of adjuvant radiation therapy have been shown to be
related to metastasis and recurrence in patients with SFTs. These
risk factors are integral, and no single factor has a predictive
value for metastasis or recurrence. Therefore, multivariable models
have been constructed for predicting metastasis, overall survival
(OS) and progression-free survival (PFS) in patients with SFTs.
The Demicco Score, which incorporates mitotic
activity, patient age and tumor size to predict the risk of
metastasis, is the most used and validated system for the risk
stratification of non-meningeal SFTs (21). The modified Demicco Score (developed
in 2017) adds tumor necrosis to the previously included risk
factors, yielding a total score of 7. Based on this, SFTs can be
divided into three groups according to the modified Demicco score:
Low-, intermediate- and high-risk SFTs (22). The modified Demicco Score was
primarily used to predict the metastasis of soft-tissue and pleural
SFTs, and may help improve the treatment strategy for high-risk
SFTs (22). However, the use of the
modified Demicco score in predicting local recurrence has not been
demonstrated. Therefore, other models should be used for predicting
local recurrence. The Salas score system (23), developed by the French Sarcoma
Group, is a frequently used model for predicting local recurrence
in SFT. The risk factors include patient age, site and history of
radiotherapy. Patients with SFT were divided into four groups
according to the Salas score: Low-, intermediate- and high-risk
SFTs (23). However, there are no
specific risk models for breast SFTs owing to the paucity of
incidence.
Due to the low incidence of SFTs and the paucity of
randomized control trials on this tumor type, the treatment
strategy varies according to the tumor location. Therefore,
multidisciplinary teams consisting of clinical and radiation
oncologists, surgeons and pathologists are formed to determine the
best individualized therapeutic strategy.
Complete en-bloc surgical resection is the mainstay
of SFT treatment, including breast SFT treatment. Similar to the
treatment approach followed for primary SFTs in other locations,
the goal of surgical management is to resect the tumor and obtain
adequate negative margins. Concurrently, surgical management can
help preserve critical surrounding organs. According to previous
case reports (6,24), 31/33 patients (93.9%) underwent
complete surgical resection and showed a negative margin, whereas 2
patients underwent mastectomy during the primary surgery. However,
despite R0 margins, 2 patients (6.3%) with breast SFTs showed
recurrence and required a second surgery; one malignant tumor was
removed using mastectomy and a second benign tumor was resected
with a negative margin (24,25).
The 5- and 10-year OS rates of re-resection in cases of local
recurrence were 86 and 77%, respectively (26). Therefore, complete surgical
resection is the primary approach in breast SFTs, with procedures
similar to those used in other breast surgeries.
Radiotherapy has not been performed for breast SFTs
to date. However, adjuvant radiotherapy was shown to reduce the
local recurrence of high-risk SFTs in other locations, including
pleural, intracranial and spinal locations, according to the Salas
score system (27). Nevertheless,
local recurrence control in response to adjuvant therapy did not
lead to an improvement in OS (27).
For patients with locally advanced or metastatic SFTs, definitive
radiotherapy yielded an objective response rate (ORR) of 67%
(28). Therefore, radiotherapy was
a supplementary treatment to surgery, especially in patients with
high-risk local recurrence and metastatic breast SFTs.
Evidence supporting the use of adjuvant chemotherapy
or neoadjuvant chemotherapy for treating breast SFTs is yet to be
reported. Some retrospective studies (29–32)
assessed the effectiveness of adjuvant chemotherapy with a
doxorubicin-based regimen or anthracycline-based regimen in SFTs in
the pleura, pelvis, meninges, limb, visceral organs, spine,
peritoneum and mediastinum. The effectiveness of chemotherapy
varied, and most studies reported a low to questionable ORR
(29,30,32).
Chemotherapy may be suitable for unresectable lung metastases or
extrapulmonary metastatic disease; however, a standard chemical
drug regimen has not been established. According to results from
small case series and retrospective studies (29–33),
an anthracycline-based regimen yielded an ORR range of 0–20%, and
the stable disease range was 26–65%, with a mPFS range of 4–5.2
months and a mOS range of 11.5-14.6 months. Other drugs such as
ifosfamide and trabectedin are effective for metastatic SFTs
(34,35). Even in the absence of a formal gold
standard, anthracycline is the first-line therapy, followed by
ifosfamide and dacarbazine as second-line therapeutics for patients
with metastatic SFTs, including breast SFTs.
Owing to the low effectiveness of chemotherapy, some
targeted therapeutic drugs for metastatic SFTS have been studied
(36–39). The inhibition of angiogenesis
pathways using the antiangiogenic agents sunitinib, sorafenib and
pazopanib may be a suitable alternative therapeutic method for
inhibiting tumor metastasis (37–39).
According to findings from previous phase I and phase II trials,
the ORR range was 0–87% with a mPFS range of 4.7-9.7 months when
antiangiogenic agents were used for treatment (37–39).
Based on these results, antiangiogenic agents are prescribed in
subsequent treatments if chemotherapy resistance is observed in
patients with metastatic SFT.
Owing to late relapse of 10–20 years after initial
presentation (40,41), continued long-term follow-up is
essential for SFTs, including breast SFTs, after surgical resection
due to the indolent nature and the potential for the late
recurrence of SFTs (40,41). Regular surveillance can help
identify local recurrence and metastatic disease at an early stage
and assist with receipt of early therapy. According to previous
case reports, the local recurrence of breast SFTs is 6.1% (2/33),
and the monitoring time range from surgery is 6–18 months. There
are no reports on the metastasis of breast SFTs likely due to the
low incidence and short follow-up time. A study performed by the MD
Anderson Cancer Center (20) showed
that patients with stronger risk factors, including age >55
years, mitotic figures >4/10 HPFs and tumors >15 cm, need
closer surveillance. Based on findings from other breast tumors,
the recommended frequency of surveillance is every 3 months for the
first 2 years after surgery, every 6 months for years 2–5 after
surgery and then at 5 years after the surgery.
Owing to the low morbidity of breast SFTs and the
availability of only retrospective data from case reports,
extensive research is needed on the condition. First, the molecular
mechanism underlying SFT development needs to be studied to
identify novel signaling pathways. Novel target therapies may lead
to high clinical effectiveness. Based on the low effectiveness of
traditional drugs, immunotherapy is another promising approach for
SFT treatment. To develop the most effective treatment strategy,
prospective, randomized and double-blinded studies may be
performed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YHW and XLS conceived the study. YHW, LQW and JNH
completed the surgery. LQW, JNH, XPL, HMS, and XMS collected and
analyzed the data. YHW wrote the manuscript. XMS and XLS revised
the manuscript. YHW and XLS confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang People's Hospital.
Patient consent for publication
The patient provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SFT
|
solitary fibrous tumor
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
References
|
1
|
Klemperer P and Coleman BR: Primary
neoplasms of the pleura. A report of five cases. Am J Ind Med.
22:1–31. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kinslow CJ and Wang TJC: Incidence of
extrameningeal solitary fibrous tumors. Cancer. 126:40672020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kazazian K, Demicco EG, de Perrot M,
Strauss D and Swallow CJ: Toward better understanding and
management of solitary fibrous tumor. Surg Oncol Clin N Am.
31:459–483. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chmielecki J, Crago AM, Rosenberg M,
O'Connor R, Walker SR, Ambrogio L, Auclair D, McKenna A, Heinrich
MC, Frank DA and Meyerson M: Whole-exome sequencing identifies a
recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet.
45:131–132. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
England DM, Hochholzer L and McCarthy MJ:
Localized benign and malignant fibrous tumors of the pleura. A
clinicopathologic review of 223 cases. Am J Surg Pathol.
13:640–658. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawaguchi S, Kinowaki K, Tamura N,
Nishikawa A, Shibata A, Tanaka K, Kobayashi Y, Ogura T, Sato J and
Kawabata H: Solitary fibrous tumor of male breast: A case report
and literature review. Medicine (Baltimore). 101:e321992022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung MJ, Alrahwan D, Dubrovsky E, Baek D,
Ayala AG and Ro JY: Solitary fibrous tumor of breast with
anaplastic areas (Malignant Solitary Fibrous Tumor): A case report
with review of literature. J Breast Cancer. 22:326–335. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spak DA, Plaxco JS, Santiago L, Dryden MJ
and Dogan BE: BI-RADS® fifth edition: A summary of
changes. Diagn Interv Imaging. 98:179–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dubois C, Nika E, Hoffmann P, Delouche A,
Michy T and Philippe AC: Solitary fibrous tumor of the breast: A
rare entity. Breast J. 26:289–290. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barthelmeß S, Geddert H, Boltze C,
Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A,
Agaimy A and Haller F: Solitary fibrous tumors/hemangiopericytomas
with different variants of the NAB2-STAT6 gene fusion are
characterized by specific histomorphology and distinct
clinicopathological features. Am J Pathol. 184:1209–1218. 2014.
View Article : Google Scholar
|
|
11
|
Kallen ME and Hornick JL: The 2020 WHO
Classification: What's new in soft tissue tumor pathology? Am J
Surg Pathol. 45:e1–e23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohajeri A, Tayebwa J, Collin A, Nilsson
J, Magnusson L, von Steyern FV, Brosjö O, Domanski HA, Larsson O,
Sciot R, et al: Comprehensive genetic analysis identifies a
pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic
imbalances, and a characteristic gene expression profile in
solitary fibrous tumor. Genes Chromosomes Cancer. 52:873–886. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vogels RJ, Vlenterie M, Versleijen-Jonkers
YM, Ruijter E, Bekers EM, Verdijk MA, Link MM, Bonenkamp JJ, van
der Graaf WT, Slootweg PJ, et al: Solitary fibrous
tumor-clinicopathologic, immunohistochemical and molecular analysis
of 28 cases. Diagn Pathol. 9:2242014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fritchie KJ, Jin L, Rubin BP, Burger PC,
Jenkins SM, Barthelmeß S, Moskalev EA, Haller F, Oliveira AM and
Giannini C: NAB2-STAT6 Gene fusion in meningeal hemangiopericytoma
and solitary fibrous tumor. J Neuropathol Exp Neurol. 75:263–271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schweizer L, Koelsche C, Sahm F, Piro RM,
Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, et al:
Meningeal hemangiopericytoma and solitary fibrous tumors carry the
NAB2-STAT6 fusion and can be diagnosed by nuclear expression of
STAT6 protein. Acta Neuropathol. 125:651–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demicco EG, Harms PW, Patel RM, Smith SC,
Ingram D, Torres K, Carskadon SL, Camelo-Piragua S, McHugh JB,
Siddiqui J, et al: Extensive survey of STAT6 expression in a large
series of mesenchymal tumors. Am J Clin Pathol. 143:672–682. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vivero M, Doyle LA, Fletcher CD, Mertens F
and Hornick JL: GRIA2 is a novel diagnostic marker for solitary
fibrous tumour identified through gene expression profiling.
Histopathology. 65:71–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magro G, Spadola S, Motta F, Palazzo J,
Catalano F, Vecchio GM and Salvatorelli L: STAT6 expression in
spindle cell lesions of the breast: An immunohistochemical study of
48 cases. Pathol Res Pract. 214:1544–1549. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doyle LA, Vivero M, Fletcher CD, Mertens F
and Hornick JL: Nuclear expression of STAT6 distinguishes solitary
fibrous tumor from histologic mimics. Mod Pathol. 27:390–395. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demicco EG, Park MS, Araujo DM, Fox PS,
Bassett RL, Pollock RE, Lazar AJ and Wang WL: Solitary fibrous
tumor: A clinicopathological study of 110 cases and proposed risk
assessment model. Mod Pathol. 25:1298–1306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demicco EG, Wagner MJ, Maki RG, Gupta V,
Iofin I, Lazar AJ and Wang WL: Risk assessment in solitary fibrous
tumors: Validation and refinement of a risk stratification model.
Mod Pathol. 30:1433–1442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demicco EG, Griffin AM, Gladdy RA, Dickson
BC, Ferguson PC, Swallow CJ, Wunder JS and Wang WL: Comparison of
published risk models for prediction of outcome in patients with
extrameningeal solitary fibrous tumour. Histopathology. 75:723–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salas S, Resseguier N, Blay JY, Le Cesne
A, Italiano A, Chevreau C, Rosset P, Isambert N, Soulie P, Cupissol
D, et al: Prediction of local and metastatic recurrence in solitary
fibrous tumor: Construction of a risk calculator in a multicenter
cohort from the French Sarcoma Group (FSG) database. Ann Oncol.
28:1979–1987. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nitta T, Kimura K, Tominaga T, Ikari A,
Takashima Y, Hirata A, Takeshita A, Ishibashi T and Iwamoto M:
Malignant solitary fibrous tumor of the breast. Breast J.
27:391–393. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lahon B, Mercier O, Fadel E, Ghigna MR,
Petkova B, Mussot S, Fabre D, Le Chevalier T and Dartevelle P:
Solitary fibrous tumor of the pleura: Outcomes of 157 complete
resections in a single center. Ann Thorac Surg. 94:394–400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haas RL, Walraven I, Lecointe-Artzner E,
van Houdt WJ, Strauss D, Schrage Y, Hayes AJ, Raut CP, Fairweather
M, Baldini EH, et al: Extrameningeal solitary fibrous
tumors-surgery alone or surgery plus perioperative radiotherapy: A
retrospective study from the global solitary fibrous tumor
initiative in collaboration with the Sarcoma Patients EuroNet.
Cancer. 126:3002–3012. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haas RL, Walraven I, Lecointe-Artzner E,
Scholten AN, van Houdt WJ, Griffin AM, Ferguson PC, Miah AB, Zaidi
S, DeLaney TF, et al: Radiation therapy as sole management for
solitary fibrous tumors (SFT): A Retrospective study from the
global SFT initiative in collaboration with the sarcoma patients
EuroNet. Int J Radiat Oncol Biol Phys. 101:1226–1233. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Bernardi A, Dufresne A, Mishellany F,
Blay JY, Ray-Coquard I and Brahmi M: Novel therapeutic options for
solitary fibrous tumor: Antiangiogenic therapy and Beyond. Cancers
(Basel). 14:10642022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park MS, Ravi V, Conley A, Patel SR, Trent
JC, Lev DC, Lazar AJ, Wang WL, Benjamin RS and Araujo DM: The role
of chemotherapy in advanced solitary fibrous tumors: A
retrospective analysis. Clin Sarcoma Res. 3:72013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stacchiotti S, Libertini M, Negri T,
Palassini E, Gronchi A, Fatigoni S, Poletti P, Vincenzi B, Dei Tos
AP, Mariani L, et al: Response to chemotherapy of solitary fibrous
tumour: A retrospective study. Eur J Cancer. 49:2376–2383. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Constantinidou A, Jones RL, Olmos D, Thway
K, Fisher C, Al-Muderis O and Judson I: Conventional
anthracycline-based chemotherapy has limited efficacy in solitary
fibrous tumour. Acta Oncol. 51:550–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levard A, Derbel O, Meeus P, Ranchère D,
Ray-Coquard I, Blay JY and Cassier PA: Outcome of patients with
advanced solitary fibrous tumors: The Centre Leon Berard
experience. BMC Cancer. 13:1092013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stacchiotti S, Tortoreto M, Bozzi F,
Tamborini E, Morosi C, Messina A, Libertini M, Palassini E,
Cominetti D, Negri T, et al: Dacarbazine in solitary fibrous tumor:
A case series analysis and preclinical evidence vis-a-vis
temozolomide and antiangiogenics. Clin Cancer Res. 19:5192–5201.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schoffski P, Timmermans I, Hompes D, Stas
M, Sinnaeve F, De Leyn P, Coosemans W, Van Raemdonck D, Hauben E,
Sciot R, et al: Clinical presentation, natural history, and
therapeutic approach in patients with solitary fibrous tumor: A
retrospective analysis. Sarcoma. 2020:13859782020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Outani H, Kobayashi E, Wasa J, Saito M,
Takenaka S, Hayakawa K, Endo M, Takeuchi A, Kobayashi H, Kito M, et
al: Clinical Outcomes of Patients with Metastatic Solitary Fibrous
Tumors: A Japanese Musculoskeletal Oncology Group (JMOG)
Multiinstitutional Study. Ann Surg Oncol. 28:3893–3901. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Pas T, Toffalorio F, Colombo P, Trifirò
G, Pelosi G, Vigna PD, Manzotti M, Agostini M and de Braud F: Brief
report: Activity of imatinib in a patient with
platelet-derived-growth-factor receptor positive malignant solitary
fibrous tumor of the pleura. J Thorac Oncol. 3:938–941. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
George S, Merriam P, Maki RG, Van den
Abbeele AD, Yap JT, Akhurst T, Harmon DC, Bhuchar G, O'Mara MM,
D'Adamo DR, et al: Multicenter phase II trial of sunitinib in the
treatment of nongastrointestinal stromal tumor sarcomas. J Clin
Oncol. 27:3154–3160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Valentin T, Fournier C, Penel N, Bompas E,
Chaigneau L, Isambert N and Chevreau C: Sorafenib in patients with
progressive malignant solitary fibrous tumors: A subgroup analysis
from a phase II study of the French Sarcoma Group (GSF/GETO).
Invest New Drugs. 31:1626–1627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stacchiotti S, Negri T, Palassini E, Conca
E, Gronchi A, Morosi C, Messina A, Pastorino U, Pierotti MA, Casali
PG and Pilotti S: Sunitinib malate and figitumumab in solitary
fibrous tumor: Patterns and molecular bases of tumor response. Mol
Cancer Ther. 9:1286–1297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gholami S, Cassidy MR, Kirane A, Kuk D,
Zanchelli B, Antonescu CR, Singer S and Brennan M: Size and
location are the most important risk factors for malignant behavior
in resected solitary fibrous tumors. Ann Surg Oncol. 24:3865–3871.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kayani B, Sharma A, Sewell MD, Platinum J,
Olivier A, Briggs TWR and Eastwood DM: A review of the surgical
management of extrathoracic solitary fibrous tumors. Am J Clin
Oncol. 41:687–694. 2018. View Article : Google Scholar : PubMed/NCBI
|