Introduction
Renal cell carcinoma (RCC) is the seventh-most
common cancer type in males and the tenth-most common cancer type
in females, with an estimated 50,290 new cases in the US alone in
2021 (1,2). Papillary RCC (PRCC) is the second-most
common heterogeneous cancer, accounting for 15% of all kidney
cancers (3). PRCC may be
morphologically subdivided into types 1 and 2, and the latter
usually has a poorer prognosis than the former (4). It has been reported that among all
patients with RCC, only 20–30% experience metastasis (5). Furthermore, RCC commonly metastasizes
to the lung (50–60%), liver (30–40%), bone (30–40%) and regional
lymph nodes (40–60%), among which the bladder is a rare site of
metastases (only 2%) (6,7). Therefore, bladder metastasis of type 2
PRCC is uncommon and rarely reported. The present study reported
the case of a 54-year-old male patient with a 6-month history of a
dull ache in the left part of the waist and gross hematuria. The
patient had undergone computed tomography (CT) and ureteroscopic
biopsy before being admitted to our hospital (the First Affiliated
Hospital of Sun Yat-sen University). Physical examination revealed
no obvious abnormality. Voided urine cytology indicated the
presence of tumor cells; however, the histological type of the
tumor cells was unclear. The fluorescence in situ
hybridization (FISH) chromosome variation detected by the
p16/CSP3/CSP7 combination probe, indicating malignancies in the
collective system. The patient underwent a series of surgical
treatments, including radical nephroureterectomy, transurethral
resection of bladder tumor (TURBT) and systemic therapy. The
patient experienced recurrent type-2 PRCC and the outcome of the
combination treatment was modest. In the end, the patient died 1.5
years later due to multiple organ failure caused by multiple organ
metastasis.
Case report
Chief complaints
A 54-year-old male patient was admitted to the First
Affiliated Hospital of Sun Yat-sen University (Guangzhou, China)
with a 6-month history of a dull ache in the left part of the waist
and gross hematuria.
History of present condition
The patient was initially admitted to a local
hospital (Meizhou Hospital of Traditional Chinese Medicine,
Meizhou, China) in March 2020 with a 6-month history of a dull ache
in the left part of the waist, gross hematuria and urinary
irritation. Prior to admission, the patient did not receive any
treatment. Soon after the admission, a series of symptomatic
treatments and examinations, including computed tomography (CT) and
ureteroscopic biopsy combined with double J tube insertion, were
performed to make an initial diagnosis. The ureteroscopic biopsy
was performed after acquiring consent from the patient; it has a
certain risk of causing implantation metastasis through the ureter.
The pathological examination revealed moderate to severe dysplasia
of the urothelium, which required to be further differentiated from
urothelial carcinoma. The patient decided to further turn to the
Department of Urology of the First Affiliated Hospital of Sun
Yat-sen University (Guangzhou, China) for further clinical
treatment based on the results.
Past medical history
The patient had a history of left kidney stones and
underwent percutaneous nephrostomy and left percutaneous
nephrolithotomy in 2018. In addition, the patient had a 10-year
history of hypertension and was taking amlodipine daily to control
his blood pressure. The patient denied a history of smoking.
Physical examination
On admission to the First Affiliated Hospital of Sun
Yat-sen University (Guangzhou, China), the patient's body
temperature was 36.5°C, the heart rate was 76 bpm, respiratory rate
was 17 breaths/min and blood pressure was 133/77 mmHg. The abdomen
was supple, without any masses or organomegaly, and no renal
tenderness or percussion pain were observed.
Laboratory examination
Routine blood tests revealed a red blood cell count
of 4.37×109/l (normal range, 4–10×109/l) and
a hemoglobin level of 130 g/l (normal range, 120–160 g/l). None of
the parameters was abnormal. Prothrombin and partial thromboplastin
times were regular. Blood biochemistry test results were within
normal ranges. The patient's serum creatinine level was 136 µmol/l
and his blood urea nitrogen level was 6.4 mmol/l, demonstrating
normal renal function. Voided urine cytology indicated the presence
of tumor cells; however, the histological type of the tumor cells
was unclear. FISH analysis (8)
revealed chromosome variation detected by the GLP p16/CSP3/CSP7
combination probe.
Imaging examination
During the patient's stay at a local hospital (March
2020), abdominal CT revealed a filling defect in the left renal
pelvis with chronic inflammation of the left kidney. However, left
renal pelvis biopsy revealed moderate to high-grade dysplasia of
the urothelial tissue and did not rule out the possibility of
urothelial carcinoma. After admission to the Department of Urology,
the First Affiliated Hospital of Sun Yat-sen University (Guangzhou,
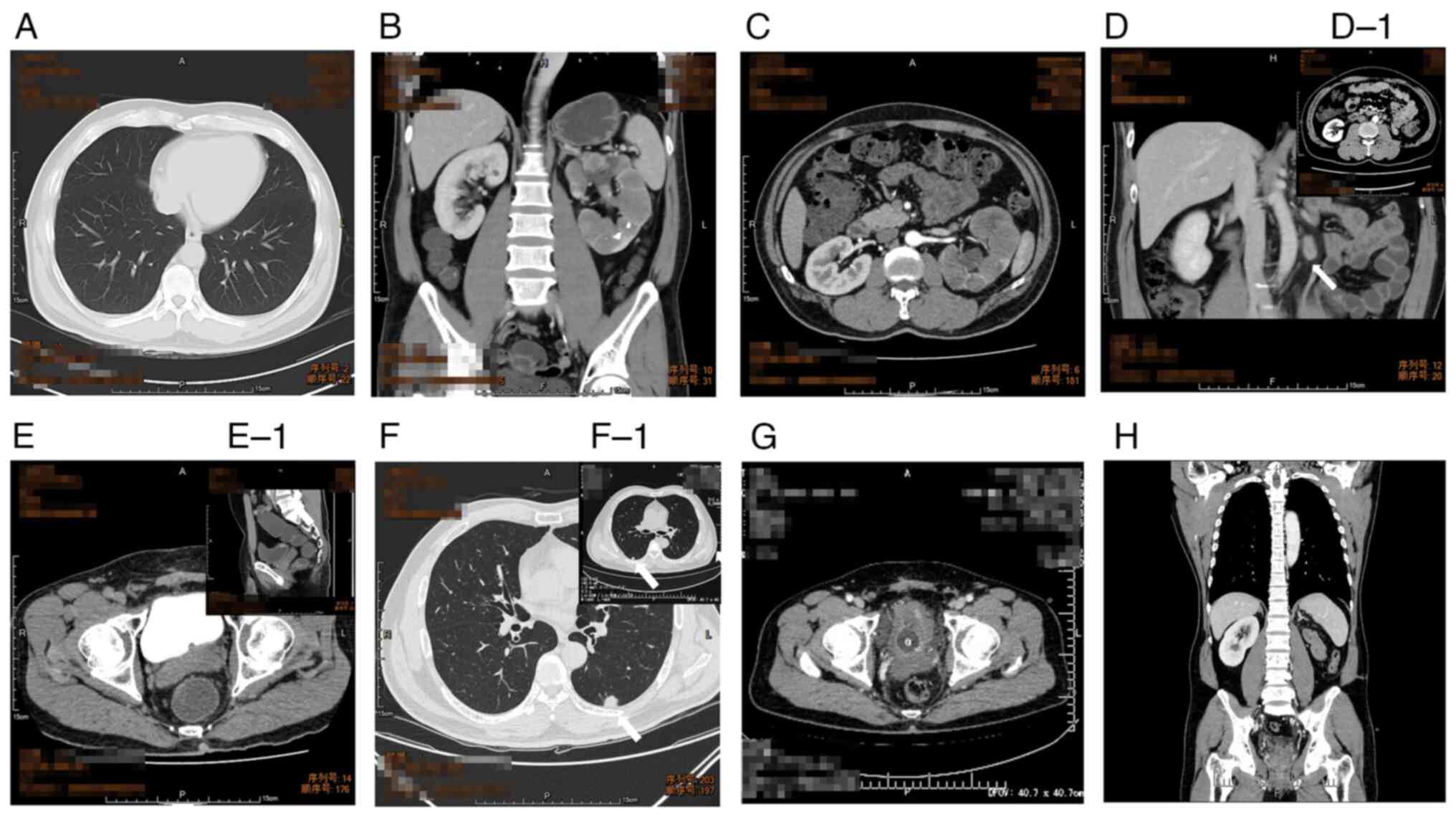
China), a chest CT scan, as well as an enhanced abdominal CT scan,
were performed, revealing no obvious abnormality in both lungs,
multiple nodular dense shadows in the left renal parenchyma and
soft tissue density shadows in the left collective system. Some of
the tissues in the middle and lower segments of the left kidney
exhibited slight enhancement. By contrast, those in the middle and
lower part of the collective system displayed moderate enhancement
(Fig. 1). A pathology consultation
was held based on the previously obtained pathological sections of
masses in the ureter (the plain sections were acquired from the
Meizhou Hospital of TCM by the patient and then stained at the
pathology laboratory of our hospital). Pathologists at our hospital
further identified the tissues as malignant tumors with a high
suspicion of urothelial carcinoma.
Final diagnosis
The final diagnosis of the present case was
carcinoma of the left renal pelvis.
Treatment
A laparoscopic radical nephroureterectomy of the
left kidney combined with hilar lymph node dissection was
performed. The postoperative specimen was nephridial tissue
measuring 14×10×9 cm and left hilar lymph nodes 3 cm in diameter.
The tumor was grey-white and measured 11.5×8×5.5 cm. Histological
examination, which was performed according to standard procedures,
revealed that the tumor was consistent with a type 2 PRCC (stage
pT1b; Fig. 2A-A1), and an
intravascular tumor thrombus was found. However, the tumor did not
invade the renal adipose capsule and no malignant tissue was found
in the lymph nodes. The patient recovered uneventfully and was
discharged from our department on the seventh postoperative
day.
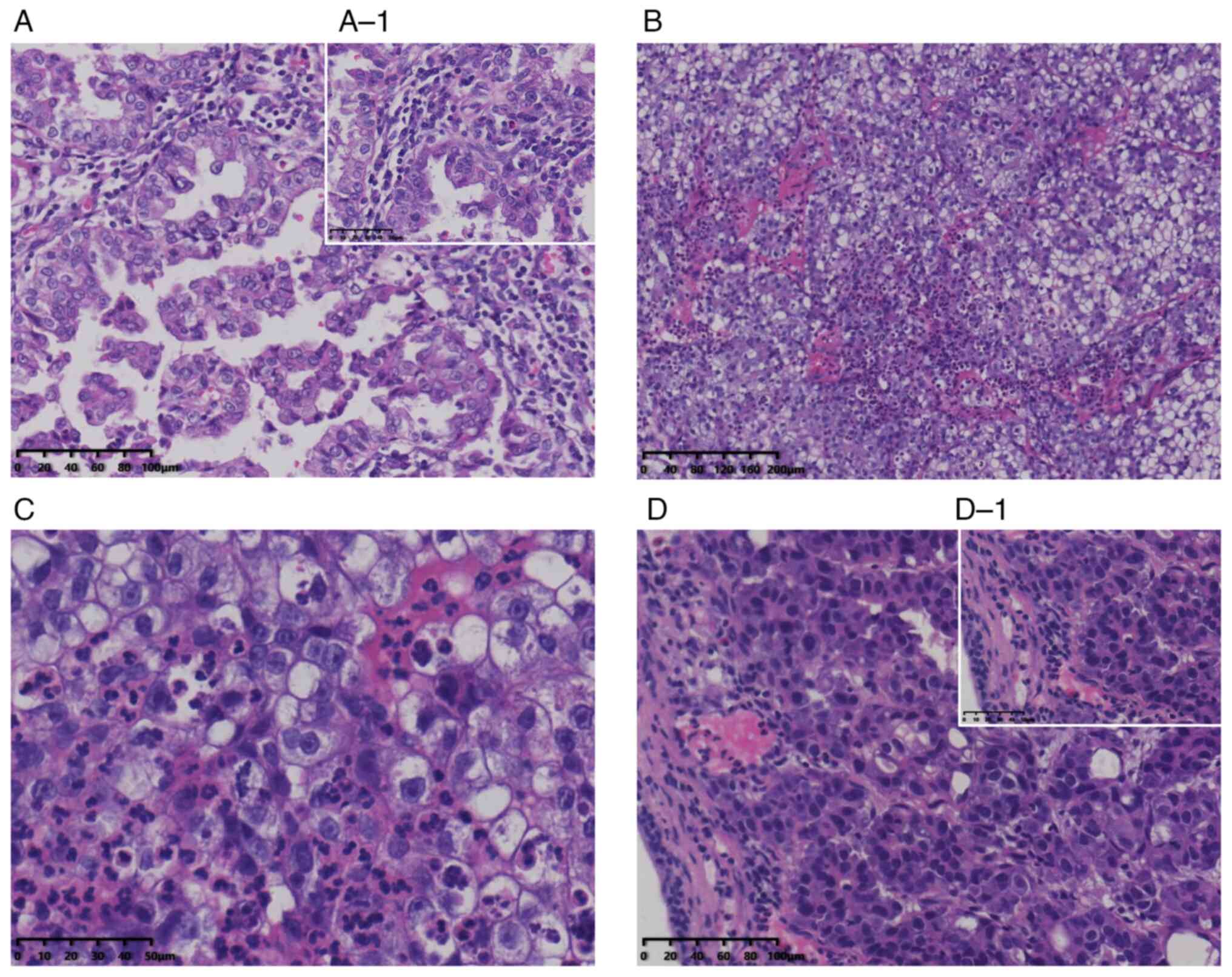 | Figure 2.Pathological results. H&E staining
of renal tumor and bladder metastases. (A) Histological examination
of resected tumor in the left kidney revealed type 2 PRCC
(magnification, ×20). (A-1) The resected tumor in the left kidney
(magnification, ×40). (B) After initial TURBT, the pathological
outcome revealed a mass of bladder metastasis of PRCC
(magnification, ×10). (C) Bladder metastasis of PRCC
(magnification, ×40). (D) Pathological outcome of the second TURBT
revealed metastasis of PRCC in the bladder (magnification, ×20).
(D-1) Recurrent bladder metastasis of PRCC (magnification, ×40)
(H&E stain; scale bars, 200 µm). H&E, hematoxylin and
eosin; PRCC, papillary renal cell carcinoma; TURBT, transurethral
resection of the bladder tumor. |
Outcome and follow-up
At three months after the surgery, routine abdominal
ultrasound indicated a spherical protuberance (1.0 cm) into the
left side of the bladder lumen, which was subsequently confirmed by
CT and combined positron-emission tomography-CT, which also
revealed a highly metabolic lymph node in the left renal hilum with
no other focal hypermetabolism (Fig.
1D, D-1). Although there were multiple scattered small nodules
in both lungs, none demonstrated hypermetabolism. To clarify the
nature of the mass in the bladder lumen, the patient underwent
TURBT and four weeks of intravesical therapy of epirubicin (40 mg
per instillation), and pathology of the resected specimen indicated
bladder metastasis of PRCC (Fig. 2B and
C). After initial TURBT, gene sequencing was performed (3DMed
Clinical Laboratory, Inc.), and the result revealed amplification
of the cyclin D (CCND1) gene. According to the European Society for
Medical Oncology (ESMO) guidelines, a targeted therapy protocol
with pazopanib (1,000 mg orally QD) was arranged.
After three months of targeted therapy, a routine CT
scan indicated local thickening with multiple calcifications in the
left and top parts of the bladder wall (Fig. 1E-E1), accompanied by enlarged
nodules in both lungs, which were most likely metastatic tumors
(Fig. 1F). After a general
discussion in our department, radical cystectomy and continuation
of targeted treatment were recommended. However, due to concerns
regarding a potential reduction in quality of life, the patient
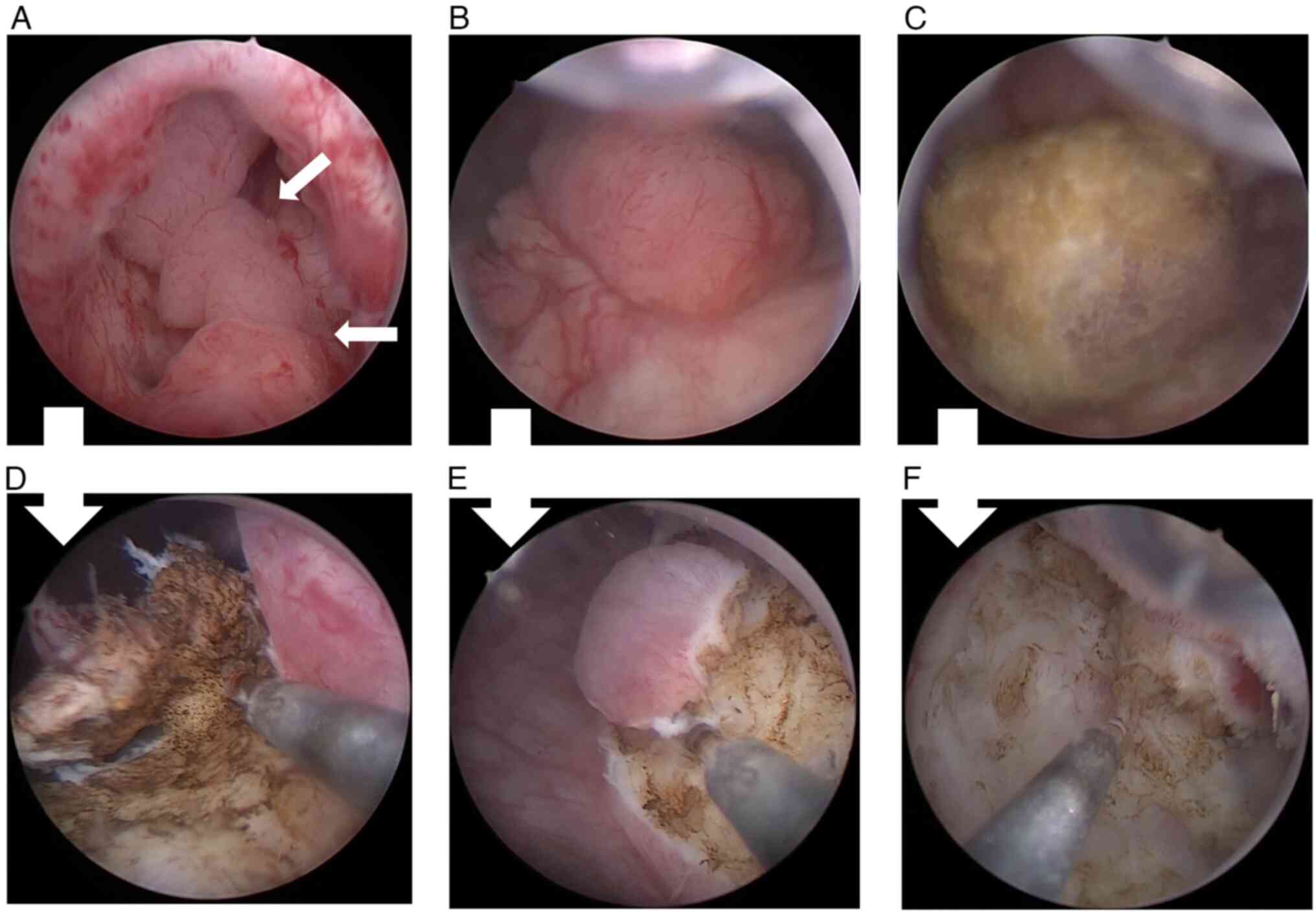
refused this suggestion. Therefore, a second TURBT was performed to
remove the metastatic tumors in the patient's bladder as thoroughly
as possible (Fig. 3A-F), and the
postoperative pathological results still revealed PRCC (Fig. 2D-D1). After the second TURBT, the
patient received four weeks of intravesical therapy of epirubicin
(40 mg per instillation) and underwent regular follow-up
examinations at our hospital.
During the 9-month follow-up, the patient underwent
TURBT three times to eliminate new recurrent tumors and received
the targeted drug (pazopanib 1,000 mg orally once a day). However,
after nine months, a CT scan revealed multiple recurrent tumors
damaging the normal structure of the bladder (Fig. 1G and H) and new nodules in both
lungs (Fig. 1F-F1), indicating the
treatment effect of the drugs was modest. Therefore, immunotherapy
with tirelizumab (200 mg administered every three weeks) was added.
However, the antitumor efficacy was still unsatisfactory.
Ultimately, the patient died in September 2021 due to multiple
organ failure caused by multiple organ metastasis.
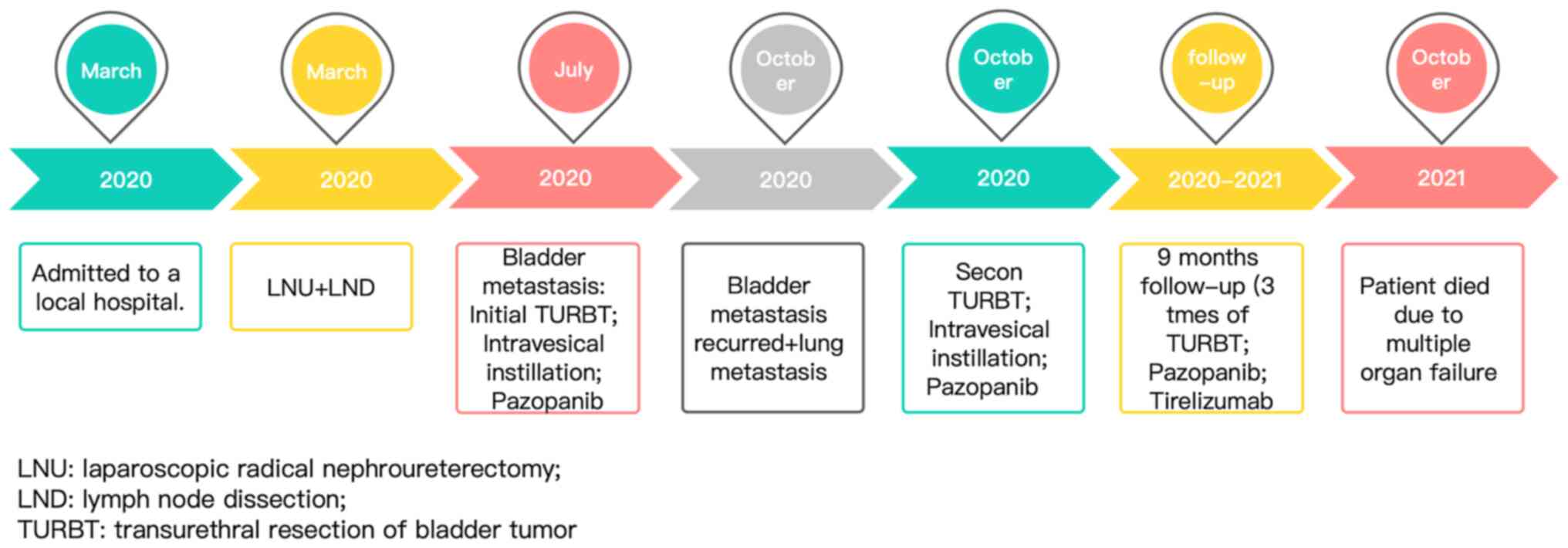
A flow chart is provided in Fig. 4 to briefly illustrate the entire
treatment process of the patient.
Discussion
RCC is the most common renal malignancy in adults
and has a high incidence of distant metastases (1). The most common organs of metastasis
include the lungs, bones, lymph nodes and skin (9), while the bladder only accounts for
approximately 2% of RCC metastases (7). Bladder metastasis of type-2 PRCC is
rare, and only four cases have been reported (10–13).
In the earliest case reported by Raviv et al (12), the patient received no adjuvant
treatment after TURBT but conservative surveillance and was alive
and free of disease six years after initial TURBT. In the study by
Gajasinghe et al (10), the
patient did not receive any adjuvant treatment until bladder
metastasis was detected. Then, although she received
interferon-alpha injections thrice a week, lung metastasis was
subsequently developed. The patient survived at least six months
after lung metastasis was diagnosed. However, it is reported that
interferon-alpha has a low response rate (12%).
Babar et al (11) reported on a patient who was
diagnosed with metachronous renal cell carcinoma and initially
given sunitinib for systemic treatment. However, the patient did
not tolerate the dosage of 25 mg; therefore, sunitinib was
substituted with nivolumab. The patient died two years later after
bladder metastasis was detected. However, in the most recent report
by Kang et al (13), no
detailed treatment schedule was mentioned. The treatment schedule
of the present case partially referred to these previous
reports.
Due to limited clinical data, there is currently no
standard treatment schedule for PRCC and the existing treatment has
largely been extrapolated from that of clear cell RCC (ccRCC). The
present review summarized the experience gained from this rare case
and certain potential mistakes were pointed out with the aim to
provide new ideas for treating bladder PRCC metastasis.
Although targeted therapy and immune checkpoint
inhibitors (ICI) have been widely utilized, surgery still has a
crucial role in treating metastatic RCC (mRCC), which usually falls
into two major categories: Cytoreductive nephrectomy and
metastasectomy (MTS). Although the effect of MTS remains
controversial, a recent systematic review has indicated that it
could improve overall survival in patients who had previously
undergone nephrectomy (14).
Therefore, when suspicious metastasis was initially discovered
during the follow-up of the present case, a TURBT was arranged. As
previously mentioned, the current treatment plan for papillary mRCC
mainly refers to ccRCC or other similar diseases. Therefore,
intravesical instillation with epirubicin (50 mg/50 ml, once a week
for eight consecutive weeks and then once a month for 10
consecutive months) was arranged following TURBTs according to
clinical experience and the patient was placed under observation,
which is a strategy conventionally pursued in non-muscle invasive
bladder cancer and has already been proven effective in eliminating
residual tumor cells in the bladder after TURBT (14,15).
Intravesical RCC metastases are usually divided into
two different types: Synchronous (present within 12 months after
nephrectomy) and metachronous (present >12 months after
nephrectomy), and the former correlates with an unfavorable
prognosis (16). However, the
mechanism of metastasis to the urinary bladder remains elusive. In
general, four different hypotheses are suggested: i) Metastasis by
dissemination through the urinary tract; ii) direct spread through
the bloodstream via invasion of the renal vein; iii) metastasis
through lymphatic vessels; and iv) retrograde perfusion due to
embolism of tumor cells from the renal vein to its venous
connections (17). In the present
case, the metastases in the bladder were superficial. The patient
was initially admitted to our hospital with a history of gross
hematuria, which suggested a potential fracture of the renal
pelvis. Therefore, drop metastasis may have been a significant
route for tumor spread to the urinary bladder. By contrast,
pathological analysis after nephrectomy revealed an intravascular
tumor thrombus, indicating that retrograde tumor dissemination may
have also had an important role in bladder metastasis in the
present case. In the case of lung metastasis occurring only three
months after bladder metastasis, a more radical treatment, namely
radical cystectomy, should be considered when bladder metastasis
was initially detected to decrease the metastasis rate and prolong
the patient's survival time (1).
Sunitinib and pazopanib are both recommended
targeted agents for non-ccRCC according to ESMO guidelines
(1). Furthermore, a previous study
revealed that pazopanib has similar efficacy to sunitinib with
better safety and quality of life (18). Therefore, the latter was selected as
the main drug in targeted therapy. Unfortunately, the effect of
pazopanib in preventing tumor progression was modest. The
insensitivity of tumor cells to targeted agents may be the most
important reason. Von Hippel-Lindau (VHL) gene mutations are
closely related to RCC and cause the accumulation of
hypoxia-inducible factors in cells and activation of downstream
hypoxia-driven genes, including vascular endothelial growth factor
(VEGF) (19). As a VEGF-receptor
tyrosine kinase inhibitor, pazopanib may target and deactivate
these downstream pathways, thereby achieving antitumor activity.
However, VHL mutations are less frequent in patients with type 2
PRCC. Instead, mutations in the cyclin-dependent kinase (CDK)
inhibitor 2A, CDK4/6 and MET genes are more common and are linked
to other targeted therapies than pazopanib (20). The result of genetic testing also
revealed amplification of the CCND1 gene, indicating the
sensitivity of the tumor to CDK4/6 inhibitors. Therefore,
inhibitors of these genes, including foretinib, crizotinib and
savolitinib, would likely show a better antitumor effect on PRCC
than pazopanib, and the survival period of the patient may be
further prolonged if treated with these drugs. However, the China
Food and Drug Administration has not approved these drugs for
treating patients with PRCC.
There were certain shortcomings in the treatment of
the present case. First, a routine cystoscopy may have been
performed prior to the laparoscopic radical nephroureterectomy,
particularly when considering left renal pelvic carcinoma. However,
the above-mentioned measure was not taken due to the negative
result of the abdominal CT scan. It may be helpful to detect
potential bladder metastasis early. Therefore, routine cystoscopy
is recommended for type 2 PRCC when renal pelvis invasion is
present. Furthermore, radical cystectomy should be performed
decisively when bladder metastasis recurs. However, the patient of
the present study refused this recommended treatment. The absence
of radical cystectomy probably causes frequent recurrence and
distant metastasis and affects the prognosis. In addition,
ureteroscopic biopsy would have been feasible at the local
hospital. However, a recent study revealed that ureteroscopic
biopsy prior to radical nephroureterectomy may be associated with
increased intravesical recurrence (21). As another limitation, adjustments
for systematic treatment should be made depending on the
therapeutic effect. In the case of the present study, genetic
testing was performed and antitumor drug sensitivity was revealed.
Although the effect was modest, out of concern regarding off-label
drug use, the patient was always treated with pazopanib. However,
there is currently no standard treatment protocol for bladder
metastasis of PRCC due to its rarity. Therefore, it is bold to
experimentally use those targeted drugs included in the
drug-sensitivity table of PRCC but that are not standard agents
permitted by the China Food and Drug Administration. Finally, there
are several novel combination treatments for ccRCC, including
immunotherapy combined with targeted therapy and targeted therapy
combined with cryoablation (22).
Although most of them were still in the clinical trial stage, these
treatment strategies may have a reference function in treating
nccRCC. Further large-scale prospective studies are still
required.
In conclusion, bladder metastasis of type 2 PRCC is
a relatively rare condition, with few reports and a lack of
standard and effective treatment schedules. Decisive and radical
metastasectomy may minimize the risk of distant metastasis. In
addition, specific targeted agents and ICIs may have better effects
in prolonging patients' survival than those with broad-spectrum
antitumor activity. More case reports and systemic and large-scale
studies are needed to guide clinical treatment of this
condition.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ: Conceptualization, revision and editing of the
manuscript. SL: Writing-original draft, investigation, revision and
editing of the manuscript. LWC: Writing-original draft,
investigation. LZC: Conceptualization, supervision. JC:
Conceptualization, methodology and supervision. QZ, SL and JC
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Human Investigation Committee/Institutional
Review Board of the First Affiliated Hospital of Sun Yat-sen
University (Guangzhou, China) approved the present study. All
procedures performed in this study involving the patient were in
accordance with the 1975 Helsinki declaration and its later
amendments or comparable ethical standards (approval no.
2020-420).
Patient consent for publication
The patient provided written informed consent
regarding publishing his case data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Escudier B, Porta C, Schmidinger M,
Rioux-Leclercq N, Bex A, Khoo V, Grunwald V, Gillessen S and
Horwich A; ESMO Guidelines Committee, : Renal cell carcinoma: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 30:706–720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flippot R, Comperat E, Tannir NM and
Malouf GG: Papillary renal cell carcinoma: A family portrait. Eur
Urol. 73:79–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akhtar M, Al-Bozom IA and Al Hussain T:
Papillary renal cell carcinoma (PRCC): An update. Adv Anat Pathol.
26:124–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ljungberg B, Campbell SC, Choi HY, Jacqmin
D, Lee JE, Weikert S and Kiemeney LA: The epidemiology of renal
cell carcinoma. Eur Urol. 60:615–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thyavihally YB, Mahantshetty U,
Chamarajanagar RS, Raibhattanavar SG and Tongaonkar HB: Management
of renal cell carcinoma with solitary metastasis. World J Surg
Oncol. 3:482005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McAchran SE, Williams DH and MacLennan GT:
Renal cell carcinoma metastasis to the bladder. J Urol.
184:726–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liem EIML, Baard J, Cauberg ECC, Bus MTJ,
de Bruin DM, Laguna Pes MP, de la Rosette JJMCH and de Reijke TM:
Fluorescence in situ hybridization as prognostic predictor of tumor
recurrence during treatment with Bacillus Calmette-Guérin therapy
for intermediate- and high-risk non-muscle-invasive bladder cancer.
Med Oncol. 34:1722017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsumoto K, Hayakawa N, Nakamura S and
Oya M: Bladder metastasis from renal cell carcinoma: Retrospective
analysis of 65 reported cases. Clin Exp Metastasis. 32:135–141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gajasinghe DG, Nazeer I, Maddumage HP,
Perera C and Abeygunasekera AM: Metachronous bladder metastases of
a type 2 papillary renal cell carcinoma: A case report and review
of the literature. World J Surg Oncol. 14:2192016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Babar M, Hamdani S, Liu C, Vedula J and
Schnapp DS: Metachronous renal cell carcinoma with metastasis to
the urinary bladder, and distant organs, 28 years after radical
nephrectomy: A case report. BMC Urol. 19:1362019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raviv S, Eggener SE, Williams DH, Garnett
JE, Pins MR and Smith ND: Long-term survival after ‘drop
metastases’ of renal cell carcinoma to the bladder. Urology.
60:6972002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung HK, Silva LN, Pereira MA, Catelli R
and Freitas RJ: Giant Abdominal Mass: Papillary Renal Cell
Carcinoma with Metastases to the Bladder, Omentum and Perigastric
tissue. J Surg Oncol. 2674–3000. 2020.
|
|
14
|
Hsieh PY, Hung SC, Li JR, Wang SS, Yang
CK, Chen CS, Lu K, Cheng CL and Chiu KY: The effect of
metastasectomy on overall survival in metastatic renal cell
carcinoma: A systematic review and meta-analysis. Urol Oncol.
39:422–430. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abern MR, Owusu RA, Anderson MR,
Rampersaud EN and Inman BA: Perioperative intravesical chemotherapy
in non-muscle-invasive bladder cancer: A systematic review and
meta-analysis. J Natl Compr Canc Netw. 11:477–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kavolius JP, Mastorakos DP, Pavlovich C,
Russo P, Burt ME and Brady MS: Resection of metastatic renal cell
carcinoma. J Clin Oncol. 16:2261–2266. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Groote R, Larcher A, Goossens M,
Hendrik R, Kris VS, De Coninck V, De Naeyer G, Schatteman P,
D'Hondt F and Mottrie A: Metachronous metastasis of renal cell
carcinoma to the urinary bladder: A case report. Ther Adv Urol.
10:29–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaelin WG Jr: The von Hippel-Lindau tumour
suppressor protein: O2 sensing and cancer. Nat Rev Cancer.
8:865–873. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Avella C, Abbosh P, Pal SK and Geynisman
DM: Mutations in renal cell carcinoma. Urol Oncol. 38:763–773.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma V, Miest TS, Juvet TS, Toussi A,
Packiam V, Chamie K, Matin SF, Boorjian SA, Thompson RH, Frank I,
et al: The impact of upper tract urothelial carcinoma diagnostic
modality on intravesical recurrence after radical
nephroureterectomy: A single institution series and updated
meta-analysis. J Urol. 206:558–567. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campbell MT, Matin SF, Tam AL, Sheth RA,
Ahrar K, Tidwell RS, Rao P, Karam JA, Wood CG, Tannir NM, et al:
Pilot study of Tremelimumab with and without cryoablation in
patients with metastatic renal cell carcinoma. Nat Commun.
12:63752021. View Article : Google Scholar : PubMed/NCBI
|