Introduction
Lung cancer was the most prevalent cause of
cancer-related death (18.4% of total cancer deaths) and the most
widely diagnosed (11.6% of total cancer cases) worldwide in 2018
(1). While the etiology of lung
cancer remains to be fully elucidated, some of the well-established
causes include tobacco use, environmental pollution and second-hand
smoking (2). Approximately 8% of
lung cancer cases are linked to a genetic predisposition to the
disease. Multiple individuals diagnosed with lung cancer in a
related family is referred to as familial lung cancer (FLC)
(3). FLC occurs in the offspring of
parents who carry lung cancer-linked genes. As a result, the
affected individuals pass on their predisposition to lung cancer to
future generations by inheriting a de novo autosomal
dominant or X-linked germline mutation.
The majority of FLC cases occur due to germline
mutations, which may include changes in the number of chromosomes
(triploidy and aneuploidy) or changes in gene dosage due to
duplications or deletions ranging in size from a small number of
base pairs to megabases (4).
Mutations may occur as duplications or deletion in microsatellites,
minisatellites and chromosomes that have been remodeled via
retrotransposition, translocation or inversion (4). Previous research into germline
mutation has revealed that P53, von Hippel-Lindau, retinoblastoma,
breast cancer-associated gene 1 (BRCA1) and BRCA2 act as ‘driver
mutations’ in carcinogenesis. Germline mutations represent a
predisposition to cancer, enabling the identification of
individuals who are at a higher risk of inheriting these mutations
(high-risk population). Furthermore, germline mutations may
facilitate the development of novel biomarkers for diagnosis and
targeted therapy (2). Tang et
al (5) reported that epidermal
growth factor receptor (EGFR) mutations are commonly observed in
the normal bronchial and bronchiolar epithelia of patients with
EGFR mutant lung adenocarcinomas and confirmed that EGFR mutations
are early events in the tumor formation process. Furthermore,
Arteaga (6) demonstrated that EGFR
signaling is necessary for the development of lung adenocarcinomas
in transgenic mice. Therefore, the present review aimed to
summarize the germline mutations of EGFR in lung cancer and
elucidate the possible underlying mechanisms.
Germline mutation of EGFR T790M
The T790M substitution somatic mutation at exon 20
is considered the primary cause of EGFR-tyrosine kinase inhibitor
(EGFR-TKI) acquired resistance in ~60% of patients with lung cancer
treated with first-generation EGFR-TKIs (7). However, the germline T790M mutation is
rare, found in only 1% of patients with lung cancer whose EGFR gene
has been sequenced, and in ~50% of patients who have baseline EGFR
T790M in their pretreatment tumor samples (8). Furthermore, T790M has been linked to
familial non-small cell lung cancer (NSCLC) (7) and in a population of never-smoking
women who carry the T790M germline mutation, there is a 31%
probability of developing lung cancer.
A study of 627 Japanese patients with lung cancer
found no T790M germline mutations, despite EGFR mutations being
present in 33.3% of patients (209/627) (9). These findings are consistent with a
gene analysis study of 503 patients with lung cancer in the US,
which found only five patients with the T790M germline mutation
(9). In addition, no patients with
a T790M germline mutation were identified in the 1,000 Genomes
Project databases or the genomes of 6,503 individuals from the
National Heart Lung and Blood Institute GO Exome Sequencing
Project. Another study performed peripheral blood screening on 369
non-smoking patients with lung adenocarcinoma and discovered only
two patients with the germline T790M mutation (10). In 52 families with high
susceptibility to lung cancer and 237 probands with FLC, no
germline T790M mutation was observed, while in the two cohorts, 86%
had a smoking history (11,12). Therefore, the prevalence of the
germline T790M mutation in the general population is ~0.5-1/7,500
individuals (13). Further
prospective evaluation of patients with lung cancer and baseline
EGFR T790M mutation is recommended to better understand familial
penetrance, lifetime lung cancer risk and germline prevalence.
To date, ~10 cases of germline EGFR T790M mutations
have been reported. Vikis et al (11) previously sequenced the germline EGFR
T790M mutation of 237 families with a predisposition toward lung
cancer but the mutation was not detected. This suggests that this
type of germline mutation is rare, even in families with a genetic
predisposition toward lung cancer. Adenocarcinomas are the
pathological types that exhibit the germline T790M mutation, which
are generally more common in females (6:3, female/male). In a study
by Gazdar et al (9), the
median age of patients with lung cancer with a T790M germline
mutation was 63 years, with the youngest proband being 29 years
old. To the best of our knowledge, prior to a study by Lu et
al (14), there were no reports
of families of East Asian origin with the germline T790M mutation.
Lu et al (14) reported a
case of a Chinese patient among 5,675 EGFR-positive patients.
Smoking was not associated with the development of lung cancer in
people with T790M germline mutation. Of the 10 cases examined, only
two were identified to have a history of smoking. It has been
hypothesized that the germline EGFR T790M mutation is a weak
oncogene that requires a common activating EGFR mutation, such as
L858R, to induce lung cancer development. Mäkinen et al
(15) used microdissection, a
targeted panel (a hybrid-capture and massively parallel sequencing
assay) and whole-exome sequencing to analyze multiple foci of
atypical adenomatous hyperplasia in situ, invasive
components of lung adenocarcinoma, normal lung tissue and whole
blood from patients at the molecular level. Their findings revealed
that each neoplastic lesion exhibited a secondary somatic EGFR
mutation, namely L858R or L861Q (15). However, conventional chemotherapy
may be utilized as the primary treatment for patients with the
germline T790M mutation, as two sisters who were administered TKI
exhibited partial responses (Table
I) (16–18).
 | Table I.Summary of patients with lung cancer
exhibiting an EGFR T790M mutation. |
Table I.
Summary of patients with lung cancer
exhibiting an EGFR T790M mutation.
|
|
|
|
|
|
|
|
| Effective for
treatment? |
|
|---|
| First author,
year | Sex (M/F) | Smoking
history | Family cancer
history | Pathology | TNM stage | Secondary somatic
mutation | Ethnicity |
|
|
|---|
| EGFR-TKI | Chemotherapy | (Refs.) |
|---|
| Bell, 2005 | M | Yes | Yes | Unknown | Unknown | EGFR L858R,
delL747-T751, G719A | Caucasian | Yes
(gefitinib) | No | (16) |
| Bell, 2005 | M | Unknown | Yes | Unknown | Unknown | EGFR G719A | Caucasian | Unknown | Unknown | (16) |
| Prudkin, 2009 | F | No | Not mentioned | 2 nodes are
adenocarcinomas, and 1 node is large-cell neuroendocrine
carcinoma | Unknown | No | N/A | Unknown | Unknown | (17) |
| Girard, 2010 | F | No | Yes | Mixed
adenocarcinoma with acinar and bronchioloalveolar features | Unknown | EGFR L858R | East
Indian/Caucasian | Unknown | Unknown | (10) |
| Girard, 2010 | M | No | Yes | Poorly
differentiated acinar and solid adenocarcinoma | IV | EGFR L858R | European | Unknown | Unknown | (10) |
| Tibaldi, 2011 | F | No | Yes | Lung
adenocarcinoma | Unknown | EGFR del
E746-A750 | Caucasian | Yes (gefitinib,
partial response for 9 months) | Yes (first line,
stable for 6 months) | (18) |
| Tibaldi, 2011 | F | No | Yes | Poorly
differentiated acinar and solid adenocarcinoma | IIIb | Unknown | Caucasian | Yes (gefitinib,
partial response for 45 months) | Yes (first line,
stable for 12 months) | (18) |
| Oxnard, 2012 | F | No | Unknown | Lung
adenocarcinoma | Advanced lung
cancer | EGFR L858R, exon 19
del | NA | No | No | (8) |
| Gazdar, 2014 | F | Yes | Yes | Adenocarcinoma
(bilateral preneoplastic and preinvasive lesions) | Unknown | EGFR L858R | Unknown | Unknown | Unknown | (9) |
| Vikis, 2007 | M | Unknown | Yes | Lung
adenocarcinoma | IV | EGFR G719S | Chinese | Unknown | Unknown | (11) |
| Mäkinen, 2021 | M | Yes | No | Lung
adenocarcinoma | IV | EGFR L858R | Chinese | Yes (icotinib) | Unknown | (15) |
| Mäkinen, 2021 | F | Yes | Yes | Lung
adenocarcinoma | NA | L861Q, G719A | Chinese | Yes (icotinib) | Unknown | (15) |
Germline mutation of EGFR V843I
Exon 21 of the EGFR gene harbors the V843I mutation
(19). Initially, V843I was not
recognized as a germline mutation until it was reported in a study
aimed at determining the feasibility of EGFR mutation analysis in
needle biopsy/aspiration paraffin-fixed specimens (19). Subsequently, Ikeda et al
(20) presented the first evidence
that V843I is a germline mutation in a study involving a
70-year-old female with multiple lung adenocarcinomas and family
members with lung cancer. The patient had a germline EGFR V843I
mutation and additional mutations, L861Q and L858R, were found in
all examined specimens (20). The
authors proposed that the V843I mutation causing lung cancer was
based on the ‘two-hit theory’, which suggests that tumors may
develop from normal tissue with congenital (first) mutations after
acquiring a second mutation.
To date, four cases of germline EGFR V843I mutations
have been reported (21) (Table II). Of note, three of the four
patients with germline EGFR V843I mutations had a second mutation,
except for patient no. 3. Ohtsuka et al (21) presented the case of patient no. 2
and revealed that the somatic secondary L858R mutation occurred
nonrandomly in cis to the germline V843I mutation. By
conducting growth inhibition assays of the tumor cells obtained
from the pleural effusion of patient no. 2, the author concluded
that the germline V843I mutation was associated with TKI
resistance, similar to the germline T790M mutation (21,22).
Patient no. 3 underwent three different types of therapy, including
erlotinib, with no success. The researchers used computer-aided
approaches to model the EGFR ATP catalytic domain in a complex with
ATP, gefitinib and erlotinib to further demonstrate that the
germline mutation V843I is associated with EGFR-TKI resistance
(23). By contrast, patient no. 4
exhibited sensitivity to erlotinib and the effectiveness lasted for
9 months (24). Thus, three of the
four reported cases of the germline V843I mutation exhibited
EGFR-TKI resistance. Recently, Song et al (25) reported that osimertinib therapy was
effective in patients with NSCLC and the germline EGFR V843I
mutation. However, due to the small number of cases included, the
finding that third-generation targeted drugs are effective for
V843I cannot be generalized. Therefore, further research studies
are required to confirm the preliminary findings.
 | Table II.Summary of patients with lung cancer
exhibiting an EGFR V843I mutation. |
Table II.
Summary of patients with lung cancer
exhibiting an EGFR V843I mutation.
|
|
|
|
|
|
|
|
| Effective for
treatment |
|
|---|
| First author,
year | Sex (M/F) | Smoking
history | Family cancer
history | Pathology | TNM stage | Secondary somatic
mutation | Ethnicity |
|
|
|---|
| EGFR-TKI | Chemotherapy | (Refs.) |
|---|
| Ikeda, 2008 | F | Unknown | Yes | 3 nodules AD; 4
nodules BAC; 3 lesions AAH | T1N1M0 (stage
IIA) | EGFR L858R,
L861Q | East Asian | Not used | Unknown | (20) |
| Ohtsuka, 2011 | F | Unknown | No | Lung
adenocarcinoma | T4N2M1 (stage
IV) | EGFR L858R | East Asian | No (gefitinib,
erlotinib) | No | (21) |
| Demierre, 2013 | F | Yes | Yes | Poorly
differentiated adenocarcinoma | Stage IV | None | Caucasian | No (erlotinib) | No
(pemetrexed/cisplatin/bevacizumab) | (23) |
| Prim, 2014 | M | Yes | No | Invasive, acinar
predominant adenocarcinoma | cT2aN3M1a (stage
IV) | EGFR L858R | Caucasian | Yes (erlotinib
adenocarcinoma 9 months) | Yes (14 months)
(pemetrexed/cisplatin) | (24) |
| Mäkinen, 2021 | F | No | No | Lung
adenocarcinoma | IV | EGFR L858R | Chinese | Yes adenocarcinoma
(gefitinib, osimertinib) | Unknown | (15) |
| Ikeda, 2008 | F | Unknown | Yes | 3 nodules AD; 4
nodules BAC; 3 lesions AAH | T1N1M0 (stage
IIA) | EGFR L858R,
L861Q | East Asian | Not used | Unknown | (20) |
| Ohtsuka, 2011 | F | Unknown | No | Lung
adenocarcinoma | T4N2M1 (stage
IV) | EGFR L858R | East Asian | No (gefitinib,
erlotinib) | No | (21) |
| Demierre, 2013 | F | Yes | Yes | Poorly
differentiated adenocarcinoma | Stage IV | None | Caucasian | No (erlotinib) | No
(pemetrexed/cisplatin/bevacizumab) | (23) |
| Prim, 2014 | M | Yes | No | Invasive,
acinar-predominant adenocarcinoma | cT2aN3M1a (stage
IV) | EGFR L858R | Caucasian | Yes (erlotinib 9
months) | Yes (14 months)
(pemetrexed/cisplatin) | (24) |
| Mäkinen, 2021 | F | No | No | Lung
adenocarcinoma | IV | EGFR L858R | Chinese | Yes (gefitinib,
osimertinib) | Unknown | (15) |
| Ikeda, 2008 | F | Unknown | Yes | 3 nodules AD; 4
nodules BAC; 3 lesions AAH | T1N1M0 (stage
IIA) | EGFR L858R,
L861Q | East Asian | Not used | Unknown | (20) |
| Ohtsuka, 2011 | F | Unknown | No | Lung
adenocarcinoma | T4N2M1 (stage
IV) | EGFR L858R | East Asian | No (gefitinib,
erlotinib) | No | (21) |
However, the underlying mechanism by which the
germline V843I mutation causes lung cancer remains elusive. One
possible mechanism is that the V843I mutation causes EGFR gene
instability, which then predisposes cells to additional mutations,
such as L858R, L861Q and L858R, all of which may collectively cause
tumorigenesis. However, the precise mechanism requires further
investigation.
Germline mutation of EGFR R776H
In 2013, a new germline mutation, R776H, was
discovered in a Caucasian mother and daughter following the
discovery of two germline-transmitted EGFR variants linked to lung
cancer (26). The mother's right
hilar tumor was diagnosed as squamous cell carcinoma, while the
daughter's right-sided lung cancer was also determined to be
squamous cell carcinoma. A codon 776 mutation was found in DNA
derived from normal lung tissue, normal lymph node, skin and blood
of the daughter, as well as a germline mutation inherited from her
mother. Of note, another EGFR mutation, G719A, was discovered in
the mother's carcinoma. However, as this mutation was not found in
the mother's normal DNA, it was classified as a somatic mutation.
Analysis from large cohorts of EGFR-mutated lung carcinomas
revealed that 25% of patients with R776H/G719A-mutated tumors were
classified as having mixed adenosquamous cell carcinoma, despite
both the mother's and daughter's tumors being squamous cell tumors
(26). This suggests that the
R776H/G719A mutation may be linked to squamous cell carcinomas.
Somatic codon 719 mutations have been linked to squamous
carcinomas. In a recent study by Guo et al (27), two patients with germline EGFR R776H
mutations were reported: A 42-year-old female with no smoking
history and her 17-year-old son. A CT scan revealed numerous
ground-glass nodes in her son's both lungs and postoperative
pathology indicated the presence of adenocarcinomas. Genetic
analysis of both patients revealed the same germline EGFR mutation,
R776H. Her son was monitored through regular CT examination
(Table III).
 | Table III.Summary of patients with lung cancer
exhibiting an EGFR R776H mutation. |
Table III.
Summary of patients with lung cancer
exhibiting an EGFR R776H mutation.
|
|
|
|
|
|
|
|
| Effective for
treatment |
|
|---|
| First author,
year | Sex (M/F) | Smoking
history | Family cancer
history | Pathology | TNM stage | Secondary somatic
mutation | Ethnicity |
|
|
|---|
| EGFR-TKI | Chemotherapy | (Refs.) |
|---|
| van Noesel,
2013 | F | No | Yes | Squamous
differentiation | Unknown | Unknown | White | Unknown | Yes | (26) |
| van Noesel,
2013 | F | No | Yes | Squamous-cell
carcinoma | Unknown | EGFR G719S | White | Yes | Unknown | (26) |
| Guo, 2021 | F | No | Yes | Lung
adenocarcinoma | IA | EGFR G719A | Chinese | Unknown | Unknown | (27) |
| Guo, 2021 | M | No | Yes | Unknown | Unknown | Unknown | Chinese | Unknown | Unknown | (27) |
| Lin, 2021 | M | Yes | Yes | Lung
adenocarcinoma | IV | EGFR T790M | Chinese | Yes
(osimertinib) | Unknown | (29) |
| van Noesel,
2013 | F | No | Yes | Squamous
differentiation | Unknown | Unknown | White | Unknown | Yes | (26) |
| van Noesel,
2013 | F | No | Yes | Squamous-cell
carcinoma | Unknown | EGFR G719S | White | Yes | Unknown | (26) |
| Guo, 2021 | F | No | Yes | Lung
adenocarcinoma | IA | EGFR G719A | Chinese | Unknown | Unknown | (27) |
| Guo, 2021 | M | No | Yes | Unknown | Unknown | Unknown | Chinese | Unknown | Unknown | (27) |
| Lin, 2021 | M | Yes | Yes | Lung
adenocarcinoma | IV | EGFR T790M | Chinese | Yes
(osimertinib) | Unknown | (29) |
| van Noesel,
2013 | F | No | Yes | Squamous
differentiation | Unknown | Unknown | White | Unknown | Yes | (26) |
| van Noesel,
2013 | F | No | Yes | Squamous-cell
carcinoma | Unknown | EGFR G719S | White | Yes | Unknown | (26) |
Germline mutation of EGFR P848L
The EGFR exon 21 P848L point mutation was first
described by de Gunst et al (28) as an infrequent mutation and could
silence polymorphisms. The mutation was initially identified as a
germline mutation in a 31-year-old Caucasian woman who was an
active smoker with a maternal grandfather diagnosed with throat
cancer in 2014. The proband was resistant to erlotinib and
chemotherapy (cisplatin and pemetrexed) (24). The morbidity of the P848L population
is currently unclear. A large-scale retrospective study involving
31,906 Chinese patients with lung cancer found 22 germline EGFR
variants in 64 patients with lung cancer, while germline EGFR P848L
account for 10.9% (7/64) of the patients with EGFR germline
mutation (29). A further study of
120 patients with colorectal or lung cancer reported that only one
patient exhibited the P848L mutation (30).
The question arises as to whether lung cancer
patients with P848L germline mutation are sensitive to EGFR-TKI.
According to current research, patients with P848L germline
mutation are not sensitive to EGFR-TKI (24,31).
Previous studies have shown that oral erlotinib treatment in
patients with the germline P848L mutation provides ~4 months of
progression-free survival (24,32).
However, Chinese patients with the P848L mutation alone or in
combination with the L858R somatic mutation were unresponsive to
EGFR-TKI, but germline P848L mutation combined with an exon 19
deletion was sensitive to gefitinib and icotinib treatment
(29). Han et al (31) showed that patients with the P848L
mutation treated with gefitinib exhibited a response similar to
that of patients with wild-type EGFR, and that patients with a
T790M and P848L double mutation exhibited higher resistance to
gefitinib. These findings provide greater insight into the response
of patients with lung cancer and rare EGFR mutations, such as the
P848L mutation, to gefitinib, regardless of whether the mutation is
somatic or germline. Sarcar et al (33) studied patients with the EGFR
germline mutation and established P848L-transformed Ba/F3 cells
that were resistant to multiple EGFR-TKIs but sensitive to a number
of Janus kinase 1/2 inhibitors (Table
IV).
 | Table IV.Summary of patients with lung cancer
exhibiting an EGFR P848L mutation. |
Table IV.
Summary of patients with lung cancer
exhibiting an EGFR P848L mutation.
|
|
|
|
|
|
|
|
| Effective for
treatment |
|
|---|
| First author,
year | Sex (M/F) | Smoking
history | Family cancer
history | Pathology | TNM stage | Secondary somatic
mutation | Ethnicity |
|
|
|---|
| EGFR-TKI | Chemotherapy | (Refs.) |
|---|
| De Gunst, 2007 | F | Yes | Yes | Lung
adenocarcinoma | Unknown | Unknown | Caucasian | No | No | (28) |
| Yang, 2021 | F | No | Unknown | Adenocarcinoma | Unknown | Unknown | Caucasian | Yes
(gefitinib) | Unknown | (34) |
| Prim, 2014 | F | Yes | Yes | Invasive,
acinar-predominant adenocarcinoma | T4N0M1b (stage
IV) | No | Caucasian | No | No | (24) |
| Guo, 2021 | F | No | Yes | Lung
adenocarcinoma | IV | L858R | Chinese | Yes
(gefitinib) | Unknown | (27) |
| Guo, 2021 | M | Yes | Yes | Lung
adenocarcinoma | IV | L858R | Chinese | No | Unknown | (27) |
| Guo, 2021 | F | No | Yes | Lung
adenocarcinoma | IV | L747_T751del | Chinese | Yes (gefitinib,
icotinib) | Unknown | (27) |
| Guo, 2021 | M | Yes | No | Lung
adenocarcinoma | IV | No | Chinese | Yes (icotinib,
afatinib) | Unknown | (27) |
| De Gunst, 2007 | F | Yes | Yes | Lung
adenocarcinoma | Unknown | Unknown | Caucasian | No | No | (28) |
| Yang, 2021 | F | No | Unknown | Adenocarcinoma | Unknown | Unknown | Caucasian | Yes
(gefitinib) | Unknown | (34) |
| Prim, 2014 | F | Yes | Yes | Invasive,
acinar-predominant adenocarcinoma | T4N0M1b (stage
IV) | No | Caucasian | No | No | (24) |
| Guo, 2021 | F | No | Yes | Lung
adenocarcinoma | IV | L858R | Chinese | Yes
(gefitinib) | Unknown | (27) |
| Guo, 2021 | M | Yes | Yes | Lung
adenocarcinoma | IV | L858R | Chinese | No | Unknown | (27) |
Discussion
Germline mutations in humans contribute to both
adaptive evolution and the genetic burden of the species. Various
types of germline mutations have been thoroughly studied, including
changes in gene dosage due to duplications or deletions ranging in
size from a few base pairs to megabases, as well as changes in the
number of chromosomes (34,35). While germline mutations inherited
from affected or carrier parents have been linked to poor health
worldwide, an increasing number of researchers are attempting to
understand the mechanisms that cause diseases. The four most common
EGFR germline mutations involved in lung cancer (T790M, V843I,
R776X and P848L) have been described previously. Furthermore, rare
germline mutations of EGFR include K757R, R831H, D1014N and L792F.
Most EGFR germline mutations are generally associated with lung
cancer susceptibility and the majority of these mutations are point
mutations, with other mutation types being uncommon.
Nachman and Crowell (36) used an indirect measurement approach
to compare human and chimpanzee data and determined that the
average neutral mutation rate in the human genome is
2.3×10−8 mutations per nucleotide site per generation.
Species divergence and diversity exhibit a strong relationship with
the mutation rate per generation. For lung cancer, most data from
western populations concluded that 3.5-8.5% of patients with lung
cancer have a pathogenic germline mutation (37,38).
BRCA2 and CHEK2 have been linked to an increased risk of lung
cancer (39). In the Chinese
population, Peng et al (40)
reported that among 1,794 patients with lung cancer, 106 had
pathogenic or likely pathogenic germline mutations, with a germline
mutation-carrying rate of 5.91%. Thus, the germline
mutation-carrying rate of Chinese patients with lung cancer is
comparable with that of the Western population (40).
Substitution mutations, such as the lung cancer EGFR
germline mutation, are genomic disorders that are likely to cause
diseases and may be initiated during the early stages of testis
development. A study comparing the mutational frequency of
self-renewing spermatogonia (SrAp) in young and old male patients
with the Apert syndrome mutation found that the fibroblast growth
factor receptor 2 mutation was significantly higher (5%) in the
testes of the four older donors. By contrast, two younger patients
did not exhibit high mutation frequencies (41). Furthermore, not all members of the
first family with the germline V843I mutation developed lung
cancer. Specifically, while the proband's father and brother died
of lung cancer, another brother and sister with the same germline
mutation did not develop the disease (20). These observations suggest that
germline mutations did not originate early during testis
development. Hence, there may be selective advantages that
facilitate the development of this genomic disorder. These benefits
may be categorized based on sex. According to studies on Apert
syndrome and achondroplasia, premeiotic testis cells carrying the
causal mutation in males showed a selective advantage (42,43).
In Apert syndrome and achondroplasia, the two most common mutations
are 755C>G and 785C>G. Researchers found that mutant SrAp
cells had an advantage over wild-type SrAp cells because they could
occasionally divide symmetrically to produce two SrAp cells,
whereas wild-type SrAp cells could only replace themselves
(41,44,45).
These germline selections may not be limited to nucleotide
substitutions. In fragile X syndrome, for instance,
trinucleotide-repeat expansion mutations could increase the
frequency of germ cells with smaller alleles as testis development
progresses because they have an advantage over cells with a disease
allele (46–52). Aside from germline selection in
males, females exhibit a higher selective advantage attributable to
hereditary disease. A study reported that ovarian germline cells
carrying trisomy 21 could influence the effect of maternal age on
the development of Down syndrome during fetal and postnatal life
(53). Based on the preceding
analysis, it may be concluded that germline mutations are not
passed on during birth but occur after birth. While individuals may
inherit susceptibility towards developing certain familial genetic
diseases at birth, the presence of these selective advantages
requires confirmation through future research.
In addition to the four common types of EGFR
germline mutation, some rare types of EGFR germline mutations have
also been reported. Li et al (54) discovered the EGFR V1010M germline
mutation in six individuals from four generations of family
members, many of whom had lung cancer. The proband had the somatic
mutation of EGFR L858R and responded to gefitinib after only 4
months. Van der Leest et al (55) reported the EGFR V834L germline
mutation in a 57-year-old woman diagnosed with stage IIIA
adenocarcinoma. While only a few cases with rare EGFR germline
mutations have been reported, Lin et al (29) sequenced 31,906 patients with lung
cancer and found 22 germline EGFR variants, including G863D,
D1014N, K757R, V769M, V774M, K757R, V897A, R831H, V769M, V765M,
R836C, G724S, T725M, V889M, V788M, A647T, D761Y, K754E, P753S and
R776S. Patients with lung cancer and EGFR germline mutations have
limited therapeutic options and new treatments should be
investigated. In addition to traditional platinum-based
chemotherapy, immunotherapy also has also received a lot of
attention and may produce a good therapeutic effect. Trabelsi Grati
et al (56) reported the
case of a patient with metastatic NSCLC and EGFR germline and KRAS
somatic mutations who exhibited a long response to immune
checkpoint inhibitors. Although there is no solid clinical evidence
indicating that immunotherapy is effective in patients with EGFR
germline mutations, this case report suggests that immunotherapy
may be efficient in patients with lung cancer with EGFR germline
mutations.
Conclusion
EGFR germline mutations, including T790M, V843I,
R776H and P848L, have been shown to impact the development of lung
cancer. The likelihood of developing lung cancer is higher for
individuals with a germline mutation if they also have a somatic
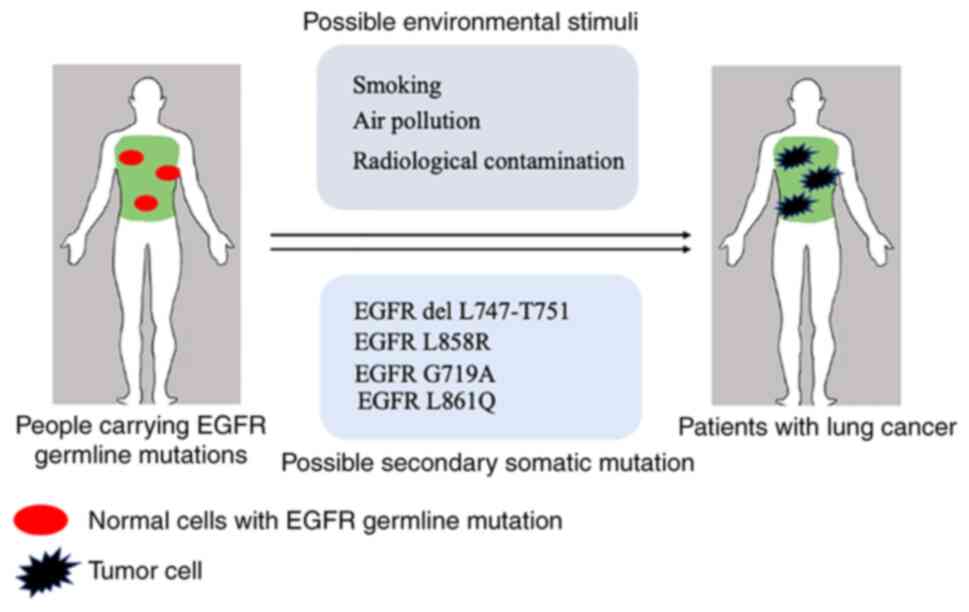
mutation or are exposed to environmental stimuli (Fig. 1). The efficacy of EGFR-TKI in
treating patients with lung cancer with EGFR germline mutations is
unclear; however, it appears to be most effective in those who have
previously received EGFR-TKI treatment. Therefore, effective and
appropriate treatment options should be investigated in future
studies. It is recommended that individuals with germline mutations
should undergo population screening as well as regular physical
examinations to detect and diagnose lung tumors early.
Acknowledgements
Not applicable.
Funding
This work was supported by Tianjin Municipal Education
Commission Natural Science Foundation (grant. no. 2019KJ202).
Availability of data and materials
Not applicable.
Authors' contributions
ML, XN designed and wrote the manuscript. JC and HL
performed the literature search and drafted the manuscript. All
authors have read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanwal M, Ding XJ, Song X, Zhou GB and Cao
Y: MUC16 overexpression induced by gene mutations promotes lung
cancer cell growth and invasion. Oncotarget. 9:12226–12239. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subramanian J, Velcheti V, Gao F and
Govindan R: Presentation and stage-specific outcomes of lifelong
never-smokers with non-small cell lung cancer (NSCLC). J Thorac
Oncol. 2:827–830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnheim N and Calabrese P: Understanding
what determines the frequency and pattern of human germline
mutations. Nat Rev Genet. 10:478–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang XN, Shigematsu H, Bekele BN, Roth JA,
Minna JD, Hong WK, Gazdar AF and Wistuba II: EGFR tyrosine kinase
domain mutations are detected in histologically normal respiratory
epithelium in lung cancer patients. Cancer Res. 65:7568–7572. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arteaga CL: EGF receptor mutations in lung
cancer: From humans to mice and maybe back to humans. Cancer Cell.
9:421–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu HA, Arcila ME, Hellmann MD, Kris MG,
Ladanyi M and Riely GJ: Poor response to erlotinib in patients with
tumors containing baseline EGFR T790M mutations found by routine
clinical molecular testing. Ann Oncol. 25:423–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oxnard GR, Miller VA, Robson ME, Azzoli
CG, Pao W, Ladanyi M and Arcila ME: Screening for germline EGFR
T790M mutations through lung cancer genotyping. J Thorac Oncol.
7:1049–1052. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gazdar A, Robinson L, Oliver D, Xing C,
Travis WD, Soh J, Toyooka S, Watumull L, Xie Y, Kernstine K and
Schiller JH: Hereditary lung cancer syndrome targets never smokers
with germline EGFR gene T790M mutations. J Thorac Oncol. 9:456–463.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Girard N, Lou E, Azzoli CG, Reddy R,
Robson M, Harlan M, Orlow I, Yatabe Y, Nafa K, Ladanyi M, et al:
Analysis of genetic variants in never-smokers with lung cancer
facilitated by an Internet-based blood collection protocol: A
preliminary report. Clin Cancer Res. 16:755–763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vikis H, Sato M, James M, Wang D, Wang Y,
Wang M, Jia D, Liu Y, Bailey-Wilson JE, Amos CI, et al: EGFR-T790M
is a rare lung cancer susceptibility allele with enhanced kinase
activity. Cancer Res. 67:4665–4670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amos CI, Gorlov IP, Dong Q, Wu X, Zhang H,
Lu EY, Scheet P, Greisinger AJ, Mills GB and Spitz MR: Nicotinic
acetylcholine receptor region on chromosome 15q25 and lung cancer
risk among African Americans: A case-control study. J Natl Cancer
Inst. 102:1199–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi D, Xu L, Luo J, You X, Huang T, Zi Y,
Li X, Wang R, Zhong Z, Tang X, et al: Germline TP53 and MSH6
mutations implicated in sporadic triple-negative breast cancer
(TNBC): A preliminary study. Hum Genomics. 13:42019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu S, Yu Y, Li Z, Yu R, Wu X, Bao H, Ding
Y, Shao YW and Jian H: EGFR and ERBB2 germline mutations in Chinese
lung cancer patients and their roles in genetic susceptibility to
cancer. J Thorac Oncol. 14:732–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mäkinen N, Zhou M, Bemus M, Nevin J, Nag
A, Chen R, Colson YL, Thorner AR, Oxnard GR, Meyerson M and Sholl
LM: Genomic evolution in a patient with lung adenocarcinoma with a
germline EGFR T790M mutation. JTO Clin Res Rep.
2:1001462021.PubMed/NCBI
|
|
16
|
Bell DW, Gore I, Okimoto RA, Godin-Heymann
N, Sordella R, Mulloy R, Sharma SV, Brannigan BW, Mohapatra G,
Settleman J and Haber DA: Inherited susceptibility to lung cancer
may be associated with the T790M drug resistance mutation in EGFR.
Nat Genet. 37:1315–1316. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prudkin L, Tang X and Wistuba II:
Germ-line and somatic presentations of the EGFR T790M mutation in
lung cancer. J Thorac Oncol. 4:139–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tibaldi C, Giovannetti E, Vasile E,
Boldrini L, Gallegos-Ruiz MI, Bernardini I, Incensati R, Danesi R,
Cappuzzo F, Peters GJ and Fontanini G: Inherited germline T790M
mutation and somatic epidermal growth factor receptor mutations in
non-small cell lung cancer patients. J Thorac Oncol. 6:395–396.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shih JY, Gow CH, Yu CJ, Yang CH, Chang YL,
Tsai MF, Hsu YC, Chen KY, Su WP and Yang PC: Epidermal growth
factor receptor mutations in needle biopsy/aspiration samples
predict response to gefitinib therapy and survival of patients with
advanced nonsmall cell lung cancer. Int J Cancer. 118:963–969.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikeda K, Nomori H, Mori T, Sasaki J and
Kobayashi T: Novel germline mutation: EGFR V843I in patient with
multiple lung adenocarcinomas and family members with lung cancer.
Ann Thorac Surg. 85:1430–1432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohtsuka K, Ohnishi H, Kurai D, Matsushima
S, Morishita Y, Shinonaga M, Goto H and Watanabe T: Familial lung
adenocarcinoma caused by the EGFR V843I germ-line mutation. J Clin
Oncol. 29:e191–e192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsushima S, Ohtsuka K, Ohnishi H,
Fujiwara M, Nakamura H, Morii T, Kishino T, Goto H and Watanabe T:
V843I, a lung cancer predisposing EGFR mutation, is responsible for
resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol.
9:1377–1384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Demierre N, Zoete V, Michielin O, Stauffer
E, Zimmermann DR, Betticher DC and Peters S: A dramatic lung cancer
course in a patient with a rare EGFR germline mutation exon 21
V843I: Is EGFR TKI resistance predictable? Lung Cancer. 80:81–84.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prim N, Legrain M, Guerin E, Mennecier B,
Weingertner N, Voegeli AC, Guenot D, Maugard CM, Quoix AE and
Beau-Faller M: Germ-line exon 21 EGFR mutations, V843I and P848L,
in nonsmall cell lung cancer patients. Eur Respir Rev. 23:390–392.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song H, Chen Y, Yan Z, Sun G and Shi Z:
Response to osimertinib in a NSCLC patient harboring EGFR V843I
germ-line mutation. Lung Cancer. 150:247–248. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Noesel J, van der Ven WH, van Os TAM,
Kunst PWA, Weegenaar J, Reinten RJA, Kancha RK, Duyster J and van
Noesel CJ: Activating germline R776H mutation in the epidermal
growth factor receptor associated with lung cancer with squamous
differentiation. J Clin Oncol. 31:e161–e164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo T, Zhu L, Li W, Lin R, Ding Y, Kang Q,
Shao L, Li C and Pan X: Two cases of non-small cell lung cancer
patients with somatic or germline EGFR R776H mutation. Lung Cancer.
161:94–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Gunst MM, Gallegos-Ruiz MI, Giaccone G
and Rodriguez JA: Functional analysis of cancer-associated EGFR
mutants using a cellular assay with YFP-tagged EGFR intracellular
domain. Mol Cancer. 6:562007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin X, Peng M, Chen Q, Yuan M, Chen R,
Deng H, Deng J, Liu O, Weng Y, Chen M and Zhou C: Identification of
the unique clinical and genetic features of chinese lung cancer
patients with EGFR germline mutations in a large-scale
retrospective study. Front Oncol. 11:7741562021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borràs E, Jurado I, Hernan I, Gamundi MJ,
Dias M, Martí I, Mañé B, Arcusa A, Agúndez JA, Blanca M and
Carballo M: Clinical pharmacogenomic testing of KRAS, BRAF and EGFR
mutations by high resolution melting analysis and ultra-deep
pyrosequencing. BMC Cancer. 11:4062011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han B, Zhou X, Zhang RX, Zang WF, Chen ZY,
Song HD, Wan HY and Zheng CX: Mutations of the epidermal growth
factor receptor gene in NSCLC patients. Oncol Lett. 2:1233–1237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klughammer B, Brugger W, Cappuzzo F,
Ciuleanu T, Mok T, Reck M, Tan EH, Delmar P, Klingelschmitt G, Yin
AY, et al: Examining treatment outcomes with erlotinib in patients
with advanced non-small cell lung cancer whose tumors harbor
uncommon EGFR mutations. J Thorac Oncol. 11:545–555. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sarcar B, Gimbrone NT, Wright G, Remsing
Rix LL, Gordian ER, Rix U, Chiappori AA, Reuther GW,
Santiago-Cardona PG, Muñoz-Antonia T and Cress WD: Characterization
of epidermal growth factor receptor (EGFR) P848L, an unusual EGFR
variant present in lung cancer patients, in a murine Ba/F3 model.
FEBS Open Bio. 9:1689–1704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Li H, Li B, Li W, Guo Q, Hu L,
Song Z and Zhou B: Profiling oncogenic germline mutations in
unselected chinese lung cancer patients. Front Oncol.
11:6475982021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shukuya T and Takahashi K: Germline
mutations in lung cancer. Respir Investig. 57:201–206. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nachman MW and Crowell SL: Estimate of the
mutation rate per nucleotide in humans. Genetics. 156:297–304.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu C, Xie M, Wendl MC, Wang J, McLellan
MD, Leiserson MD, Huang KL, Wyczalkowski MA, Jayasinghe R, Banerjee
T, et al: Patterns and functional implications of rare germline
variants across 12 cancer types. Nat Commun. 6:100862015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang KL, Mashl RJ, Wu Y, Ritter DI, Wang
J, Oh C, Paczkowska M, Reynolds S, Wyczalkowski MA, Oak N, et al:
Pathogenic germline variants in 10,389 adult cancers. Cell.
173:355–370.e14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, McKay JD, Rafnar T, Wang Z,
Timofeeva MN, Broderick P, Zong X, Laplana M, Wei Y, Han Y, et al:
Rare variants of large effect in BRCA2 and CHEK2 affect risk of
lung cancer. Nat Genet. 46:736–741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng W, Li B, Li J, Chang L, Bai J, Yi Y,
Chen R, Zhang Y, Chen C, Pu X, et al: Clinical and genomic features
of Chinese lung cancer patients with germline mutations. Nat
Commun. 13:12682022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi SK, Yoon SR, Calabrese P and Arnheim
N: A germ-line-selective advantage rather than an increased
mutation rate can explain some unexpectedly common human disease
mutations. Proc Natl Acad Sci USA. 105:10143–10148. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tiemann-Boege I, Navidi W, Grewal R, Cohn
D, Eskenazi B, Wyrobek AJ and Arnheim N: The observed human sperm
mutation frequency cannot explain the achondroplasia paternal age
effect. Proc Natl Acad Sci USA. 99:14952–14957. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kan SH, Elanko N, Johnson D,
Cornejo-Roldan L, Cook J, Reich EW, Tomkins S, Verloes A, Twigg SR,
Rannan-Eliya S, et al: Genomic screening of fibroblast
growth-factor receptor 2 reveals a wide spectrum of mutations in
patients with syndromic craniosynostosis. Am J Hum Genet.
70:472–486. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qin J, Calabrese P, Tiemann-Boege I,
Shinde DN, Yoon SR, Gelfand D, Bauer K and Arnheim N: The molecular
anatomy of spontaneous germline mutations in human testes. PLoS
Biol. 5:e2242007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Crow JF: Age and sex effects on human
mutation rates: An old problem with new complexities. J Radiat Res.
47 (Suppl B):B75–B82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Malter HE, Iber JC, Willemsen R, de Graaff
E, Tarleton JC, Leisti J, Warren ST and Oostra BA: Characterization
of the full fragile X syndrome mutation in fetal gametes. Nat
Genet. 15:165–169. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moutou C, Vincent MC, Biancalana V and
Mandel JL: Transition from premutation to full mutation in fragile
X syndrome is likely to be prezygotic. Hum Mol Genet. 6:971–979.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Silveira I, Alonso I, Guimarães L,
Mendonça P, Santos C, Maciel P, Fidalgo De Matos JM, Costa M,
Barbot C, Tuna A, et al: High germinal instability of the (CTG)n at
the SCA8 locus of both expanded and normal alleles. Am J Hum Genet.
66:830–840. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Delatycki MB, Paris D, Gardner RJ, Forshaw
K, Nicholson GA, Nassif N, Williamson R and Forrest SM: Sperm DNA
analysis in a Friedreich ataxia premutation carrier suggests both
meiotic and mitotic expansion in the FRDA gene. J Med Genet.
35:713–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
De Temmerman N, Sermon K, Seneca S, De
Rycke M, Hilven P, Lissens W, Van Steirteghem A and Liebaers I:
Intergenerational instability of the expanded CTG repeat in the
DMPK gene: Studies in human gametes and preimplantation embryos. Am
J Hum Genet. 75:325–329. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Moseley ML, Schut LJ, Bird TD, Koob MD,
Day JW and Ranum LP: SCA8 CTG repeat: En masse contractions in
sperm and intergenerational sequence changes may play a role in
reduced penetrance. Hum Mol Genet. 9:2125–1230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
De Michele G, Cavalcanti F, Criscuolo C,
Pianese L, Monticelli A, Filla A and Cocozza S: Parental gender,
age at birth and expansion length influence GAA repeat
intergenerational instability in the X25 gene: Pedigree studies and
analysis of sperm from patients with Friedreich's ataxia. Hum Mol
Genet. 7:1901–1906. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hultén MA, Patel SD, Westgren M,
Papadogiannakis N, Jonsson AM, Jonasson J and Iwarsson E: On the
paternal origin of trisomy 21 Down syndrome. Mol Cytogenet.
3:42010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li N, Liu C, Xiong L, Huang D and Jiang Y:
Pedigree analysis of the EGFR p.V1010M germline mutation in a
family with a family history of non-small-cell lung cancer. Ann
Transl Med. 10:1542022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
van der Leest C, Wagner A, Pedrosa RM,
Aerts JG, Dinjens WNM and Dubbink HJ: Novel EGFR V834L germline
mutation associated with familial lung adenocarcinoma. JCO Precis
Oncol. 2:PO.17.00266. 2018.PubMed/NCBI
|
|
56
|
Trabelsi Grati O, Zemoura L, Nhy C, Daniel
C, Melaabi S, Callens C, Gauthier Villars M, Bièche I and Girard N:
Long response to immune checkpoint inhibitors in metastatic NSCLC
despite EGFR germline mutation. A case report. Lung Cancer.
174:186–187. 2022. View Article : Google Scholar : PubMed/NCBI
|