Introduction
PAAD is among the deadliest malignant tumors of the
digestive system, with a high rate of recurrence, a high fatality
rate, and a poor prognosis (1). In
the early stages, there are few characteristic clinical signs and
no reliable screening tools; up to 85% of patients with PAAD are
diagnosed at advanced stages or develop distant metastases
(2), and it is the seventh leading
cause of cancer death (3). Despite
ongoing advances in therapy, PAAD is still one of the most
difficult cancers to treat, with a 5-year survival rate of less
than 10% (4). The incidence of PAAD
is projected to rise to 18.6‰ in 2050, with an average yearly
increase of 1.1% (5). Therefore,
the underlying mechanisms of PAAD should be thoroughly
investigated, and the search for potential therapeutic targets
becomes crucial.
Myosins are classifiable into 24 classes based on
the amino acid sequence of the ATP hydrolytic region, and they are
involved in various cellular functions, including organelle
transport, actin recombination, and cell signal transduction
(6). Class I myosin consists of
Myo1a~Myo1h (7), an actin-dependent
molecular motor expressed in various organisms, from yeast to
humans (8). Class I myosins may
interact with actin filaments and cell membranes through their
N-terminal motor structural domain and C-terminal tail homology 1
(TH1) structural domain, respectively; in addition to the TH1
structural domain, class I myosins also include the proline-rich
TH2 structural domain and the SH3 structural domain (9,10).
According to previous research, MYO1E contributes to the
progression of breast cancer and affects breast tumor cell
differentiation and proliferation (11). The role of MYO1E in PAAD has yet to
be reported, and its related mechanisms still need further
investigation.
In this research, we found that MYO1E was
substantially expressed in PAAD and negatively correlated with PAAD
patient survival prognosis. Functional and pathway enrichment
analyses revealed that MYO1E was linked to tumor-associated
pathways. In addition, MYO1E was involved in multiple tumor immune
cell infiltrates in the TME. Further validation of the impact of
MYO1E on the proliferation, invasion, and migration of PAAD cells
was provided by in vitro tests. Our results reveal the
clinical significance, potential function, and immune relevance of
MYO1E in PAAD, which may provide new strategies for the early
diagnosis and prognosis of PAAD. The study flowchart is shown in
Fig. 1.
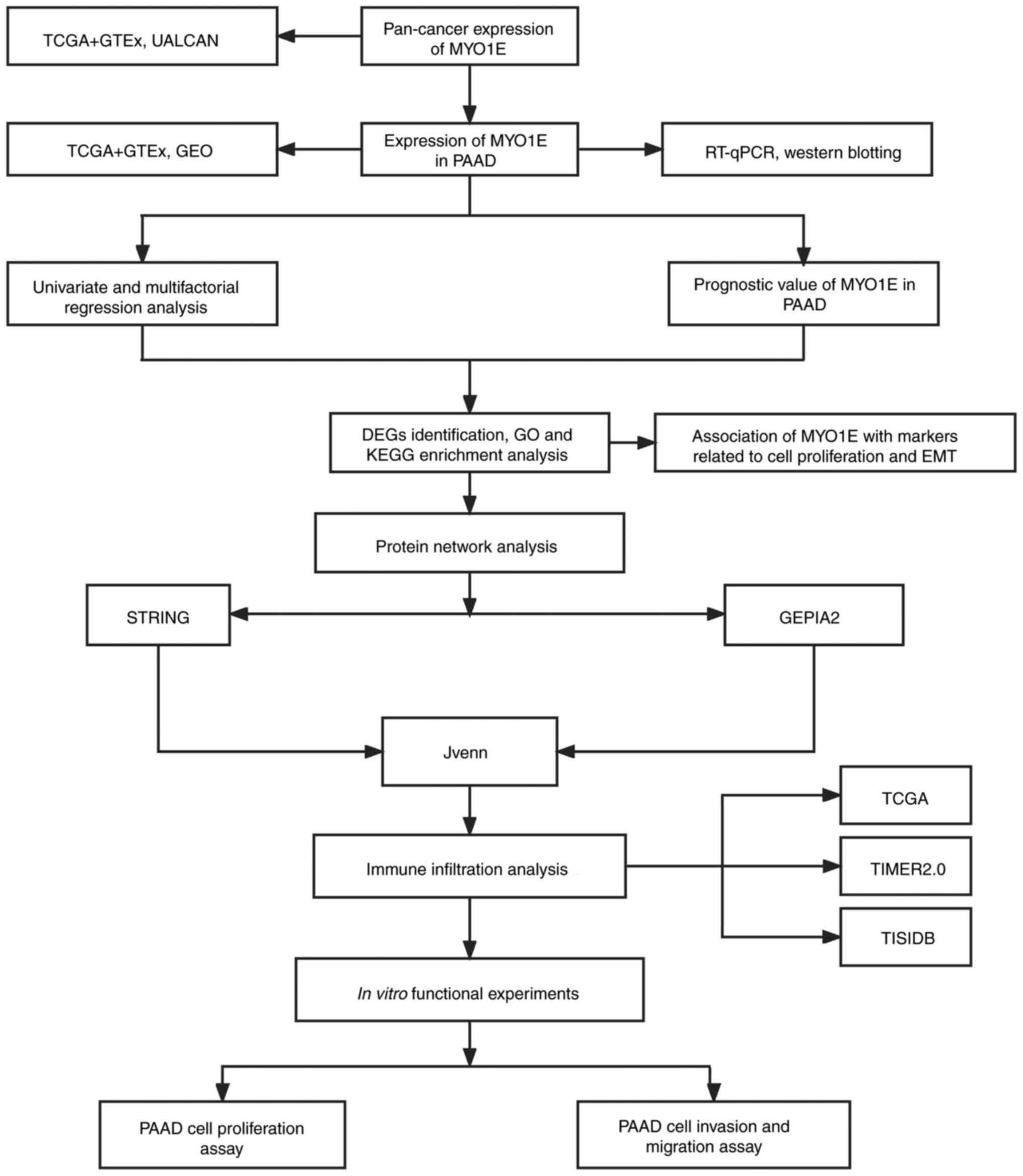 | Figure 1.Flowchart of the study design. DEGs,
differentially expressed genes; EMT, epithelial-mesenchymal
transition; GEO, Gene Expression Omnibus; GEPIA2, Gene Expression
Profiling Interactive Analysis 2; GO, Gene Ontology; GTEx,
Genotype-Tissue Expression; KEGG, Kyoto Encyclopedia of Genes and
Genomes; MYO1E, myosin 1E; PAAD, pancreatic adenocarcinoma;
RT-qPCR, reverse transcription-quantitative PCR; STRING, Search
Tool for the Retrieval of Interacting Genes/Proteins; TCGA, The
Cancer Genome Atlas; TIMER2.0, Tumor Immune Estimation Resource
2.0; UALCAN, The University of Alabama at Birmingham Cancer Data
Analysis Portal. |
Materials and methods
MYO1E gene expression analysis
We used RNAseq data in FPKM format from The Cancer
Genome Atlas Project (12) (TCGA)
(https://portal.gdc.cancer.gov/, Version
35) and Genotype-Tissue Expression (13) (GTEx) (https://www.gtexportal.org/, Version 8) processed
uniformly by the UCSC XENA (University Of California Sisha Cruz,
https://xenabrowser.net/datapages/,
July 20, 2019) database to extract the TCGA corresponding to 33
tumors data and normal tissue data in GTEx. The calculation was
performed using the ‘stats’ package in R (Version 4.2.1)
(https://cran.r-project.org/), the
Wilcoxon rank sum test was used, and the results were visualized
using the ‘ggplot2’ package. The CPTAC module of the UALCAN
database (http://ualcan.path.uab.edu/, May 13, 2022) analyzed
the total protein expression of MYO1E in different cancers.
Finally, we compared MYO1E expression in PAAD tissues and normal
tissues in the GEO (GSE16515, GSE62165, and GSE15471) cohort
(https://www.ncbi.nlm.nih.gov/geo/).
Tissue samples
Fifteen cases of pancreatic adenocarcinoma and
matched adjacent normal tissues were obtained from the Affiliated
Hospital of Guizhou Medical University and the Cancer Hospital
Affiliated with Guizhou Medical University. Patients were informed
and signed an informed consent form agreeing to use their tissues
for scientific research. This study was approved by the ethics
committees of the Affiliated Hospital of Guizhou Medical University
and the Cancer Hospital Affiliated with Guizhou Medical University.
All specimens were frozen and stored at −80°C before western blot
and reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis.
Survival analysis
GEPIA2 (http://gepia2.cancer-pku.cn/, Version 2) is a platform
for gene expression analysis based on tumor and normal samples from
TCGA and GTEx databases (14). We
obtained the overall survival (OS) and disease free survival (DFS)
of the MYO1E gene in PAAD using the ‘Survival analysis’ panel of
the GEPIA2 database with a 95% confidence interval.
Univariate and multifactorial
regression analysis
RNAseq data and corresponding clinical information
for pancreatic adenocarcinoma were obtained from TCGA dataset
(TCGA-PAAD, Version 35). The influence of MYO1E and clinical
features of PAAD patients (age, gender, M-stage, pTNM-stage, and
grading) on ‘OS’ was evaluated using univariate and multivariate
regression models (P<0.05) using the ‘forestplot’ package.
Following this analysis's findings, we created a Nomogram using the
‘rms’ package to forecast the overall recurrence rate at 1, 2, and
3 years. Using the ‘stage plot’ panel of GEPIA2, the link between
MYO1E and the pathological stage of PAAD was assessed. P<0.05
was considered significant.
Differentially expressed genes
analysis
In the TCGA database, the median expression of MYO1E
was separated between high and low expression groups, and 237
differential genes were obtained and analyzed for differential
expression using the ‘Limma’ package of R. Differentially expressed
genes (DEGs) were considered as the threshold value with log2 (fold
change) >1 and adjusted P<0.05.
String protein network analysis
We built a protein-protein interaction network (PPI)
of MYO1E-binding proteins through the STRING website (http://STRING-DB.org/); using the main settings
‘evidence’, ‘experimental’, and ‘low confidence’, the top 50
MYO1E-interacting proteins were obtained. Next, using the ‘similar
genes detection’ module of GEPIA2, the top 100 target genes
connected with MYO1E were selected. We used an interactive Venn
diagram viewer (Jvenn) (15) to
intersect the two sets of data.
Enrichment analysis
We used R's ‘clusterProfiler’, ‘enrichplot’, and
‘org.Hs.eg.db’ packages for Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) enrichment analysis and
‘ggplot2’ for bubble and histogram plots. P<0.05 is considered
to be a meaningful pathway.
Immunological characterization
We downloaded RNAseq data from the TCGA database for
PAAD. We used the TIMER algorithm for the immune scoring of B
cells, CD4+ T cells, CD8+ T cells,
neutrophils, macrophages, and myeloid dendritic cells via the
‘immunedeconv’ package. We continued to explore the expression and
infiltration of MYO1E in these cells using the ‘immunegene’ module
in the TIMER2.0 database (https://cistrome.shinyapps.io/Timer/) (16). Immunoinfiltration of
tumor-associated fibroblasts was assessed by the MCPCOUNTER and
EPIC algorithms. In addition, TISIDB (http://cis.hku.hk/TISIDB/) is a database that can
query the immune interactions of specific genes with tumors
(17), and we evaluated the
relationship between MYO1E and chemokines/receptors through the
‘chemokine’ module. Finally, we extracted the expression of CD274,
CTLA4, HAVCR2, LAG3, PDCD1, PDCD1LG2, TIGIT, and SIGLEC15 and
analyzed the expression of MYO1E and immune checkpoints by
‘pheatmap’ package. Adjusted P<0.05 was considered
significant.
Cell culture and transfection
Human normal pancreatic ductal epithelial cells
(HPDE) from Cellosaurus cell bank (https://www.cellosaurus.org/) and pancreatic
adenocarcinoma cell lines (ASPC-1, BxPC-3, MIA PaCa-2, and PANC-1)
were obtained from the Chinese Academy of Sciences (https://www.cellbank.org.cn/). HPDE, ASPC-1, and
BxPC-3 cells were cultured in RPMI1640 (Gibco) containing 10% fetal
bovine serum and 1% P/S; MIA PaCa-2 and PANC-1 cells were cultured
in DMEM under the same conditions, and all cells were cultured at
37°C in a 5% CO2 incubator. The si-MYO1E target sequence
was si-MYO1E#1 (sense 5′-CAGAAGCAACUACCUCUGAAA-3′; antisense
5′-UUUCAGAGGUAGUUGCUUCUG-3′), si-MYO1E#2 (sense
5′-CCUCAUAGAGAACAAAGUGAA-3′; antisense 5′-UUCACUUUGUUCUCUAUGAGG-3′)
(Sangon Biotech) were transfected using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific), and all steps were
performed strictly according to the instructions.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
PAAD tissues or cell lines were treated with TRIzol
(Invitrogen; Thermo Fisher Scientific) to extract total RNA. RNA
quality and concentration were determined using a NanoDrop
spectrophotometer (Thermo Fisher Scientific), and reverse
transcription was performed using the PrimeScript™ RT Reagent kit
(Takara). RT-qPCR analysis was performed using TB Green®
Premix Ex Taq TM (Takara). The MYO1E primer sequence was sense
5′-GCAGCAGTCTACCAGTTC-3′ and antisense 5′-GAGCGTCATAGGCATACAA-3′.
GAPDH (sense 5′-CCACAGTCCATGCCATCACTG-3′; antisense
5′-GTCAGGTCCACCACTGACACG-3′) was selected as the endogenous
reference. The 2−ΔΔCq method (18) was used to calculate the experimental
results.
Western blot assay
Total proteins from PAAD tissues or cell lines were
extracted using radio immunoprecipitation assay (RIPA) lysate
(Merck Millipore), and protein quantification was performed using
the BCA kit. Then, 5× loading buffer was added and boiled for 10
min at 95°C. The proteins were separated by electrophoresis using
10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and then
transferred to a 0.45 µm PVDF membrane. Five percent skim milk was
blocked at room temperature for 2 h, incubated with the
corresponding primary antibodies MYO1E, Cyclin E2, GAPDH, CDK4,
CDK2, P27, E-cadherin, vimentin, N-cadherin (all the above
antibodies were from Proteintech, China) overnight at 4°C. TBST was
washed 3 times and incubated with the corresponding species;
secondary antibodies were incubated at room temperature for 2 h,
and ECL reagent (Boster) was used for exposure imaging.
Cell proliferation, migration and
invasion assays
CCK-8 experiment: CCK-8 chromogenic solution
(GlpBio) was added to 96-well plates containing 3×103
cells per well and incubated for 2 h at 37°C, and absorbance values
at 450 nm were measured.
EDU incorporation experiment
Using the Click-iT EDU-555 kit (Servicebio), 20 µM
EDU storage solution was added and incubated for 2 h, and the
fluorescent dye iF555 was used for staining. Photographs were taken
under a fluorescence microscope (Nikon Japan).
Wound healing assay
When cell fusion in the 6-well plate reached 100%,
the cells were scratched using the tip of a 200 µl pipette, and the
wound area was recorded after 0 h, 24 h, and 48 h incubation in
serum-free medium.
Cell migration assay
In an upper chamber of a Transwell plate containing
200 µl of serum-free media (NEST Biotechnology Co.),
1×104 cells were seeded, and 800 µl of medium containing
20% fetal bovine serum was added to the bottom chamber. Migrating
cells were stained with 0.5% crystal violet. The same method was
used for cell invasion experiments, except that matrix gel was
added at a concentration of 50 mg/l in the upper chamber of the
Transwell plate (R&D Systems).
Statistical analysis
Pan-cancer comparative analysis was performed using
the Mann-Whitney U test. A paired Student's t-test was used to
compare the RT-qPCR data for the collected pancreatic
adenocarcinoma tissues and their adjacent normal tissues. For
datasets containing multiple groups, one-way ANOVA with Tukey and
least significant difference post hoc multiple comparison tests was
used. Survival analysis was performed using the Kaplan-Meier method
and log-rank test. The impact of MYO1E and clinical features of
PAAD on OS was analyzed using univariate and multifactorial
regression, and nomograms were constructed to predict the OS of
PAAD at 1, 2, and 3 years. The association between MYO1E and tumor
pathways was evaluated using Spearman's correlation coefficients.
All statistical analyses were performed using R. The statistical
threshold is P<0.05, and continuous data were reported as the
mean ± standard deviation.
Results
Expression analysis of MYO1E in
different cancers
To explore the expression of MYO1E in different
cancers, we analyzed the expression level of MYO1E in 33
malignancies using the TCGA and GTEx datasets. The findings
demonstrated that MYO1E expression kurtosis was elevated in tumor
tissues relative to normal tissues in the vast majority of
malignancies, including PAAD (Fig.
2A). Next, we used CPTAC to assess the levels of MYO1E protein
expression in each tumor. The findings indicated that MYO1E
expression was higher in lung adenocarcinoma (LUAD), glioblastoma
(GBM), PAAD, colon adenocarcinoma (COAD), breast invasive carcinoma
(BRCA), and head and neck squamous carcinoma (HNSC) than in normal
tissues (Fig. 2B). Combining the
data of each database, we found that the elevated expression of
MYO1E in PAAD was more stable; thus, we further investigated the
specific role of MYO1E in PAAD.
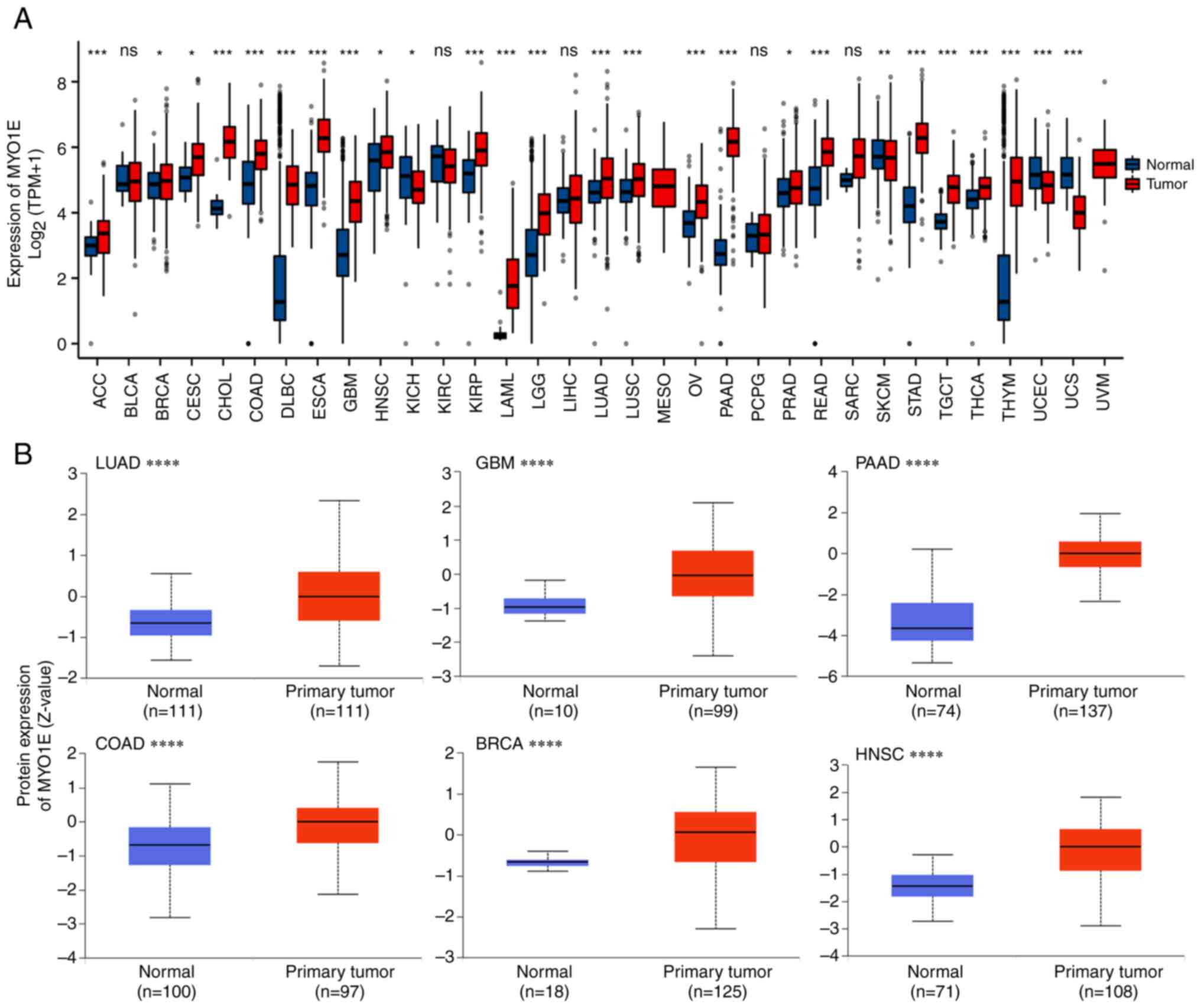 | Figure 2.Expression levels of MYO1E in
different tumor tissues and normal tissues. (A) Analysis of MYO1E
expression in 33 tumors based on TCGA (version 35) and GTEx
(version 8) data using R (version 4.2.1). In some cases,
statistical analysis could not be performed because only tumor
tissue data but not normal tissue data were provided. (B) Based on
Clinical Proteomic Tumor Analysis Consortium and the International
Cancer Proteogenome Consortium data, using UALCAN (May 13, 2022)
database analysis of MYO1E protein expression in LUAD, GBM, PAAD,
COAD, BRCA and HNSC. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001. The normal tissues shown in (A) include unpaired
healthy control tissues from TCGA and GTEx; The normal tissues in
(B) are from the Clinical Proteomic Tumor Analysis Consortium and
the International Cancer Proteogenome Consortium datasets in the
UALCAN database. BRCA, breast invasive carcinoma; COAD, colon
adenocarcinoma; GBM, glioblastoma; GTEx, Genotype-Tissue
Expression; HNSC, head and neck squamous carcinoma; LUAD, lung
adenocarcinoma; MYO1E, myosin 1E; ns, not significant; PAAD,
pancreatic adenocarcinoma; TCGA, The Cancer Genome Atlas; TPM,
transcripts per million. |
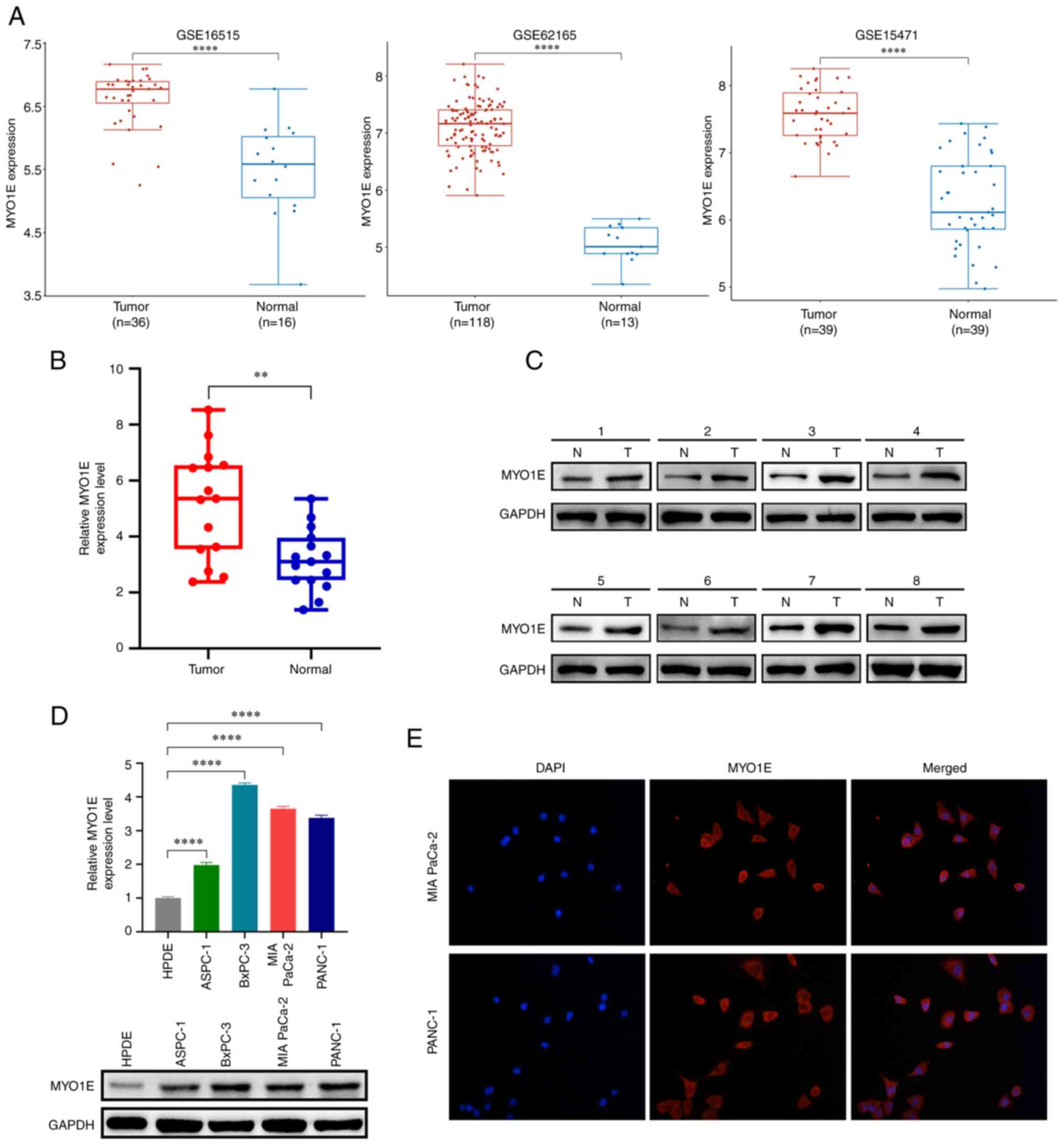
Expression of MYO1E in PAAD
Based on the above screening results, to verify the
expression of MYO1E in PAAD, we collected three datasets (GSE16515,
GES62165, and GSE15471) through the GEO database. We found that
MYO1E expression was considerably greater in PAAD tissues than in
normal tissues (Fig. 3A). We
verified this by RT-qPCR and western blotting experiments using
collected PAAD tissues and paired adjacent normal tissues. We found
that the mRNA and protein expression levels of MYO1E in PAAD
tissues were higher than those in normal tissues (Fig. 3B and C). Next, we used RT-qPCR and a
Western blot assay to measure the amount of MYO1E expression in
PAAD cell lines. The findings revealed that MYO1E was substantially
expressed in PAAD cell lines (Fig.
3D). In addition, the immunofluorescence colocalization assay
showed that MYO1E was localized in the cytoplasm (Fig. 3E).
Clinical prognostic correlation
between MYO1E and PAAD
MYO1E is abundantly expressed in PAAD tissues, so we
wanted to analyze the relationship between MYO1E and PAAD patients.
We downloaded the RNAseq data and clinical information of PAAD
patients from the TCGA database. Through univariate and
multivariate regression analyses, MYO1E might function as a
standalone prognostic factor for PAAD (Fig. 4A and B). The nomogram further
indicated that MYO1E could be used as an independent factor
affecting PAAD patients' OS and predict the prognosis at 1, 2, and
3 years (Fig. 4C and D). To clarify
the connection between MYO1E and PAAD survival prognosis, we
demonstrated by Kaplan-Meier analysis that high MYO1E expression
was inversely connected with OS and DFS in PAAD patients (Fig. 4E). In addition, the GEPIA2 database
also found that MYO1E was associated with the pathological stage of
PAAD (Fig. 4F). These data
indicated that MYO1E might have a cancer-promoting function in
PAAD.
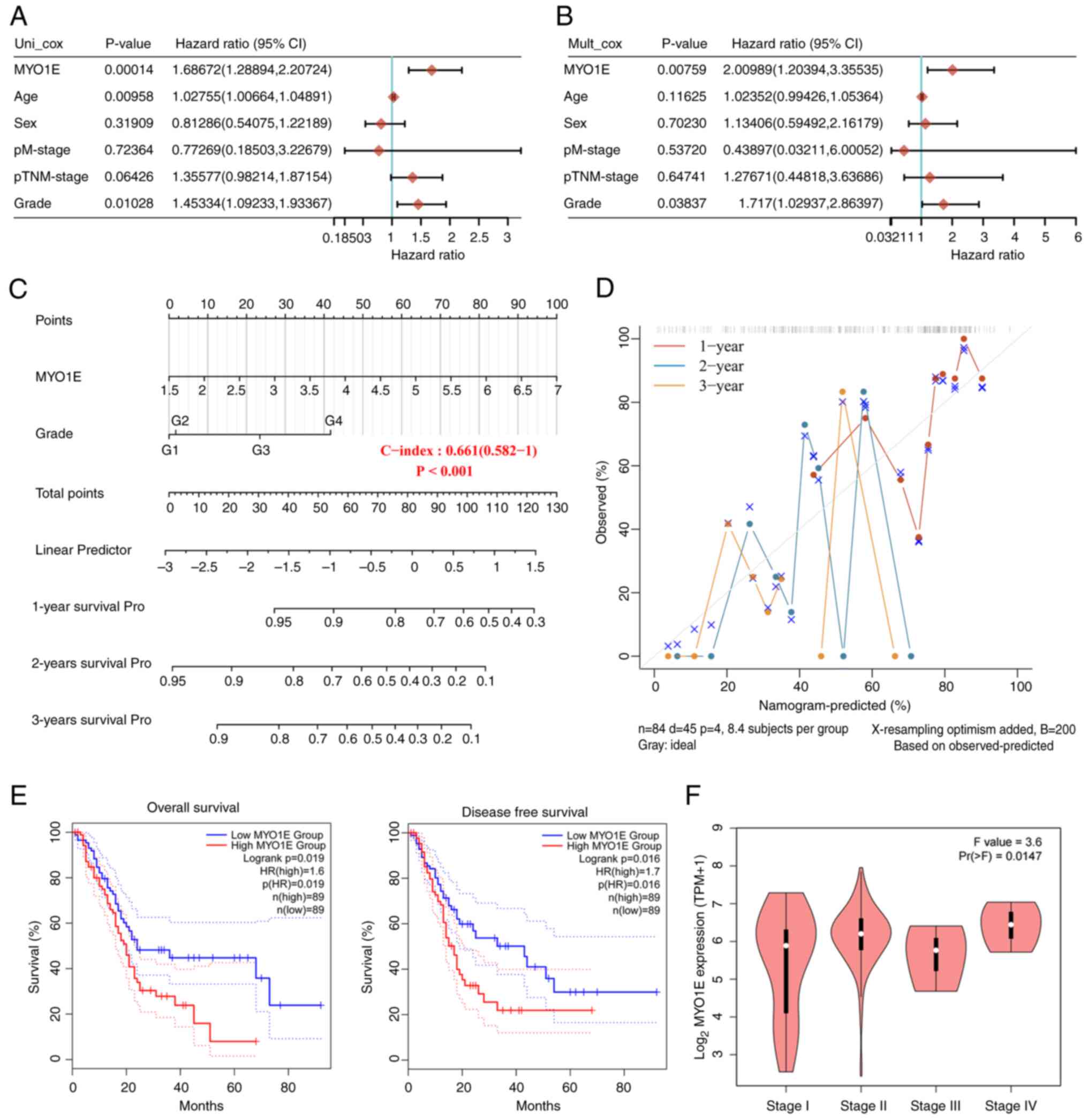 | Figure 4.Association between MYO1E expression
and pancreatic adenocarcinoma survival prognosis. (A-D) Based on
TCGA (version 35) data, the association between MYO1E and survival
and prognosis of patients with PAAD was analyzed. (A) Univariate
and (B) multifactorial regression analyses of the interaction
between MYO1E and clinical features of PAAD. (C) A nomogram of
MYO1E and grade was established to predict 1-, 2- and 3-year
survival rates of patients with PAAD. (D) OS of patients with PAAD
at 1, 2 and 3 years was predicted using calibration curves. (E)
Based on TCGA and GTEx data, the OS and disease-free survival
curves of groups of patients with PAAD based on MYO1E expression
were obtained using the GEPIA2 database. (F) GEPIA2 was used to
explore the connection between pathological stage and MYO1E
(one-way ANOVA with Tukey post hoc test). GEPIA2, Gene Expression
Profiling Interactive Analysis 2; GTEx, Genotype-Tissue Expression;
HR, hazard ratio; MYO1E, myosin 1E; OS, overall survival; PAAD,
pancreatic adenocarcinoma; TCGA, The Cancer Genome Atlas; TPM,
transcripts per million; survival Pro, survival probability; p,
pathology. |
Potential function of MYO1E in
PAAD
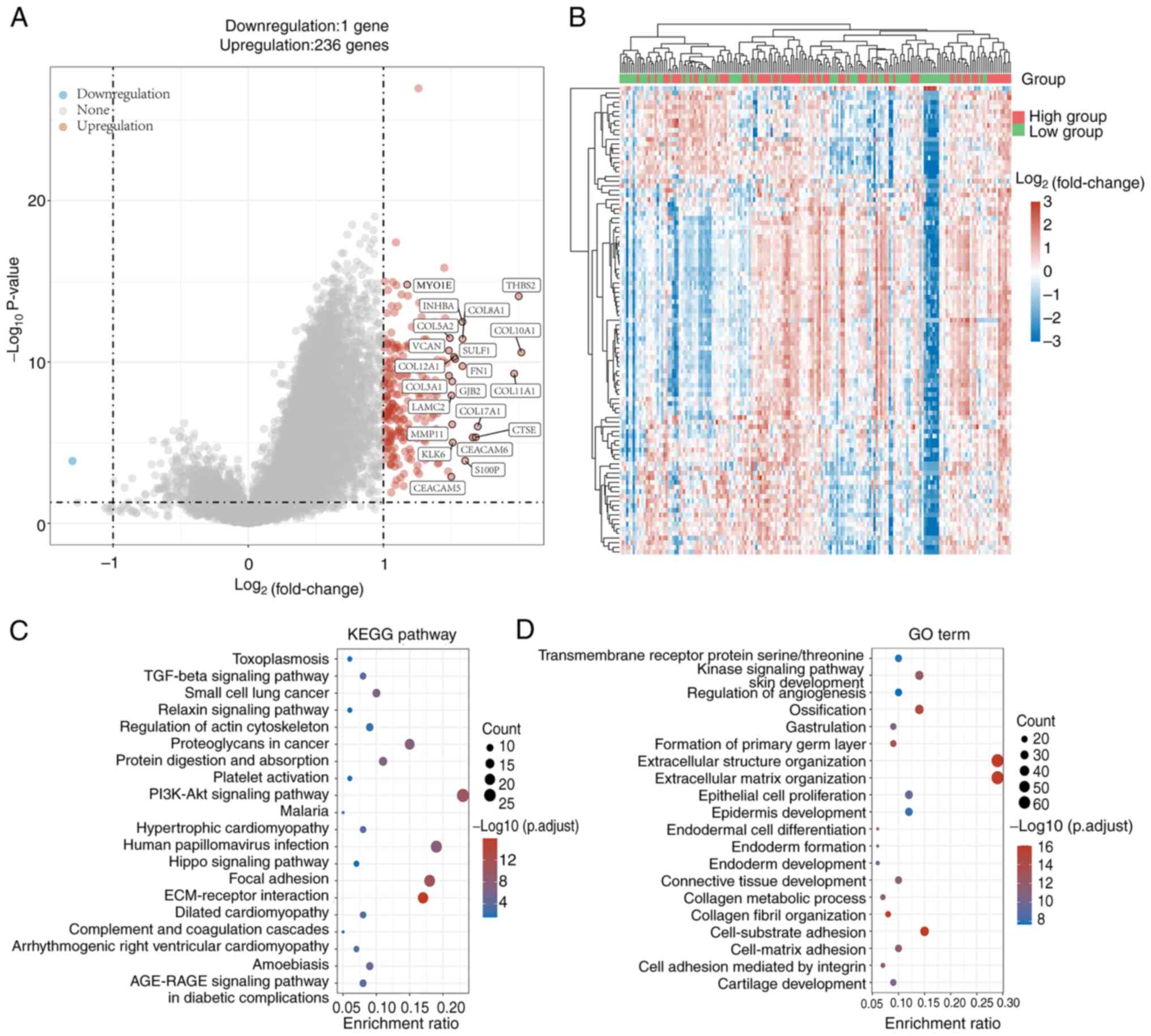
To analyze the potential biological functions of
MYO1E in PAAD, we performed enrichment analysis by DEGs. We
downloaded the RNAseq data and clinical information of PAAD
patients from TCGA database. Patients were into high and low
expression groups based on the median expression of MYO1E in PAAD.
The two groups of DEGs were compared using |log2FC|>1. We found
237 genes with differential expression, including 236 upregulated
genes and 1 downregulated gene (Fig.
5A). The heatmap (Fig. 5B)
shows the expression of these DEGs in different tissues. As there
is only one downregulated gene, it is not being analyzed. Then, we
performed KEGG and GO enrichment analyses on the upregulated DEGs.
The findings of the KEGG enrichment analysis demonstrated that
upregulated DEGs were mostly related to the PI3K-AKT signaling
pathway, ECM-receptor interaction, and proteoglycans (Fig. 5C). In addition, the GO enrichment
analysis showed that upregulated DEGs were associated with
extracellular structural organization, extracellular matrix
organization, epithelial cell proliferation, and cell-substrate
adhesion (Fig. 5D). So we analyzed
the markers related to MYO1E and cell proliferation and migration
through the TCGA database. The results showed that MYO1E was
positively correlated with CDK2, CDK4, CDK6, CCNB1 (Cyclin B1),
CCND1 (Cyclin D1), CCNE2 (Cyclin E2), FN1, SNAIL, and VIM
(Vimentin) (Fig. 6A-C). These
results indicated that MYO1E might regulate cell proliferation and
Epithelial-Mesenchymal Transition (EMT).
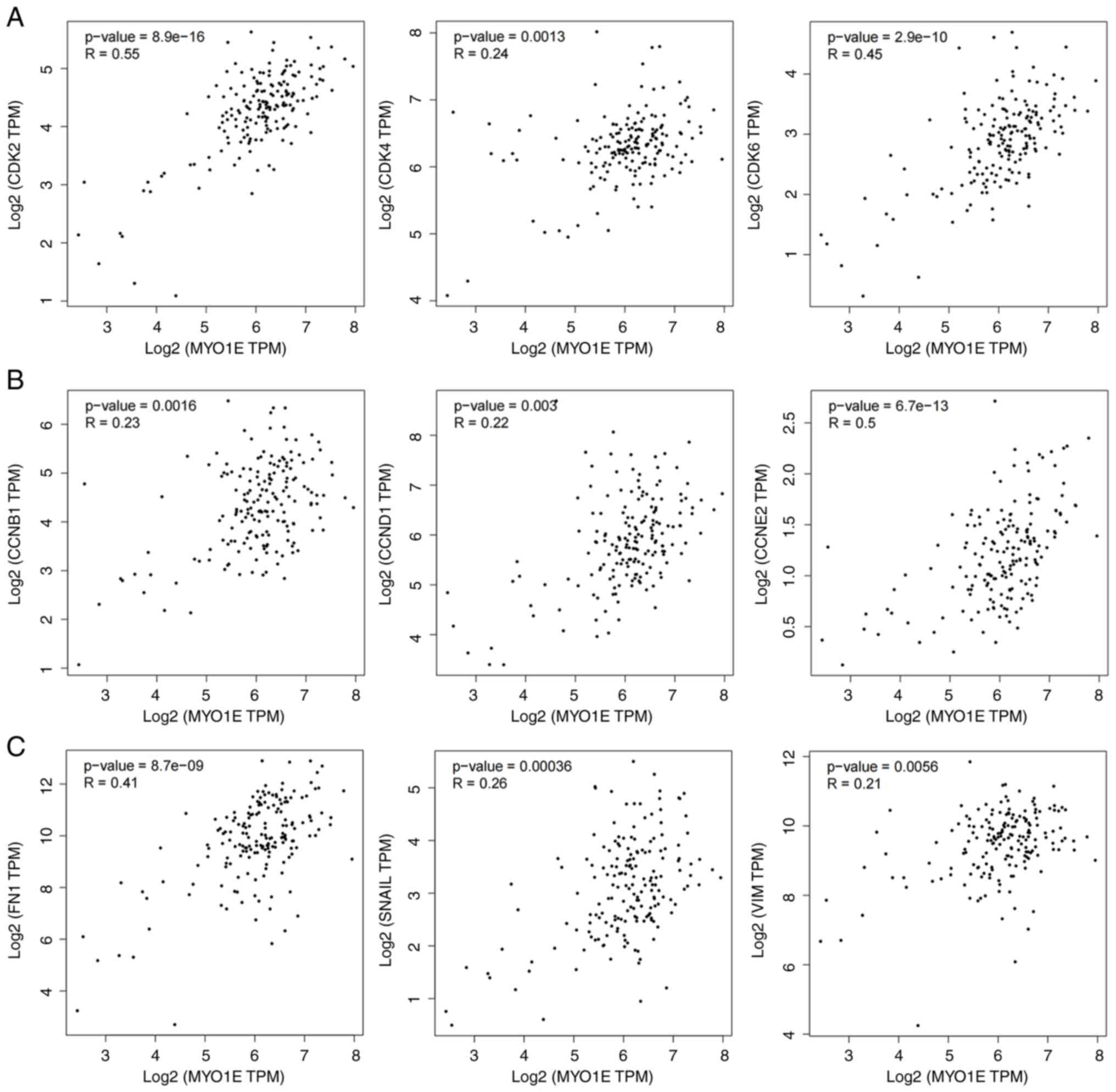 | Figure 6.Based on The Cancer Genome Atlas
data, scatter plots of MYO1E expression and cell proliferation and
EMT marker expression were obtained using the Gene Expression
Profiling Interactive Analysis 2 (version 2) database. (A)
Relationship between MYO1E and CDK2, CDK4 and CDK6. (B)
Relationship between MYO1E and CCNB1, CCND1 and CCNE2. (C)
Relationship between MYO1E and FN1, SNAIL and VIM. CCNB1, cyclin
B1; CCND1, cyclin D1; CCNE2, cyclin E2; FN1, fibronectin 1; MYO1E,
myosin 1E; TPM, transcripts per million; VIM, vimentin. |
Molecular interactions of MYO1E in
PAAD
To further investigate the intrinsic mechanism of
MYO1E gene in tumorigenesis, we screened the top 50 proteins bound
to MYO1E using the STRING database (Fig. 7A) and identified the top 100 genes
related to MYO1E expression in PAAD utilizing the GEPIA2 database.
Cross-tabulation examination of the two datasets above revealed
that ARPC5 and ARPC2 crossed each other (Fig. 7B). We performed an enrichment
analysis on both datasets. The KEGG enrichment analysis indicated
that MYO1E might participate in the Ras signaling pathway,
phosphatidylinositol signaling system, and Hippo signaling pathway
(Fig. 7C). The GO enrichment
analysis showed that MYO1E was involved in adhesion, EMT,
epithelial cell proliferation, and migration (Fig. 7D). Furthermore, we analyzed ARPC5
and ARPC2 proteins. GEPIA2 database analysis revealed that ARPC5
and ARPC2 are also highly expressed in PAAD (Fig. 7E and F). ARPC5 and ARPC2 are members
of the actin-related protein 2/3 complex (Arp2/3) (19,20)
and are involved in tumor development. Examples include multiple
myeloma (21), breast cancer
(22), and gastric cancer (23). Notably, related literature reported
the analysis of ARPC5 and ARPC2 in immunology, suggesting that
ARPC5 and ARPC2 may be crucial for tumor immunity (24–26).
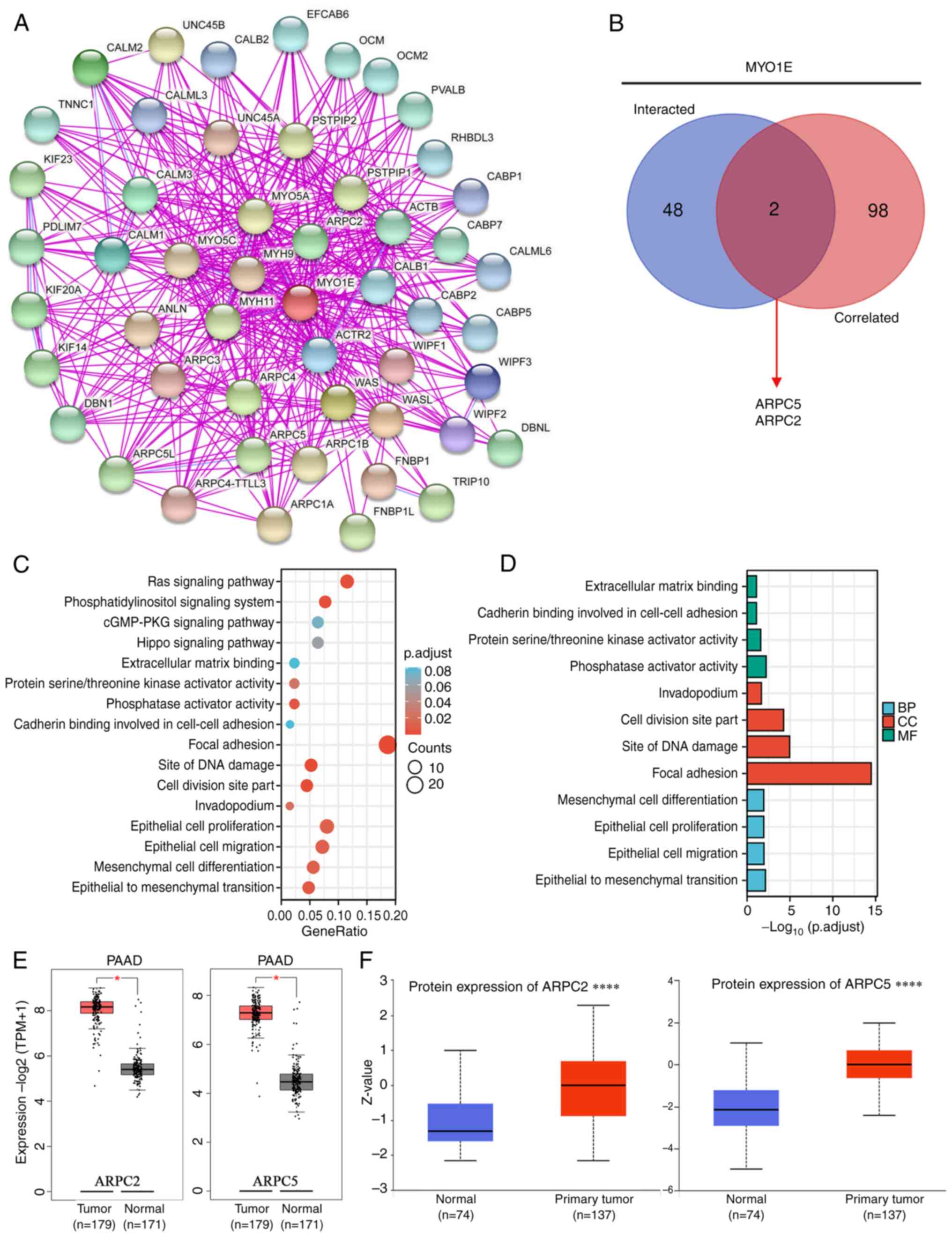 | Figure 7.Potential mechanism of MYO1E in PAAD.
(A) Top 50 genes interacting with MYO1E were obtained from the
STRING database. (B) Venn diagram of the intersection between the
top 50 genes interacting with MYO1E and the top 100 genes
correlated with MYO1E (as determined using STRING and GEPIA2,
respectively). (C) Kyoto Encyclopedia of Genes and Genomes and (D)
Gene Ontology enrichment analysis of potential biological processes
of MYO1E in PAAD. (E) Boxplots of ARPC5 and ARPC2 mRNA expression
were obtained using the GEPIA2 database. (F) Boxplots of ARPC5 and
ARPC2 protein expression were obtained using the UALCAN database
(May 13, 2022). *P<0.05, ****P<0.0001. ARPC, actin related
protein 2/3 complex subunit; BP, biological process; CC, cellular
component; GEPIA2, Gene Expression Profiling Interactive Analysis
2; MF, molecular function; MYO1E, myosin 1E; PAAD, pancreatic
adenocarcinoma; STRING, Search Tool for the Retrieval of
Interacting Genes/Proteins; TPM, transcripts per million; p.adjust,
adjusted P-value; PKG, protein kinase G; UALCAN, The University of
Alabama at Birmingham Cancer Data Analysis Portal. |
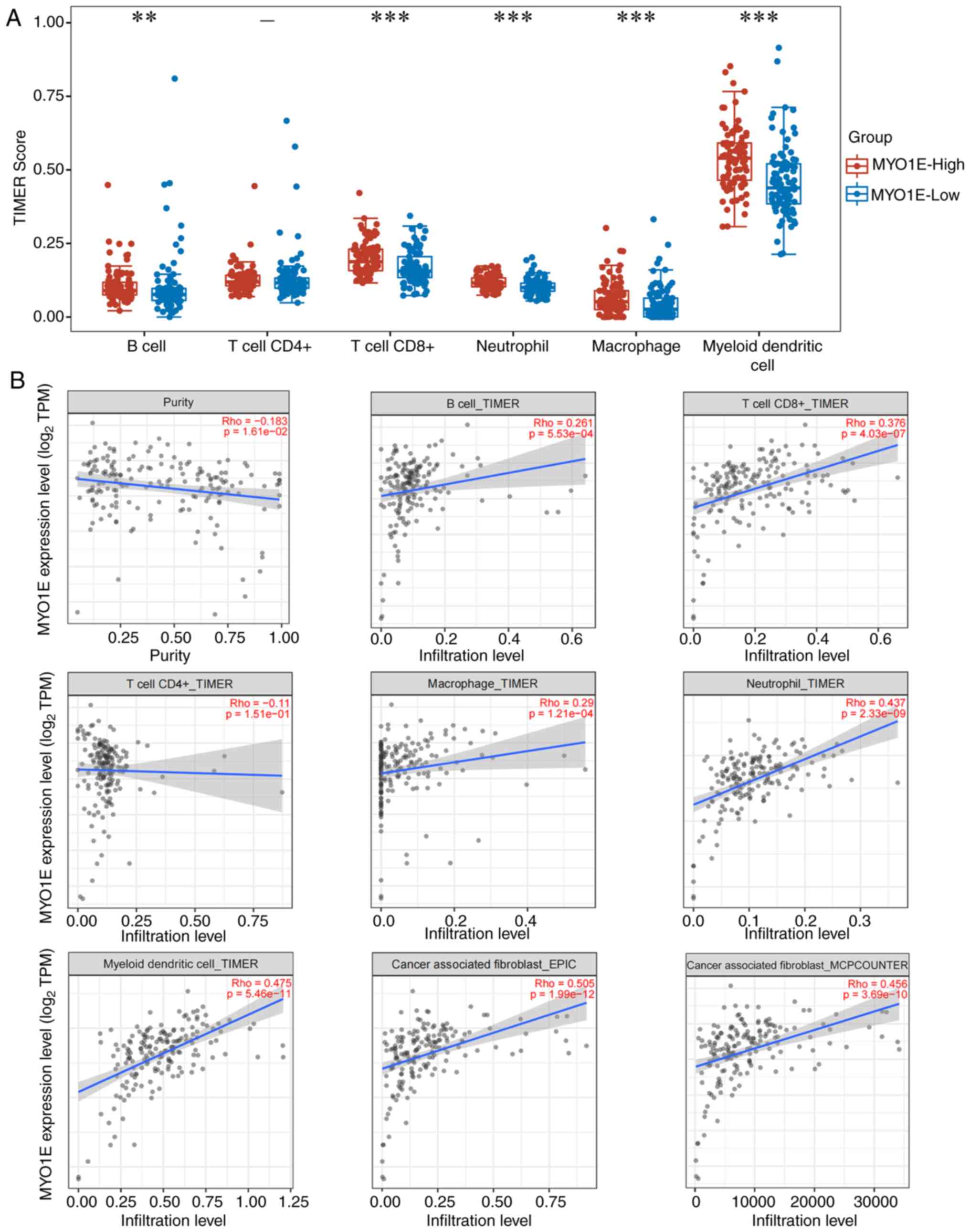
Relationship between MYO1E expression
and immune characteristics
MYO1E is a potential interacting protein of ARPC5
and ARPC2, and we next investigated MYO1E in immunological aspects.
As a major component of the TME, tumor-infiltrating immune cells
are essential for tumor growth (27,28).
Using the TIMER method, we evaluated the connection between MYO1E
and immune cell infiltration with the ‘immunedeconv’ package. We
found that MYO1E was substantially linked to higher B-cell scores,
CD8+T cells, neutrophils, macrophages, and dendritic
cells (Fig. 8A). Additional
investigation found that MYO1E expression was negatively correlated
with tumor purity (cor=−0.183, P=1.61e−02) and
positively correlated with CD8+T cells (cor=0.376,
P=4.03e-07), B cells (cor=0.261 P=5.53e-04), macrophages (cor=0.29,
P=1.21e-04), neutrophils (cor=0.437, P=2.33e-09), and dendritic
cells (cor=0.475, P=5.46e-11). At the same time, there was no
significant relationship with CD4+T. Cancer-associated
fibroblasts regulate tumor-infiltrating immune cells (29,30),
and we further confirmed whether MYO1E has a relationship with
cancer-associated fibroblasts. Using the TIMER2.0 database with
MCPCOUNTER and EPIC algorithms, we found that MYO1E was
significantly associated with cancer-associated fibroblasts (EPIC:
Rho=0.505, P=1.99e−12, MCPCOUNTER: Rho=0.456,
P=3.69e−10) (Fig. 8B).
Furthermore, chemokines can be expressed by cells, including immune
cells and stromal cells in TME (31), regulating the phenotype and function
of immune cells by modulating their localization and cellular
interactions in lymphoid tissue and TME (32). We found that MYO1E was positively
correlated with CCL7, CCL24, CXCL14, CCR1, CCR3, and CCR8 through
the TISIDB database analysis (Fig. 9A
and B), and MYO1E may be implicated in immune cell migration to
TME. The primary purpose of immune checkpoint molecules associated
with tumor cells is to mediate immune evasion and play a crucial
role in maintaining several malignant tendencies (33). Finally, we investigated the
expression of high and low MYO1E groups with immune checkpoints. We
downloaded RNAseq data and clinical information of PAAD patients
from TCGA database and used the ‘ggplot2’ package for analysis. The
results showed that CD274 (p=9.67e−08), HAVCR2
(p=1.39e−04, PDCD1LG2 (p=4.26e−08, and
SIGLEC15 (p=5.00e−02) were significantly elevated in the
high expression group of MYO1E (Fig.
9C).
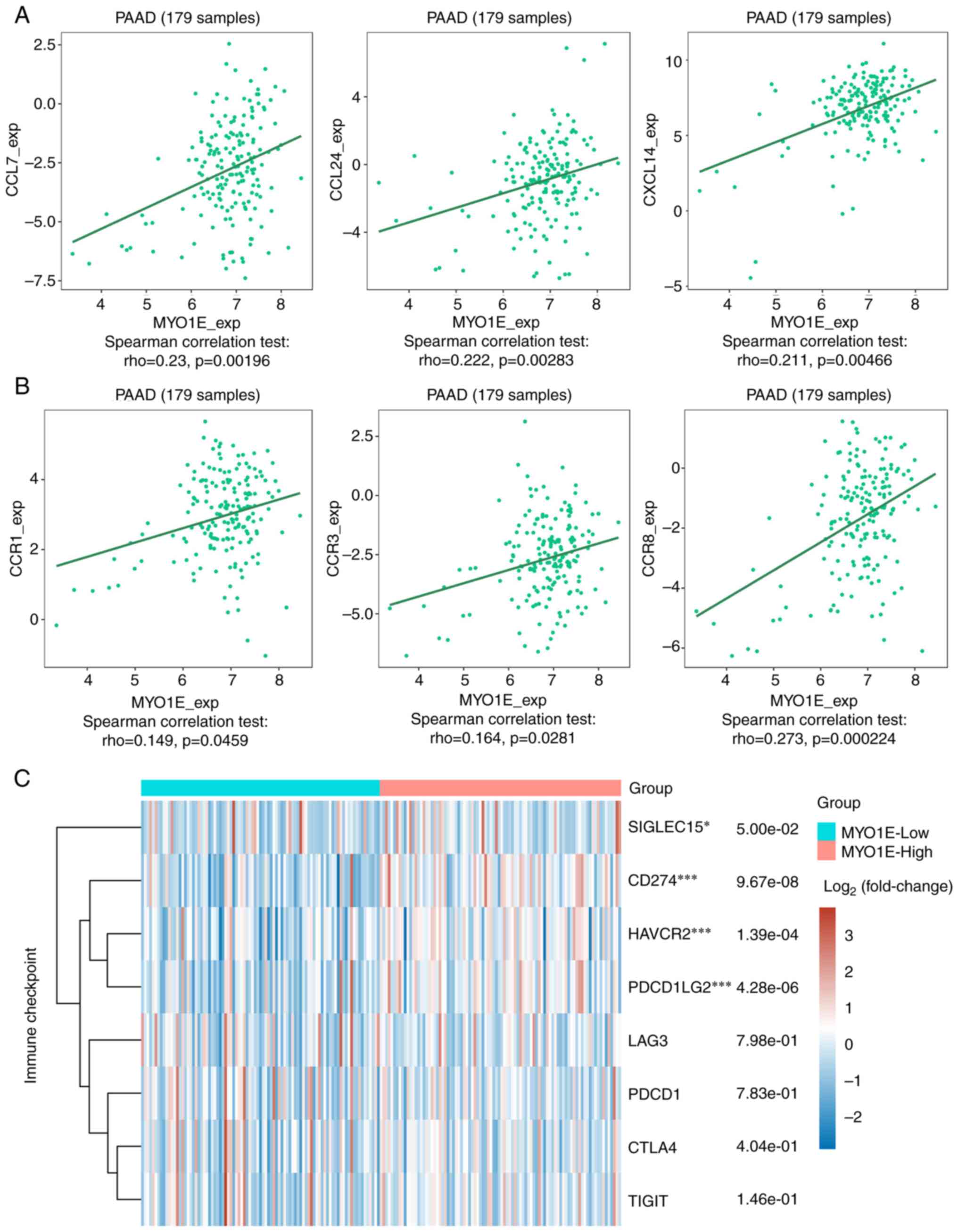 | Figure 9.Association of MYO1E expression with
chemokines/receptors and immune checkpoints. Scatter plots of MYO1E
and (A) CCL7, CCL24, CXCL14, (B) CCR1, CCR3 and CCR8 generated
using the TISIDB database. (C) Based on The Cancer Genome Atlas
data (version 35), patients were divided into MYO1E high and low
expression groups. The expression levels of CD274, CTLA4, HAVCR2,
LAG3, PDCD1, PDCD1LG2, TIGIT and SIGLEC15 in the two groups were
analyzed using the ‘ggplot2’ package, and the ‘pheatmap’ package
was used to obtain the expression heat map. *P<0.05,
***P<0.001 (high vs. low expression groups). CCL, C-C motif
chemokine ligand; CCR, C-C motif chemokine receptor; CXCL14, C-X-C
motif chemokine ligand; MYO1E, myosin 1E; PAAD, pancreatic
adenocarcinoma. |
Silencing of MYO1E inhibits
proliferation, invasion and migration of pancreatic adenocarcinoma
cells in vitro
Based on the above preliminary bioinformatics
analysis, we conducted in vitro tests to further confirm the
impact of MYO1E on PAAD cells. Through the above validation on PAAD
cell lines, we selected MIA PaCa-2 and PANC-1 for experimental
studies. We constructed MYO1E small interfering RNA (Si-NC,
Si-MYO1E#1, and Si-MYO1E#2) and performed RT-qPCR and Western
blotting for validation (Fig. 10A and
B). The CCK-8 and EDU incorporation experiment demonstrated
that MYO1E downregulation effectively suppressed the proliferation
ability of PAAD cells (Fig.
10C-E). We subsequently studied the impact of MYO1E on cyclins,
and the Western blot analysis results indicated that downregulation
of MYO1E led to reduced expression of Cyclin E2, CDK4, and CDK2 and
increased expression of P27 (Fig.
10F). A wound healing test and Transwell assay were conducted
to confirm the impact of MYO1E on the invasion and migration of
PAAD cells. The wound healing experiment revealed that MYO1E
downregulation reduced PAAD cell migration ability (Fig. 11A-D). The Transwell experiment
revealed that the number of invading and migrating PAAD cells
decreased when MYO1E was downregulated compared with the control
group (Fig. 11E-H). We validated
the EMT protein by Western blot assay. The downregulation of MYO1E
resulted in decreased expression of N-cadherin and vimentin and
increased expression of E-cadherin (Fig. 11I). These findings imply that
silencing MYO1E decreases PAAD cell proliferation, invasion, and
migration.
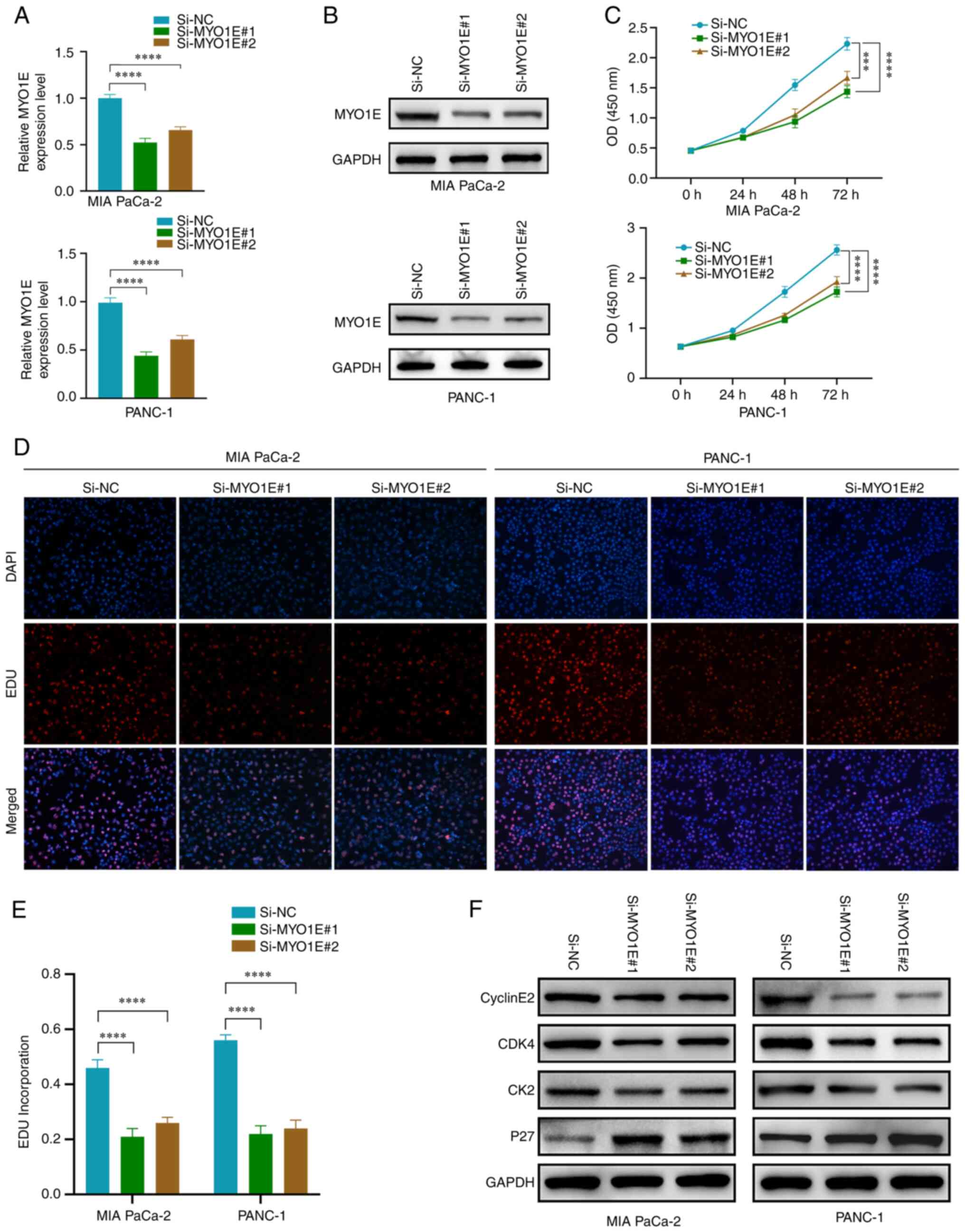 | Figure 10.MYO1E silencing reduces the
proliferation of PAAD cells. (A) Reverse transcription-quantitative
PCR and (B) western blotting were performed to verify the
transfection efficiency. (C) Cell Counting Kit-8 assays were
performed to examine the effects of MYO1E knockdown on PAAD cell
viability. (D) EDU assays were performed to examine the effects of
MYO1E knockdown on PAAD cell proliferation. Magnification, ×100.
(E) Quantitative analysis of PAAD cell proliferation rate. (F)
Expression levels of CyclinE2, CDK4, CDK2 and P27 after knockdown
of MYO1E. (A, C and E) The least significant difference post hoc
test was used. ***P<0.001, ****P<0.0001. EDU,
5-ethynyl-2-deoxyuridine; MYO1E, myosin 1E; NC, negative control;
OD, optical density; PAAD, pancreatic adenocarcinoma; Si, small
interfering RNA. |
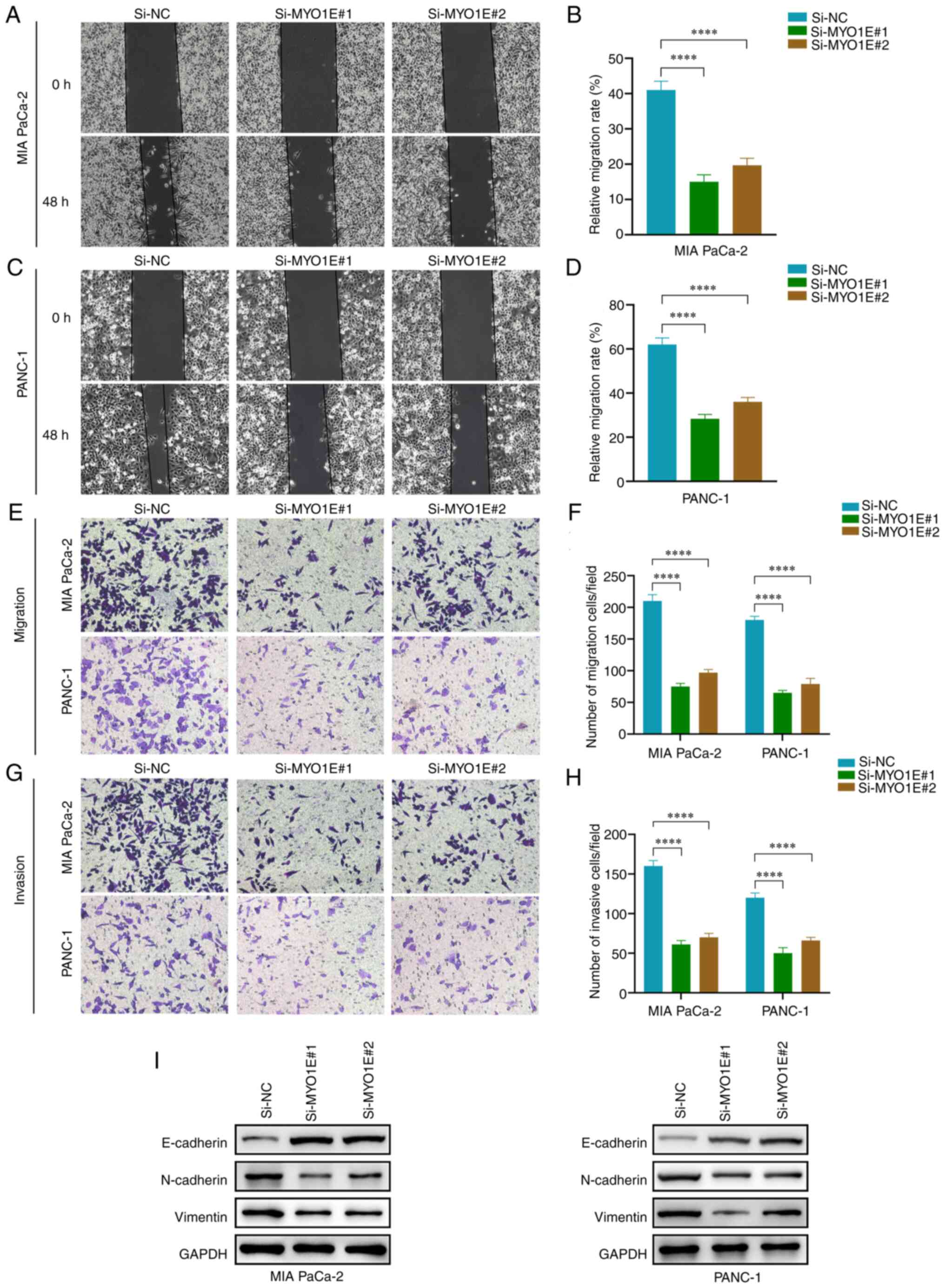 | Figure 11.Silencing MYO1E inhibits PAAD cell
invasion and migration. (A) Evaluation of MIA PaCa-2 cell migration
using a wound-healing experiment (magnification, ×100). (B)
Quantitative analysis of wound healing rate of MIA PaCa-2 cells.
The percentage of the wound healing was calculated as: (width of
wound at 0 h-width of wound at 48 h)/width of wound at 0 h. (C)
Evaluation of PANC-1 cell migration using a wound-healing
experiment (magnification, ×100). (D) Quantitative analysis of
wound healing rate of PANC-1 cells. (E) Transwell assay of PAAD
cell migration (magnification, ×100). (F) Quantitative analysis of
PAAD cell migration. (G) Transwell assay of PAAD cell invasion
(magnification, ×100). (H) Quantitative analysis of PAAD cell
invasion. (I) Epithelial-mesenchymal transition protein expression
after knockdown of MYO1E. (B, D, F and H) The least significant
difference post hoc test was used. ****P<0.0001. MYO1E, myosin
1E; NC, negative control; PAAD, pancreatic adenocarcinoma; Si,
small interfering RNA. |
Discussion
PAAD is one of the solid tumors with the worst
prognosis. American Society of Clinical estimates that there will
be approximately 57,600 new cases and 47,050 PAAD deaths in the
United States in 2020, with a mortality rate almost similar to the
incidence rate and second only to colon cancer among
gastrointestinal tumors (34,35).
Therefore, early diagnosis and treatment of PAAD are crucial, and
the search for efficient biomarkers and fresh treatment targets is
crucial.
MYO1E is a member of the class I myosins.
Localization studies have shown that MYO1E is present in regions of
high actin concentration (36) and
can bind ATP and the motor head structural domain,
calmodulin-binding neck region, and tail structural domain of
F-actin (37). MYO1E has been
reported to promote cellular endocytosis, migration, and cell
motility in various ways (38,39).
This protein's functional abnormalities are found in tumor
progression and various pathological states of renal disease
(40). Given the limited studies of
the MYO1E gene in tumors, we further examined the biological roles
and possible regulation mechanisms of MYO1E in PAAD using
bioinformatics and in vitro functional tests.
We evaluated the mRNA and protein expression levels
of MYO1E in several cancer types using TCGA, GTEx, and CPTAC
datasets and found that MYO1E was substantially expressed in many
tumors. This indicates that MYO1E may have a pro-cancer function in
the formation of tumors. The GEO databases revealed that MYO1E
expression in PAAD tissues was considerably greater than in normal
tissues. We used RT-qPCR and Western blot for validation, and the
results were consistent with bioinformatics analysis. In addition,
Kaplan-Meier evaluated the predictive significance of MYO1E in
PAAD. We found that MYO1E was closely associated with poor OS and
poor DFS in PAAD, and MYO1E may function as a PAAD oncogene and
prognostic biomarker. We subjected 236 upregulated DEGs to KEGG and
GO enrichment analysis and found that upregulated DEGs were linked
in the PI3K-Akt-mTOR signaling pathway, ECM-receptor interaction,
proteoglycan, and cell-substrate adhesion. We further analyzed
MYO1E's potential value in PAAD. We investigated the potential
binding protein of MYO1E in PAAD through the PPI protein
interaction network. The KEGG and GO enrichment analyses revealed
that MYO1E and its interacting proteins were mainly associated with
the Ras signaling pathway, phosphatidylinositol signaling system,
Hippo signaling pathway, adhesion class, EMT, epithelial cell
proliferation, and migration. In addition, we identified two genes,
ARPC5 and ARPC2, by cross-tabulation analysis of the dataset. The
literature review found that ARPC2 and ARPC5 were involved in
migrating invasive tumors (41,42),
suggesting that MYO1E may promote PAAD progression through related
synergistic effects.
Currently, surgery remains the only radical
treatment for patients with PAAD. However, without additional
treatment, more than 90% of patients will recur after surgery
(43). Immunotherapy, alongside
surgery, radiation, and chemotherapy, has been recognized as the
fourth pillar of cancer treatment (44). Therefore, we analyzed and studied
tumor-infiltrating immune cells, tumor-associated fibroblasts,
chemokines, and immune checkpoints in TME. We evaluated the MYO1E
score with immune infiltrating cells using the TIMER method and
found that high MYO1E expression was positively connected with B
cells, CD8+ T cells, neutrophils, macrophages, and
dendritic cells in PAAD. Using the MCPCOUNTER and EPIC algorithms,
we found that MYO1E is closely related to tumor-associated
fibroblasts. Chemokines are crucial for immune cell migration;
thus, we analyzed them using by TISIDB database and found that
MYO1E was closely associated with CCL7, CCL24, and CXCL14. MYO1E
may be involved in migrating immune cells in the TME. Next, we
evaluated immune checkpoints and immune checkpoint inhibitors
(ICIS), a class of immunotherapies that modulate tumor immune
tolerance by blocking specific inhibitory receptor-ligand
interactions on the surface of immune cells (45). However, this study was not performed
to experimentally validate the role of MYO1E in tumor immunology,
which is a limitation of this paper. In the future, we would like
to validate the effect of MYO1E on immune cells by flow cytometry
and immunohistochemistry experiments. These experiments can further
validate the role of MYO1E in tumor immunology. Taken together,
MYO1E is implicated in tumor immune modulation and may provide a
novel PAAD immunotherapy method. To verify in vitro the
impact of MYO1E on PAAD cells, we found that suppressing MYO1E
decreased the proliferation, invasion, and migration of PAAD cells
using CCK-8, EDU, wound healing, Transwell, and Western blot
assays.
In this work, we initially revealed the potential
functions and possible mechanisms of MYO1E in PAAD through a
comprehensive analysis of bioinformatics and in vitro
Assays, but there are still some shortcomings of this study. First,
the MYO1E analysis was derived from tumor databases, and errors
existed between databases. Second, although we performed functional
in vitro trials, in vivo experimental confirmation is
lacking. Finally, clinical data were not assessed as there was a
small clinical sample of PAAD in the database. We will do more
research to determine the mechanism of action of MYO1E in PAAD.
In conclusion, we performed a bioinformatics-based
investigation of the expression, prognosis, and potential pathways
of MYO1E in PAAD. We found that MYO1E may regulate the
proliferation and migration of PAAD cells and participate in tumor
immunology. In vitro experiments showed that silencing MYO1E
inhibits PAAD cellular proliferation, invasion, and migration.
MYO1E may function as a biomarker for PAAD and provide a new
strategy for diagnosing and treating PAAD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (NSFC; grant nos. 81960431 and 81960535), and
Hospital-level Science and Technology Plan Project of Guizhou
Cancer Hospital (grant no. YJ2019016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL, XW and YP were responsible for the conception
and design of the experiments. PL, XF, CZ and JH contributed
substantially to data acquisition, analysis and interpretation. SL
wrote the manuscript, XW participated in the drafting of the
manuscript and critically revised important intellectual content.
YP and XW approved the final version of the manuscript. SL and YP
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
Affiliated Hospital of Guizhou Medical University and the
Affiliated Cancer Hospital of Guizhou Medical University (approval
no. 2022-138; Guiyang, China). The patients provided written
informed consent for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao H, Xie R, Huang R, Wang C, Wang Y,
Wang D, Liu K, Yang C, Yang Q and Chen L: CIRBP regulates
pancreatic cancer cell ferroptosis and growth by directly binding
to p53. J Immunol Res. 2022:25272102022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garg SK and Chari ST: Early detection of
pancreatic cancer. Curr Opin Gastroenterol. 36:456–461. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stanciu S, Ionita-Radu F, Stefani C,
Miricescu D, Stanescu S II, Greabu M, Ripszky Totan A and Jinga M:
Targeting PI3K/AKT/mTOR signaling pathway in pancreatic cancer:
From molecular to clinical aspects. Int J Mol Sci. 23:101322022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tonini V and Zanni M: Pancreatic cancer in
2021: What you need to know to win. World J Gastroenterol.
27:5851–589. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu JX, Zhao CF, Chen WB, Liu QC, Li QW,
Lin YY and Gao F: Pancreatic cancer: A review of epidemiology,
trend, and risk factors. World J Gastroenterol. 27:4298–321. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krendel M, Kim SV, Willinger T, Wang T,
Kashgarian M, Flavell RA and Mooseker MS: Disruption of myosin 1e
promotes podocyte injury. J Am Soc Nephrol. 20:86–94. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen H, Bao Y, Feng C, Fu H and Mao J:
Overexpression of Myo1e promotes albumin endocytosis by mouse
glomerular podocytes mediated by Dynamin. PeerJ. 8:e85992020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krendel M, Osterweil EK and Mooseker MS:
Myosin 1E interacts with synaptojanin-1 and dynamin and is involved
in endocytosis. FEBS Lett. 581:644–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng J, Grassart A and Drubin DG: Myosin
1E coordinates actin assembly and cargo trafficking during
clathrin-mediated endocytosis. Mol Biol Cell. 23:2891–2904. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feeser EA, Ignacio CM, Krendel M and Ostap
EM: Myo1e binds anionic phospholipids with high affinity.
Biochemistry. 49:9353–9360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ouderkirk-Pecone JL, Goreczny GJ, Chase
SE, Tatum AH, Turner CE and Krendel M: Myosin 1e promotes breast
cancer malignancy by enhancing tumor cell proliferation and
stimulating tumor cell de-differentiation. Oncotarget.
7:46419–46432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chandran UR, Medvedeva OP, Barmada MM,
Blood PD, Chakka A, Luthra S, Ferreira A, Wong KF, Lee AV, Zhang Z,
et al: TCGA Expedition: A Data Acquisition and Management System
for TCGA Data. PLoS One. 11:e01653952016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
GTEx Consortium: The Genotype-tissue
expression (GTEx) project. Nat Genet. 45:580–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47:W556–W560. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bardou P, Mariette J, Escudie F, Djemiel C
and Klopp C: Jvenn: An interactive Venn diagram viewer. BMC
Bioinformatics. 15:2932014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res. 48:W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ru B, Wong CN, Tong Y, Zhong JY, Zhong
SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, et al: TISIDB: An
integrated repository portal for tumor-immune system interactions.
Bioinformatics. 35:4200–4202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lui JW, Moore SPG, Huang L, Ogomori K, Li
Y and Lang D: YAP facilitates melanoma migration through regulation
of actin-related protein 2/3 complex subunit 5 (ARPC5). Pigment
Cell Melanoma Res. 35:52–65. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mei P, Tey SK, Wong SWK, Ng TH, Mao X,
Yeung CLS, Xu Y, Yu L, Huang Q, Cao P, et al: Actin-related protein
2/3 complex subunit 2-enriched extracellular vesicles drive liver
cancer metastasis. Hepatol Int. 16:603–613. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kinoshita T, Nohata N, Watanabe-Takano H,
Yoshino H, Hidaka H, Fujimura L, Fuse M, Yamasaki T, Enokida H,
Nakagawa M, et al: Actin-related protein 2/3 complex subunit 5
(ARPC5) contributes to cell migration and invasion and is directly
regulated by tumor-suppressive microRNA-133a in head and neck
squamous cell carcinoma. Int J Oncol. 40:1770–1778. 2012.PubMed/NCBI
|
|
22
|
Cheng Z, Wei W, Wu Z, Wang J, Ding X,
Sheng Y, Han Y and Wu Q: ARPC2 promotes breast cancer proliferation
and metastasis. Oncol Rep. 41:3189–3200. 2019.PubMed/NCBI
|
|
23
|
Zhang J, Liu Y, Yu CJ, Dai F, Xiong J, Li
HJ, Wu ZS, Ding R and Wang H: Role of ARPC2 in human gastric
cancer. Mediators Inflamm. 2017:54328182017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang S, Li D, Zhuang L, Sun L and Wu J:
Identification of Arp2/3 complex subunits as prognostic biomarkers
for hepatocellular carcinoma. Front Mol Biosci. 8:6901512021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang S, Sun L, Hou P, Liu K and Wu J: A
comprehensively prognostic and immunological analysis of
actin-related protein 2/3 complex subunit 5 in pan-cancer and
identification in hepatocellular carcinoma. Front Immunol.
13:9448982022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang S, Dong C, Li D, Xu Y and Wu J:
ARPC2: A Pan-Cancer prognostic and immunological biomarker that
promotes hepatocellular carcinoma cell proliferation and invasion.
Front Cell Dev Biol. 10:8960802022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He Z, Gu J, Luan T, Li H, Li C, Chen Z,
Luo E, Wang J, Huang Y and Ding M: Comprehensive analyses of a
tumor-infiltrating lymphocytes-related gene signature regarding the
prognosis and immunologic features for immunotherapy in bladder
cancer on the basis of WGCNA. Front Immunol. 13:9739742022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang D, He W, Wu C, Tan Y, He Y, Xu B,
Chen L, Li Q and Jiang J: Scoring system for tumor-infiltrating
lymphocytes and its prognostic value for gastric cancer. Front
Immunol. 10:712019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barrett RL and Pure E: Cancer-associated
fibroblasts and their influence on tumor immunity and
immunotherapy. Elife. 9:e572432020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagarsheth N, Wicha MS and Zou W:
Chemokines in the cancer microenvironment and their relevance in
cancer immunotherapy. Nat Rev Immunol. 17:559–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozga AJ, Chow MT and Luster AD: Chemokines
and the immune response to cancer. Immunity. 54:859–874. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y and Zheng J: Functions of immune
checkpoint molecules beyond immune evasion. Adv Exp Med Biol.
1248:201–226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tonini V and Zanni M: Early diagnosis of
pancreatic cancer: What strategies to avoid a foretold catastrophe.
World J Gastroenterol. 28:4235–4248. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El Mezgueldi M, Tang N, Rosenfeld SS and
Ostap EM: The kinetic mechanism of Myo1e (human myosin-IC). J Biol
Chem. 277:21514–21521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mele C, Iatropoulos P, Donadelli R,
Calabria A, Maranta R, Cassis P, Buelli S, Tomasoni S, Piras R,
Krendel M, et al: MYO1E mutations and childhood familial focal
segmental glomerulosclerosis. N Engl J Med. 365:295–306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin X, Wang W, Mao J, Shen H, Fu H, Wang
X, Gu W, Liu A, Yu H, Shu Q and Du L: Overexpression of Myo1e in
mouse podocytes enhances cellular endocytosis, migration, and
adhesion. J Cell Biochem. 115:410–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanimura S, Hashizume J, Arichika N,
Watanabe K, Ohyama K, Takeda K and Kohno M: ERK signaling promotes
cell motility by inducing the localization of myosin 1E to
lamellipodial tips. J Cell Biol. 214:475–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Navines-Ferrer A and Martin M: Long-tailed
unconventional class I myosins in health and disease. Int J Mol
Sci. 21:25552020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoon YJ, Han YM, Choi J, Lee YJ, Yun J,
Lee SK, Lee CW, Kang JS, Chi SW, Moon JH, et al: Benproperine, an
ARPC2 inhibitor, suppresses cancer cell migration and tumor
metastasis. Biochem Pharmacol. 163:46–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kinoshita T, Nohata N, Watanabe-Takano H,
Yoshino H, Hidaka H, Fujimura L, Fuse M, Yamasaki T, Enokida H,
Nakagawa M, et al: Actin-related protein 2/3 complex subunit 5
(ARPC5) contributes to cell migration and invasion and is directly
regulated by tumor-suppressive microRNA-133a in head and neck
squamous cell carcinoma. Int J Oncol. 40:1770–1778. 2012.PubMed/NCBI
|
|
43
|
Zhu H, Li T, Du Y and Li M: Pancreatic
cancer: Challenges and opportunities. BMC Med. 16:2142018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sunami Y and Kleeff J: Immunotherapy of
pancreatic cancer. Prog Mol Biol Transl Sci. 164:189–216. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jagodinsky JC, Bates AM, Clark PA,
Sriramaneni RN, Havighurst TC, Chakravarty I, Nystuen EJ, Kim K,
Sondel PM, Jin WJ and Morris ZS: Local TLR4 stimulation augments in
situ vaccination induced via local radiation and anti-CTLA-4
checkpoint blockade through induction of CD8 T-cell independent Th1
polarization. J Immunother Cancer. 10:e0051032022. View Article : Google Scholar : PubMed/NCBI
|