Introduction
According to the GLOBOCAN 2020 estimates of cancer
incidence and mortality produced by the International Agency for
Research on Cancer, there were 19.3 million new cancer cases and
~10.0 million cancer-associated deaths worldwide in 2020 (1). In addition, the incidence of new cases
of cancer in 2020 was 10.1 million in males and 9.2 million in
females, and that of cancer-associated death was 5.5 million in
males and 4.4 million in females (1). Therefore, one in two patients with
cancer globally will die of cancer. In particular, gastric cancer
is the third leading cause of cancer-related death, accounting for
>1 million patients newly diagnosed with gastric cancer
worldwide each year (2). The 5-year
survival rate for patients with gastric cancer is <40%; gastric
cancer has long been regarded as an aggressive malignancy (3). Furthermore, according to Ye et
al (4), unresectable cases of
gastric cancer account for 10% of the total number of cases in
China. The median survival time is 5–12 months and the 5-year
survival rate is ~9.4%.
Due to its poor prognosis and the advanced stage at
which most cases are diagnosed, gastric cancer is a disease in
which mortality accounts for ~70% of its incidence (1). Survival rates for gastric cancer have
increased due to improved treatment strategies during the past
decade; gastric cancer is by no means incurable. Early gastric
cancer is difficult to diagnose immediately due to latent and
nonspecific clinical symptoms. In general, numerous examinations,
such as endoscopic examination, computed tomography (CT), magnetic
resonance imaging (MRI), endoscopic ultrasound (EUS), positron
emission tomography, explorative laparoscopy and cytological
examination are required to make a definitive diagnosis. Various
examinations are useful for the early detection and diagnostic
differentiation of gastric cancer and may improve patient
prognosis. Indeed, detection of gastric cancer at the early stage
substantially improves the 5-year disease-specific survival rate to
99.3% for mucosal cancer and 97.2% for submucosal cancer,
suggesting that detection of early gastric cancer may result in a
good prognosis (5); however, valid
screening procedures for early gastric cancer are lacking, even in
high-incidence areas (Asia, Russia and South America).
The poor prognosis of gastric cancer is due to the
non-specific symptoms and lack of reliable early-stage biomarkers.
The most effective solution to improve the prognosis of gastric
cancer is early detection and diagnosis; the prognosis of early
gastric cancer is significantly more favorable than that of gastric
cancer discovered in the late stages. In addition, the requirement
for additional examinations for the definitive diagnosis of early
gastric cancer may become a barrier to early detection. Endoscopic
mucosal resection is a surgical method that may completely resect
early gastric cancer that is limited to the mucosa (6). Therefore, effective identification of
T1a stage cancer is crucial in the planning of endoscopic resection
of early gastric cancer. Thus, there is an urgent requirement for
new diagnostic imaging strategies to detect early gastric cancer.
In the present study, the CT diagnosis of T1a gastric cancers
potentially caused by gastric morphological abnormalities
(double-track sign) was retrospectively investigated.
Materials and methods
Study design and patient
population
The present retrospective study was approved by the
institutional review board of the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China). The requirement for
written informed consent was waived. The T1a stage was determined
according to the American Joint Committee on Cancer (AJCC, 8th
edition) (7): Tumor invasion of the
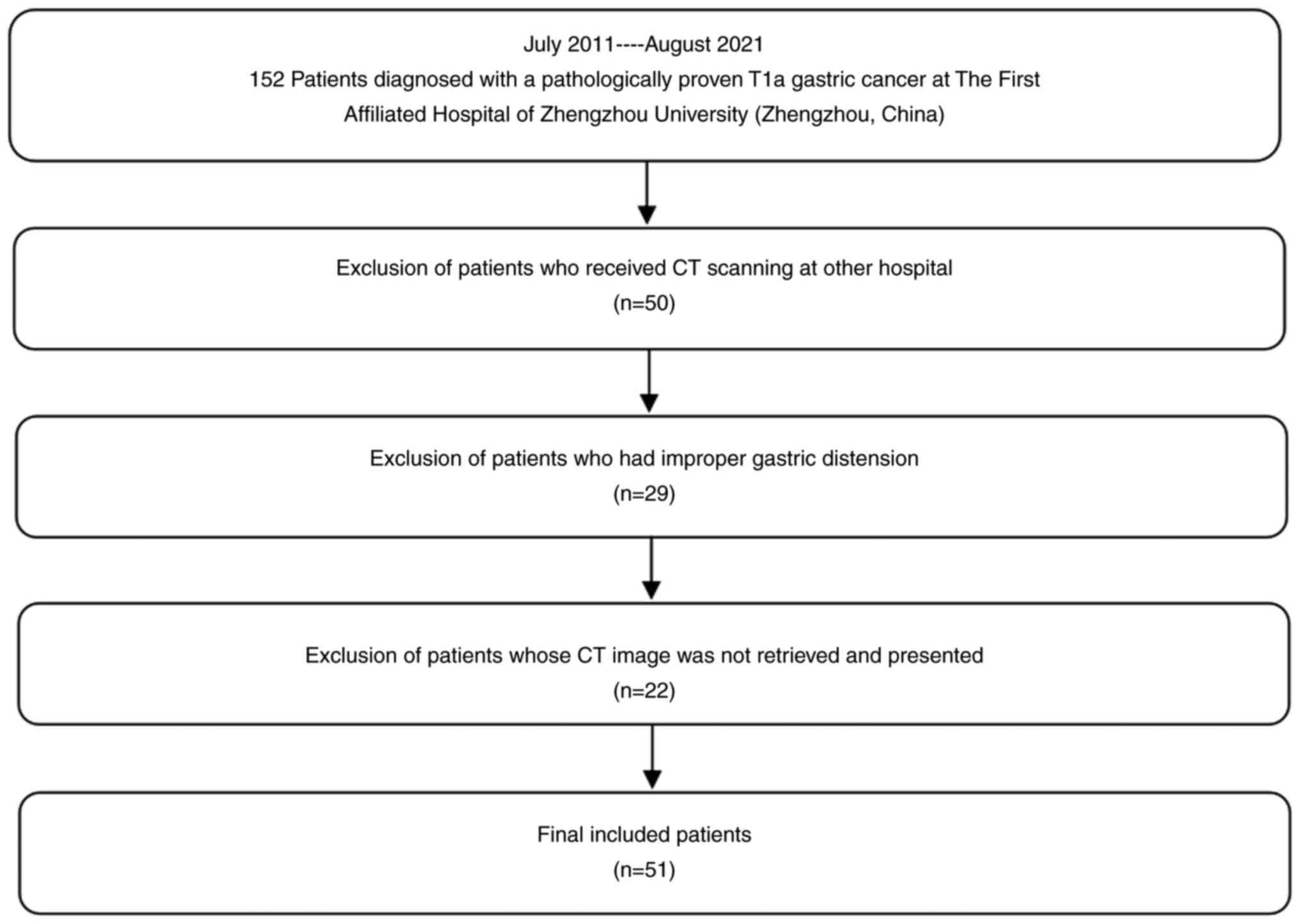
lamina propria or muscularis mucosae is considered T1a (7). A total of 152 patients diagnosed with
pathologically proven T1a gastric cancer at the First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China) between July
2011 and August 2021 were retrospectively reviewed. Surgical (n=20)
or endoscopic mucosal resection (n=132) was performed within one
week of CT image acquisition. The inclusion criteria were as
follows: i) Diagnosis of early gastric cancer (T1a) based on
postoperative pathology, ii) surgical or endoscopic mucosal
dissection, and iii) T1a gastric cancer as the only primary tumor.
A total of 50 patients were excluded because they underwent CT at
another hospital. Another 29 patients were excluded because of
improper gastric distension, which resulted in images being
inadequate for evaluation. A further 22 patients were excluded
because their CT images could not be retrieved or presented.
Ultimately, 51 patients were included in the present study. The
control group consisted of 2,926 patients with gastritis who had
undergone endoscopic examination at the First Affiliated Hospital
of Zhengzhou University (Zhengzhou, China) between July 2011 and
August 2021. Subjects were retrospectively selected according to
the following inclusion criteria: i) Diagnosis of gastritis based
on pathology, ii) newly diagnosed patients without any therapy, and
iii) CT examination acquired within 2 weeks before endoscopic
examination. A flowchart of the study is presented in Fig. 1.
CT protocol
A 600–1,000 ml oral dose of water was used to dilate
the gastric cavity immediately before CT examination. CT
examinations were performed using a 64 multidetector CT scanner
(Discovery CT750HD; GE Healthcare). A conventional axial scan (120
kV; 350 mA; field of view, 500 mm; matrix, 512×512; section
thickness, 0.75 mm) was performed before and after intravenous
injection of nonionic iohexol (iopromide; 370 mg/ml; GE Medical
Systems; 1.5 ml/kg and 3 ml/sec) using a dual-head pump injector
(Medrad®; Bayer AG). Finally, 20 ml of saline flush was
injected at a rate of 3 ml/sec. Contrast-enhanced CT scans were
performed with scanning delays of 30 sec (arterial phase) and 70
sec (portal venous phase) after the start of intravenous (i.v.)
injection of iopromide. The CT dose index volume for all three
phases was 15 mSv.
Statistical analysis
All data were analyzed using Excel spreadsheet
(version no. 2302; build 1601613020332; Microsoft Corporation).
Statistical analyses were performed by SPSS software version 21.0
(IBM Corporation). The chi-square test or Mann-Whitney U-test was
used for comparison between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Population characteristics
Detailed clinical characteristics of the included
patients are presented in Table I.
The patients of T1a gastric cancer consisted of 40 males and 11
females (age range, 32–86 years; mean age, 63.19 years). The
control subjects consisted of 1,609 males and 1,317 females (age
range, 5–90 years; mean age, 55.49 years). The proportion of males
among patients with T1a gastric cancer was higher than that of the
control subjects and the difference was statistically significant
(Z=12.072, P=0.001). The age distribution in patients with T1a
gastric cancer was different from that of the control subjects and
the difference was statistically significant (Z=4.644,
P<0.001).
 | Table I.Clinicopathological features of T1a
gastric cancer patients in the present study (n=51). |
Table I.
Clinicopathological features of T1a
gastric cancer patients in the present study (n=51).
| Feature | N |
|---|
| Age, years |
|
| ≤63 | 24 |
|
>63 | 27 |
| Gender |
|
| Male | 40 |
|
Female | 11 |
| Involved segment |
|
| Upper
1/3 | 23 |
| Middle
1/3 | 7 |
| Lower
1/3 | 21 |
| Type of
histology |
|
|
Well-differentiated | 6 |
|
Moderately differentiated | 39 |
| Poorly
differentiated | 6 |
A representative case and
classification by specific abnormality of the stomach
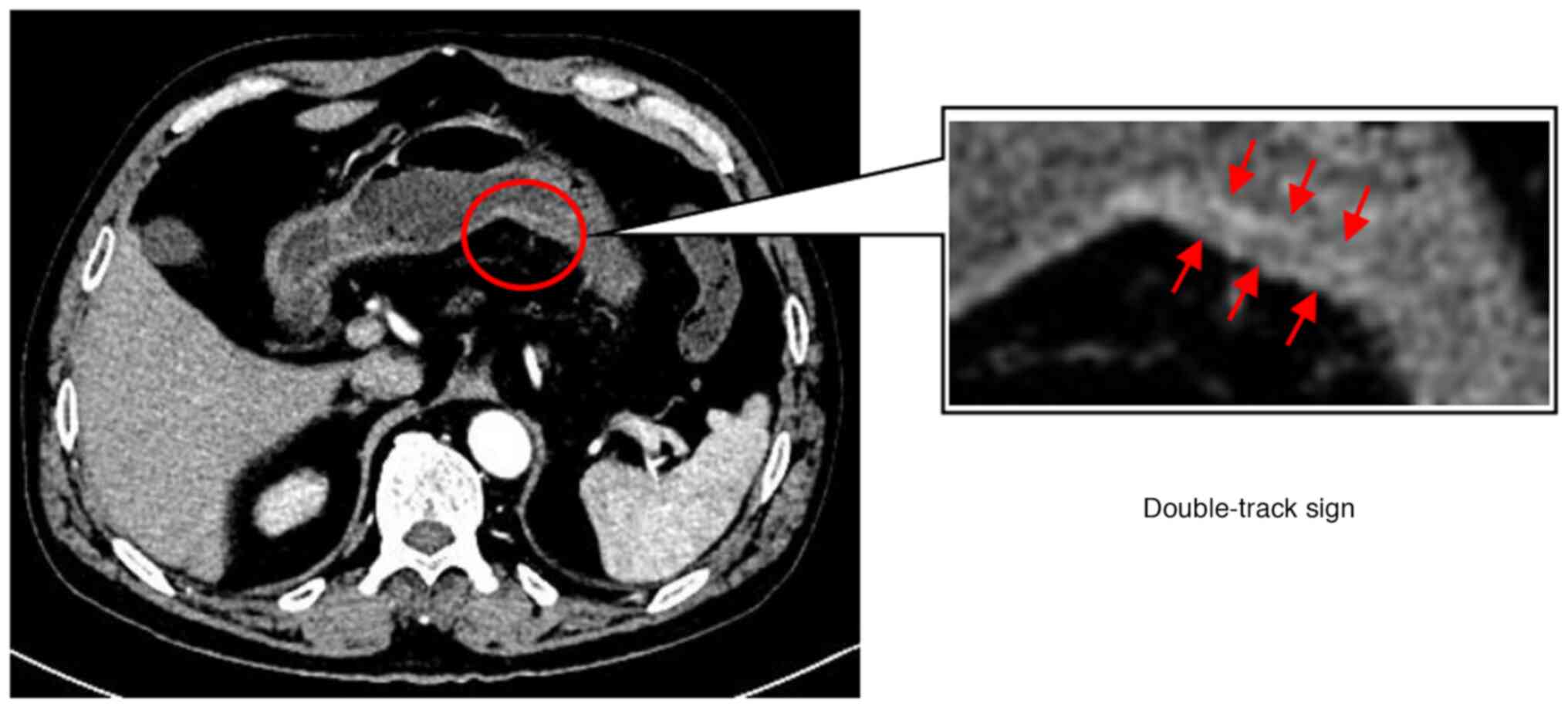
CT images may reveal two specific morphological
abnormalities of the stomach: Local double-track enhancement
(double-track sign; Fig. 2) and
well-enhanced mucosal thickening of the gastric wall. For example,
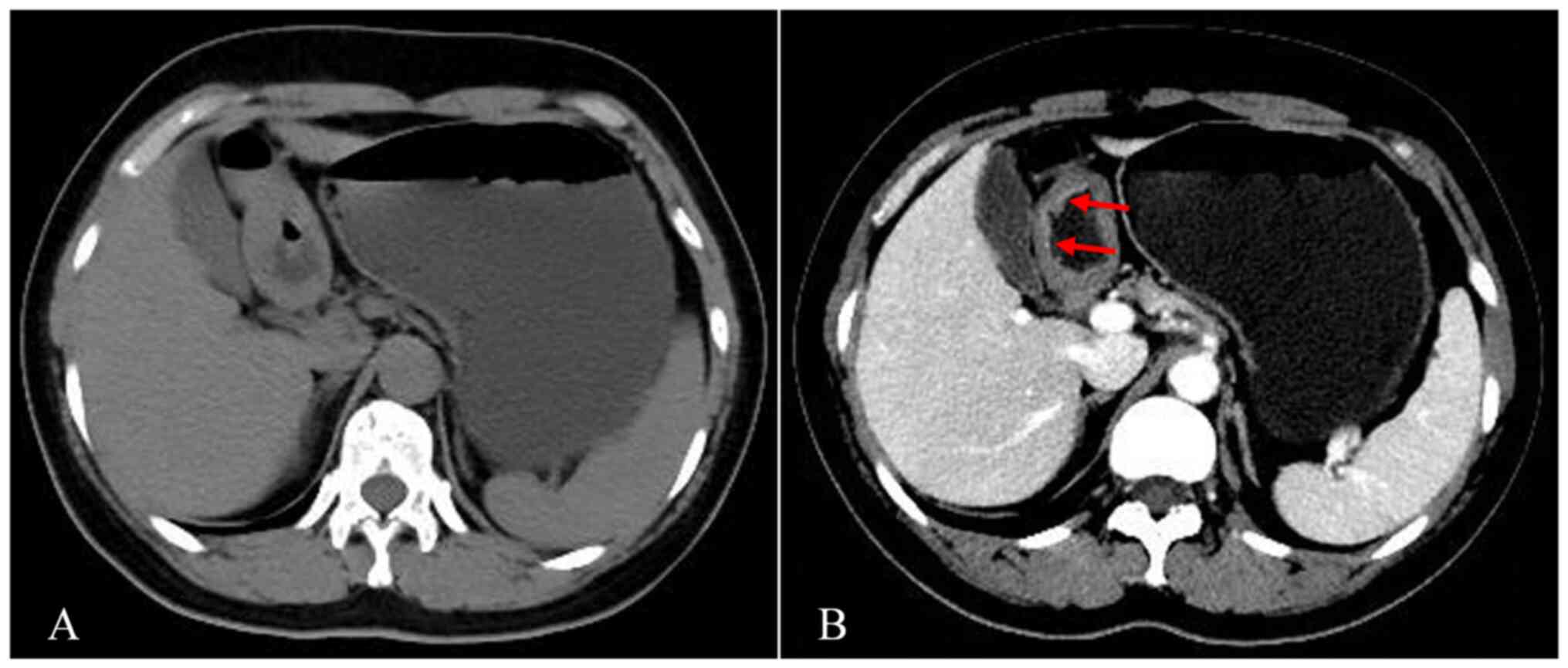
Fig. 3 presents a typical case of a
patient (male, 60 years old) with a double-track sign. The patient
was diagnosed with T1a gastric cancer by pathology within one week
of undergoing a CT, which revealed a double-track sign.
Based on CT findings and interpretations, patients
were divided into the following three groups: Group A-double-track
sign. Enhanced CT images with two or more consecutive layers
indicate the double-track change; group B-well-enhanced mucosal
thickening of the gastric wall on CT image; and group C-no
abnormalities. Contrast-enhanced CT images did not reveal any
abnormal gastric lesions.
The classification results are presented in Table II. According to the classification
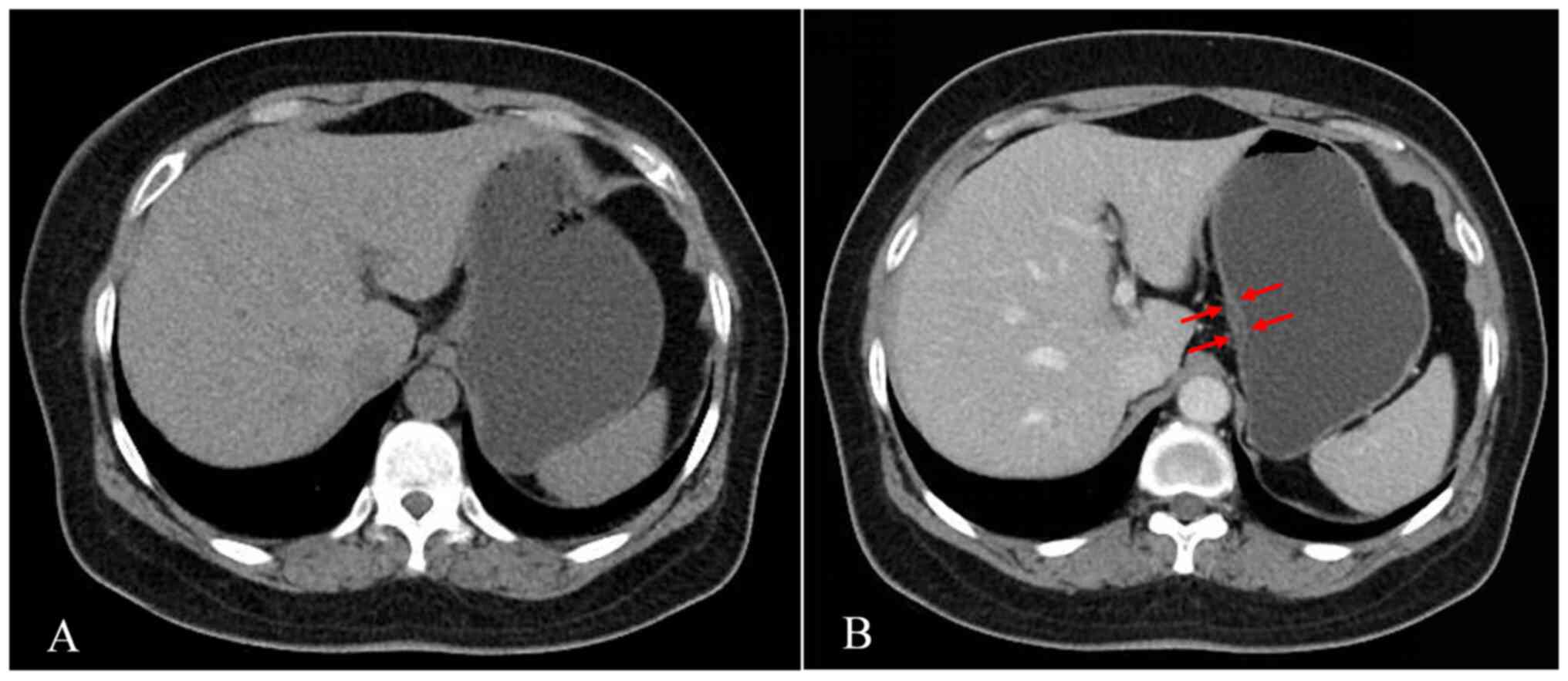
of CT manifestations in all patients, 31 patients were assigned to
group A (60.8%), 4 to group B (7.8%; representative case; female,
51 years old; provided in Fig. 4)
and 16 to Group C (31.4%). Regarding the specific locations of the
manifestations in the patients, 23 cases had manifestations in the
upper 1/3 of the stomach, 7 patients in the middle 1/3 and 21
patients in the lower 1/3. In addition, in Group A, the percentages
of T1a gastric cancer with the double-track sign in the upper 1/3,
middle 1/3 and lower 1/3 of the stomach were 51.6, 11.8 and 17.6%,
respectively. In Group B, the percentages of well-enhanced mucosal
thickening of the gastric wall in the middle 1/3 and lower 1/3 of
the stomach were 25 and 75%, respectively. Group C included seven
patients with gastric cancer in the upper 1/3 of the stomach and
nine patients with gastric cancer in the lower 1/3 of the stomach.
All sixteen patients in Group C had no local mucosal thickening of
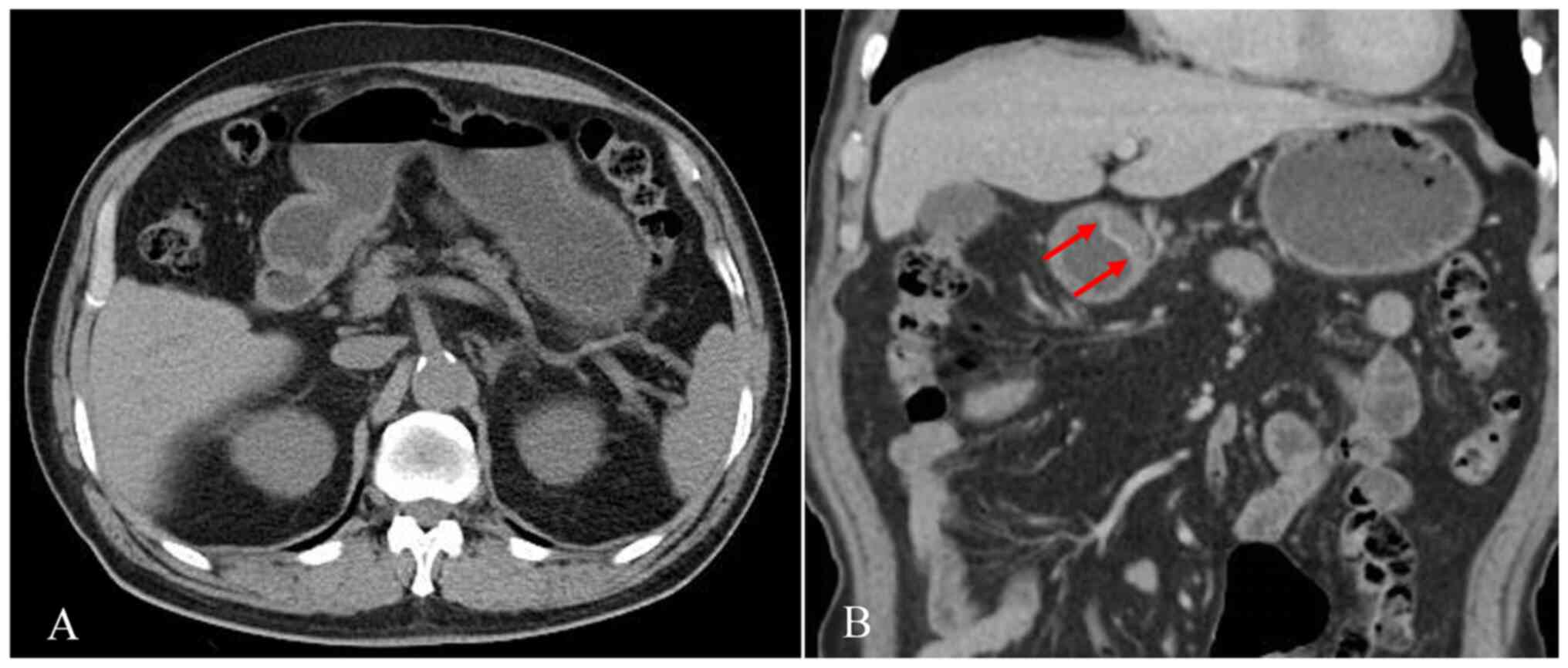
the gastric wall or abnormal enhancement changes. Of the 2,926
control subjects, none of the patients exhibited a double-track
sign and six patients (0.2%) had local gastric wall thickening with
abnormally strengthened enhancement (representative case; female,
50 years old; provided in Fig.
5).
 | Table II.Classification of CT findings of
patients in the study (n=51). |
Table II.
Classification of CT findings of
patients in the study (n=51).
|
|
|
| Location of early
gastric cancer |
|---|
|
|
|
|
|
|---|
| Group | CT finding | N | Upper 1/3 | Middle 1/3 | Lower 1/3 |
|---|
| A | Double-track
sign | 31 | 16 | 6 | 9 |
| B | Well-enhanced mucosal
thickening | 4 | 0 | 1 | 3 |
| C | No abnormality | 16 | 7 | 0 | 9 |
Discussion
The early detection and accurate preoperative
staging of T1a gastric cancer enables endoscopic resection or
minimally invasive surgery in certain patients, leading to a better
prognosis (8). However, detecting
gastric cancer in the T1a stage may be important; one report
suggested that most gastric cancers are not diagnosed until the
cancer is at the progressive stage (9). In addition, <40% of patients with
diagnosable early gastric cancer have typical malignant disease
symptoms, indicating that early gastric cancer may be serious and
difficult to diagnose (10).
However, several risk factors have been noted to have a significant
impact on T1a gastric cancer, such as family history, diet, alcohol
consumption and smoking, as well as Helicobacter pylori and
Epstein-Barr virus infections (11). Early gastric cancer screening has
reached a consensus on the aforementioned high-risk groups;
however, the need for further screening of the general population
remains under discussion (5).
Performing highly invasive examinations on all potential patients
with T1a gastric cancer may lead to disadvantages, which would
offset any advantages. The detection of appropriate tumor markers
for T1a gastric cancer may be performed as a feasible scheme for
general population screening (12).
In particular, carbohydrate antigen (CA72-4) is superior for
diagnosing early gastric cancer. A previous study indicated that
when the cutoff value of CA72-4 was 18.34 IU/ml, its sensitivity
and specificity were 65 and 90%, respectively (13). In addition, a novel molecular
marker, dihydropyrimidinase-like 3, has been noted for its high
sensitivity and specificity in diagnosing early gastric cancer (75
and 94%, respectively) (13).
However, digestive system inflammation and certain drug-related
diseases may lead to false-positives. The sensitivity of tumor
markers is unclear. Therefore, the practice of using tumor markers
for early diagnosis remains questionable. To date, various valuable
tumor markers have been identified in the clinic; however, no
notable tumor markers have been found that may achieve specific
sensitivity and meet the screening criteria for T1a gastric cancer.
Thus, discovering specific morphological changes for the diagnosis
of T1a gastric cancer using non-invasive imaging modalities has
become increasingly important.
Various imaging methods are typically used for
diagnosing T1a gastric cancer, including endoscopic ultrasound, CT
and MRI. However, only a small number of studies have investigated
the diagnosis of T1a gastric cancer by detecting distinct signs on
imaging. Abnormal gastric morphology is considered one of the
contributing factors to poor prognosis of gastric cancer. One study
addressing this issue suggested that indications for the diagnosis
of early gastric cancer may be thickening and enhancement of the
gastric wall (14). In addition,
one report demonstrated that a tumor invading a low-density stripe
layer at <50% of the thickness is a criterion used for
diagnosing early gastric cancer (8). The two studies did not investigate the
morphological or contour abnormalities of the stomach in detail. In
the present study, CT findings indicated that morphological or
enhancement abnormalities of the stomach, such as thickening and
enhancement of the gastric wall, were present. This is consistent
with a previous report that found a correlation between the
diagnosis of T1a gastric cancer and morphological or contour
abnormalities of the stomach (8).
Kim et al (15) also
reported that the hyperattenuating serosa sign may be a useful CT
finding in differentiating between T4a and less-advanced gastric
cancers. This further confirms the feasibility and value of gastric
morphological characteristics in staging and diagnosing gastric
cancer. The double-track sign is a localized morphological CT sign
of T1a gastric cancer and may be caused by the direction of primary
gastric cancer growth along and perpendicular to the stomach wall
(16). In the present study, it was
speculated that the enhancement of the serous layer may be related
to the inflammation that accompanies the tumor. In the enhanced CT
images, the significantly enhanced mucosal and outer layers of the
gastric wall, with the middle low-density band completely
displayed, form the double-track sign of T1a gastric cancer. The
underlying mechanism of the double-track sign remains elusive.
However, the present study provided important insight, i.e.,
patients without local double-track enhancement changes of the
stomach have unobvious morphological abnormalities compared to
those reported in previous studies. Furthermore, these patients
have minimal to no abnormalities (31.4%) or well-enhanced mucosal
thickening of the gastric wall on CT images (7.8%). Although it is
difficult to compare previous research results with those of the
present study, the current results indicated that ~60.8% of
patients with T1a gastric cancer exhibited a double-track sign,
suggesting that these signs may provide a new indication for the
diagnosis of T1a gastric cancer. In addition, although these CT
findings may not reflect a direct relationship with the diagnosis
of T1a gastric cancer, the present study demonstrated that the
presence of the double-track sign is useful in diagnosing T1a
gastric cancer.
The AJCC Cancer Staging Manual proposes CT criteria
used for T staging of gastric cancer (8). The differentiation between T1 and T2
stage gastric cancer may be well distinguished on the enhanced CT
image and it is also easy to find the abnormal enhancement of the
gastric wall of T2 stage gastric cancer. Therefore, T2 stage
gastric cancer was not included in the present study; T1a gastric
cancer is frequently neglected in clinical practice, so it is
important to propose better CT-enhanced signs for diagnosis and T1b
gastric cancer may be diagnosed according to the AJCC Cancer
Staging Manual. The present study is a supplement to this guideline
because for T1a gastric cancer, good results may be achieved
through endoscopic mucosal resection. The present results suggested
that the ‘double-track sign’ may be used for the diagnosis of T1a
gastric cancer.
In the present study, the requirement for the
imaging diagnosis of T1a gastric cancer was that all patients
involved had surgical pathology results. For the diagnostic
differentiation of T1a gastric cancer, endoscopic examination is
the first choice of screening modality. However, there are ‘blind
areas’ in endoscopic examinations; the determination of lesion
location is frequently inaccurate. In addition, patients undergoing
these examinations experience obvious discomfort. The rate of
missed diagnoses by endoscopic examination is ~20–30% (17). Most of these cases are of cardiac
cancer. This may be related to patient compliance, gastroscopic
performance and the special anatomical structure of the upper
digestive tract. CT reveals the double-track sign, which is
difficult to display on EUS and may be useful for accurately
diagnosing T1a gastric cancer. In general, CT examinations include
various imaging methods, such as plain scan, enhanced and energy
CT. Several studies reported that using the gastric window in CT
provides more accurate staging for early gastric cancer than that
of the conventional abdominal window (8). In addition, the multiplanar
reformation image provided by CT post-processing reconstruction
technology is useful for accurately detecting early gastric cancer
(18). However, the sensitivity of
CT for early gastric cancer is relatively low. To diagnose T1a
gastric cancer, more accurate diagnostic imaging is required.
Although there may be challenges related to the diagnostic
differentiation of T1a gastric cancer in facilities with different
imaging modes, the double-track sign as detected in the present
study has additional advantages as an early diagnostic indicator.
Under the capturing conditions of abdominal CT, such as contrast
and non-contrast CT and multidetector CT, the double-track sign may
be detected in all parts of the stomach. Therefore, the
double-track sign detected on any CT scan may be a meaningful
diagnostic imaging finding, suggesting the presence of T1a gastric
cancer.
The current study had certain limitations. First, it
was a retrospective single-institution study. Furthermore, the
visibility of T1a gastric cancer was not assessed based on gastric
distension. In addition, quantifying the local contraction of the
stomach is difficult because the double-track sign is a
‘still-imaging’ finding. A subsequent prospective study on the
exact time of developing T1a gastric cancer in patients with the
double-track sign will provide a further theoretical basis for
using this sign in diagnostic differentiation. Finally, as a
retrospective analysis, the present study did not compare
histopathological results and CT images; these variables should be
investigated in future studies.
In conclusion, the double-track sign is an important
CT manifestation of stomach morphological abnormalities and may be
used as a reliable indicator for diagnosing T1a gastric cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Outstanding Youth Project in
Henan Province for Young and Middle-aged Health and Health
Technology Innovation (grant no. YXKC2020053).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL and JG designed the study. JG critically reviewed
the manuscript and revised it. PL, BZ, XR, DL and MC performed the
database search and literature review. ZH and LY performed data
analysis, the database search and the literature review. BZ
performed the data collation/processing. PL, BZ, XR, DL and MC
analysed the data. PL and JG confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of the First Hospital of Zhengzhou University (Zhengzhou,
China). Any requirement of written informed consent was waived by
the Institutional Review Board due to the retrospective nature of
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thrift AP and El-Serag HB: Burden of
gastric cancer. Clin Gastroenterol Hepatol. 18:534–542. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niu PH, Zhao LL, Wu HL, Zhao DB and Chen
YT: Artificial intelligence in gastric cancer: Application and
future perspectives. World J Gastroenterol. 26:5408–5419. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye Z, Zeng Y, Wei S, Wang Y, Lin Z, Chen
S, Wang Z, Chen S and Chen L: Short-term survival and safety of
apatinib combined with oxaliplatin and S-1 in the conversion
therapy of unresectable gastric cancer. BMC Cancer. 21:7022021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida N, Doyama H, Yano T, Horimatsu T,
Uedo N, Yamamoto Y, Kakushima N, Kanzaki H, Hori S, Yao K, et al:
Early gastric cancer detection in high-risk patients: A multicentre
randomised controlled trial on the effect of second-generation
narrow band imaging. Gut. 70:67–75. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee IJ, Lee JM, Kim SH, Shin CI, Lee JY,
Kim SH, Han JK and Choi BI: Diagnostic performance of 64-channel
multidetector CT in the evaluation of gastric cancer:
Differentiation of mucosal cancer (T1a) from submucosal involvement
(T1b and T2). Radiology. 255:805–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan Y, Ren S, Wang T, Shen F, Hao Q and
Lu J: Differentiating T1a-T1b from T2 in gastric cancer lesions
with three different measurement approaches based on
contrast-enhanced T1W imaging at 3.0 T. BMC Med Imaging.
21:1402021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang ZL, Li YL, Tang L, Li XT, Bu ZD and
Sun YS: Utility of the gastric window in computed tomography for
differentiation of early gastric cancer (T1 stage) from muscularis
involvement (T2 stage). Abdom Radiol (NY). 46:1478–1486. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel TH and Cecchini M: Targeted
therapies in advanced gastric cancer. Curr Treat Options Oncol.
21:702020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Everett SM and Axon AT: Early gastric
cancer in Europe. Gut. 41:142–150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21:40122020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Z, Bian H, Chen C, Chen W and Li Q:
Application of serum pepsinogen and carbohydrate antigen 72-4
(CA72-4) combined with gastrin-17 (G-17) detection in the
screening, diagnosis, and evaluation of early gastric cancer. J
Gastrointest Oncol. 12:1042–1048. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong H and Luo X: Serum
dihydropyrimidinase-like 3 concentration in patients with gastric
cancer and its diagnostic value. Iran J Public Health.
50:1789–1795. 2021.PubMed/NCBI
|
|
14
|
Park KJ, Lee MW, Koo JH, Park Y, Kim H,
Choi D and Lee SJ: Detection of early gastric cancer using
hydro-stomach CT: Blinded vs unblinded analysis. World J
Gastroenterol. 17:1051–1057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim TU, Kim S, Lee JW, Lee NK, Jeon TY and
Park DY: MDCT features in the differentiation of T4a gastric cancer
from less-advanced gastric cancer: Significance of the
hyperattenuating serosa sign. Br J Radiol. 86:201302902013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Jia Y, Peng Z and Wang G: The
prognostic role of tumor size in stage T1 gastric cancer. World J
Surg Oncol. 20:1352022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahn HS, Lee HJ, Yoo MW, Kim SG, Im JP, Kim
SH, Kim WH, Lee KU and Yang HK: Diagnostic accuracy of T and N
stages with endoscopy, stomach protocol CT, and endoscopic
ultrasonography in early gastric cancer. J Surg Oncol. 99:20–27.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YN, Choi D, Kim SH, Kim MJ, Lee SJ,
Lee WJ, Kim S and Kim JJ: Gastric cancer staging at isotropic MDCT
including coronal and sagittal MPR images: Endoscopically diagnosed
early vs. advanced gastric cancer. Abdom Imaging. 34:26–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|