Introduction
In 2020, ~73,750 new cases of kidney cancer were
diagnosed in the United States, with >20% of patients succumbing
to the disease (1). Patients with
distant metastasis demonstrated a 5-year survival of ~10% (1). Renal cell carcinoma (RCC) is the most
common type of kidney cancer, and the most common histologic
subtype of RCC is clear cell RCC (ccRCC) (2). The prognosis and treatment of
metastatic ccRCC is a current problem (2). Existing knowledge of the mechanisms of
metastatic ccRCC has indicated the critical role of the
immune-related pathways (3).
Immunotherapy a promising treatment strategy alternative to
targeted therapy for metastatic renal cancer (3).
Long non-coding (lnc)RNA is involved in the
regulation of gene expression at epigenetic, transcriptional and
post-transcriptional levels at all stages of tumorigenesis and
cancer progression. lncRNAs serve a key role in a variety of
cellular processes and molecular signaling pathways. For example,
the activation of the lncRNA gene EPIC1 enhances tumorigenesis by
promoting the binding of MYC to its target genes (e.g., CDKN1A),
which indicates that it has a carcinogenic effect (4), while the inactivation of the lncRNA
gene growth arrest specific 5 promotes cell proliferation and tumor
formation in cancer, which indicates its tumor inhibitory effect
(5). Moreover, lncRNA also
participates in tumor progression and metastasis, which is closely
related to prognosis (6).
lncMX1-215 is upregulated by IFNα and inhibits the proliferation
and metastasis of head and neck squamous carcinoma cells (7). lncMX1-215 has been reported to
negatively regulate PD-L1 expression to inhibit immune escape by
the suppression of H3K27 acetylation via binding to H3K27 acetylase
GCN5 (also known as KAT2A, lysine acetyltransferase 2A) (7). Moreover, lncRNA HOXA-AS2 promoted
proliferation, invasion and migration of nasopharyngeal carcinoma
by promoting hypoxia inducible factor-1α and PD-L1 expression via
the direct targeting of miR-519 (8). However, the role of long non-coding
RNAs (lncRNA) in metastatic ccRCC remains unclear.
Competing endogenous RNA (ceRNA) is a type of
regulatory network. It is known that microRNAs (miRNAs) can cause
gene silencing by binding to mRNA, while lncRNAs can regulate gene
expression by competitively binding miRNA (9). Previous studies have reported that the
ceRNA mechanism exists in a wide variety of signaling pathways and
serves an important role in metastatic ccRCC (10). However, the immune-related ceRNA
regulatory networks are poorly understood. The lncRNAs could be
biomarkers for the prognosis of ccRCC and could pave the way for
further investigation of the immune-related mechanisms and
therapeutic potentials of these lncRNAs in ccRCC.
Materials and methods
Transcriptome data
The transcriptome data were downloaded from The
Cancer Genome Atlas project (https://portal.gdc.cancer.gov/) and Gene Expression
Omnibus (https://www.ncbi.nlm.nih.gov/geo/) databases. The raw
(.CEL) files of microarray (Affymetrix Human Genome U133 Plus 2.0
Array platform) datasets GSE53757 (11) and GSE66270 (12) were downloaded. A total of 72 tumor
samples and 72 normal samples were downloaded in the GSE53757
dataset, and 14 tumor samples and 14 normal samples were downloaded
in the GSE66270 dataset. Moreover, the read count expression data
and the corresponding clinical data of the TCGA-KIRC (n=603)
dataset were downloaded from the Genomic Data Commons data portal
(https://portal.gdc.cancer.gov/repository). The RNA-Seq
data (read count) and the corresponding clinical information for
ccRCC (557 donors, accession number KIRC-US) from the International
Cancer Genome Consortium (ICGC) project were downloaded from the
Xena data hub (https://xena.ucsc.edu/public). The annotation file
(.gtf) from the GENCODE (v23) database was used for the gene
annotation for TCGA-KIRC dataset and ICGC KIRC-US dataset.
Differential expression analysis
The edgeR package (13) and limma package (14) in R software (version 3.6.1; R core
team) were used to perform differential expression (DE) analysis
for the read count data and microarray expression data,
respectively. The genes and probes with Benjamini-Hochberg (BH)
adjusted P<0.05 were considered as statistically significant.
The DE lncRNAs, DE miRNAs and DE coding genes commonly screened
from all three datasets were identified as high-confidence DE
genes.
ceRNA network analysis
The DE lncRNAs, DE miRNAs and DE coding genes were
used to construct the ceRNA network based on the strategy
previously reported by Zhang et al (15). Based on the ceRNA theory, lncRNA may
interact with miRNA to prevent the interaction of miRNA with its
targets. This strategy identified the lncRNAs and miRNAs with
interactions. lncRNA-miRNA regulation networks were predicted using
the miRcode database (16).
Similarly, miRNA-mRNA regulation networks were predicted using the
miRDB database (17). The predicted
networks based on miRcode and miRDB databases were used to
construct the ceRNA networks. The ceRNA network was constructed
using Cytoscape (version 3.7.4; http://cytoscape.org/).
Identification of prognostic,
metastasis-related and immune-related lncRNAs
We identified metastasis-related lncRNAs based on a
previously reported algorithm (18), which leveraged the change in gene
differential expression status, in different stages of the disease
of interest, to identify the significant dynamic expression changes
of genes. Specifically, the delta value of each lncRNA was
calculated. The lncRNAs with the absolute value of delta >0 were
determined as metastasis-related. To identify immune-related
lncRNAs, the gene sets of immune pathways (https://www.kegg.jp/kegg/pathway.html; pathways of
‘5.1 immune system’) from the Kyoto Encyclopedia of Genes and
Genomes (KEGG) (19) were
downloaded. The prognostic lncRNAs were identified using univariate
and multivariate Cox regression analysis. To determine whether the
lncRNA was an independent prognostic factor, multivariate analysis
was performed using the clinical parameters as covariates. Both the
overall survival (OS) and progression-free survival (PFS) were
evaluated. The log-rank test was used to assess whether the lncRNA
was prognosis-related.
To identify immune-related lncRNAs, for each lncRNA,
Pearson correlation analysis was first performed to identify all
associated protein-coding genes in the KEGG database. Then, pathway
enrichment analysis was performed to determine whether these coding
genes were overrepresented in the immune pathway. The Pearson
correlation was performed between lncRNAs expression and the KEGG
genes. Gene pairs with coefficient |r|>0.4 and BH-adjusted
P<0.05 were considered significant.
Functional enrichment analysis
Function analysis of lncRNAs was performed based on
the correlated protein-coding genes. The significant lncRNA-coding
gene correlation was defined by the expression correlation (Pearson
correlation |r|≥0.4 with BH-adjusted P<0.05). KEGG and Gene
Ontology (GO) terms were analyzed.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was used to assess the relative expression
of β-actin and DSCR9 on commercial cDNA chips to compare DSCR9 gene
expression between cancer and adjacent samples. A total of 30 pairs
of ccRCC samples were analyzed. Total RNA was extracted from
tissues or cells using the Eastep® Super Total RNA
Extraction Kit (Promega Corporation), and the reverse transcription
was performed using the PrimeScript™ RT Master Mix
(Takara Biotechnology Co., Ltd.) by Shanghai Outdo Biotech Co.,
Ltd., according to the manufacturer's instructions. The ccRCC
samples were collected by Shanghai Outdo Biotech Co., Ltd. and the
cDNA of ccRCC sample tissues generated by Shanghai Outdo Biotech
Company was purchased and used in the present study. qPCR was
performed using the SYBR Green PCR Kit (Bio-Rad Laboratories, Inc.)
in triplicate in three independent experiments using the
2−ΔΔCq method (20). The
qPCR conditions were as follows: 95°C for 30 sec, followed by 39
cycles at 95°C for 5 sec and 60°C for 30 sec. The relative
expression of DSCR9 was normalized to β-actin and assessed using a
RT-PCR Quantitation Kit (cat. no. E21006; Shanghai GenePharma Co.,
Ltd.). Informed consent was obtained from all participating
patients by Shanghai Outdo Biotech Co., Ltd. Ethical approval for
the use of ccRCC samples was provided. The levels of DSCR9 were
assessed by qPCR on the Step One Plus Real-Time PCR system, and
β-actin was used as endogenous control. The primer sequences used
for qPCR were as follows: DSCR9 forward (F),
5′-AGGAAGGAACTGAGAACACC-3′ and reverse (R),
5′-CAGTCCATTTCTACCGTCAC-3′; and β-actin F,
5′-CCTTCCTGGGCATGGAGTC-3′ and R, 5′-TGATCTTCATTGTGCTGGGTG-3′.
Statistical analysis
The BH adjustment was performed for multiple tests
in differential expression analysis, enrichment analysis and
expression correlation analysis. Cox regression analysis was
performed to investigate the correlation between lncRNAs and
patient survival. Log-rank test was used and hazard ratios were
calculated using survival/R package (version 3.6.1; http://www.r-project.org/). The statistical test used
for two-group comparison in differential expression of TCGA
samples, using the edgeR package in R (version 3.6.1) was empirical
Bayes quasi-likelihood F-tests. The statistical test for two-group
comparison of the qPCR results was a two-tailed Student's t-test.
P<0.05 was considered to indicate a statistically significant
diffference.
Results
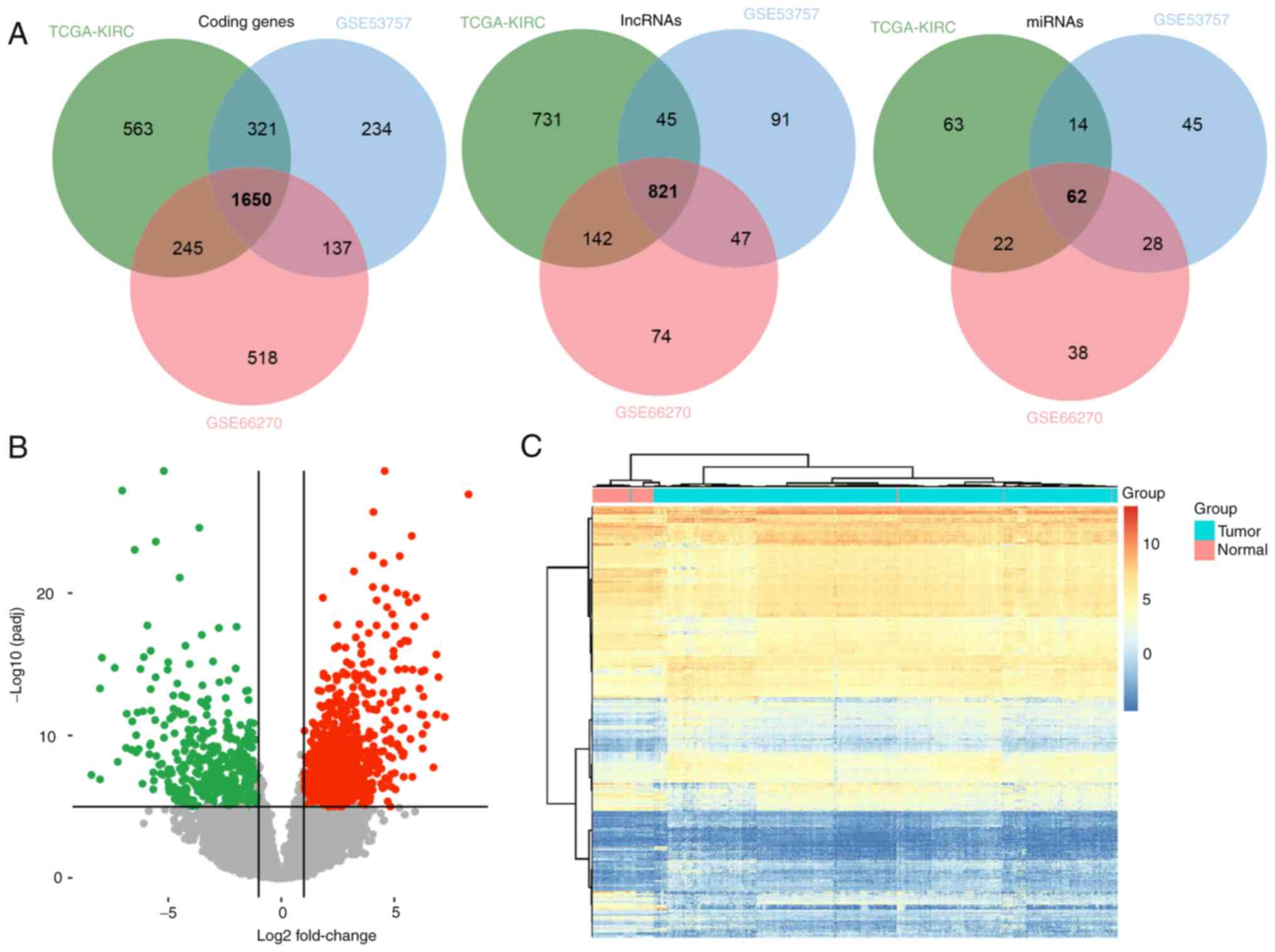
Differential expression analysis
Differential expression analysis was performed to
screen ccRCC-related genes in three independent datasets
(TCGA-KIRC, GSE53757 and GSE66270). The miRNAs, lncRNAs, and coding
genes were annotated using the GENCODE database. Commonly
deregulated genes with BH-adjusted P<0.01 were retained. A total
of 1650 coding genes, 821 lncRNAs and 62 miRNAs were identified as
differentially expression genes between normal and tumor samples
(Fig. 1A). A volcano plot and
heatmap of the deregulated genes in the TCGA dataset were generated
(Fig. 1B and C).
Identification of prognosis-related
and metastasis-related lncRNAs
To identify lncRNAs associated with patient
survival, univariate and multivariate Cox regression analyses were
performed based on the TCGA dataset. The candidate lncRNAs
considered in this step were obtained from the aforementioned
differential expression analysis. A total of 522 lncRNAs associated
with OS or PFS were identified (Table
SI). Among the identified lncRNAs, multivariate analysis based
on the lncRNA expression and clinical parameters indicated that 408
lncRNAs were independent factors for the prognosis of ccRCC
patients. Whether the prognosis-related lncRNAs were associated
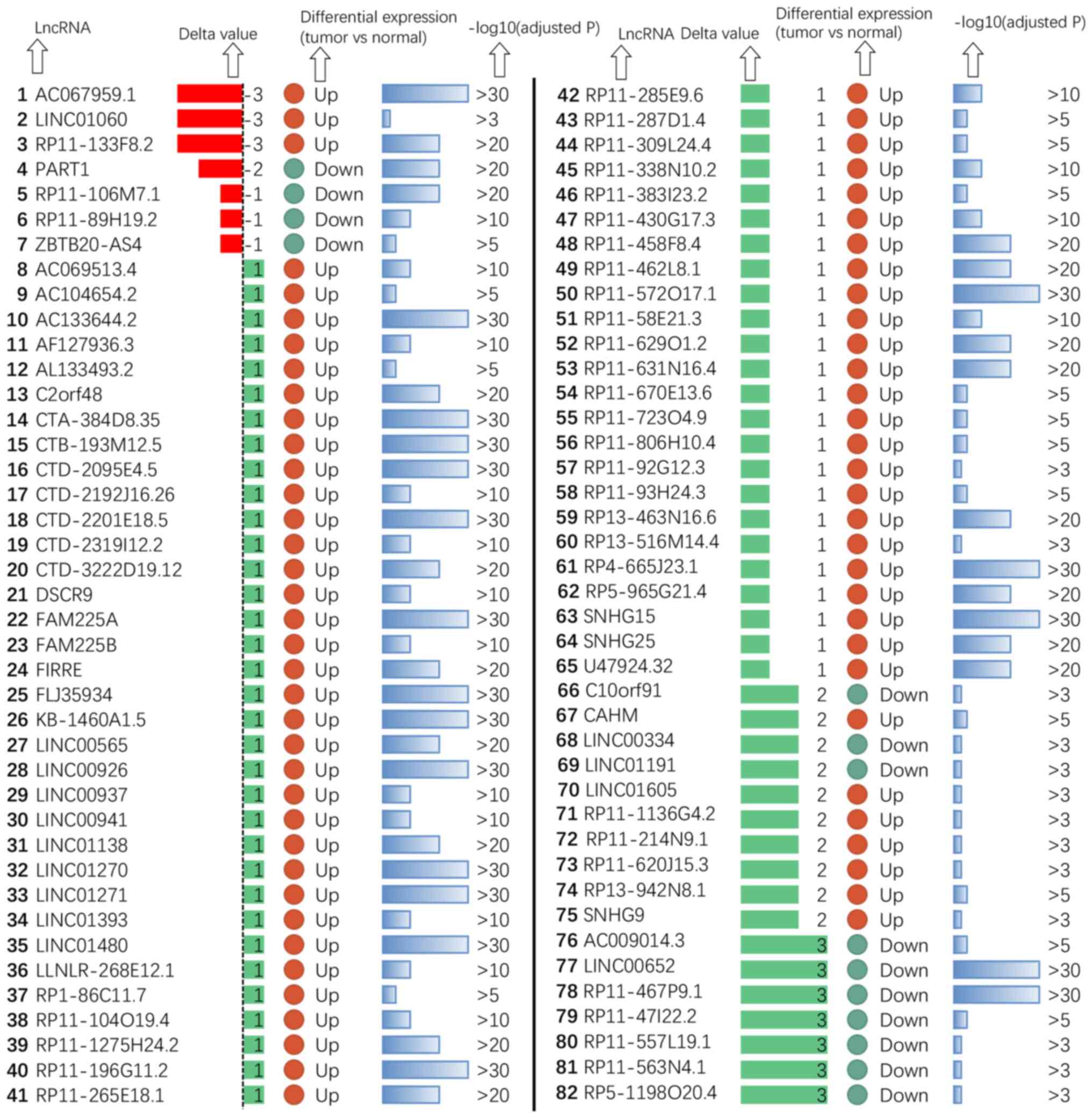
with distant metastasis of ccRCC was evaluated by calculating the
delta values (|delta|>0), which indicated that 82 of the lncRNAs
were distant metastasis-related (Fig.
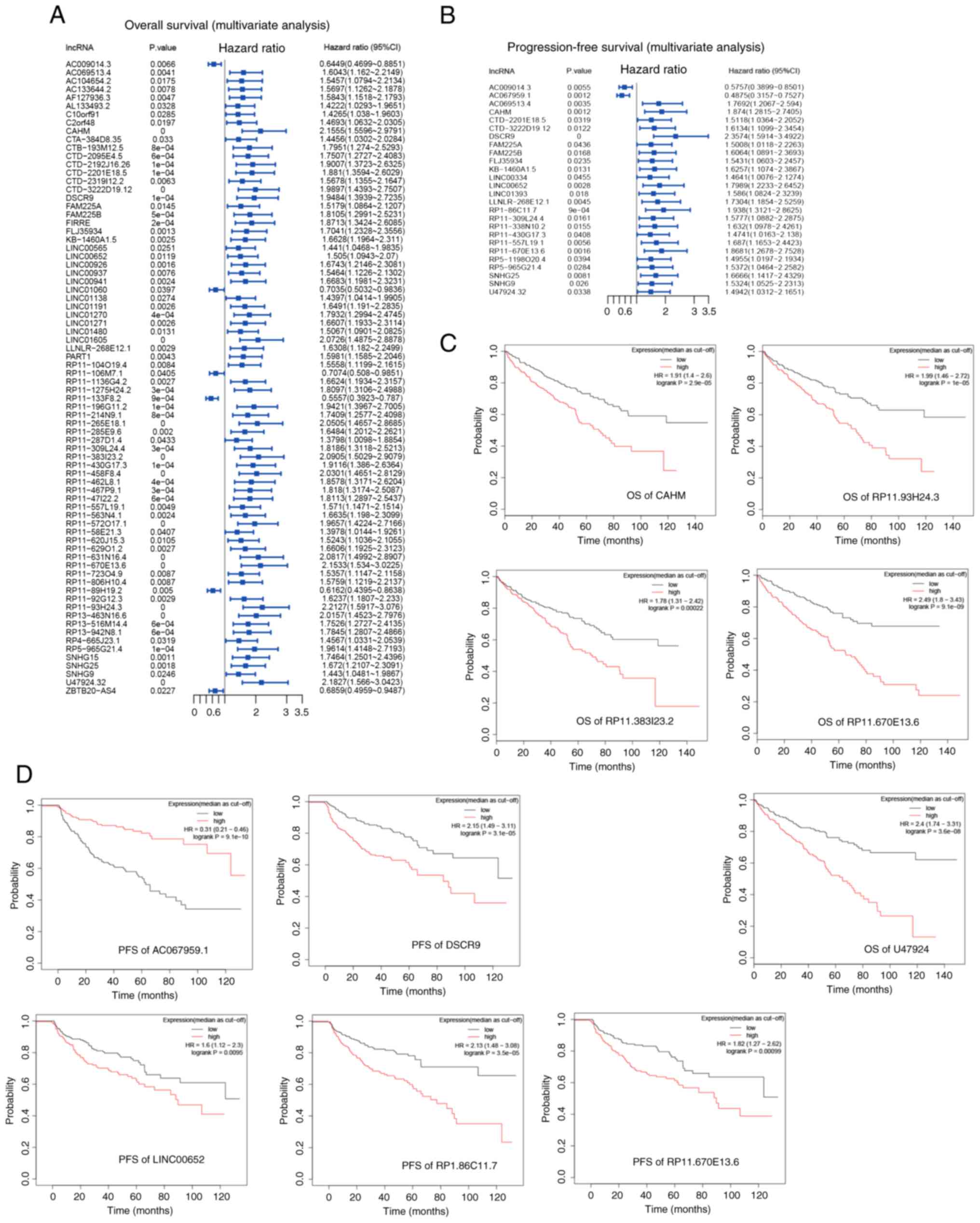
2). The 82 lncRNAs were assessed using multivariate models of
OS or PFS (Fig. 3A and B). The
Kaplan Meier plots of the top 5 lncRNAs associated with OS (CAHM,
RP11.93H24.3, RP11.383I23.2, RP11.670E13.6 and U47924; Fig. 3C) and the top 5 lncRNAs associated
with PFS (DSCR9, AC067959.1, RP1.86C11.7, LINC00652 and
RP11.670E13.6; Fig. 3D) were
presented.
ceRNA networks based on immune-related
lncRNAs
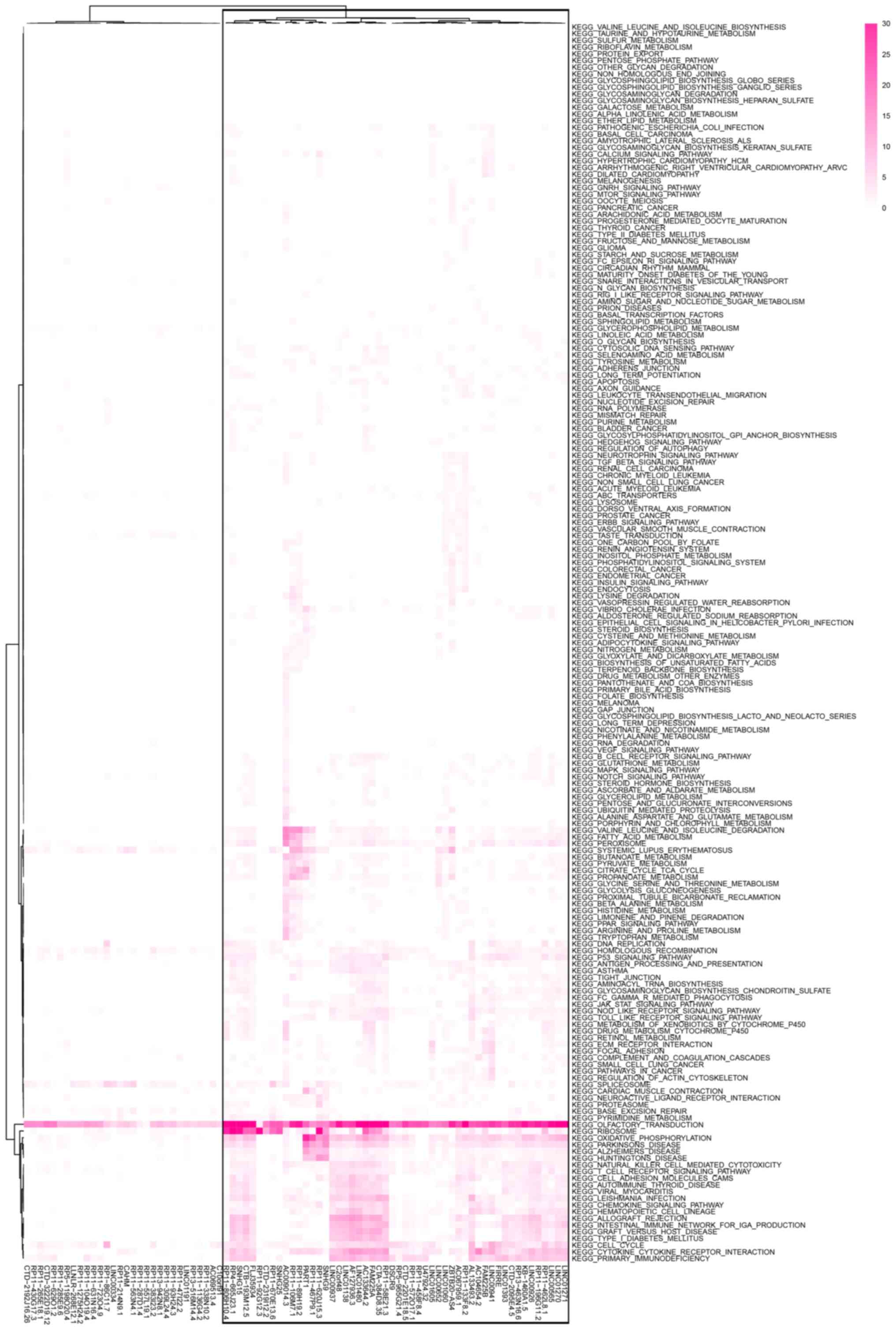
To evaluate the functions of the distant
metastasis-related lncRNAs, Pearson correlation analysis was
performed to screen the correlated protein-coding genes. Then,
pathway enrichment analysis was performed for each lncRNA to
identify the involved pathways. The results demonstrated that the
lncRNAs were associated with cell adhesion and cytoskeleton-related
pathways, such as tight junction, focal adhesion, regulation of
actin cytoskeleton and cell adhesion molecule cams (Fig. 4), which supported the association of
these lncRNAs and metastasis. Moreover, it was observed that most
of the lncRNAs were involved in immune-related pathways, such as
the T cell receptor signaling pathway, NK cell-mediated
cytotoxicity and chemokine signaling pathway. Thus, a group of 52
lncRNAs were identified (bottom, 52 lncRNAs from right to left,
Fig. 4). To evaluate the function
of lncRNAs, GO enrichment analysis was performed for each lncRNA
based on the lncRNA-correlated proteins. This demonstrated that
DSCR9 was involved in T cell activation regulation (biological
process), protein complexes (such as inflammasome complex and cell
adhesion-related complex; cellular components) and ATPase activity
(molecular function; Fig. 5A).
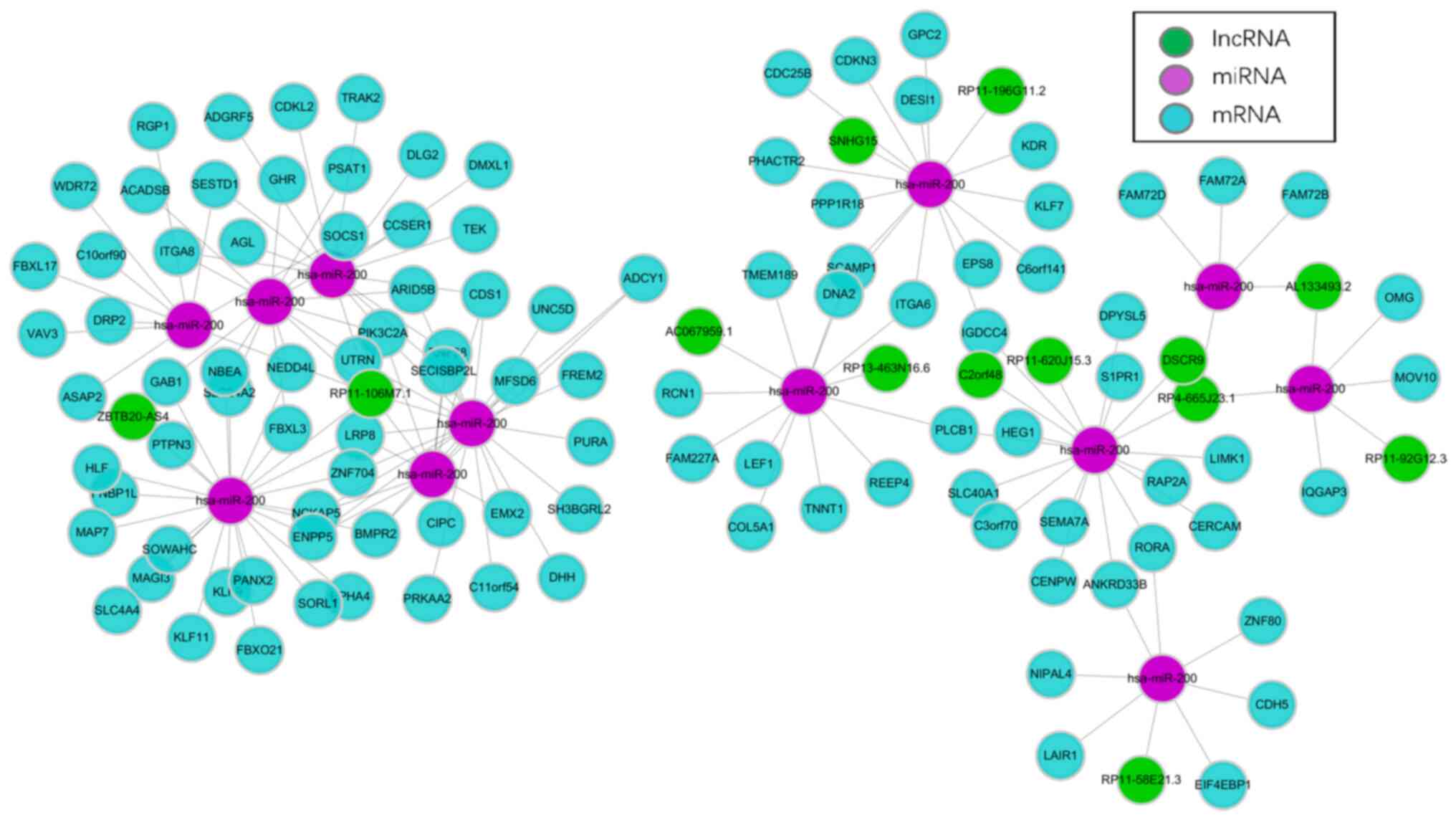
A ceRNA network was constructed based on the 52
lncRNAs, 62 DE miRNAs and 1,650 DE mRNAs. To improve the confidence
of the regulatory network, only the lncRNA-miRNA pairs and the
miRNA-mRNA pairs with opposite DE directions were retained. This
resulted in ceRNA networks which included 14 lncRNAs, 13 miRNAs,
and 107 mRNAs (Fig. 5).
DSCR9 could be an independent
prognostic biomarker
In the ceRNA networks, lncRNA DSCR9 demonstrated a
significant association with PFS of patients with ccRCC (Fig. 6A). Therefore, another independent
cohort was used to validate the prognostic value of DSCR9 in ccRCC.
The univariate and multivariate Cox analyses concordantly
demonstrated that DSCR9 was an independent risk factor for PFS of
ccRCC patients (Figs. 3B and
6B; Table I). Considering that the clinical
stage was evaluated based on the TNM stage, we used the TNM stage
instead of the clinical stage in the multivariate analysis based on
the methods previously reported (21). The subnetwork of DSCR9 was extracted
(Fig. 6C). DSCR9 was associated
with the immune pathway, so the correlation between DSCR9 and
immunotherapeutic markers was assessed. This demonstrated that
DSCR9 was significantly associated with programmed cell death
protein 1 (PDCD1, also known as PD-1) and correlated with CTLA4
(Fig. 6D), which implied that DSCR9
might be related to immunotherapeutic response. However, no
significant association was demonstrated between DSCR9 and CD274
(P=0.45).
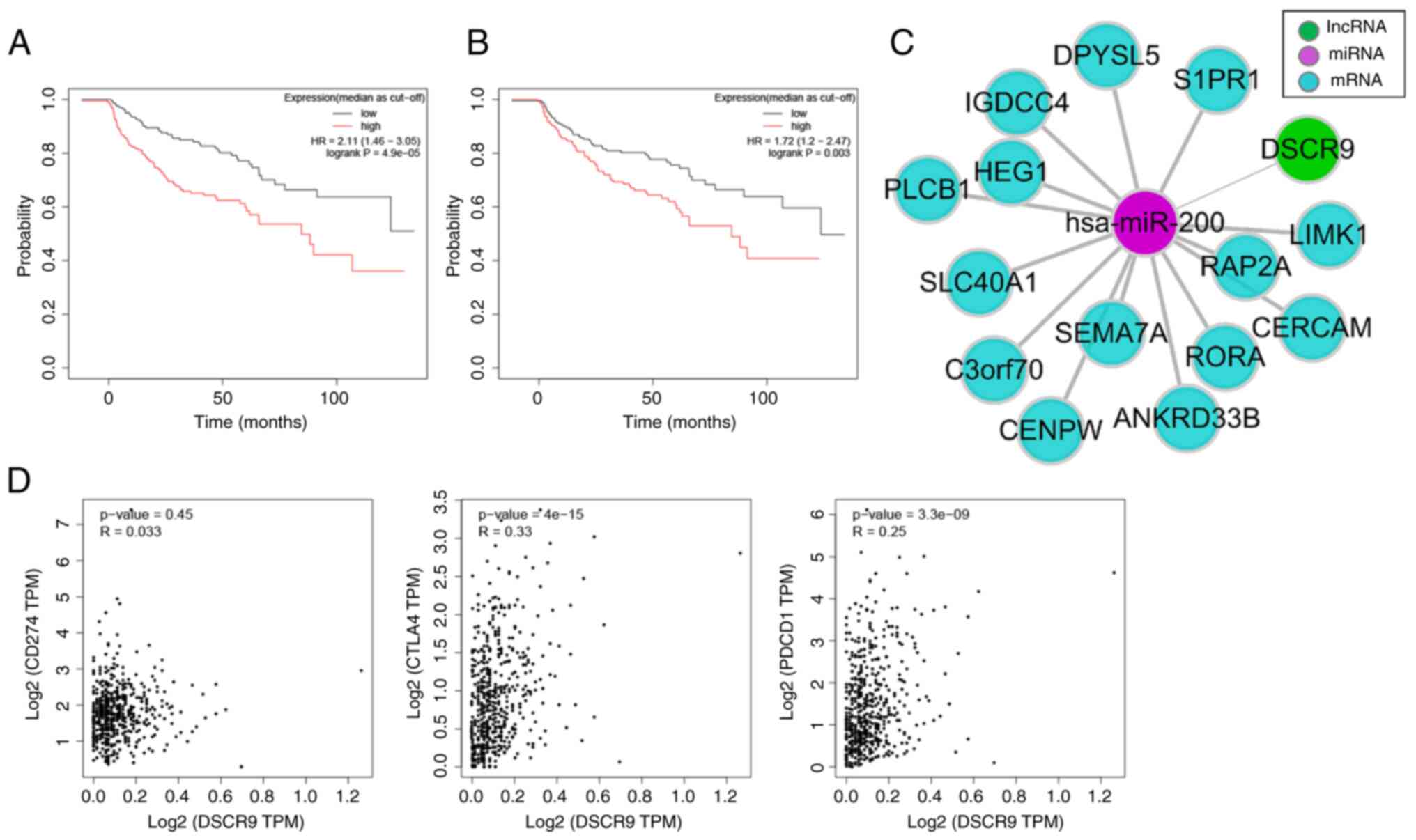 | Figure 6.DSCR9 could be a novel prognostic
marker. Kaplan Meier curves of PFS and DSCR9 in the (A) TCGA cohort
and (B) ICGC cohort. (C) The sub-network of DSCR9 from the
competing endogenous RNA network. (D) Pearson correlation of DSCR9
and three immunotherapeutic markers, CD274 (also known as PD-L1;
P=0.45), CTLA4 (P=4×10−15) and PDCD1 (also known as
PD-1; P=3.3×10−9), respectively from left to right.
lncRNA, long non-coding RNA; miRNA, micro RNA; OS, overall
survival; PFS, progression-free survival; TCGA, The Cancer Genome
Atlas; ICGC, International Cancer Genome Consortium; TPM,
transcripts per million; PDCD1, programmed cell death protein
1. |
 | Table I.Univariate and multivariate Cox
regression model. |
Table I.
Univariate and multivariate Cox
regression model.
| A, TCGA_KIRC
n=434 |
|---|
|
|---|
|
| Univariate
regression | Multivariate
regression |
|---|
|
|
|
|
|---|
| Variable | P-value | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) |
|---|
| DSCR9 (high vs.
low) | 0.000049 | 2.11
(1.46-3.05) | 0.000012 | 2.32
(1.57-3.36) |
| Age (ref, ≤59
years) |
|
|
|
|
|
>59 | 0.083 | 1.36
(0.96-1.94) | 0.11 | 1.36
(0.93-1.99) |
| Gender (ref,
female) |
|
|
|
|
|
Male | 0.060 | 1.46
(0.98-2.17) | 0.039 | 1.58
(1.02-2.45) |
| Grade (ref,
G1) |
|
|
|
|
| G2 | 0.66 | 1.57
(0.21-11.56) | 0.79 | 0.76
(0.10-5.78) |
| G3 | 0.20 | 3.60
(0.49-26.03) | 0.69 | 1.50
(0.19-11.33) |
| G4 | 0.0079 | 14.79
(2.02-107.97) | 0.28 | 3.10
(0.39-24.17) |
| Unknown | 0.79 | 1.44
(0.09-23.15) | 0.79 | 1.46
(0.08-24.51) |
| Stage (ref=I) |
|
|
|
|
| II | 0.037 | 2.13
(1.04-4.36) | 0.085 | 4.97
(0.80-30.84) |
|
III |
1.39×10−8 | 4.38
(2.61-7.20) | 0.037 | 5.69
(1.10-29.31) |
| IV |
1.39×10−30 | 18.17
(11.08-29.80) |
8.67×10−6 | 180.68
(18.29-1784.09) |
|
Unknown | 0.99 | NA | 0.99 | NA |
| pM (ref, M0) |
|
|
|
|
| M1 |
1.72×10−29 | 8.66
(5.95-12.61) | 0.040 | 0.15
(0.02-0.92) |
|
Unknown | 0.87 | 0.90
(0.28-2.88) | 0.98 | 1.02
(0.26-3.97) |
| pN (ref, N0) |
|
|
|
|
| N1 | 0.00001 | 4.90
(2.40-9.99) | 0.0069 | 3.38
(1.39-8.22) |
|
Unknown | 0.15 | 0.76
(0.53-1.18) | 0.14 | 0.74
(0.57-1.10) |
| pT (ref, T1) |
|
|
|
|
| T2 | 0.00007 | 3.26
(1.82-5.84) | 0.18 | 0.31
(0.05-1.64) |
| T3 |
1.45×10−15 | 6.16
(3.96-9.64) | 0.33 | 0.45
(0.09-2.18) |
|
| B, ICGC_KIRC
n=384 |
|
|
| Univariate
regression | Multivariate
regression |
|
|
|
|
|
Variable | P-value | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) |
|
| DSCR9 (high vs.
low) | 0.003 | 1.72
(1.2-2.47) | 0.0009 | 1.89
(1.29-2.76) |
| Age (ref, ≤59) |
|
|
|
|
|
>59 | 0.090 | 1.28
(0.91-1.99) | 0.040 | 1.49
(1.01-2.18) |
| Gender (ref,
female) |
|
|
|
|
|
Male | 0.067 | 1.41
(0.99-2.04) | 0.16 | 1.36
(0.88-2.10) |
| Grade (ref,
G1) |
|
|
|
|
| G2 | 0.68 | 1.55
(0.32-10.25) | 0.91 | 0.89
(0.11-6.78) |
| G3 | 0.30 | 3.51
(0.47-21.25) | 0.61 | 1.68
(0.22-12.77) |
| G4 | 0.0092 | 12.14
(2.32-71.96) | 0.27 | 3.18
(0.40-24.81) |
|
Unknown | 0.80 | 1.41
(0.09-16.39) | 0.83 | 1.36
(0.08-23.02) |
| Stage (ref, I) |
|
|
|
|
| II | 0.041 | 2.13
(1.08-4.36) | 0.13 | 4.23
(0.65-27.19) |
|
III |
1.10×10−5 | 4.33
(2.61-7.20) | 0.035 | 5.98
(1.13-31.46) |
| IV |
1.22×10−25 | 18.17
(11.08-29.80) | 0.00004 | 112.98
(11.81-1080.33) |
|
Unknown | 0.99 | NA | 0.99 | NA |
| pM (ref, M0) |
|
|
|
|
| M1 |
1.72×10−25 | 8.10
(4.97-10.5) | 0.13 | 0.25
(0.04-1.44) |
|
Unknown | 0.95 | 0.90
(0.36-2.87) | 0.70 | 0.74
(0.16-3.37) |
| pN (ref, N0) |
|
|
|
|
| N1 | 0.00005 | 4.20
(2.54-8.65) | 0.014 | 3.15
(1.26-7.87) |
|
Unknown | 0.18 | 0.88
(0.61-1.14) | 0.32 | 0.82
(0.56-1.21) |
| pT (ref, T1) |
|
|
|
|
| T2 | 0.00009 | 3.01
(1.54-5.12) | 0.36 | 0.44
(0.07-2.4) |
| T3 |
1.36×10−12 | 5.69
(3.24-8.21) | 0.32 | 0.44
(0.09-2.17) |
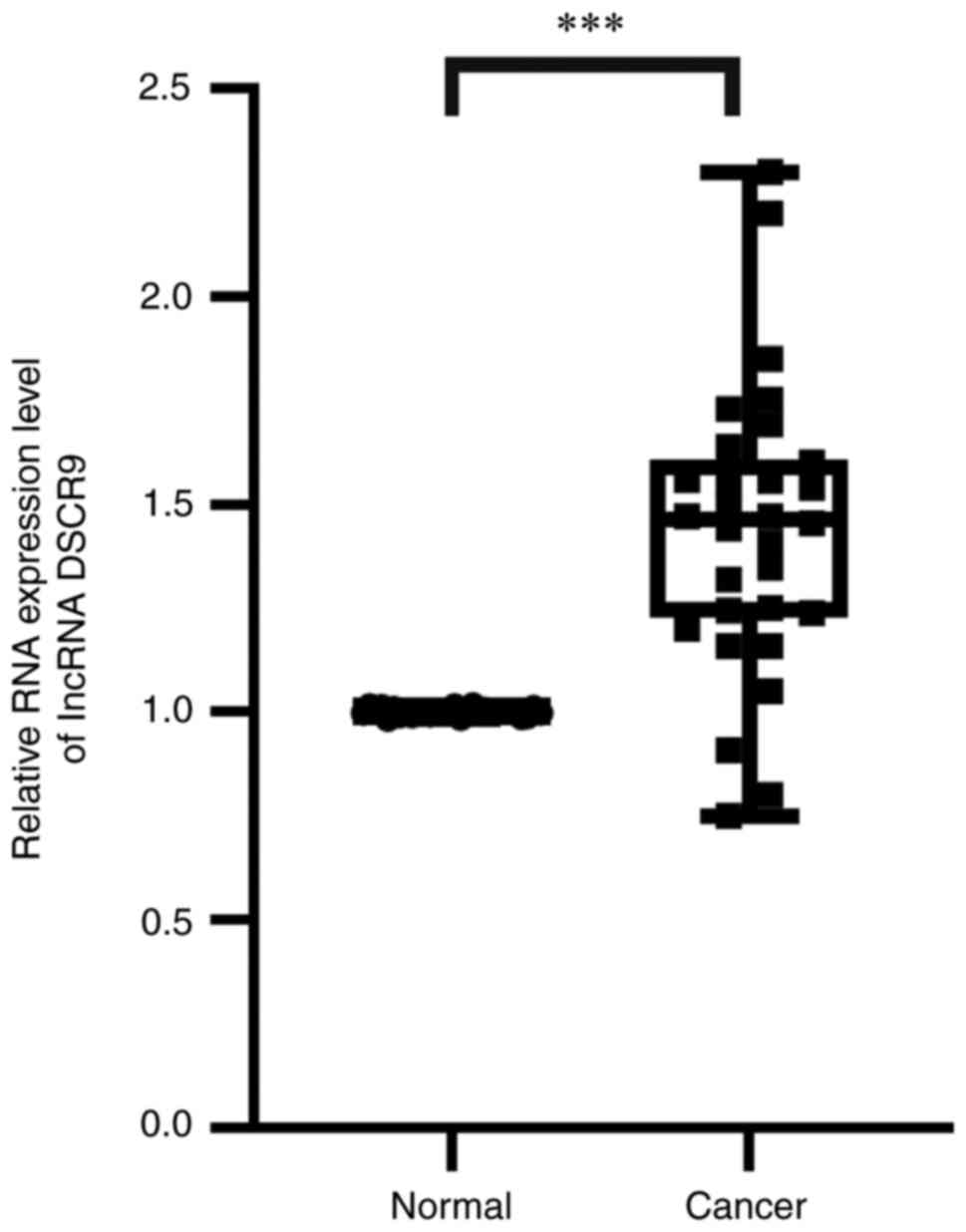
DSCR9 was upregulated in ccRCC
tissues
lncRNA DSCR9 was further assessed to validate it's
expression in tumor and normal tissues. This was because DSCR9 was
identified in the top 5 lncRNAs associated with PFS and had the
highest hazard ratio (HR) (HR=2.15). Moreover, further analysis of
regulatory network showed that DSCR9 was the only one of the top 5
PFS-related lncRNAs to be included in the final network.
A total of 30 pairs of ccRCC tissues and normal
kidney samples were used to evaluate the expression of DSCR9 in
ccRCC. The results demonstrated that the RNA expression level of
DSCR9 was significantly upregulated in ccRCC compared with normal
kidney samples (Fig. 7).
Discussion
The present study identified the key immune-related
lncRNAs in the process of the distant metastasis of ccRCC and
constructed ceRNA networks based on the immune-related lncRNAs.
First, differentially expressed lncRNAs, miRNAs and mRNAs between
ccRCC and normal samples were screened using three independent
datasets, which identified 408 prognostic lncRNAs and 82 distant
metastasis-related lncRNAs. Pathway analysis demonstrated that the
82 lncRNAs were mainly involved in immune-related pathways. Based
on the clustering, 52 immune-related lncRNAs were identified.
Finally, ceRNA networks including 14 lncRNAs, 13 DE miRNAs and 107
DE mRNAs were constructed. DSCR9 may serve an important role in the
regulatory network. It was demonstrated that DSCR9 could be an
independent risk factor for the PFS prognosis of patients with
ccRCC. Further analyses indicated DSCR9 might be associated with
immunotherapeutic response, and the association between DSCR9 and
immunotherapy response could be an area for future study. The
present study demonstrated a significant association between DSCR9
and ccRCC prognosis based on Cox regression analysis. Furthermore,
it was demonstrated that DSCR9 was an unfavorable prognostic factor
of PFS for ccRCC (log-rank P<3.1×10−5; HR=2.15; 95%
CI of HR, 1.49-3.11). The association between DSCR9 and
immune-related pathways was demonstrated based on correlation
analysis and pathway enrichment analysis. The expression of DSCR9
was positively associated with the expression of the immune
checkpoint blockade therapy target PDCD1 and positively correlated
with CTLA-4. These data implied that DSCR9 might play a role in
immune response, which could be a future direction of study.
Gene expression in most cancers is a dynamic process
involving all stages of tumor progression. Genes might have
different expression statuses (upregulated or downregulated) in
different stages of cancer progression. For example, loss of the
H3K36me3 demethylase SETD2 has been reported in both primary ccRCC
and metastases of ccRCC (22),
while decreased methylation in regional H3K36me3 was only reported
in lesions of distant metastases (23), to the best of our knowledge, which
indicated that identification of dynamically expressed genes is
crucial for understanding ccRCC metastasis. Thus, the present study
applied a previously reported algorithm (18) to identify distant metastasis-related
lncRNA events, including lncRNAs with reversed expression change
and lncRNAs with consistent expression change. For example,
LINC00652 (Delta=−3) demonstrated upregulation in non-distant
metastatic ccRCC and downregulation in distant metastatic ccRCC.
Further studies should be conducted to investigate the mechanism of
LINC00652 in in non-distant metastatic ccRCC and distant metastatic
ccRCC in the future. Another lncRNA, DSCR9 (Delta=1), demonstrated
consistent upregulation in both non-metastatic and metastatic
ccRCC. These examples indicated the dynamic behaviors of the
prognosis-related modulators in ccRCC distant metastasis. The
present study identified 82 distant metastasis-related lncRNAs,
certain of which were also associated with patient OS and PFS in
ccRCC. For example, lncRNAs FAM225A/B, SNHG9 and SNHG25 (24) were unfavorable prognostic factors,
while lncRNAs AC067959.1, DSCR9 and AC009014.3 were favorable
prognostic factors.
Enrichment analysis is usually used to evaluate the
main functions and pathways of a set of non-coding genes. In the
present study, this method was used to distinguish whether a single
lncRNA was associated with immune-related signaling pathways. It
was demonstrated that the 82 lncRNAs were mainly involved in
immune-related pathways, such as T-cell receptor signaling pathway,
NK cell-mediated cytotoxicity, cytokine-cytokine receptor
interactions and chemokine signaling pathway. A recent study
reported the role of lncRNA in T-cell and NK-cell immunology; the
lncRNA HOTAIR facilitated the induction of IκBα phosphorylation by
suppressing the expression of the NF-κB upstream protein UBXN1,
which promoted NF-κB phosphorylation and nuclear translocation in
gliomas. In vivo, HOTAIR reduction decreased PD-L1 protein
expression, which indicated that cells may have been targeted by
immune T cells (25). lncRNA NCAL1
enhanced the cytotoxicity of NK cells toward tumor cells through
the GAB2-PI3K-AKT pathway (26).
Moreover, the results of the present study also demonstrated that
the 82 lncRNAs were distant-metastasis-related because numerous
metastasis-related functions and pathways, such as cell
junction/adhesions and CAMs, were identified. Furthermore, ceRNA
regulatory networks were inferred based on the immune-related
lncRNAs using miRcode (16) and
miRDB (17) databases. The
miR-200-centered regulation network was identified. mir-200 is a
critical miRNA in the progression of ccRCC (27). The specific mechanism of DSCR9 in
regulating miR-200 in ccRCC needs to be elucidated in the
future.
In the constructed ceRNA networks, two cohorts were
used to validate that DSCR9 was an independent unfavorable factor
for PFS in patients with ccRCC. DSCR9 was first reported to express
preferentially in testis with unknown function in 2002 (28). Studies in subsequent years reported
that it was expressed in numerous tissues, such as the kidney
(29), prostate (30), testis (29) and breast (31). The genetic locus of DSCR9 (21q22.13)
was linked to the human eye color phenotype (32). DSCR9 was also reported to be
associated with prostate cancer (30), Down's syndrome (33) and patients with rheumatoid arthritis
(34). Recent studies reported that
DSCR9 was significantly associated with immune infiltration and
survival, in conditions including pancreatic cancer (35) and triple-negative breast cancer
(31). The present study
demonstrated the DSCR9 was associated with CTLA4 and PDCD1, which
indicated that it may be related to immunotherapeutic
responses.
In the present study, correlation between lncRNA
DSCR9 and tumor metastasis and immune-related pathways was
demonstrated based on a series of data analyses. It is demonstrated
that DSCR9 could be an independent risk factor for the PFS
prognosis of patients with ccRCC. Patients with a higher level of
DSCR9 demonstrated worse PFS. It is essential to determine whether
more lncRNAs could serve as biomarkers and therapeutic targets.
Moreover, how DSCR9 regulates ccRCC metastasis, for example, by the
T cell receptor signaling pathway and the specific molecular
mechanism require further study and elucidation. Addressing these
points is crucial for understanding the biological function of
DSCR9 in ccRCC metastasis.
The limitations of the present study should be
disclosed. First, although the PFS prognostic potential of DSCR9 in
ccRCC was discovered, validation using further independent cohorts
and prospective clinical trials are needed in the future. Second,
the predicted regulation network implied a role for DSCR9 in ccRCC
progression potentially by interacting with miRNAs. However, to
validate the role of DSCR9, fluorescence in situ
hybridization dual-luciferase reporter experiments should be
performed in the future to explore the specific binding site of
DSCR9 with miR-200.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by the Natural Science Foundation of
Jiangxi (grant no. 20212BAB206038) and the Jiangxi Provincial
Health Technology Project (grant no. 202210339).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available as follows: GSE53757 (https://www.ncbi.nlm.nih.gov/geo), GSE66270
(https://www.ncbi.nlm.nih.gov/geo),
TCGA-KIRC (https://portal.gdc.cancer.gov/) and KIRC-US dataset of
ICGC downloaded from UCSC Xena (https://xenabrowser.net/hub/).
Authors' contributions
JX and WL conceived and designed the study. BF, YL,
JL, JZ and LY downloaded, processed, analyzed and interpreted the
data. JX and YL confirm the authenticity of all the raw data. WL
and LY provided constructive advice for conception and data
analyses. JX wrote the manuscript. BF revised the manuscript
critically for important intellectual content. JX supervised the
study. All authors were responsible for reviewing the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all participating
patients by Shanghai Outdo Biotech Co., Ltd. Ethical approval for
the use of ccRCC samples was provided.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonasch E, Walker CL and Rathmell WK:
Clear cell renal cell carcinoma ontogeny and mechanisms of
lethality. Nat Rev Nephrol. 17:245–261. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Terry S, Dalban C, Rioux-Leclercq N, Adam
J, Meylan M, Buart S, Bougoüin A, Lespagnol A, Dugay F, Moreno IC,
et al: Association of AXL and PD-L1 expression with clinical
outcomes in patients with advanced renal cell carcinoma treated
with PD-1 blockade. Clin Cancer Res. 27:6749–6760. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang
Y, Jia L, Li S; Cancer Genome Atlas Research Network, ; Xie W and
Yang D: lncRNA epigenetic landscape analysis identifies EPIC1 as an
oncogenic lncRNA that interacts with MYC and promotes cell-cycle
progression in cancer. Cancer Cell. 33:706–720.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Xie Z, Lei X and Gan R: Long
non-coding RNA GAS5 in human cancer. Oncol Lett. 20:2587–2594.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Z, Li X, Yang Y, He Z, Qu X and Zhang
Y: Long noncoding RNAs in the progression, metastasis, and
prognosis of osteosarcoma. Cell Death Dis. 7:e23892016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma H, Chang H, Yang W, Lu Y, Hu J and Jin
S: A novel IFNα-induced long noncoding RNA negatively regulates
immunosuppression by interrupting H3K27 acetylation in head and
neck squamous cell carcinoma. Mol Cancer. 19:42020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, You H and Yu S: Long non-coding
RNA HOXA-AS2 promotes the expression levels of hypoxia-inducible
factor-1α and programmed death-ligand 1, and regulates
nasopharyngeal carcinoma progression via miR-519. Oncol Lett.
20:2452020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Braga EA, Fridman MV, Moscovtsev AA,
Filippova EA, Dmitriev AA and Kushlinskii NE: LncRNAs in ovarian
cancer progression, metastasis, and main pathways: ceRNA and
alternative mechanisms. Int J Mol Sci. 21:88552020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao K, Zhang Q, Wang Y, Zhang J, Cong R,
Song N and Wang Z: The construction and analysis of competitive
endogenous RNA (ceRNA) networks in metastatic renal cell carcinoma:
A study based on the cancer genome atlas. Transl Androl Urol.
9:303–311. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
von Roemeling CA, Radisky DC, Marlow LA,
Cooper SJ, Grebe SK, Anastasiadis PZ, Tun HW and Copland JA:
Neuronal pentraxin 2 supports clear cell renal cell carcinoma by
activating the AMPA-selective glutamate receptor-4. Cancer Res.
74:4796–4810. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wotschofsky Z, Gummlich L, Liep J, Stephan
C, Kilic E, Jung K, Billaud JN and Meyer HA: Integrated microRNA
and mRNA signature associated with the transition from the locally
confined to the metastasized clear cell renal cell carcinoma
exemplified by miR-146-5p. PLoS One. 11:e01487462016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Z, Irizarry RA, Gentleman R,
Martinez-Murillo F and Spencer F: A model-based background
adjustment for oligonucleotide expression arrays. J Am Stat Assoc.
99:909–917. 2004. View Article : Google Scholar
|
|
15
|
Zhang H, Chen X, Zhang D, Liu L, Song J,
Xu Y and Tian J: Identification of a novel six-long noncoding RNA
signature for molecular diagnosis of dilated cardiomyopathy. DNA
Cell Biol. Nov 3–2020.(Epub ahead of print). View Article : Google Scholar
|
|
16
|
Jeggari A, Marks DS and Larsson E:
miRcode: A map of putative microRNA target sites in the long
non-coding transcriptome. Bioinformatics. 28:2062–2063. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:(Database Issue). D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song J, Song F, Liu K, Zhang W, Luo R,
Tang Y and Ran L: Multi-omics analysis reveals
epithelial-mesenchymal transition-related gene FOXM1 as a novel
prognostic biomarker in clear cell renal carcinoma. Aging (Albany
NY). 11:10316–10337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanehisa M: The KEGG database. Novartis
Found Symp. 247:91–103. 119–128. 244–252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Han D, Zhao Y, Zhang C, Shi X and
Gu W: Multi-omics analysis of the prognosis and biological function
for TRPV channel family in clear cell renal cell carcinoma. Front
Immunol. 13:8721702022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hakimi AA, Ostrovnaya I, Reva B, Schultz
N, Chen YB, Gonen M, Liu H, Takeda S, Voss MH, Tickoo SK, et al:
Adverse outcomes in clear cell renal cell carcinoma with mutations
of 3p21 epigenetic regulators BAP1 and SETD2: A report by MSKCC and
the KIRC TCGA research network. Clin Cancer Res. 19:3259–3267.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho TH, Park IY, Zhao H, Tong P, Champion
MD, Yan H, Monzon FA, Hoang A, Tamboli P, Parker AS, et al:
High-resolution profiling of histone h3 lysine 36 trimethylation in
metastatic renal cell carcinoma. Oncogene. 35:1565–1574. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang W, Zhang K, Li L, Ma K, Hong B, Gong
Y and Gong K: Discovery and validation of the prognostic value of
the lncRNAs encoding snoRNAs in patients with clear cell renal cell
carcinoma. Aging (Albany NY). 12:4424–4444. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Yi K, Liu X, Tan Y, Jin W, Li Y,
Zhou J, Wang H and Kang C: HOTAIR up-regulation activates NF-κB to
induce immunoescape in gliomas. Front Immunol. 12:7854632021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niu C, Li M, Chen Y, Zhang X, Zhu S, Zhou
X, Zhou L, Li Z, Xu J, Hu JF, et al: LncRNA NCAL1 potentiates
natural killer cell cytotoxicity through the Gab2-PI3K-AKT pathway.
Front Immunol. 13:9701952022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao C, Peng FH and Peng LK: MiR-200c
sensitizes clear-cell renal cell carcinoma cells to sorafenib and
imatinib by targeting heme oxygenase-1. Neoplasma. 61:680–689.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takamatsu K, Maekawa K, Togashi T, Choi
DK, Suzuki Y, Taylor TD, Toyoda A, Sugano S, Fujiyama A, Hattori M,
et al: Identification of two novel primate-specific genes in DSCR.
DNA Res. 9:89–97. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodriguez-Sanchez IP, Garza-Rodríguez ML,
Tejero ME, Cole SA, Comuzzie AG and Barrera-Saldaña HA: DSCR9 gene
simultaneous expression in placental, testicular and renal tissues
from baboon (Papio hamadryas). BMC Res Notes. 5:2982012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yegnasubramanian S, Wu Z, Haffner MC,
Esopi D, Aryee MJ, Badrinath R, He TL, Morgan JD, Carvalho B, Zheng
Q, et al: Chromosome-wide mapping of DNA methylation patterns in
normal and malignant prostate cells reveals pervasive methylation
of gene-associated and conserved intergenic sequences. BMC
Genomics. 12:3132011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Z, Mi M, Li X, Zheng X, Wu G and Zhang
L: lncRNA OSTN-AS1 may represent a novel immune-related prognostic
marker for triple-negative breast cancer based on integrated
analysis of a ceRNA network. Front Genet. 10:8502019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu F, Wollstein A, Hysi PG, Ankra-Badu
GA, Spector TD, Park D, Zhu G, Larsson M, Duffy DL, Montgomery GW,
et al: Digital quantification of human eye color highlights genetic
association of three new loci. PLoS Genet. 6:e10009342010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen M, Wang J, Luo Y, Huang K, Shi X, Liu
Y, Li J, Lai Z, Xue S, Gao H, et al: Identify down syndrome
transcriptome associations using integrative analysis of microarray
database and correlation-interaction network. Hum Genomics.
12:22018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wen J, Liu J, Jiang H, Wan L, Xin L, Sun
Y, Zhang P, Sun Y, Zhang Y, Du X, et al: lncRNA expression profiles
related to apoptosis and autophagy in peripheral blood mononuclear
cells of patients with rheumatoid arthritis. FEBS Open Bio.
10:1642–1654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhuang H, Huang S, Zhou Z, Ma Z, Zhang Z,
Zhang C and Hou B: A four prognosis-associated lncRNAs (PALnc)
based risk score system reflects immune cell infiltration and
predicts patient survival in pancreatic cancer. Cancer Cell Int.
20:4932020. View Article : Google Scholar : PubMed/NCBI
|