Introduction
Lung cancer is the most common cancer worldwide and
is the leading cause of cancer deaths, with low survival and high
morbidity rates (1,2). The major forms of lung cancer are
non-small cell lung cancer (NSCLC) and small-cell lung cancer
(SCLC) (3,4). NSCLC accounts for 85% of all lung
cancers, compared with 15% SCLC. A survey showed that nearly 14
million people die from NSCLC every year, and this number will
increase in developing countries during the next few decades
(5). Aggressive surgery,
conventional chemotherapy, and targeted therapeutics are currently
the standard treatment options for NSCLC (6–8).
However, neoplasm recurrence, insufficient effectiveness, acquired
drug resistance, and inevitable side effects have limited the
application of those therapies, resulting in unsatisfactory
clinical outcomes. Therefore, new drug candidates with high
therapeutic efficacy and less adverse effects are urgently needed
for patients with NSCLC (9,10).
In recent years, natural products have attracted
increasing attention as promising candidates of anti-cancer drugs
(11,12). Polyphenolic compounds that exist in
natural foods exert beneficial effects on human health by promoting
gastrointestinal digestion, preventing excessive thrombosis,
lowering blood pressure, and also possess preventive effects
against cancers (13–16). Green tea is a commonly consumed
beverage containing a large number of bioactive ingredients.
Epidemiological studies have discovered that intake of green tea
(12 cups per day) can decrease the risk of cancers, and many
studies have demonstrated the anti-cancer ability of green tea
extract (17). Theabrownin (TB),
the bioactive component of green tea, has been reported to possess
anti-NSCLC activity via a p53-dependent mechanism in our previous
studies (18,19). The in vivo data showed that
TB clearly inhibited the growth of NSCLC cell mass in the zebrafish
xenograft model with inhibitory rates from 27 to 34%, and its
anti-NSCLC efficacy was even better than that of cisplatin (26.1%).
Moreover, no toxicity or side effects of TB were observed in its
effective dose range (the LD0, 213 µg/ml), while cisplatin induced
apoptosis of normal tissues (20).
These results suggested that TB was effective and safe for treating
NSCLC.
TB contains various active components, such as
epigallocatechin gallate (EGCG), epigallocatechin (ECG) and gallic
acid (GA) (Fig. S1). After the
literature search, EGCG and GA were selected as potential
candidates of anti-cancer components (21,22).
Following the preliminary screening with CCK-8 (data not shown), we
found that GA exerted stronger activity than EGCG. Therefore, this
study was conducted to in-depth evaluate the anti-NSCLC effect of
GA. NSCLC cell lines (H1299 and A549) were employed to evaluate the
effects of GA. This study determined GA's anti-NSCLC properties and
validated its efficacy in the p53-mutated NSCLC (H1299) cells,
which might facilitate the development of a new anti-NSCLC drug
from natural products.
Materials and methods
Materials
Gallic acid (GA, >99% of purity) was obtained
from Micklin (Shanghai, China). Roswell Park Memorial Institute
(RPMI) 1640 medium was purchased from Hyclone (Logan, USA). Fetal
bovine serum (FBS) was purchased from CellMax (Beijing, China).
Phosphate buffered saline (PBS) was purchased from Basal Media
(Shanghai, China). RIPA lysis buffer and trypsin were purchased
from Thermo Fisher Scientific (MA, USA). CCK-8 was obtained from
Bimake (TX, USA). DAPI was purchased from Invitrogen (CA, USA). All
antibodies were purchased from Cell Signaling Technology Inc (MA,
USA).
Cell culture
Human NSCLC H1299 and A549 cell lines were obtained
from the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). H1299 was cultured in RPMI 1640 medium
containing 10% fetal bovine serum and A549 was cultured in
Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine
serum, in a 5% CO2 cell culture incubator. Cells were
passaged every 2–3 days and subcultured to 90% confluence.
Cell viability assay
The CCK-8 was used to detect the effect of GA on
H1299 cells. Briefly, the cells were seeded in 96-well plates at a
density of 6×103 cells/well in 200 µl medium overnight
and treated with GA at various concentrations (0, 10, 13, 17, 20,
23, 27, 30 µg/ml) for 24 h. Then, CCK-8 working solution was added
to each well and incubated at 37°C for 2 h. And the optical density
(OD) value at 450 nm was measured by microplate reader (Synergy H1,
Bio Tek Instruments, Inc). The formula selected: Survival rate
(%)=(GA-treated OD/untreated OD) ×100%. According to the
IC50 value of 24 h, three doses were chosen for further
use.
DAPI staining
The DAPI staining was performed to determine the
apoptosis of H1299 cells induced by GA. The cells were seeded in
24-well plates at a density of 3×104 cells/well in 1 ml
medium overnight and treated with GA at low, medium, and high doses
for 24 h. After GA treatment, the cells were washed twice in 400 µl
PBS and fixed with 4% paraformaldehyde for reaction in 30 min at
37°C. Then 0.5% Triton X-100 was used to permeabilize the cells in
the dark. Cells were mounted with DAPI staining and finally
observed staining cells under a fluorescence microscope (Carl
Zeiss, Gottingen, Germany). Apoptotic nuclei were visualized and
counted on three cover slips for each group.
Flow cytometry
The Annexin-V/PI staining-based flow cytometry was
performed to determine the cell apoptosis of H1299 cells induced by
GA. The cells were seeded with a density of 5×105
cells/well in 10 ml medium overnight and treated with GA at low,
medium, and high doses for 24 h. After GA treatment, the cells were
harvested and washed twice with cold PBS, then resuspended with
binding buffer. Then the cells were labeled with FITC Annexin-V and
PI respectively in the dark. Fluorescence intensity was detected by
flow cytometry (BD Biosciences, CA, USA). Apoptosis cell rate
(%)=(early apoptotic cells + late apoptotic cells)/total cell
number ×100%; Living cell rate (%)=normal cells/total cell number
×100%.
Wound-healing assay
The wound-healing assay was performed to test the
anti-migratory effect of H1299 cells induced by GA. The cells were
seeded in 6-well plates at a density of 3×105 cells/well
with 3 ml medium, which only contained 1.5% FBS to minimize the
proliferation component of cell migration. The cells were treated
with low, medium, and high doses of GA, followed by artificial
scratch being made in a cross from using a micropipette tip. The
cells were observed and imaged at 0, 12, 24, and 48 h under an
inverted microscope (Olympus, Tokyo, Japan).
Western blot (WB) analysis
The protein level of the treated GA was explored by
WB analysis. Total proteins of H1299 cells were extracted by RIPA
buffer with proteinase and phosphatase inhibitor cocktail (Bimake,
Houston, USA), and then determined concentration by Bradford
protein assay kit. The membrane was blocked with 5% non-fat milk
for 2 h and incubated by primary antibodies overnight. And then the
membrane was washed and incubated with a secondary antibody for 2
h. Ultimately, the nitrocellulose membrane was visualized by
Western Lightning® Plus 8 ECL (PerkinElmer, Inc,
Waltham, MA, USA), and detected using a chemiluminescence analyzer
(Bio-Rad, Inc, CA. USA). β-actin was employed as a control
protein.
Xenografted experiment
Female nude mice aged 6–8 weeks, weighing 18–22 g,
were selected for the experiment (Animal production license number:
202204-0284). After one week of adaptive feeding, 5×106
H1299 cells were injected subcutaneously which have highly invasive
abilities (23), and the mice were
randomly divided into four groups: normal control group treated
with normal saline for comparison (NC), GA-low dose group treated
with 10 mg/kg GA solution (L), GA-high dose group treated with 40
mg/kg GA solution (H), and cisplatin group treated with 2 mg/kg
cisplatin (CIS). After tumor formation, the mice were treated every
4 days by intraperitoneal injection, meanwhile, the tumor size and
nude mouse weight were measured. Tumor volumes were calculated as
V=1/2 (l) × (w)2, where l and w mean the tumor's longest
and shortest diameters, respectively. After the experiment, mice
were euthanized (the disappearance of reflex, respiratory arrest
and cardiac arrest) by intraperitoneal sodium pentobarbital
injection (100 mg/kg) and the tumor tissues were immediately fixed
in 4% paraformaldehyde and embedded in paraffin for future
experiments. All the experiments on mice were approved by the
Medical Norms and Ethics Committee of Zhejiang Chinese Medical
University (Approval number: IACUC-20220418-07).
TUNEL assay
The TUNEL staining using in situ cell death
detection kit (Roche) was performed to determine the tumor tissue
paraffin sections apoptosis. After dewaxing and rehydration, the
sections were repaired with sodium citrate buffer (pH=6) for 4 h in
60°C oven, then permeabilized using TritionX-100 (Biyuntian,
China). Add 50 µl TUNEL working solution which consists of TUNEL
enzyme solution and TUNEL label mix on the circled tissue and
incubated at 4°C overnight. The next day, after washing with PBS,
the nucleus was counterstained with DAPI. Images were acquired
using fluorescence microscope (Carl Zeiss, Gottingen, Germany).
Apoptotic cell rate=(TUNEL positive nuclei/DAPI positive nuclei)
×100%.
Immunohistochemistry analysis
Immunohistochemistry (IHC) was performed using an
immunohistochemistry kit (Zhongshan Golden Bridge, China). After
conventional dewaxing and rehydration, the sections were repaired
with sodium citrate buffer (pH=6) for 4 h in a 60°C oven, then
permeabilized using TritionX-100. A goat serum from the kit was
used to block slides before the primary antibody (PCNA: dilution
1:4,000; Cell Signaling; ATG5: dilution 1:100; Immunoway; p-AMPK:
dilution 1:100; Cell Signaling) incubation overnight at 4°C. The
next day, the slides were washed with PBS, followed by secondary
antibodies incubation for 30 min. After incubation, the slides were
washed in PBS, then the freshly prepared diaminobenzidine (DAB)
color developing solution was used. The color-development time was
checked under the microscope and the slides were washed in
ultrapure water to stop the reaction.
Statistical analyses
The mean and standard deviation (SD) of the data was
calculated. One-way ANOVA was used for data comparison among
multiple groups, followed by Dunnett test. When the P-value was
less than 0.05, the difference was judged significant; when the
P-value was less than 0.01, the difference was regarded to be of
higher importance.
Results and Discussion
The inhibitory effect of TB on NSCLC
cell lines
Previously, our team has proven that TB has a strong
pro-apoptotic effect on NSCLC through p53 signaling pathway
(18). At the same time, a high
content of GA in TB was detected by UPLC-Q/TOF-MS/MS and its
pro-apoptotic effects on NSCLC cells were demonstrated, indicating
that GA is a major active ingredient of TB (Fig. S1). While comparing the efficacies
of TB on A549 (p53-wild type) and H1299 (p53-null) cells, we found
that TB exerted stronger effects on H1299 cells (Fig. 1A). As shown in Fig. 1B and C, flow cytometry analysis
revealed that TB at 20, 40, and 60 µg/ml induced obvious apoptosis
of H1299 cells in a dose-dependent manner. The observed necrosis
might be the secondary necrosis caused by apoptosis, and this study
mainly focused on the apoptosis induction other than necrosis
occurrence. In addition, another study has shown that GA could
induce both apoptosis and necrosis on tumor cells (24), which was consistent with our
results. These results indicated the selective effect of TB on
H1299 cells. Whether GA also has better effects on the p53-null
NSCLC cells than on the p53-wild type NSCLC cells, remains
undetermined. Further studies are needed to explore the selective
effects of GA.
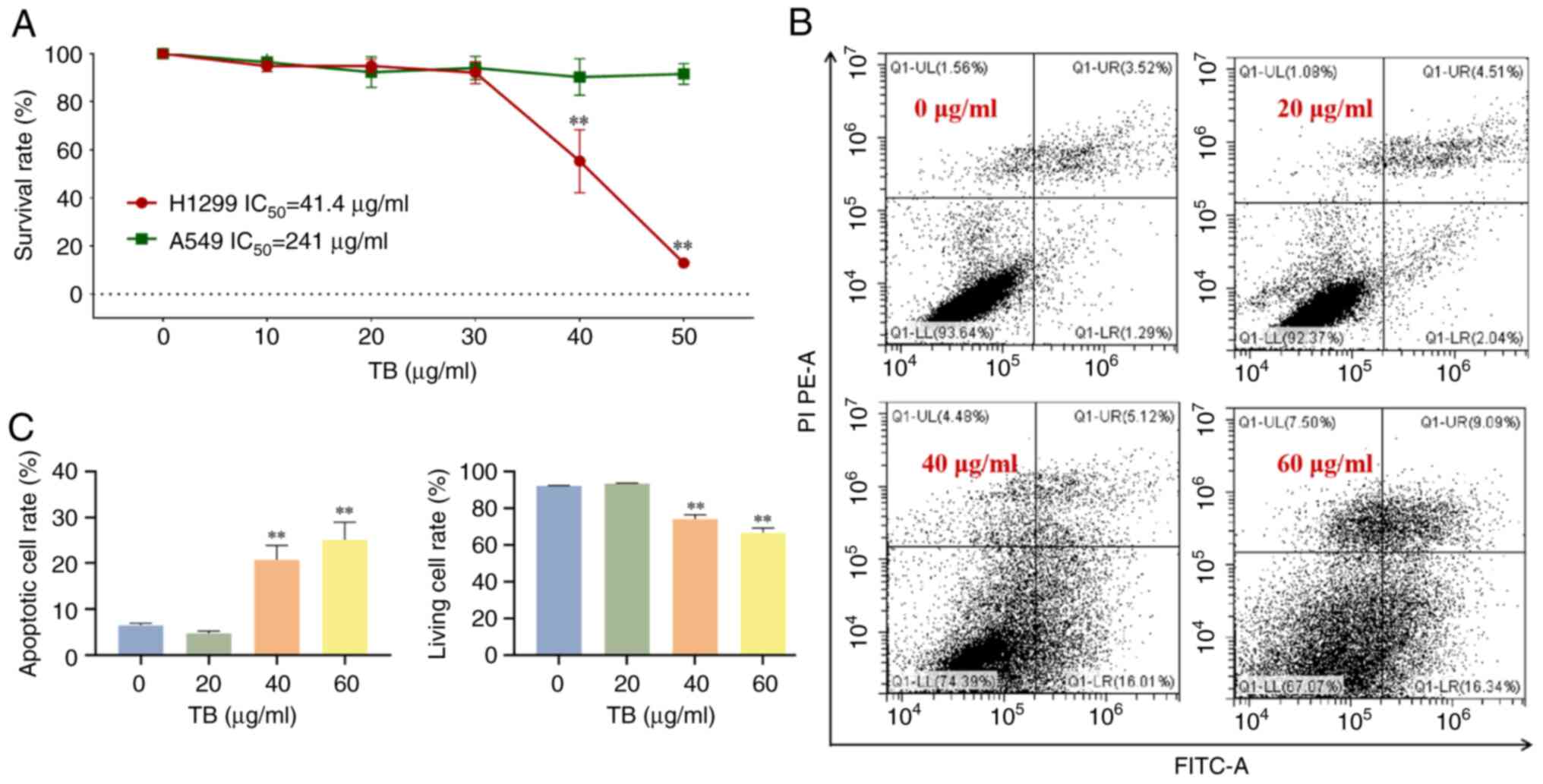 | Figure 1.(A) Survival rate of TB (0, 20, 40,
60 µg/ml) on H1299 and A549 cells at 24 h. (B) Flow cytometry
analysis on H1299 cells with TB (0, 20, 40, 60 µg/ml) treatment for
24 h. UR quadrant: Early apoptotic cells; LR quadrant: Late
apoptotic cells; UL quadrant: Necrosis cells; LL quadrant: Normal
cells. (C) Statistical data. Values were presented as the mean ± SD
(n=3). **P<0.01 vs. 0 µg/ml. LL, lower-left; LR, lower-right;
TB, theabrownin; UL, upper-left; UR, upper-right. |
The anti-NSCLC effects of GA in vitro
Anti-proliferative effect of GA
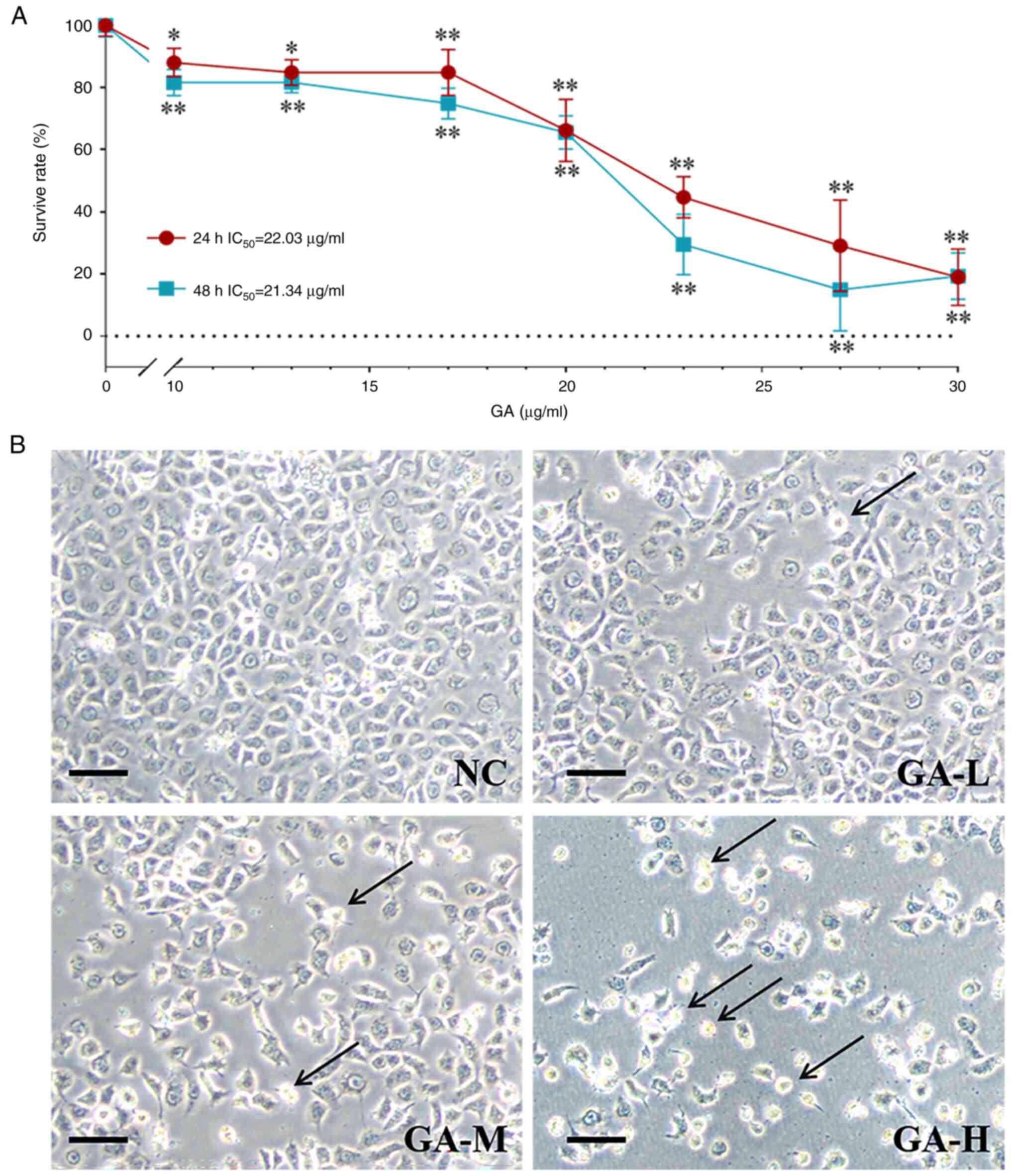
To evaluate the anti-proliferative effect of GA on
H1299 cells, CCK-8 assay and morphological observation were
performed. As shown in Fig. 2A, GA
significantly inhibited the viability of H1299 cells at a dose
range from 10 to 30 µg/ml. The IC50 values were 22.03
and 21.34 µg/ml for 24 and 48 h, respectively. Accordingly, 10, 13,
and 20 µg/ml were selected as low, medium, and high doses for
subsequent experiments. Through light microscopic, an increased
number of abnormal H1299 cells were observed to be irregular and
had shrunk (Fig. 2B).
Pro-apoptotic effect of GA
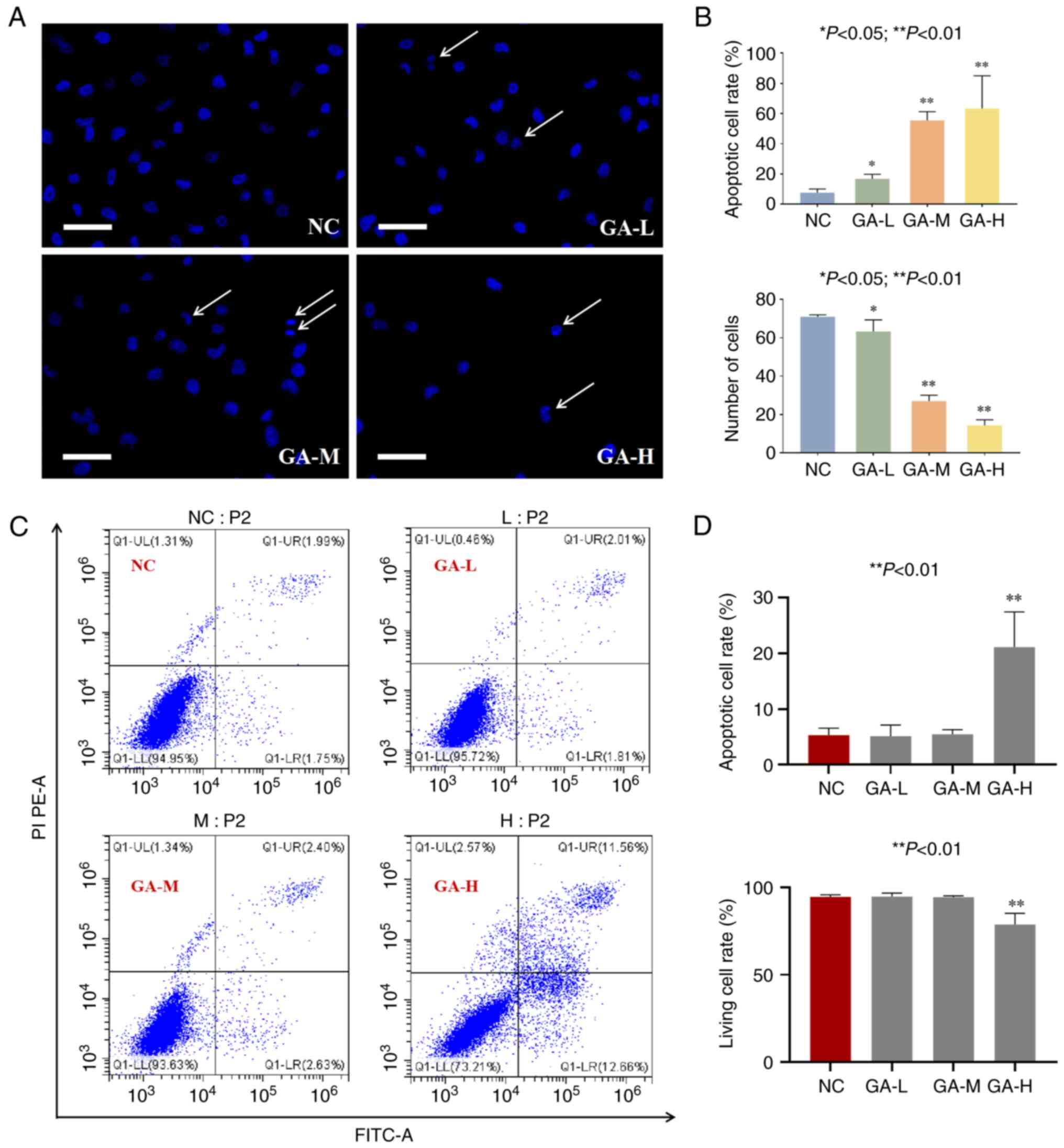
To observe the apoptosis of H1299 cells induced by
GA, DAPI staining and flow cytometry were performed. As shown in
Fig. 3A and B, GA significantly
increased the number of apoptotic cells as seen by nuclear
shrinkage (indicated by arrows) and decreased the number of normal
cells (each P<0.05 or 0.01 vs. NC).
We conducted the Annexin-V assay at the same time
point (24 h) with the DAPI test. It is because that the Annexin-V
result at an earlier time (6 h) could not show significant
difference between the groups (data not shown), suggesting that 24
h was a better time point to determine the pro-apoptotic effect of
GA. Another reason to select 24 h was that the other assays were
conducted at 24 h, so that we could obtain the results at a
consistent time point (20). As
shown in Fig. 3C and D, early
apoptosis, late apoptosis, and necrosis rates were increased
following the treatment with GA, suggesting that GA exerted a
pro-apoptotic effect on H1299 cells in a dose-dependent manner.
Anti-migratory effect of GA
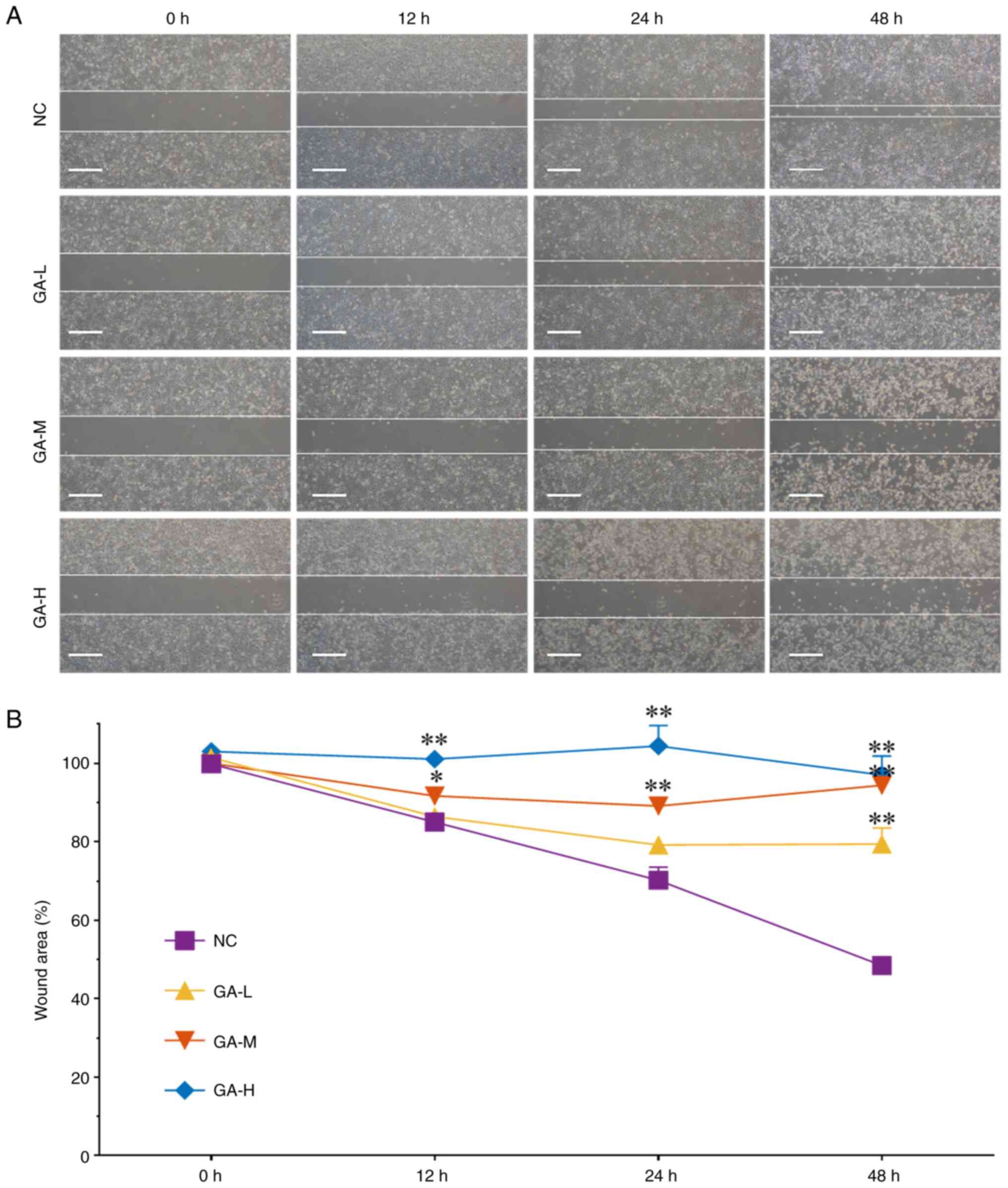
To observe the anti-migratory effect of GA, wound
healing assay was performed. As shown in Fig. 4A, GA significantly inhibited the
wounding area ratio of H1299 cells following treatment for 12, 24,
and 48 h at low, medium, and high doses (each P<0.05 or 0.01 vs.
NC), suggesting that GA inhibited H1299 cells migration in dose-
and time-dependent manners.
Molecular actions of GA
It is now widely believed that apoptosis is a
hindrance in cancer proliferation since it halts the physiological
process, preventing progression to further steps such as
embryogenesis, morphogenesis, and tissue homeostasis (25–27).
The primary physiological function of apoptosis is to eliminate
damaged cells early in development or to maintain somatic
homeostasis later.
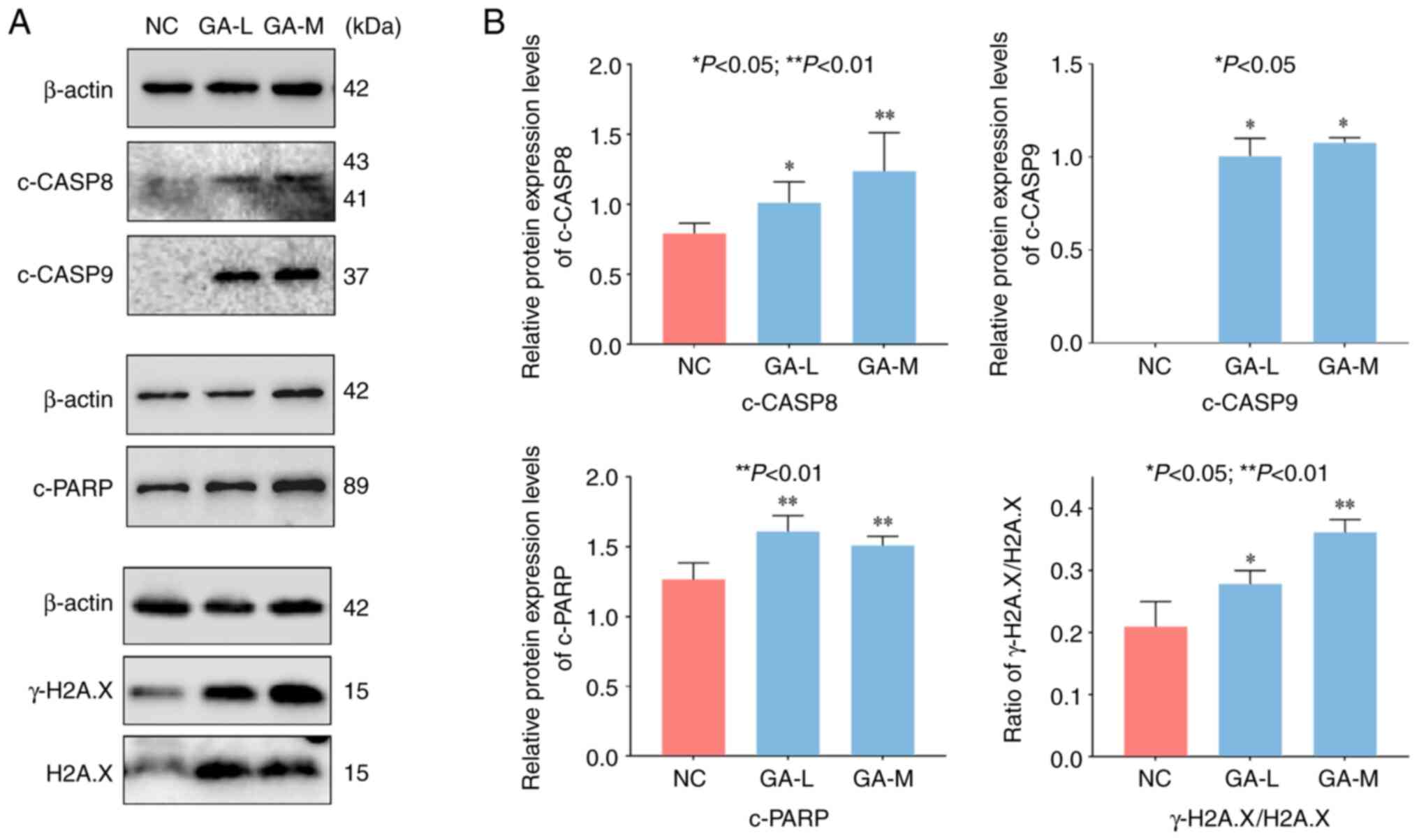
In this study, the results of cell experiments
showed that GA induced apoptosis of H1299 cells by increasing the
ratio of γ-H2A.X/H2A.X, and cleaved (c-)PARP, c-caspase-8 and
c-caspase-9 levels (Fig. 5). The
ratio of γ-H2A.X/H2A.X is a strong indicator of apoptosis-induced
DNA fragmentation, participating in the earliest cellular responses
to DNA damage (28). PARP is
responsible for DNA repair and maintaining cell viability during
external stress. It can be cleaved into two fragments (89 and 25
kDa), which serves as a marker of apoptosis (29). The WB data showed that the ratio of
γ-H2A.X/H2A.X and c-PARP protein levels were significantly
up-regulated, indicating that GA triggered DNA damage to induce
apoptosis of H1299 cells. Meanwhile, in caspase-mediated apoptosis,
caspase-8 and caspase-9 are the molecules responsible for
initiating apoptosis (30,31). Caspase-8 is the main executor of
apoptosis, which releases cytochrome c through the intracellular
pathway to trigger apoptosis (32,33),
while caspase-9, as an essential promoter in the apoptosis
signaling pathway, participates in compound-activated apoptosis
(34). The activation of these
molecules revealed that GA induced H1299 cells apoptosis in a
dose-dependent manner. In addition, no cell survived in the
high-dose group due to the highly inhibitory effect of GA, and
thereby the protein level was too low to be detected. Therefore,
the result of the high-dose group was not shown. In sum, these data
indicated that GA exerted significant anti-proliferative,
pro-apoptotic, and anti-migratory effects on H1299 cells in
vitro.
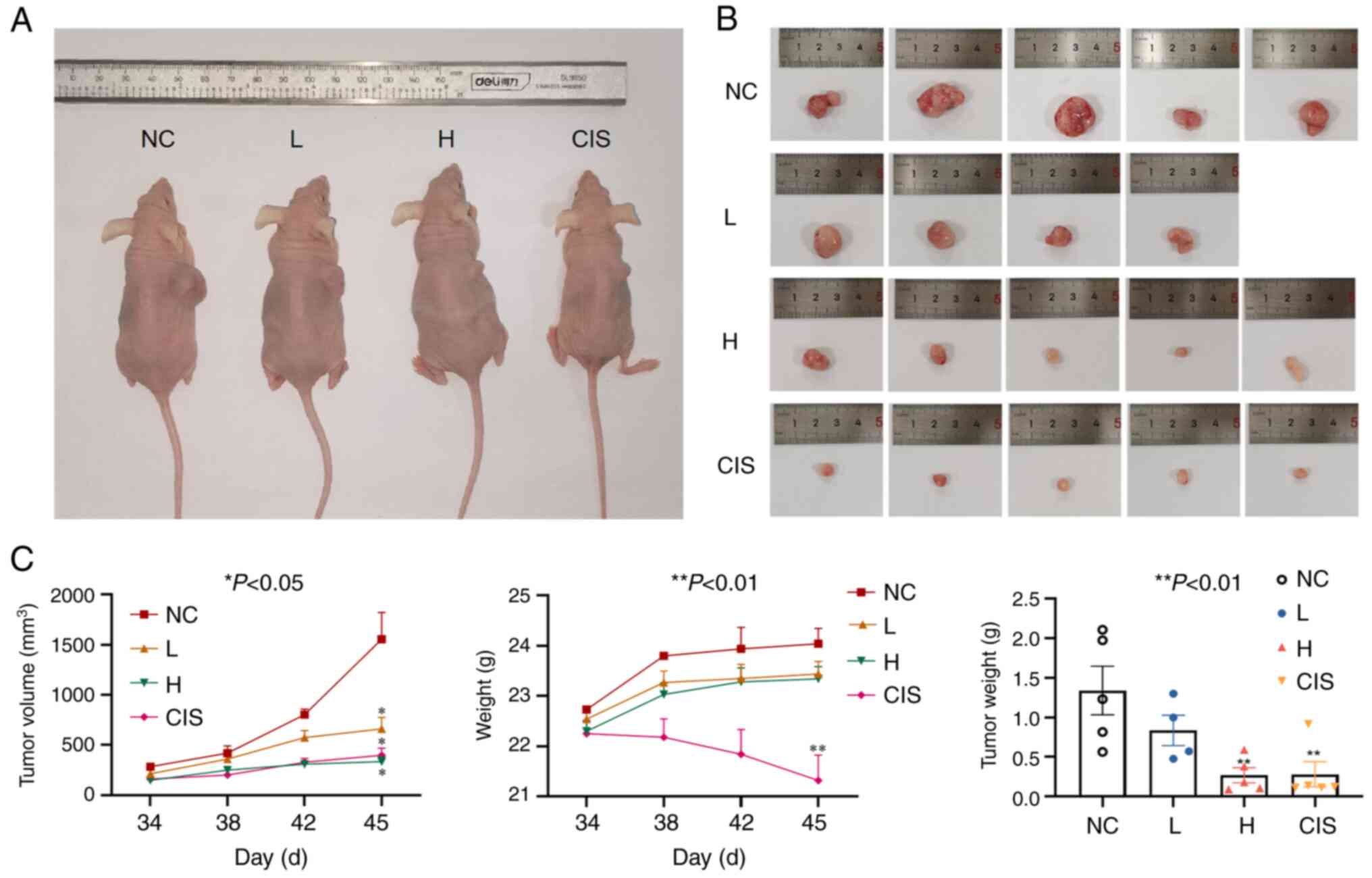
The anti-tumor effects of GA in
vivo
The nude mouse xenograft model was established to
assess the anti-tumor potential of GA in vivo. As shown in
Fig. 6, GA significantly reduced
the tumor size of nude mice in a dose-dependent manner. Meanwhile,
the high dose group of GA had tumors of approximately the same size
as those in the cisplatin control group, but the mice of the
cisplatin group lost much more weight.
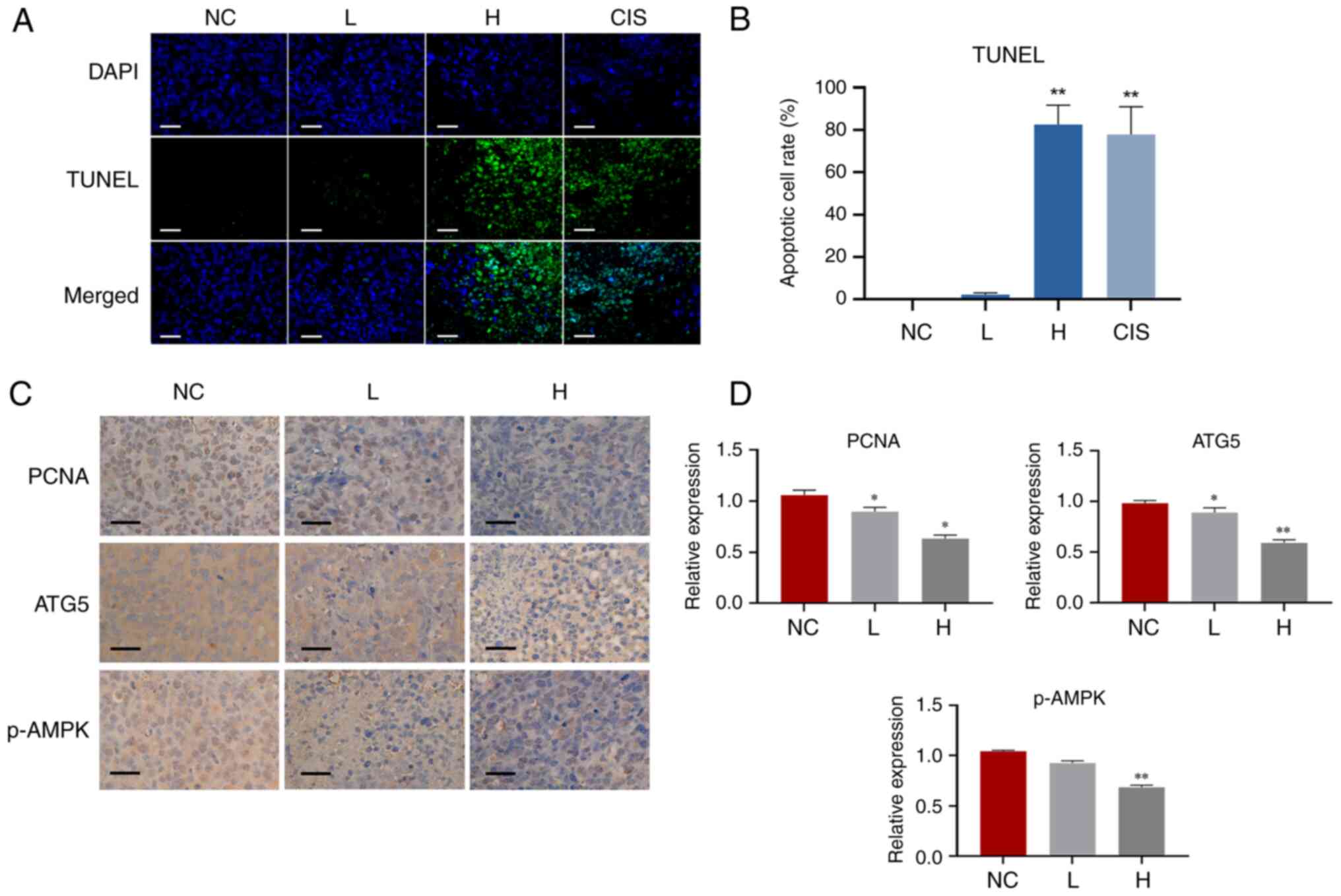
The apoptotic cells in tumor were assessed using
TUNEL assay. As shown in Fig. 7A and
B, cisplatin was included as positive control, and the number
of apoptotic cells (green fluorescence) increased after GA
treatment, demonstrating that GA induced apoptosis in H1299 cells.
Meanwhile, PCNA expression was verified to determine cell
proliferation rate in vivo through IHC analysis (Fig. 7C and D), and the data showed that GA
reduced the expression of PCNA.
In addition, accumulated research has shown that
apoptosis can be activated through autophagy inhibition (35). Therefore, we conjectured that GA's
pro-apoptotic effect was associated with autophagy. Autophagy is a
mechanism that controls cellular homeostasis through the autolysis
of lysosomal enzymes, which is considered as a tumor survival
mechanism that helps tumor cells resist drugs (36). However, accumulating evidence
suggests that autophagy can also promote tumor cell apoptosis by
degrading the reticulum, Golgi apparatus, and other organelles,
causing precancerous cells to have a negative protein balance and
thereby suppressing uncontrolled proliferation (37,38).
Autophagy related 5 (ATG5) is a key initiator of autophagy required
for autophagosome formation and has an important effect on the
occurrence and changes in autophagy phases (39,40).
Adenosine monophosphate (AMP) activated protein kinase (AMPK) is a
trigger of autophagy and phosphorylation of AMPK has in fact been
shown to promote autophagy induction (41–43).
In the present study, the level of ATG5 and p-AMPK were all reduced
by GA (Fig. 7C and D), indicating
the pro-apoptotic efficacy of GA was mediated through autophagy
inhibition.
For decades, polyphenols have been used as natural
products to protect plants from oxidative stress and UV damage, and
to attract pollen and animal material for seed dispersal. The
anti-oxidant, anti-inflammatory and anti-cancer effects of
polyphenols are receiving increasing attention in recent years
(44,45). Previous studies reported that the
combination of ascorbic acid, lysine, proline and at least one
phenolic compound can be used as an agent for cancer prevention and
treatment. As the most common derivatives of the hydroxybenzoic
acid, GA received increasing attention in this regard. Numerous
in vitro and in vivo studies have shown that GA has
broad pharmacological and therapeutic effects on a variety of
cancer cells through a pleiotropic molecular mechanism (e.g., cell
apoptotic processes, cell cycle, angiogenesis and invasion)
(46,47). In this study, the anti-NSCLC effects
of GA was evaluated.
Our previous research mainly focused on the
p53-dependent mechanism of TB, and the IC50 value of TB
against A549 cells was 239.9 µg/ml at 24 h (48). Consistently, the IC50
value of TB against A549 cells was 241 µg/ml at 24 h in the present
study, which was higher than the IC50 value of TB
against H1299 cells (41.4 µg/ml), indicating that the
anti-proliferation effect of TB on H1299 cells was better than that
of A549. Since H1299 is a p53-null cell line and A549 is a p53-wild
type cell line, we hypothesize that the inhibition difference of TB
on both cells may be related to the P53 pathway. Meanwhile, this
study demonstrates for the first time that GA can induce apoptosis
in NSCLC cells by inhibiting autophagy. It provides new insights
into the anti-NSCLC efficacy of GA and lays the foundation for our
next mechanism study, contributing to the application prospects of
GA as a therapeutic agent for NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation
of China (grant no. 81893049), Zhejiang traditional Chinese
medicine science and technology plan project (grant no. 2015ZA194),
and Science and Technology Development Project of Hangzhou (grant
no. 2020ZDSJ0900).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT, JX and LS conceptualized the study. XT and JX
analyzed data. JC, XD, QD, LZ, QY and LS provided financial
support. LZ and QY designed the method and JC, XD, and QD
participated in the method design. YY, XX, LY and SY designed and
performed experiments that generated the preliminary data. LZ, QY
and LS supervised the study. XT and JX wrote the original draft. XT
and JX confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments on the mice were approved by the
Medical Norms and Ethics Committee of Zhejiang Chinese Medical
University (approval number: IACUC-20220418-07).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hua Q, Jin M, Mi B, Xu F, Li T, Zhao L,
Liu J and Huang G: LINC01123, a c-Myc-activated long non-coding
RNA, promotes proliferation and aerobic glycolysis of non-small
cell lung cancer through miR-199a-5p/c-Myc axis. J Hematol Oncol.
12:912019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruiz EJ, Diefenbacher ME, Nelson JK,
Sancho R, Pucci F, Chakraborty A, Moreno P, Annibaldi A, Liccardi
G, Encheva V, et al: LUBAC determines chemotherapy resistance in
squamous cell lung cancer. J Exp Med. 216:450–465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Liu A, Wang Z, Wang B, Chai X, Lu
W, Cao T, Li R, Wu M, Lu Z, et al: LINC00173.v1 promotes
angiogenesis and progression of lung squamous cell carcinoma by
sponging miR-511-5p to regulate VEGFA expression. Mol Cancer.
19:982020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng W, Xu T, Xu Y, Wang Y, Liu X, Zhao Y,
Yang P and Liao Z: Survival patterns for patients with resected n2
non-small cell lung cancer and postoperative radiotherapy: A
prognostic scoring model and heat map approach. J Thorac Oncol.
13:1968–1974. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SM, Khan I, Upadhyay S, Lewanski C,
Falk S, Skailes G, Marshall E, Woll PJ, Hatton M, Lal R, et al:
First-line erlotinib in patients with advanced non-small-cell lung
cancer unsuitable for chemotherapy (TOPICAL): A double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 13:1161–1170.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho J, Min HY, Lee HJ, Hyun SY, Sim JY,
Noh M, Hwang SJ, Park SH, Boo HJ, Lee HJ, et al: RGS2-mediated
translational control mediates cancer cell dormancy and tumor
relapse. J Clin Invest. 131:e1367792021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karnthaler-Benbakka C, Groza D, Kryeziu K,
Pichler V, Roller A, Berger W, Heffeter P and Kowol CR:
Tumor-targeting of EGFR inhibitors by hypoxia-mediated activation.
Angew Chem Int Ed Engl. 53:12930–12935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phuchareon J, McCormick F, Eisele DW and
Tetsu O: EGFR inhibition evokes innate drug resistance in lung
cancer cells by preventing Akt activity and thus inactivating Ets-1
function. Proc Natl Acad Sci USA. 112:E3855–E3863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong Y, Hellyer JA, Stehr H, Hoang NT,
Niu X, Das M, Padda SK, Ramchandran K, Neal JW, Wakelee H and Diehn
M: Role of KEAP1/NFE2L2 mutations in the chemotherapeutic response
of patients with non-small cell lung cancer. Clin Cancer Res.
26:274–281. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nokihara H, Lu S, Mok TSK, Nakagawa K,
Yamamoto N, Shi YK, Zhang L, Soo RA, Yang JC, Sugawara S, et al:
Randomized controlled trial of S-1 versus docetaxel in patients
with non-small-cell lung cancer previously treated with
platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer).
Ann Oncol. 28:2698–2706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F,
Han X, Feng Y, Zheng C, Wang Z, et al: VGLL4 functions as a new
tumor suppressor in lung cancer by negatively regulating the
YAP-TEAD transcriptional complex. Cell Res. 24:331–343. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engelen M, Safar AM, Bartter T, Koeman F
and Deutz NEP: High anabolic potential of essential amino acid
mixtures in advanced nonsmall cell lung cancer. Ann Oncol.
26:1960–1966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu HF, Mao XY and Du M: Prevention of
breast cancer by dietary polyphenols-role of cancer stem cells.
Crit Rev Food Sci Nutr. 60:810–825. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gunther S, Ruhe C, Derikito MG, Bose G,
Sauer H and Wartenberg M: Polyphenols prevent cell shedding from
mouse mammary cancer spheroids and inhibit cancer cell invasion in
confrontation cultures derived from embryonic stem cells. Cancer
Lett. 250:25–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thangapazham RL, Singh AK, Sharma A,
Warren J, Gaddipati JP and Maheshwari RK: Green tea polyphenols and
its constituent epigallocatechin gallate inhibits proliferation of
human breast cancer cells in vitro and in vivo. Cancer Lett.
245:232–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adhami VM, Malik A, Zaman N, Sarfaraz S,
Siddiqui IA, Syed DN, Afaq F, Pasha FS, Saleem M and Mukhtar H:
Combined inhibitory effects of green tea polyphenols and selective
cyclooxygenase-2 inhibitors on the growth of human prostate cancer
cells both in vitro and in vivo. Clin Cancer Res. 13:1611–1619.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shanafelt TD, Call TG, Zent CS, LaPlant B,
Bowen DA, Roos M, Secreto CR, Ghosh AK, Kabat BF, Lee MJ, et al:
Phase I trial of daily oral Polyphenon E in patients with
asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin
Oncol. 27:3808–3814. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu F, Zhou L, Jin W, Yang W, Wang Y, Yan
B, Du W, Zhang Q, Zhang L, Guo Y, et al: Anti-proliferative and
apoptosis-inducing effect of theabrownin against non-small cell
lung adenocarcinoma A549 cells. Front Pharmacol. 7:4652016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Xiao X, Yan B, Yuan Q, Dong X, Du Q,
Zhang J, Shan L, Ding Z, Zhou L and Efferth T: Green tea-derived
theabrownin induces cellular senescence and apoptosis of
hepatocellular carcinoma through p53 signaling activation and
bypassed JNK signaling suppression. Cancer Cell Int. 22:392022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao X, Guo L, Dai W, Yan B, Zhang J, Yuan
Q, Zhou L, Shan L and Efferth T: Green tea-derived theabrownin
suppresses human non-small cell lung carcinoma in xenograft model
through activation of not only p53 signaling but also MAPK/JNK
signaling pathway. J Ethnopharmacol. 291:1151672022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Almatroodi SA, Almatroudi A, Khan AA,
Alhumaydhi FA, Alsahli MA and Rahmani AH: Potential therapeutic
targets of epigallocatechin gallate (EGCG), the most abundant
catechin in green tea, and its role in the therapy of various types
of cancer. Molecules. 25:31462020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang Y, Pei J, Zheng Y, Miao YJ, Duan BZ
and Huang LF: Gallic acid: A potential anti-cancer agent. Chin J
Integr Med. 28:661–671. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rios-Doria J, Stevens C, Maddage C, Lasky
K and Koblish HK: Characterization of human cancer xenografts in
humanized mice. J Immunother Cancer. 8:e0004162020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsieh SC, Wu CC, Hsu SL and Yen JH:
Molecular mechanisms of gallic acid-induced growth inhibition,
apoptosis, and necrosis in hypertrophic scar fibroblasts. Life Sci.
179:130–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun G, Ding XA, Argaw Y, Guo X and Montell
DJ: Akt1 and dCIZ1 promote cell survival from apoptotic caspase
activation during regeneration and oncogenic overgrowth. Nat
Commun. 11:57262020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marquez-Jurado S, Diaz-Colunga J, das
Neves RP, Martinez-Lorente A, Almazán F, Guantes R and Iborra FJ:
Mitochondrial levels determine variability in cell death by
modulating apoptotic gene expression. Nat Commun. 9:3892018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, He Y, Li F, Huang Q, Kato TA, Hall
RP and Li CY: Caspase-3 promotes genetic instability and
carcinogenesis. Mol Cell. 58:284–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peron S, Pan-Hammarstrom Q, Imai K, Du L,
Taubenheim N, Sanal O, Marodi L, Bergelin-Besançon A, Benkerrou M,
de Villartay JP, et al: A primary immunodeficiency characterized by
defective immunoglobulin class switch recombination and impaired
DNA repair. J Exp Med. 204:1207–1216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nick AM, Stone RL, Armaiz-Pena G, Ozpolat
B, Tekedereli I, Graybill WS, Landen CN, Villares G, Vivas-Mejia P,
Bottsford-Miller J, et al: Silencing of p130cas in ovarian
carcinoma: A novel mechanism for tumor cell death. J Natl Cancer
Inst. 103:1596–1612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaudhuri AR and Nussenzweig A: The
multifaceted roles of PARP1 in DNA repair and chromatin
remodelling. Nat Rev Mol Cell Biol. 18:610–621. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei H and Yu X: Functions of PARylation in
DNA damage repair pathways. Genomics Proteomics Bioinformatics.
14:131–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salvesen GS: Caspase 8: Igniting the death
machine. Structure. 7:R225–R229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fritsch M, Gunther SD, Schwarzer R, Albert
MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H,
Seeger JM, et al: Caspase-8 is the molecular switch for apoptosis,
necroptosis and pyroptosis. Nature. 575:683–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng J, Zhu S, Hu L, Ye P, Wang Y, Tian Q,
Mei M, Chen H and Guo X: Wild-type rabies virus induces autophagy
in human and mouse neuroblastoma cell lines. Autophagy.
12:1704–1720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim KH and Lee MS: Autophagy-a key player
in cellular and body metabolism. Nat Rev Endocrinol. 10:322–337.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hippert MM, O'Toole PS and Thorburn A:
Autophagy in cancer: Good, bad, or both? Cancer Res. 66:9349–9351.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hait WN, Jin S and Yang JM: A matter of
life or death (or both): Understanding autophagy in cancer. Clin
Cancer Res. 12:1961–1965. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu Z, Zhang J and Zhang Q: Expression
pattern and functions of autophagy-related gene atg5 in zebrafish
organogenesis. Autophagy. 7:1514–1527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu H, Liu S, Qiu X, Yang X, Bao L, Pu F,
Liu X, Li C, Xuan K, Zhou J, et al: Donor MSCs release apoptotic
bodies to improve myocardial infarction via autophagy regulation in
recipient cells. Autophagy. 16:2140–2155. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kumar D, Shankar S and Srivastava RK:
Rottlerin-induced autophagy leads to the apoptosis in breast cancer
stem cells: Molecular mechanisms. Mol Cancer. 12:1712013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Awan FM, Obaid A, Ikram A and Janjua HA:
Mutation-structure-function relationship based integrated strategy
reveals the potential impact of deleterious missense mutations in
autophagy related proteins on hepatocellular carcinoma (HCC): A
comprehensive informatics approach. Int J Mol Sci. 18:1392017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu L, Yue Q, Bian F, Sun H, Zhai H and Yao
Y: Melatonin enhances phenolics accumulation partially via ethylene
signaling and resulted in high antioxidant capacity in grape
berries. Front Plant Sci. 8:14262017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Speranza S, Knechtl R, Witlaczil R and
Schonlechner R: Reversed-phase HPLC characterization and
quantification and antioxidant capacity of the phenolic acids and
flavonoids extracted from eight varieties of sorghum grown in
Austria. Front Plant Sci. 12:7691512021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jang YG, Ko EB and Choi KC: Gallic acid, a
phenolic acid, hinders the progression of prostate cancer by
inhibition of histone deacetylase 1 and 2 expression. J Nutr
Biochem. 84:1084442020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bernhaus A, Fritzer-Szekeres M, Grusch M,
Saiko P, Krupitza G, Venkateswarlu S, Trimurtulu G, Jaeger W and
Szekeres T: Digalloylresveratrol, a new phenolic acid derivative
induces apoptosis and cell cycle arrest in human HT-29 colon cancer
cells. Cancer Lett. 274:299–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou L, Wu F, Jin W, Yan B, Chen X, He Y,
Yang W, Du W, Zhang Q, Guo Y, et al: Theabrownin inhibits cell
cycle progression and tumor growth of lung carcinoma through
c-myc-related mechanism. Front Pharmacol. 8:752017. View Article : Google Scholar : PubMed/NCBI
|