Introduction
Neuroblastoma (NB) is the most common type of
pediatric extracranial neurogenic tumor. The most common location
for the primary development of NB is in the adrenal glands. NB can
also develop anywhere along the sympathetic nervous system from the
neck to the pelvis. The frequencies in different locations are:
neck (1%), chest (19%), abdomen (30% nonadrenal), or pelvis (1%).
The current standard therapy for high-risk NB consists of 5–7
cycles of high-dose chemotherapy, surgery, autologous stem cell
transplantation, radiation therapy, and monoclonal antibody
immunotherapy with anti-GD2 antibodies (1,2). These
treatment options are fairly toxic and patients still have low
survival rates, with NB still accounting for 15% of all childhood
cancer deaths (3). NB can result in
central nervous system (CNS) metastasis, which makes treatment more
difficult. Tumor microenvironment hypoxia and blood-brain barrier
(BBB) functioning drive resistance to several cancer therapies
(4). Novel transcriptome analysis
(5), advanced 2D and 3D in
vitro models (6), and drug
design approaches (7) are thus
needed for the improved management of NB. Activating receptor
(transmembrane protein) NKG2D belongs to the NKG2 family of C-type
lectin-like receptors (CLRs) (8,9). It is
typically expressed on natural killer (NK) cells, all activated
CD8+ T cells, and certain activated CD4+ T
cells (10,11). As the primary activating receptor of
NK cells, activating NKG2D can induce cytotoxicity, cytokine
secretion, and proliferation of NK cells (12).
T cell surface NKG2D is an effective co-stimulator
of T cell receptor (TCR)-mediated effector function and can
upregulate antigen-specific T cell-mediated cytotoxic effects
against cells or tissues expressing stress-induced NKG2D ligand
(NKG2DL), particularly in the presence of suboptimal TCR (13). The NKG2DLs include major
histocompatibility complex (MHC) class-I-related chain (MIC) A/B,
and the UL-16 binding proteins 1–6 (ULBP1-6) (14), which is typically expressed on
‘stressed’ cells such as infected or malignant cells. The majority
of NKG2DLs are membrane-bound glycoproteins, whereas certain ULBPs
are GPI-anchored, which can be shed under certain circumstances
(15–17). It has been found that NB can
downregulate NKG2DL expression to prevent the recognition and
elimination mediated by NK cells (18). Additionally, NKG2DLs released by NB
cells can impair NK or T cell-mediated antitumor immunity by
promoting the internalization of NKG2D receptors in NK or T cells
(19).
Adoptive transfer cell therapy with chimeric antigen
receptor (CAR)-T cells is a relatively novel and promising approach
for the management of NB (20).
First-generation CAR-T cells have been used in two clinical studies
in patients with NB (21,22). CAR-NK cells also have been assessed,
and they were shown to achieve increased killing of tumor cell
lines in vitro (23).
Indeed, the efficacy of CAR-T/NK-cell therapy in solid tumors
depends on the local infiltration efficiency of CAR-engineered
cells, the tumor immunosuppressive microenvironment, and the
long-term viability of these CAR cells. Considering the T-cell
immune suppressive conditions induced by NKG2DL release, the
clinical efficacy of CAR-engineered cells in NB tumors may likely
be inhibited.
In the present study, it was shown that high levels
of soluble MICA and ULBP2 (NKG2DLs that activate the NK cell
receptor NKG2D) in the sera of patients with NB was closely
associated with poorer outcome and T cell exhaustion. Additionally,
these tumor-derived NKG2DLs were cleaved by a disintegrin and
metalloproteinases (ADAM)10 and ADAM 17. Consequently, sNKG2DLs
impaired the proliferation, IFN-γ production, and cytotoxicity of
CD8+ T cells through NKG2D receptor. The blockade of
these tumor-derived sNKG2DLs increased CD8+ T cells
antitumor activity, highlighting a novel potential strategy for
improving T cell-mediated immunotherapy.
Materials and methods
Patient samples
Peripheral blood from patients with NB was collected
from 35 stage IV NB patients pre and post-surgery between January
2021 and December 2021. Of the 35 patients, 24 were male and 11
were female, and the mean age at diagnosis was 26.11±9.13 months
(range, 9–43 months). Gross total resection was achieved in 27
cases and subtotal resection in 8 cases. Peripheral blood was
equally obtained from 10 healthy control patients (range, 22–32
months; mean age, 25.4±3.23 months) with benign pediatric surgical
disease (oblique inguinal hernia) at the Children's Hospital of
Fudan University (Shanghai, China) between January 2021 and
December 2021.
Mononuclear cells were isolated using a Lymphocyte
Separation Medium 1077 (PromoCell GmbH) from peripheral blood.
Fresh or cryopreserved mononuclear cells were used for
immunophenotype analysis. Plasma was used for the analysis of
soluble NKG2D ligands.
The use of human material was approved (approval no.
2020238) by the Local Ethics Committee of Fudan University
(Shanghai, China).
Cell culture
Flow cytometry was used to analyze the cell purity
of isolated subpopulations, and the T cell purity was >95%. T
cells were re-suspended in complete medium at a density of
1×106 cells/ml. For immune function analysis, T cells
were cultured with anti-CD28 (2 µg/ml) and IL-2 (10 U/ml) in 24
well plates (pre-coated with 5 µg/ml anti-CD3). Before detection
and treatment, T cells were harvested, washed twice with PBS, and
counted.
NB cell lines, including SH-SY5Y, SK-N-BE (2), and SK-N-SH, were purchased from the
Cell Bank of the Chinese Academy of Science. SH-SY-5Y cell lines
were authenticated by short tandem repeat (STR) testing provided by
the Cell Bank of the Chinese Academy of Science. The other two cell
lines, CHLA-15 and LA-N-5 cells were a gift from Professor Mujie Ye
of Fudan University. All NB cells were cultured under the same
conditions: DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and maintained in a 37°C humidified incubator supplied with 5%
CO2.
Flow cytometry
NB tumor cells (1×106) were stained with
unconjugated antibodies (1:100) according to the manufacturer's
instructions: MICA/B, ULBP1-3 (cat. nos. ab54413, ab256515, ab88645
and ab280087, respectively; all from Abcam), ADAM10, ADAM17 (cat.
nos. ab252234 and ab57484; both from Abcam) or with the appropriate
isotype-matched control IgG (cat. no. ab37355; Abcam).
T cells were stained for the surface markers CD3,
CD8, CD4, CD25, CD45RO, CD69, CCR7, NKG2D, CD107a, PD-1, and TIM-3
(cat. nos. 11-0038-42, 12-0088-42, 25-0049-42, 17-0257-42,
17-0457-42, 12-0699-42, 46-1979-42, 12-5878-42, 53-1079-42,
46-9969-42 and 17-3109-42; all from eBioscience; Thermo Fisher
Scientific, Inc.), and the intracellular markers Foxp3, IFN-γ, and
Ki67 (cat. nos. 12-4776-42, 12-7319-42 and 11-5698-82; all from
eBioscience; Thermo Fisher Scientific, Inc.) with
fluorescence-conjugated antibodies according to the manufacturer's
instructions (eBioscience; Thermo Fisher Scientific, Inc.). For
flow cytometric analysis, cells were counted, preincubated with an
Fc blocker (cat. no. 14-9161-73; eBioscience; Thermo Fisher
Scientific, Inc.) for blocking non-specific Fc receptors, then
incubated with conjugated antibodies (1:100) in the dark at 4°C for
30 min for surface marker analysis. For the intracellular cytokine
analysis, cells were pre-stimulated with Cell Stimulation Cocktail
(cat. no. 00-4975-93; eBioscience; Thermo Fisher Scientific, Inc.)
in vitro. After stimulation for 5 h at 37°C, an
Intracellular Fixation and Permeabilization Buffer Set was used for
intracellular staining and flow cytometry analysis (eBioscience;
Thermo Fisher Scientific, Inc.). For cell markers,
CD3+CD4+CD25+Foxp3+ was
used to characterize regulatory T cells (Tregs),
CD3+CD4+ was used to characterize
CD4+ T cells, and CD3+CD8+ was
used to characterize CD8+ T cells. The cells were
examined by flow cytometry (BD FACSCanto™ II; BD Biosciences) and
the data were analyzed using FlowJo software (version 10.0; FlowJo
LLC).
ELISA
NB tumor cells (1×106/ml) in 2-ml
complete medium were cultured in a six-well culture plate for 48 h
as aforementioned. Then, tumor cell-free supernatant (Tumor sup)
was obtained by centrifugation at 12,000 g at 4°C for 10 min.
Soluble MICA and ULBP1-3 levels were determined by ELISA (EHMICA;
cat. nos. EH476RB, EH477RB, and EH478RB; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions.
Inhibition or activation of ADAMs
Tumor cell lines were treated for 24 h with the
ADAM10 inhibitor GI254023X (0, 10, 20, 30, or 40 µM), ADAM17
inhibitor TAPI-1 (0, 15, 30, or 60 µM), or both. The ADAM promotor
PMA (0, 2.5, 5, or 10 ng/ml) was added for 40 min before collecting
supernatants and tumor cell lines. GI254023X (cat. no. A4436),
TAPI-1 (cat. no. B4686), and PMA (cat. no. N2060) were purchased
from APeXBIO Technology LLC. Soluble NKG2DLs (sMICA, sULBP2, and
sULBP3) levels were measured by ELISA in the supernatants. The
expression levels of NKG2DLs on tumor cells (MICA/B, ULBP2, and
ULBP3) were assessed by flow cytometry.
T-cell function assays
T cells were resuspended in complete medium with a
density of 1×106 cells/ml and were cultured with
anti-CD28 (2 µg/ml) and IL-2 (10 U/ml) in a 24-well plate
pre-coated with 5 µg/ml anti-CD3. Subsequently, T cells were
co-cultured with or without 100 µl serial diluted tumor sup for 24
h. For neutralizing sNKG2DL, single and combined soluble NKG2DL
neutralizing antibodies (1, 2, or 5 µg/ml anti-MICA/B, ULBP1,
ULBP2, and/or ULBP3) were added to T cells and to the tumor cell
supernatant co-culture system. Then, these T cells were harvested
and prepared for flow cytometric analysis. Neutralized antibodies
(anti-MICA/B, anti-ULBP1, anti-ULBP2, and anti-ULBP3) were
purchased from R&D Systems, Inc. (cat. nos. MAB13001, MAB1380,
MAB1298 and MAB1517, respectively).
Statistical analysis
Normally distributed data are presented as the mean
± SD, whereas for non-normally distributed data, the median and
interquartile range (IQR) are used. Clinically relevant immune
parameters were analyzed using a Mann-Whitney non-parametric test.
NKG2D/NKG2DL expression levels on the cell surface, sNKG2DL levels
in the tumor sup and in the patient's plasma, immune parameters of
T cells were analyzed using a one-way ANOVA followed by Tukey's
post hoc test. GraphPad Prism version 6.0 (Dotmatics) was used for
all analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Tumor-derived sNKG2DLs are initially
cleaved by ADAM10 and ADAM17
To investigate the role of NKG2DLs, first ELISA kits
were used to detect the levels of sMICA, and sULBP2 in serum
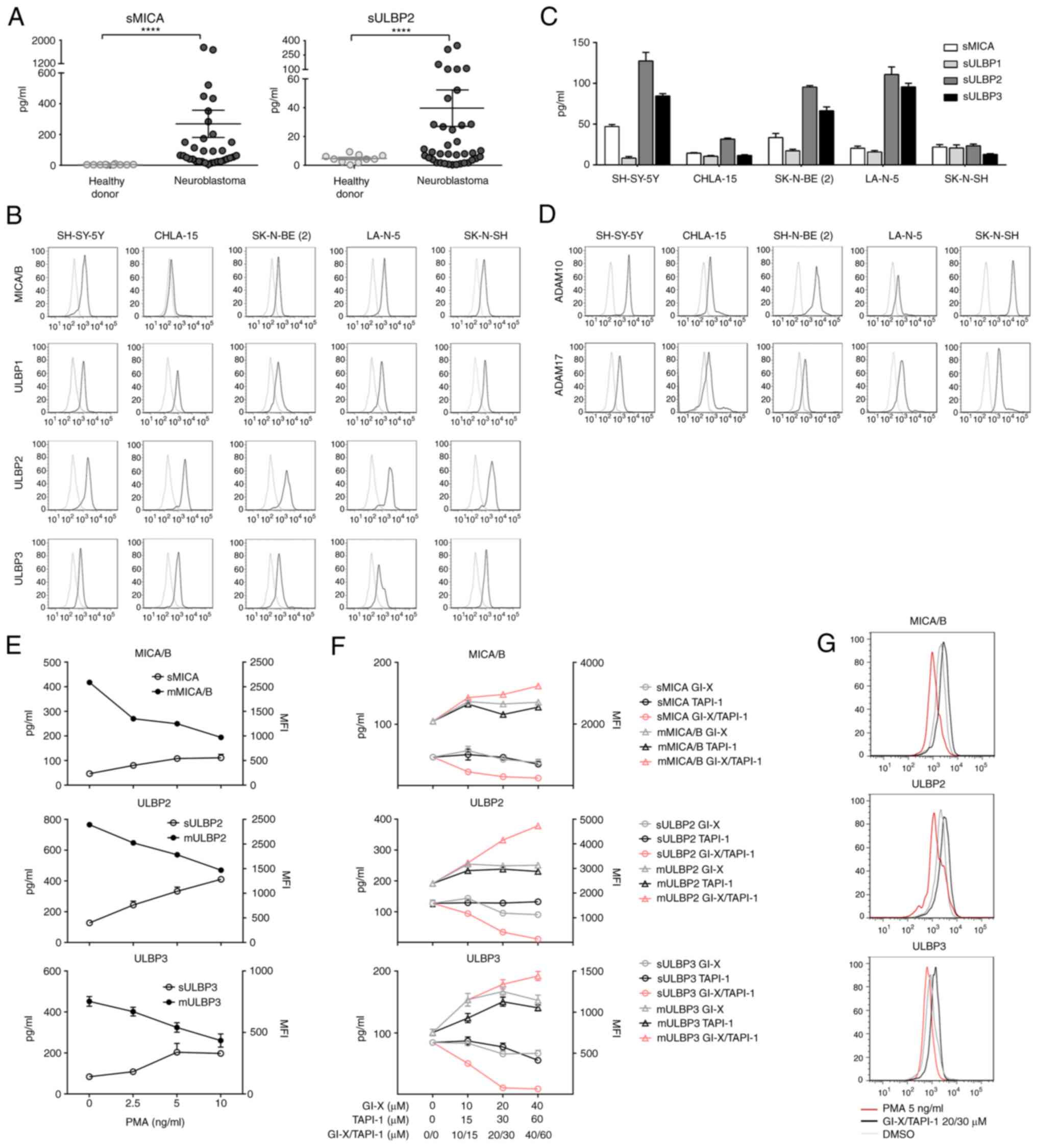
samples from 35 patients with NB and 10 healthy donors. sMICA
levels in the serum from NB patients were 269.2±88.3 pg/ml compared
with 3.94±0.73 pg/ml in healthy donors (Fig. 1A). sULBP2 was 42.61±13.63 compared
with 4.69±0.87 in healthy donors (Fig.
1A). There was a positive association between sMICA with
sULBP2, indicating that immune escape in NB involves multiple
soluble NKG2D ligands (Fig. S1).
Based on the Mann-Whitney U test, there was a statistically
significant difference between patients with NB and healthy donors
in the concentrations of sMICA and sULBP-2 (P<0.0001; Fig. 1A). Consistently, the expression of
MICA/B and ULBP 1–3 was detectable in NB cell lines, SH-SY-5Y,
CHLA-15, SK-N-BE (2), LA-N-5, and
SK-N-SH using flow cytometry (Fig.
1B). Additionally, the presence of sMICA and sULBP 1–3 in
culture supernatants from the NB cell lines was assessed using
ELISA. After 48 h of culture, there were high levels of sMICA,
sULBP-2, and sULBP-3 detected in the supernatants of SH-SY-5Y,
SK-N-BE (2), and LA-N-5 (Fig. 1C). sULBP-1 levels were lower in all
cell lines (Fig. 1C). Additionally,
the surface expression of ADAM10 and ADAM17 was assessed in all NB
cells using FACS analysis (Fig.
1D).
To evaluate whether ADAM10 and ADAM17 expression was
associated with sNKG2DL release, SH-SY-5Y NB cells were treated
with selective ADAM10 or ADAM17 promoters and inhibitors.
SH-SY-5Y cells were cocultured with the ADAM10
inhibitor GI–X (10–40 µM), ADAM 17 inhibitor TAPI-1 (15–60 µM), or
the solvent alone (DMSO) for 24 h. Then, the supernatant was
collected and sMICA, sULBP2, and sULBP3 levels were assessed using
ELISA. For enhancing ADAM analysis, the ADAM promotor PMA was added
and cells were co-cultured for 40 min before the supernatant and
tumor cells were collected for MICA/B, ULPB2, and ULBP3 expression
analysis on the tumor cell surface using FACS. PMA enhanced the
enzymatic effects of ADAM10 and ADAM17, thus inducing the releasing
of sNKG2DLs into the supernatant and shielding NKG2DLs on the
SH-SY-5Y cell surface (Fig. 1E and
G). The combined ADAM10 inhibitor and ADAM 17 inhibitor could
inhibit the shedding of NKG2DLs and increase its cell surface
expression in NB cells in a concentration-dependent manner
(Fig. 1F and G). Neither ADAM10
inhibitors GI–X nor ADAM 17 inhibitors TAPI-1 could produce similar
effects (Fig. 1F). Thus, the
production of sNKG2DL is a characteristic of primary NB and NB cell
lines, and these tumor-derived sNKG2DLs were cleaved by ADAM10 and
ADAM17 allowing its release into the supernatant.
sNKG2DLs levels are negatively
correlated with patient prognosis and the low-exhausted phenotype
of T cells
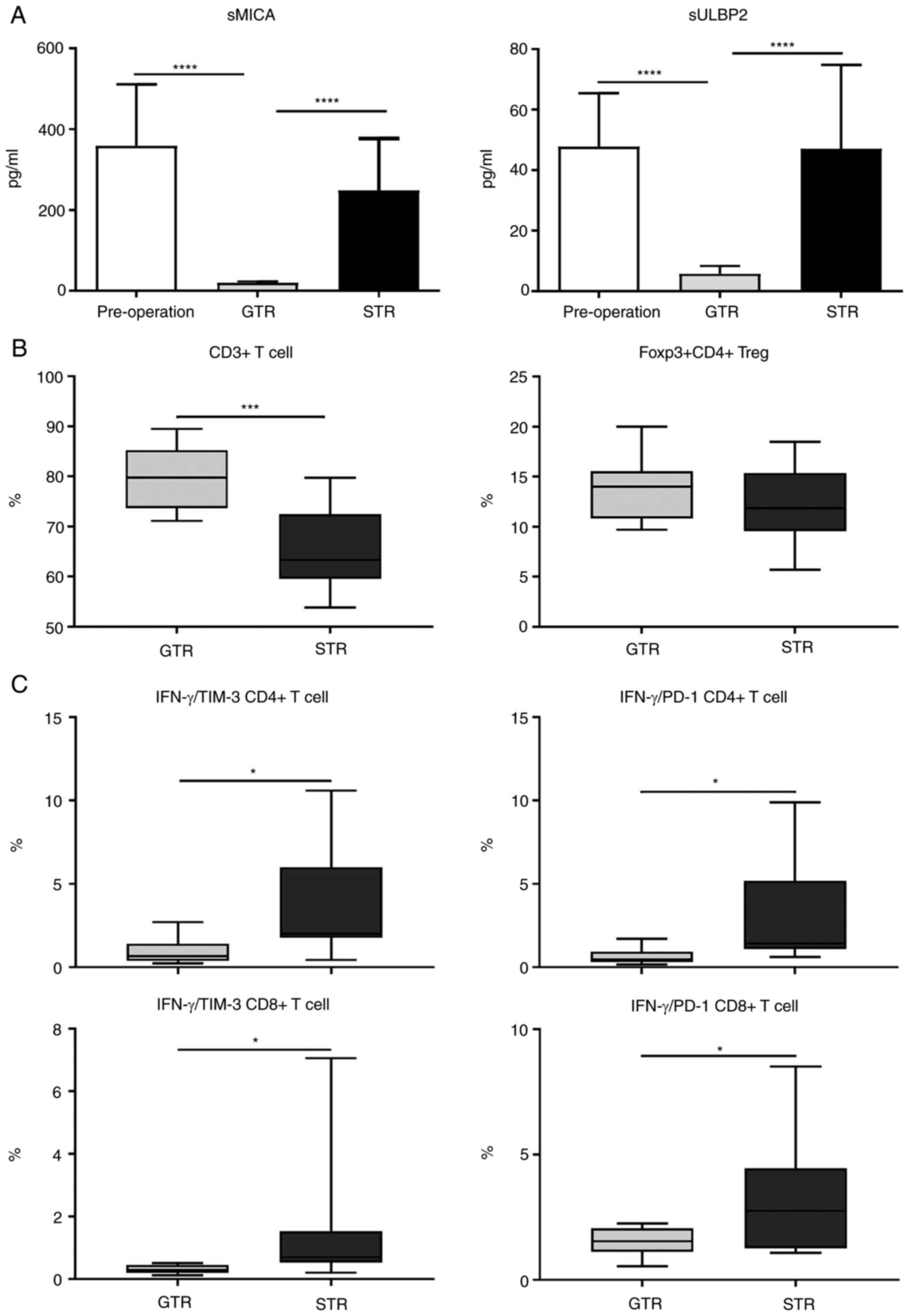
Serum samples from patients pre- and post-surgery,
and the levels of both sMICA and sULBP-2 levels were determined.
Compared with the pre-operative and subtotal resection (STR)
groups, sMICA and sULBP-2 levels were significantly lower in the
gross total resection (GTR) group (Fig.
2A), indicating that MICA/B and ULBP-2 expression levels may be
negatively associated with patient prognosis. Next, given that both
MICA/B and ULBP-2 were immune-related molecules, whether the
decreased expression of sULBP2 and sMICA affected the immune
pattern of patients was assessed. Peripheral blood mononuclear
cells were isolated from patients after standard treatment and
stained with different immunophenotypic markers. There was no
difference in the percentages in Tregs, B cells, NK cell, and NKT
lymphocyte subpopulations percentages between the GTR and STR
groups (data not shown). However, there was a significant decrease
in the percentage in CD3+ T cells in the STR group
compared with that in the GTR group (Fig. 2B). On further analysis of
IFN-γ+CD4+ and
IFN-γ+CD8+ T cell subpopulations,
significantly increased percentages of PD-1+, and
Tim-3+ T cell subpopulations were found in the STR group
compared with the GTR group (Fig.
2C). These results imply that downregulation of sULBP2 and
sMICA, and the upregulated frequency of T cells with a
low-exhausted phenotype were associated with each other.
sNKG2DLs impair CD8+ T cell
function and memory formation
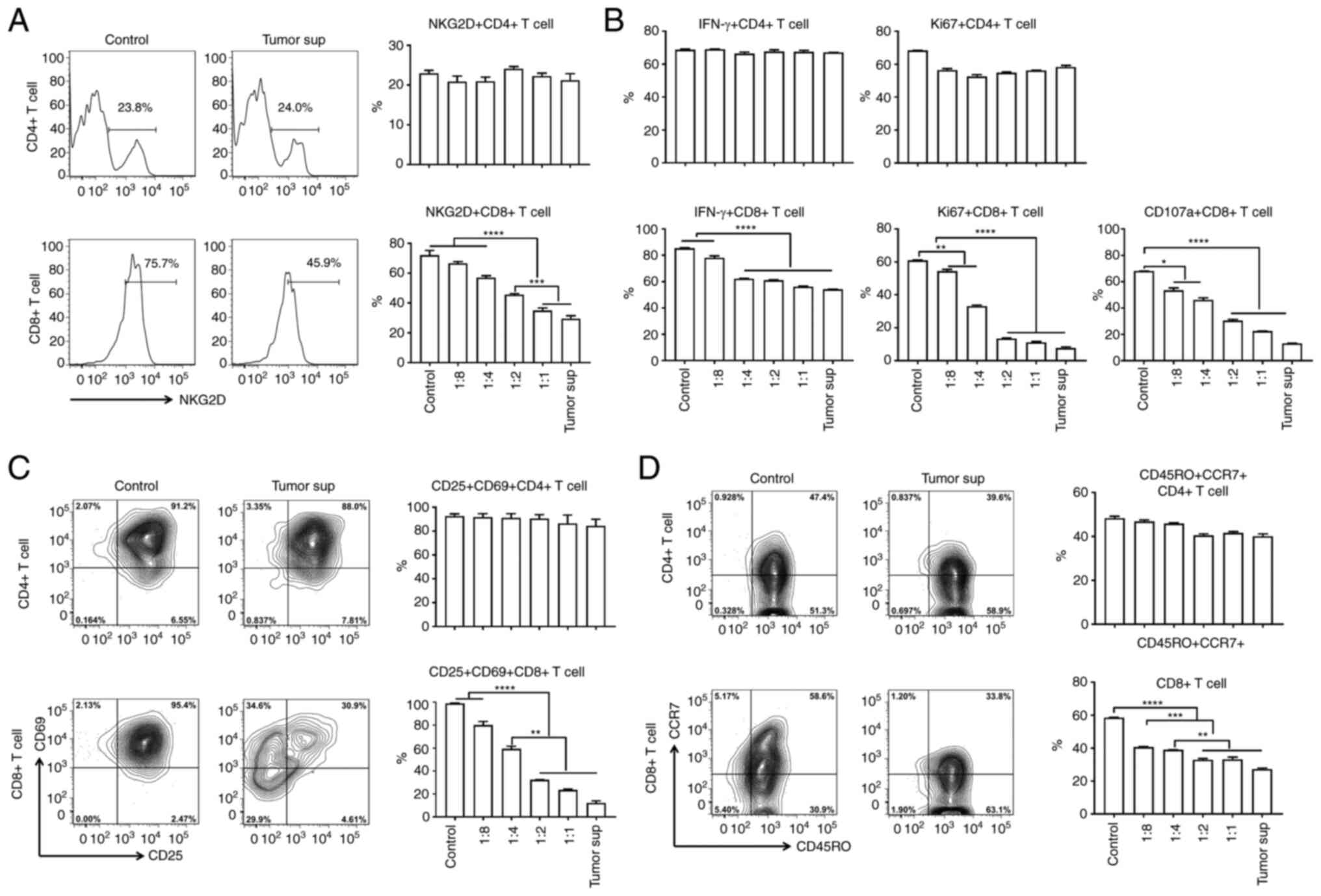
In humans, NKG2D was reported to be expressed on
several types of lymphocytes, including activated NK, NKT, and
CD8+T cells (15). To
evaluate whether soluble NKG2DLs separately affected
CD8+ T cells and whether this was associated with the
NKG2D receptor, NKG2D expression was analyzed using FACS. After
culturing cells with different concentrations of tumor cell
supernatants for 12 h, NKG2D receptor downregulation was observed
in the CD8+ T cells (Fig.
3A), and the degree of NKG2D downregulation was associated with
the supernatant concentration (Fig.
3A). To determine whether NKG2D downregulation affected
CD8+ T cell function, human T cell function was assessed
after culturing with the different concentrations of supernatants
from the NB cells for 12 h. When separately assessing
CD8+ and CD4+ T lymphocyte presence, it was
observed that sNKG2DL selectively downregulated CD8+ T
cell function, including proliferation, IFN-γ production and
cytotoxicity (Fig. 3B). The
percentage of IFN-γ+, Ki67+, and
CD107a+ in CD8+ T cells was significantly
decreased, particularly in the cells treated with the higher
concentrations of tumor culture supernatant (Fig. 3B).
NKG2D has been shown to act as a co-stimulatory
receptor in CD8+ T cells, and was demonstrated to be
involved in the function of CD8+ T cell memory formation
(11). Thus, whether T cells
treated with sNKG2DL exhibited reduced effector functions and
memory formation was assessed. Cytofluorimetric analysis of the
expression of the early (CD69+) and late activation
markers (CD25+) on T cells was performed in T cells
treated with sNKG2DL. The cultured T cell memory profiling was
defined based on CD45RO and CCR7 expression. The percentage of
activated CD8+ T cells
(CD69+CD25+) was significantly decreased in
cells treated with the high concentration of tumor cell
supernatants (Fig. 3C), indicating
that there was an inverse correlation between tumor cell
supernatant concentration and CD8+ T cell activation. A
similar trend was observed in memory formation of CD8+ T
cells. The difference in the concentration of the tumor cell
supernatants induced a decrease in the percentage of
CD45RO+CCR7+ memory CD8+ T cells
(Fig. 3D). Activation and memory
profiling of CD4+ T cells was not affected. Taken
together, sNKG2DLs impaired the antitumor effects and memory
formation of CD8+ T cells, including cell activation,
proliferation, cytokine production and cytotoxicity.
Blockage of tumor-derived sNKG2DLs
results in increased antitumor function of T cells
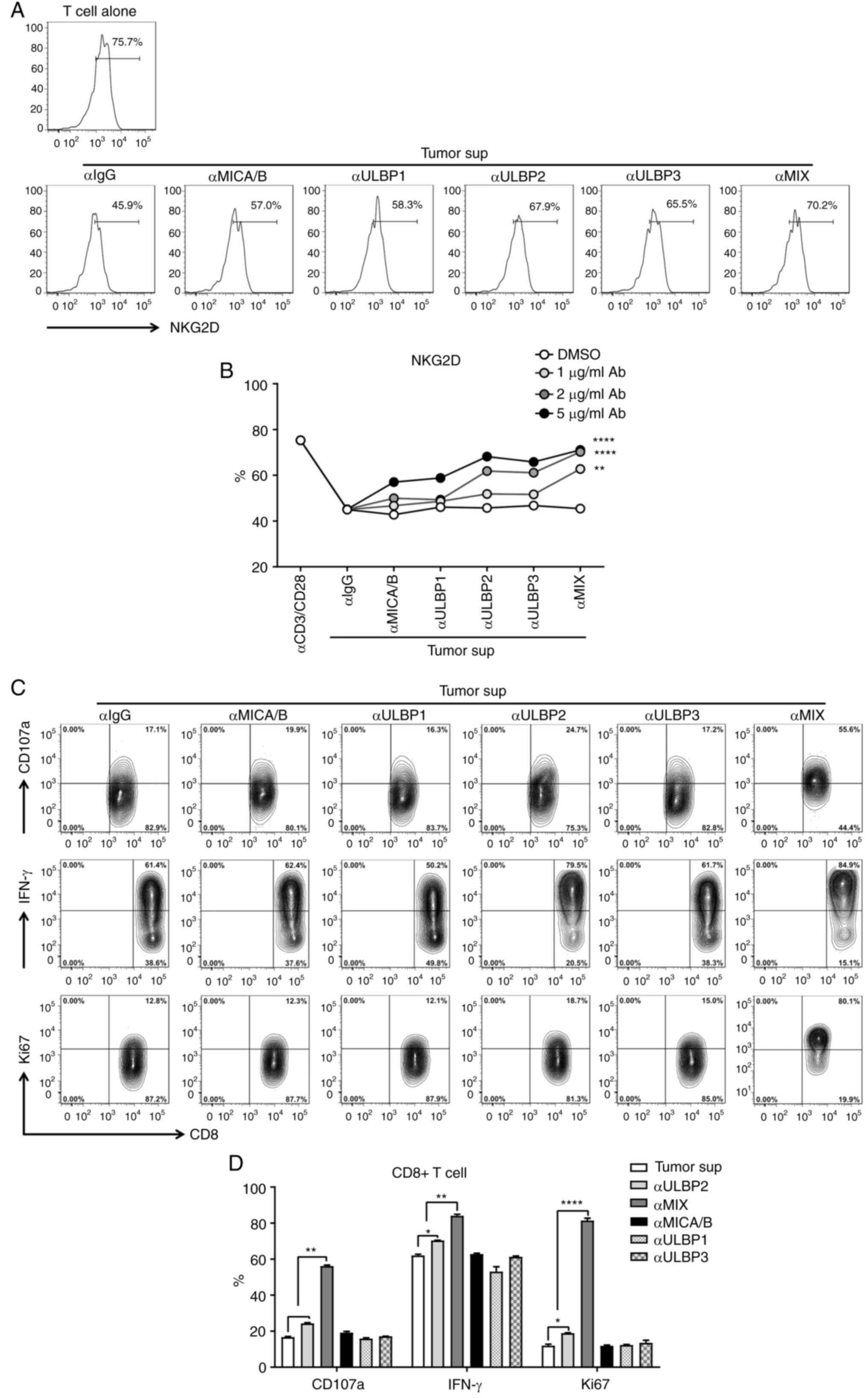
It was found that the combination of anti-MICA/B and
ULPB2-3 increased NKG2D receptor expression on CD8+ T
cells, suggesting a cooperative therapeutic effect of targeting all
the different types of sNKG2DLs (Fig.
4A and B). Furthermore, to investigate whether targeting
sNKG2DLs could result in the recovery of CD8+T cell
function, a single and combined sNKG2DL neutralizing antibody was
added to the T cells treated with sNKG2DL. Therapy with the single
anti-ULBP2 antibody significantly increased the percentage of
IFN-γ+, Ki67+, and CD107a+ in
CD8+ T cells, whereas the use of the anti-MICA/B or
anti-ULBP3 antibodies alone did not have a notable impact (Fig. 4C and D). Combined therapy with all
the sNKG2DL neutralizing antibodies resulted in a significant
increase in the antitumor function of CD8+ T cells
compared with the monotherapy (Fig. 4C
and D). Therefore, blockage of the tumor-derived sNKG2DLs
resulted in the recovery of CD8+ T function and this may
serve as a novel tumor immunotherapy approach.
Discussion
Immune cell-based antitumor therapy has achieved
incredible clinical outcomes, and tumor-derived NKG2DLs are a key
factor involved in immunosuppression to facilitate tumor cell
escape from antitumor immunity. However, the underlying mechanisms
require further elucidation. In the present study, using both
clinical data and in vitro analysis, it was found that the
immunosuppressive effects of NKG2D and its soluble ligands on
CD8+ T cells were achieved by impairing CD8+
T cell activation, proliferation, IFN-γ production, cytotoxicity
and memory formation.
There were several mechanisms in NB immune escape of
immune surveillance mediated by NK cells, including increasing the
expression of ligands for inhibitory receptors and downregulating
the expression of ligands for activation of NK cell receptors. The
ligands for inhibitory receptors include PD1L for PD1 (24) and human leukocyte antigen-E (HLA-E)
for NKG2A (25). Ligands that
reduced activation of NK cell receptors include PVR and Nectin-2
for DNAM1 (26), CD137L (4-1BBL)
for 4–1BB (27), and soluble MICA/B
and ULBPs for NKG2D. These soluble molecules not only passively
block NKG2D but also downregulate NKG2D on the surface of NK cells
by inducing receptor internalization. These effects eventually lead
to a decline in NK cell function (28). Diefenbach et al (29) indicated that NKG2D served a
costimulatory function in CD8+ T cells. It has also been
revealed that the effect of NKG2D has also been contested with
regard to CD8+ T cells, and the impact of sNKG2DL on
antitumor function in CD8+ subtype T cells has not been
elucidated. Additionally, the relevance of NKG2DLs in NB and NKG2D
on T cell function have not been determined. A previous study
reported that MICA/B in sera of most patients with NB and the
downregulated NKG2D expression on the surface of CD8, after which
the reduced cytotoxicity of IL-2-activated NK cells against
MICA+ target cells was shown (30). Based on analysis of the sera of
patients with NB in the present study, it was found that MICA/B and
ULBP-2 were the major sNKG2DLs present in patients with NB,
characterized by the high concentration of sMICA and sULBP2 in the
patient's plasma. Cell surface expression of MICA/B and ULBP1, 2
and 3 for activation of the NK cell receptor NKG2D, was observed in
the majority of NB tumor cell lines, whereas high levels of sMICA,
sULBP-2, and sULBP-3 were detected in the supernatants of SH-Y5Y,
SK-N-BE (2), and LA-N-5 cells
accompanied by low levels of sULBP-1 was observed in all the NB
cell lines. sMICA has been used as a prognostic marker in certain
solid tumors, showing a positive association between expression and
a poor prognosis (31). In the
present study, a similar phenomenon was observed in that sMICA and
sULBP2 were associated with an immunosuppressive microenvironment
in patients with NB, and this was indicative of a poor clinical
prognosis.
NKG2D ligands are cleaved by several proteases for
shedding, including different members of the metalloprotease family
and ADAM family (32). Amongst the
members of the ADAM family, ADAM10 and ADAM17 are involved in the
migration and differentiation of tumor cells through cleavage of
Notch (33). While direct ADAMs
knockout may be reminiscent of the restoration of microRNA (miR).
In hepatocellular carcinoma, ADAM17 knockout restored miR122 both
in vitro and in vivo. In gastric cancer, modulating
the miR-140/ADAM10 axis promoted GC cell proliferation and
invasion. The net effect of MicroRNA restoration represented an
overall response from all target genes and produced extensive
impact. Previous studies indicated that knockout of ADAM10 and
ADAM17 decreased tumor growth and invasiveness, and altered the
behavior of the tumorigenic cells (34,35).
The relevant functions of ADAM10 and ADAM17 in NKG2DLs cleavage
remain undefined (36,37). In glioblastoma initiating cells,
ADAM10 and ADAM17 have been found to be involved in the process of
ULBP-2 cleavage, and the ADAM inhibitor significantly increased the
secretion of IFN-γ by NK cells (38). In Hodgkin's lymphoma cells, ADAM10
showed higher specificity for the shedding of MICA/B and ULBP-3
over ADAM17 (39). Inhibition of
ADAM10 and ADAM17 with small-molecule inhibitors is a common
experimental method used in this type of research and without
altered the tumorigenic behavior of the tumor cells. ADAM10 and
ADAM17 small-molecule inhibitors also could be used to develop
specific therapeutic applicability (40,41).
Thus, in the present study, by inhibiting ADAM10 and ADAM17 with
small-molecule inhibitors, it was shown that both ADAM10 and ADAM17
contributed to the cleavage of MICA/B, ULBP2, and ULBP3 from NB
cells.
It has been reported that sNKG2DL suppressed NK cell
antitumor function by disturbing its peripheral maintenance and
activation (42). In preclinical
models, it has been shown that NK cell function is enhanced by
preventing sNKG2DL release (43).
sNKG2DL was shown to induce immune-suppressive effects through
other mechanisms, including destabilization of CD3ζ in TCR/CD3
pathways (44), and increasing the
percentage of MDSCs and tumor-associated macrophages (45). Consistently, in the present study it
was revealed that sMICA and sULBP-2 selectively downregulated NKG2D
expression on the surface of CD8+ T cells and impaired
the proliferation, IFN-γ production, and cytotoxicity of these T
cells. In a mouse model, it was identified that NKG2D malfunction
critically impaired the effector response of tumor-specific memory
T cells to NKG2DL-expressing tumors (46). With regard to the NKG2D receptor, it
has been reported to serve as a costimulatory molecule responsible
for memory formation in CD8+ T cells (47), and in the current study it was
reported that sNKG2DL significantly downregulated the percentage of
activated CD8+ T cells
(CD69+CD25+) and CD45RO+
CCR7+ memory CD8+ T cells.
In humans, NKG2D is abundantly expressed on the
surface of NK cells, activated CD8+ T cells, certain
activated CD4+ T cells, and other subtypes of T cells,
including NKT and Tregs (10). In
the present study, >75% of CD4+ T cells did not
express NKG2D in vitro after anti-CD3 and anti-CD28
treatment. Thus, there was no change in cytokine secretion (IFN-γ),
proliferation (Ki67), activation (CD69 and CD25), and memory
phenotypes (CCR7 and CD45RO) of CD4+T cells after
treatment with the tumor cell culture supernatant containing
sNKG2DL in vitro. However, in the CD4+ T cells
derived from patients with NB, it was revealed that the high levels
of sNKG2DLs (sMICA and sULBP2) were accompanied by a decrease in
the frequency of CD4+ T cells with a high-exhausted
phenotype. On account of the low NKG2D expression on
CD4+ T cells, it is hypothesized that sNKG2DLs may
mediate a suppressive immune environment, which changed the
phenotype of CD4+ T cells but did not directly impact
CD4+ T cells.
Adoptive transfer therapies applied in high-risk NB
patients in addition to current standard treatments, include NK
cells and CAR-T cells directed against NB-associated antigens (such
as GD2), and CAR-NK cells (20,48,49).
The immunosuppressive effects of sNKG2DL are
achieved by acting on the NKG2D receptor to directly or indirectly
suppress the antitumor effects of NK and CD8+ T cells,
thus reducing the effectiveness of immunotherapy based on these
types of cells (50). Immunotherapy
strategies targeting NKG2DL include interventions that result in
the upregulation of NKG2DL expression on tumor cell surfaces,
reduced tumor cell shedding, and the formation of sNKG2DL or
antibodies that neutralize already produced sNKG2DL (51). Kloess et al (52) found that soluble NKG2D ligands
drastically reduce the cytotoxicity of activated dNK cells in
pediatric patients suffering from NB and demonstrated a strategy
that used overexpression of NKG2D to maintain the antitumor
function of dNK cells. However, they suggested that the excess of
NKG2D leads to clearance of sMICA and preserves the cytotoxicity of
dNK cells via non-occupied NKG2D. The aforementioned study did not
assess the cytotoxicity of CD8+ T cells or use
neutralizing antibodies of soluble NKG2D ligands. The results of
the present study showed that the clearance of sNKG2DL rescued
CD8+ T cell function and enhanced the sensitivity to the
antitumor effects in the NB. The antitumor effect of
CD8+ T cells potentiated by clearing sNKG2DL plays an
important role in cellular immunotherapy, and it may serve as an
adjunct to CAR T therapy to enhance the therapeutic effect.
NB metastasis to the CNS is rare and often occurs in
recurrent or progressive cases (53). Even with aggressive treatment
strategies, the treatment outcomes of patients with NB with CNS
metastasis remain poor. An increasing number of studies have
confirmed that a therapeutic strategy in which the tumor hypoxic
microenvironment is targeted may be a potential treatment for
high-risk NB. Hypoxia has been also shown to affect CNS metastatic
spread of NB by affecting the BBB integrity (54). In the lymphatic system, the BBB
aquaporins (AQPs) play an important role. Treatment targeting AQP4
may allow an increase in the entry of immune molecules and cells
into the CNS (55).
Inhibitors of ADAM10 and 17 in the present study
indicated that small-molecule drugs may serve as a novel
therapeutic option for NB; however, the development of therapeutic
methods for clinical needs is still an urgent problem that remains
to be solved. Computer-aided drug design tools (56) and High-Throughput Screening
(57) platforms are required to
develop new drugs. AQPs are required for MMP-dependent migration of
cancer cells. Hypoxia-dependent upregulation of AQPs has been
demonstrated, and targeting AQP trafficking as a potential drug
therapeutic target has been suggested (58). Regarding AQP0-5 relocation, novel
drug targets have been discovered by identifying the downstream
proteins involved in the underlying molecular mechanism (59). Recent studies on neurodegenerative
diseases described that the use of brain organoid systems generated
from human pluripotent stem cells demonstrated considerable
potential in recapitulating key features of diseases'
pathophysiology. Organoid systems are defined as self-organized and
self-patterning three-dimensional (3D) structures that share
certain similarities with complex organs. Additionally, the use of
small-molecule drugs in this system was found to regulate key
disease markers phosphorylation. Thus, it was identified that stem
cell-derived 3D in vitro systems can potentially serve as
drug treatment platforms against neurodegenerative diseases
(60). An in vitro
Microvessel-on-a-Chip open microfluidic model, designed and
implemented by using human brain microvascular endothelial cells,
was validated permeability of fluorescent dextran and a human
monoclonal antibody (61). These
advanced systems not only provide the possibility for in
vitro research involving the treatment of NB with
small-molecule drugs and monoclonal antibodies, but also are
amenable for advanced imaging which are enable real-time monitoring
of cancer cell spread and invasion. The high-throughput screening
and computer-aided drug design will be applied in future research,
and inability to test the effects of NB-derived sNKG2DL on these
systems is a limitation of the present study.
In conclusion, it was found that high levels of
soluble MICA and ULBP2 in the sera of patients with NB was closely
associated with poorer outcome and T cell exhaustion. These
tumor-derived NKG2DLs were cleaved by both ADAM10 and ADAM17 in NB.
NB-derived sNKG2DL induced degradation of NKG2D on CD8+
T cells and impaired the proliferation, IFN-γ production and
cytotoxicity of CD8+ T cells. Blockage of sNKG2DL may
highlight a novel strategy to recover T-cell function and enhance
antitumor immunotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported from the National Natural
Science Foundation of China (grant nos. 81771633 and 81572324).
Availability of data and materials
The datasets used and/or analyzed during current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KD and FL designed the study. YZ collected the data
and performed experiments. YZ and FL analyzed and interpreted the
data. YZ and FL wrote the main manuscript text. YZ, FL and KD
confirm the authenticity of all the raw data. DK and FL were
involved in critical reviewing of the manuscript. All authors
reviewed, read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no
.2020238) by the Institute Research Ethics Committee at the
Children's Hospital of Fudan University (Shanghai, China). Informed
consent was acquired from every patient's legal guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park JA and Cheung NKV: Targets and
antibody formats for immunotherapy of neuroblastoma. J Clin Oncol.
38:1836–1848. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janoueix-Lerosey I, Schleiermacher G and
Delattre O: Molecular pathogenesis of peripheral neuroblastic
tumors. Oncogene. 29:1566–1579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sylvain NJ, Salman MM, Pushie MJ, Hou H,
Meher V, Herlo R, Peeling L and Kelly ME: The effects of
trifluoperazine on brain edema, aquaporin-4 expression and
metabolic markers during the acute phase of stroke using
photothrombotic mouse model. Biochim Biophys Acta Biomembr.
1863:1835732021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salman MM, Kitchen P, Woodroofe MN, Bill
RM, Conner AC, Heath PR and Conner MT: Transcriptome analysis of
gene expression provides new insights into the effect of mild
therapeutic hypothermia on primary human cortical astrocytes
cultured under hypoxia. Front Cell Neurosci. 11:3862017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ndunge OB, Kilian N and Salman MM:
Cerebral malaria and neuronal implications of plasmodium falciparum
infection: From mechanisms to advanced models. Adv Sci (Weinh).
9:e22029442022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salman MM, Kitchen P, Yool AJ and Bill RM:
Recent breakthroughs and future directions in drugging aquaporins.
Trends Pharmacol Sci. 43:30–42. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
López-Soto A, Huergo-Zapico L,
Acebes-Huerta A, Villa-Alvarez M and Gonzalez S: NKG2D signaling in
cancer immunosurveillance. Int J Cancer. 136:1741–1750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiossone L, Dumas PY, Vienne M and Vivier
E: Natural killer cells and other innate lymphoid cells in cancer.
Nat Rev Immunol. 18:671–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bauer S, Groh V, Wu J, Steinle A, Phillips
JH, Lanier LL and Spies T: Activation of NK cells and T cells by
NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prajapati K, Perez C, Rojas LBP, Burke B
and Guevara-Patino JA: Functions of NKG2D in CD8+ T cells: An
opportunity for immunotherapy. Cell Mol Immunol. 15:470–479. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mujal AM, Delconte RB and Sun JC: Natural
killer cells: From innate to adaptive features. Annu Rev Immunol.
39:417–447. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zloza A, Kohlhapp FJ, Lyons GE, Schenkel
JM, Moore TV, Lacek AT, O'Sullivan JA, Varanasi V, Williams JW,
Jagoda MC, et al: NKG2D signaling on CD8+ T cells represses T-bet
and rescues CD4-unhelped CD8+ T cell memory recall but not effector
responses. Nat Med. 18:422–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raulet DH: Roles of the NKG2D
immunoreceptor and its ligands. Nat Rev Immunol. 3:781–790. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Wang S, Xin J, Wang J, Yao C and
Zhang Z: Role of NKG2D and its ligands in cancer immunotherapy. Am
J Cancer Res. 9:2064–2078. 2019.PubMed/NCBI
|
|
16
|
Salih HR, Rammensee HG and Steinle A:
Cutting edge: Down-regulation of MICA on human tumors by
proteolytic shedding. J Immunol. 169:4098–4102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Groh V, Wu J, Yee C and Spies T:
Tumour-derived soluble MIC ligands impair expression of NKG2D and
T-cell activation. Nature. 419:734–738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pistoia V, Morandi F, Bianchi G, Pezzolo
A, Prigione I and Raffaghello L: Immunosuppressive microenvironment
in neuroblastoma. Front Oncol. 3:1672013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fuertes MB, Domaica CI and Zwirner NW:
Leveraging NKG2D ligands in immuno-oncology. Front Immunol.
12:7131582021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morandi F, Sabatini F, Podestà M and
Airoldi I: Immunotherapeutic strategies for Neuroblastoma: Present,
past and future. Vaccines (Basel). 9:432021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JR, Digiusto DL, Slovak M, Wright C,
Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg
JR and Jensen MC: Adoptive transfer of chimeric antigen receptor
re-directed cytolytic T lymphocyte clones in patients with
neuroblastoma. Mol Ther. 15:825–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pule MA, Savoldo B, Myers GD, Rossig C,
Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al:
Virus-specific T cells engineered to coexpress tumor-specific
receptors: Persistence and antitumor activity in individuals with
neuroblastoma. Nat Med. 14:1264–1270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kailayangiri S, Altvater B, Spurny C,
Jamitzky S, Schelhaas S, Jacobs AH, Wiek C, Roellecke K, Hanenberg
H, Hartmann W, et al: Targeting Ewing sarcoma with activated and
GD2-specific chimeric antigen receptor-engineered human NK cells
induces upregulation of immune-inhibitory HLA-G. Oncoimmunology.
6:e12500502017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pathania AS, Prathipati P, Olwenyi OA,
Chava S, Smith OV, Gupta SC, Chaturvedi NK, Byrareddy SN, Coulter
DW and Challagundla KB: miR-15a and miR-15b modulate natural killer
and CD8+T-cell activation and anti-tumor immune response
by targeting PD-L1 in neuroblastoma. Mol Ther Oncolytics.
25:308–329. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhen Z, Yang K, Ye L, You Z, Chen R, Liu Y
and He Y: HLA-E inhibitor enhances the killing of neuroblastoma
stem cells by co-cultured dendritic cells and cytokine-induced
killer cells loaded with membrane-based microparticles. Am J Cancer
Res. 7:334–345. 2017.PubMed/NCBI
|
|
26
|
Brandetti E, Veneziani I, Melaiu O,
Pezzolo A, Castellano A, Boldrini R, Ferretti E, Fruci D, Moretta
L, Pistoia V, et al: MYCN is an immunosuppressive oncogene
dampening the expression of ligands for NK-cell-activating
receptors in human high-risk neuroblastoma. Oncoimmunology.
6:e13164392017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan X, Johnson BD and Orentas RJ: Murine
CD8 lymphocyte expansion in vitro by artificial antigen-presenting
cells expressing CD137L (4-1BBL) is superior to CD28, and CD137L
expressed on neuroblastoma expands CD8 tumour-reactive effector
cells in vivo. Immunology. 112:105–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zingoni A, Molfetta R, Fionda C, Soriani
A, Paolini R, Cippitelli M, Cerboni C and Santoni A: NKG2D and its
ligands: ‘One for All, All for One’. Front Immunol. 9:4762018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diefenbach A, Tomasello E, Lucas M,
Jamieson AM, Hsia JK, Vivier E and Raulet DH: Selective
associations with signaling proteins determine stimulatory versus
costimulatory activity of NKG2D. Nat Immunol. 3:1142–1149. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raffaghello L, Prigione I, Airoldi I,
Camoriano M, Levreri I, Gambini C, Pende D, Steinle A, Ferrone S
and Pistoia V: Downregulation and/or release of NKG2D ligands as
immune evasion strategy of human neuroblastoma. Neoplasia.
6:558–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paschen A, Sucker A, Hill B, Moll I,
Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, et
al: Differential clinical significance of individual NKG2D ligands
in melanoma: Soluble ULBP2 as an indicator of poor prognosis
superior to S100B. Clin Cancer Res. 15:5208–5215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zingoni A, Vulpis E, Loconte L and Santoni
A: NKG2D ligand shedding in response to stress: Role of ADAM10.
Front Immunol. 11:4472020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsia HE, Tüshaus J, Brummer T, Zheng Y,
Scilabra SD and Lichtenthaler SF: Functions of ‘A disintegrin and
metalloproteases (ADAMs)’ in the mammalian nervous system. Cell Mol
Life Sci. 76:3055–3081. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng X, Jiang F, Katakowski M, Zhang ZG,
Lu QE and Chopp M: ADAM17 promotes breast cancer cell malignant
phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther.
8:1045–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo W, Huang J, Lei P, Guo L and Li X:
LncRNA SNHG1 promoted HGC-27 cell growth and migration via the
miR-140/ADAM10 axis. Int J Biol Macromol. 122:817–823. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Waldhauer I and Steinle A: Proteolytic
release of soluble UL16-binding protein 2 from tumor cells. Cancer
Res. 66:2520–2526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waldhauer I, Goehlsdorf D, Gieseke F,
Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG
and Steinle A: Tumor-associated MICA is shed by ADAM proteases.
Cancer Res. 68:6368–6376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wolpert F, Tritschler I, Steinle A, Weller
M and Eisele G: A disintegrin and metalloproteinases 10 and 17
modulate the immunogenicity of glioblastoma-initiating cells. Neuro
Oncol. 16:382–391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tosetti F, Venè R, Camodeca C, Nuti E,
Rossello A, D'Arrigo C, Galante D, Ferrari N, Poggi A and Zocchi
MR: Specific ADAM10 inhibitors localize in exosome-like vesicles
released by Hodgkin lymphoma and stromal cells and prevent sheddase
activity carried to bystander cells. Oncoimmunology.
7:e14218892018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Buchanan PC, Boylan KLM, Walcheck B,
Heinze R, Geller MA, Argenta PA and Skubitz APN: Ectodomain
shedding of the cell adhesion molecule Nectin-4 in ovarian cancer
is mediated by ADAM10 and ADAM17. J Biol Chem. 292:6339–6351. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang J, LeBlanc ME, Cano I, Saez-Torres
KL, Saint-Geniez M, Ng YS and D'Amore PA: ADAM10 and ADAM17
proteases mediate proinflammatory cytokine-induced and constitutive
cleavage of endomucin from the endothelial surface. J Biol Chem.
295:6641–6651. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu G, Lu S, Wang X, Page ST, Higano CS,
Plymate SR, Greenberg NM, Sun S, Li Z and Wu JD: Perturbation of NK
cell peripheral homeostasis accelerates prostate carcinoma
metastasis. J Clin Invest. 123:4410–4422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Andrade LF, Tay RE, Pan D, Luoma AM,
Ito Y, Badrinath S, Tsoucas D, Franz B, May KF Jr, Harvey CJ, et
al: Antibody-mediated inhibition of MICA and MICB shedding promotes
NK cell-driven tumor immunity. Science. 359:1537–1542. 2018.
View Article : Google Scholar
|
|
44
|
Zhang J, Liu D, Li G, Staveley-O'Carroll
KF, Graff JN, Li Z and Wu JD: Antibody-mediated neutralization of
soluble MIC significantly enhances CTLA4 blockade therapy. Sci Adv.
3:e16021332017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xiao G, Wang X, Sheng J, Lu S, Yu X and Wu
JD: Soluble NKG2D ligand promotes MDSC expansion and skews
macrophage to the alternatively activated phenotype. J Hematol
Oncol. 8:132015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
André MC, Sigurdardottir D, Kuttruff S,
Pömmerl B, Handgretinger R, Rammensee HG and Steinle A: Impaired
tumor rejection by memory CD8 T cells in mice with NKG2D
dysfunction. Int J Cancer. 131:1601–1610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Perez C, Prajapati K, Burke B, Plaza-Rojas
L, Zeleznik-Le NJ and Guevara-Patino JA: NKG2D signaling certifies
effector CD8 T cells for memory formation. J Immunother Cancer.
7:482019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Quamine AE, Olsen MR, Cho MM and Capitini
CM: Approaches to enhance natural killer cell-based immunotherapy
for pediatric solid tumors. Cancers (Basel). 13:27962021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu S, Galat V, Galat Y, Lee YKA,
Wainwright D and Wu J: NK cell-based cancer immunotherapy: From
basic biology to clinical development. J Hematol Oncol. 14:72021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Basher F, Dhar P, Wang X, Wainwright DA,
Zhang B, Sosman J, Ji Z and Wu JD: Antibody targeting tumor-derived
soluble NKG2D ligand sMIC reprograms NK cell homeostatic survival
and function and enhances melanoma response to PDL1 blockade
therapy. J Hematol Oncol. 13:742020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ullrich E, Koch J, Cerwenka A and Steinle
A: New prospects on the NKG2D/NKG2DL system for oncology.
Oncoimmunology. 2:e260972013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kloess S, Huenecke S, Piechulek D, Esser
R, Koch J, Brehm C, Soerensen J, Gardlowski T, Brinkmann A, Bader
P, et al: IL-2-activated haploidentical NK cells restore
NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by
scavenging of plasma MICA. Eur J Immunol. 40:3255–3267. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mastronuzzi A, Colafati GS, Carai A,
D'Egidio M, Fabozzi F, Bufalo FD, Villani MF, Baldo GD, Vennarini
S, Canino C, et al: Central nervous system metastasis in
neuroblastoma: From three decades clinical experience to new
considerations in the immunotherapy era. Cancers (Basel).
14:62492022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Salman MM, Kitchen P, Halsey A, Wang MX,
Törnroth-Horsefield S, Conner AC, Badaut J, Iliff JJ and Bill RM:
Emerging roles for dynamic aquaporin-4 subcellular relocalization
in CNS water homeostasis. Brain. 145:64–75. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kitchen P, Salman MM, Halsey AM,
Clarke-Bland C, MacDonald JA, Ishida H, Vogel HJ, Almutiri S, Logan
A, Kreida S, et al: Targeting aquaporin-4 subcellular localization
to treat central nervous system edema. Cell. 181:784–799.e19. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Salman MM, Al-Obaidi Z, Kitchen P, Loreto
A, Bill RM and Wade-Martins R: Advances in applying computer-aided
drug design for neurodegenerative diseases. Int J Mol Sci.
22:46882021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Aldewachi H, Al-Zidan RN, Conner MT and
Salman MM: High-throughput screening platforms in the discovery of
novel drugs for neurodegenerative diseases. Bioengineering (Basel).
8:302021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wagner K, Unger L, Salman MM, Kitchen P,
Bill RM and Yool AJ: Signaling mechanisms and pharmacological
modulators governing diverse aquaporin functions in human health
and disease. Int J Mol Sci. 23:13882022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Markou A, Unger L, Abir-Awan M, Saadallah
A, Halsey A, Balklava Z, Conner M, Törnroth-Horsefield S, Greenhill
SD, Conner A, et al: Molecular mechanisms governing aquaporin
relocalisation. Biochim Biophys Acta Biomembr. 1864:1838532022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Papaspyropoulos A, Tsolaki M, Foroglou N
and Pantazaki AA: Modeling and targeting Alzheimer's disease With
Organoids. Front Pharmacol. 11:3962020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Salman MM, Marsh G, Kusters I, Delincé M,
Di Caprio G, Upadhyayula S, de Nola G, Hunt R, Ohashi KG, Gray T,
et al: Design and validation of a human brain endothelial
microvessel-on-a-chip open microfluidic model enabling advanced
optical imaging. Front Bioeng Biotechnol. 8:5737752020. View Article : Google Scholar : PubMed/NCBI
|