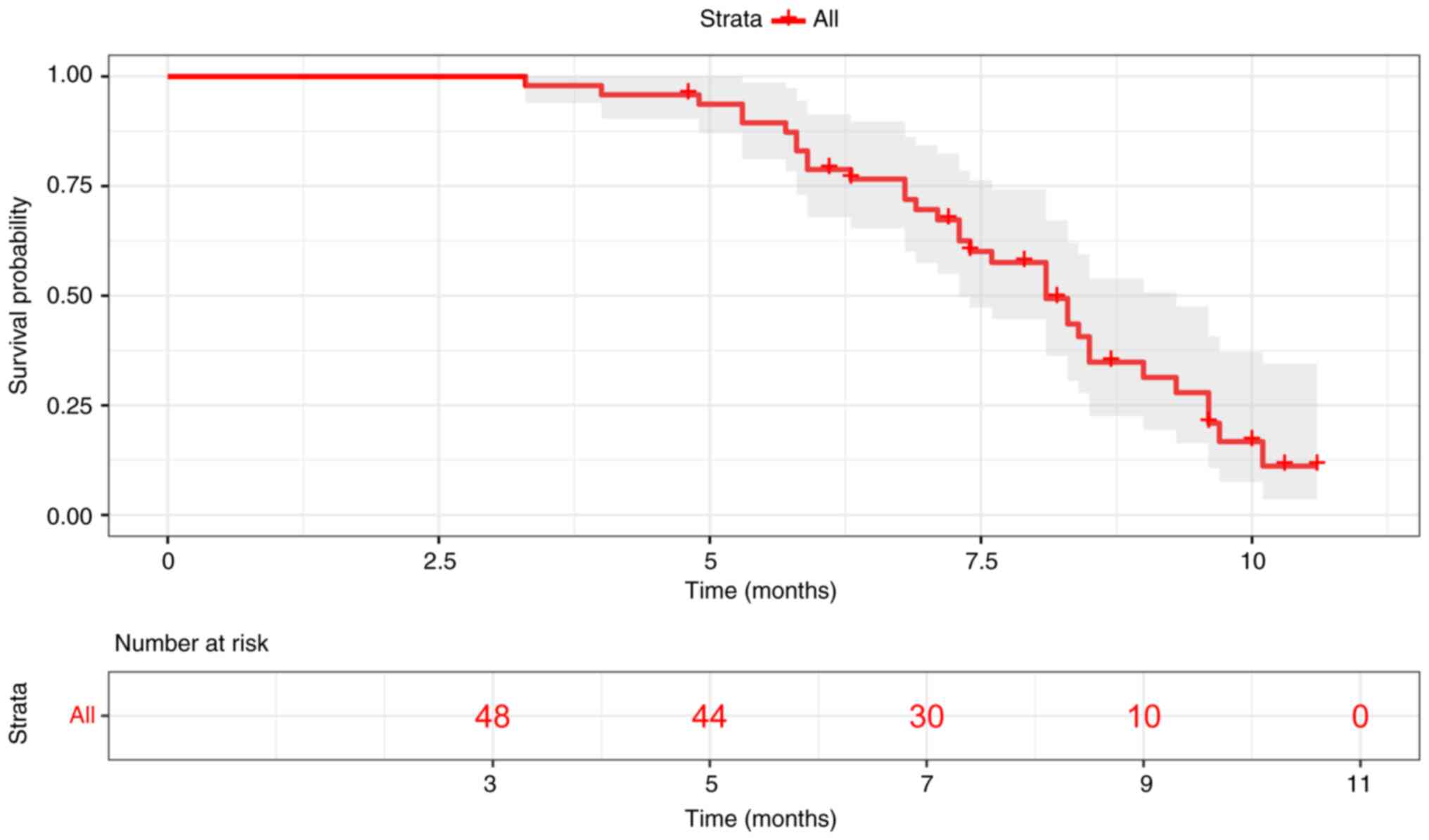
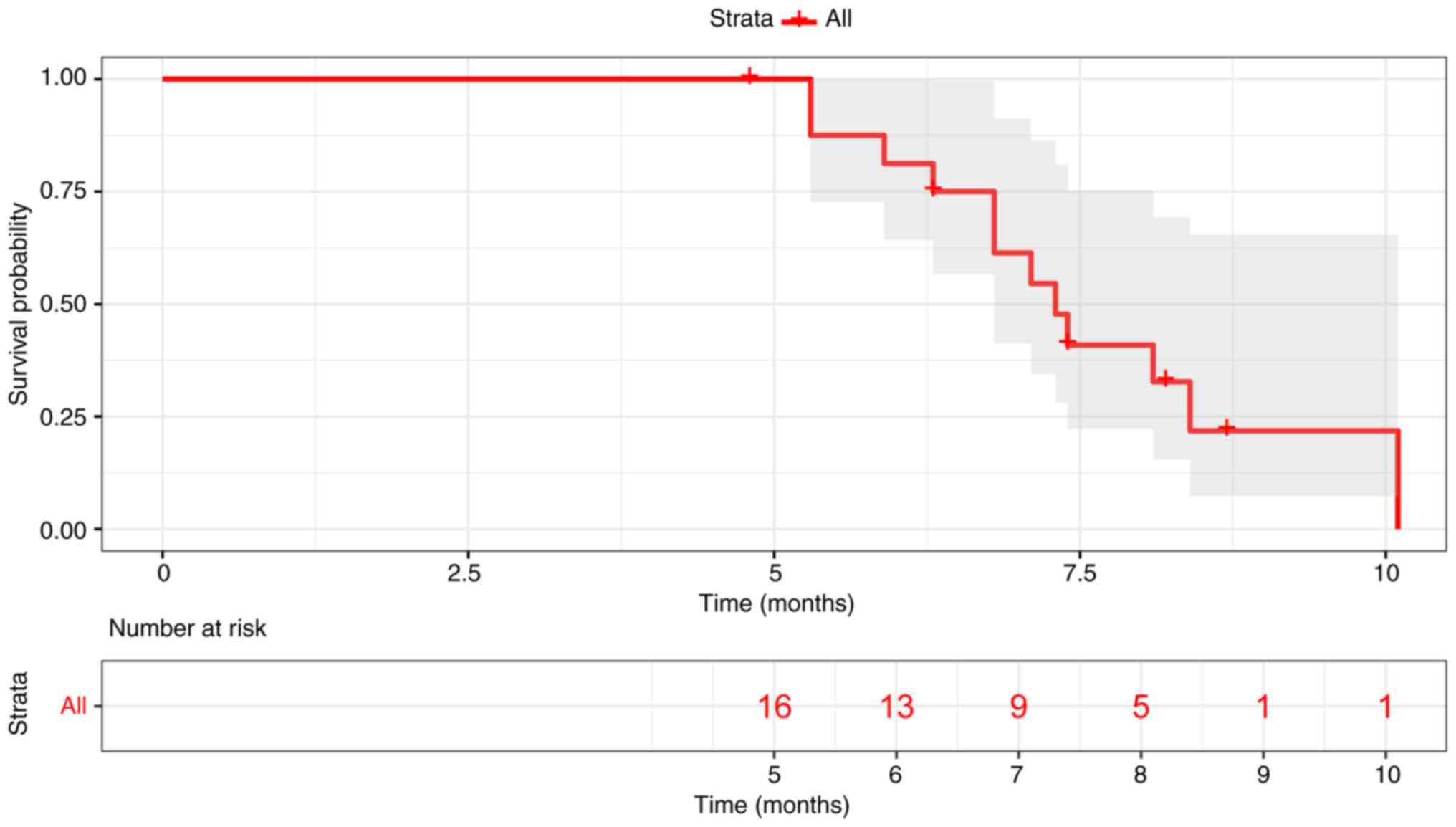
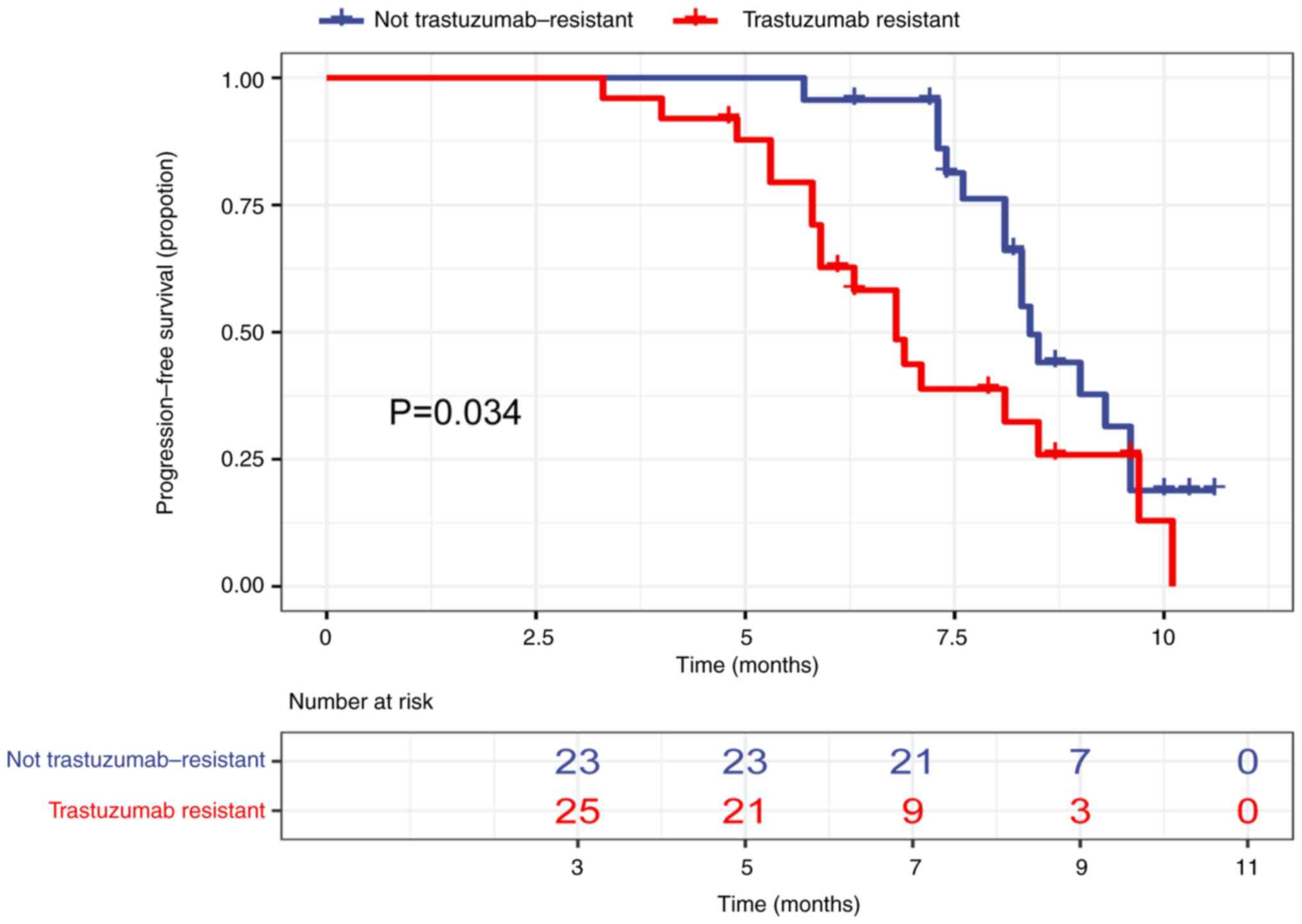
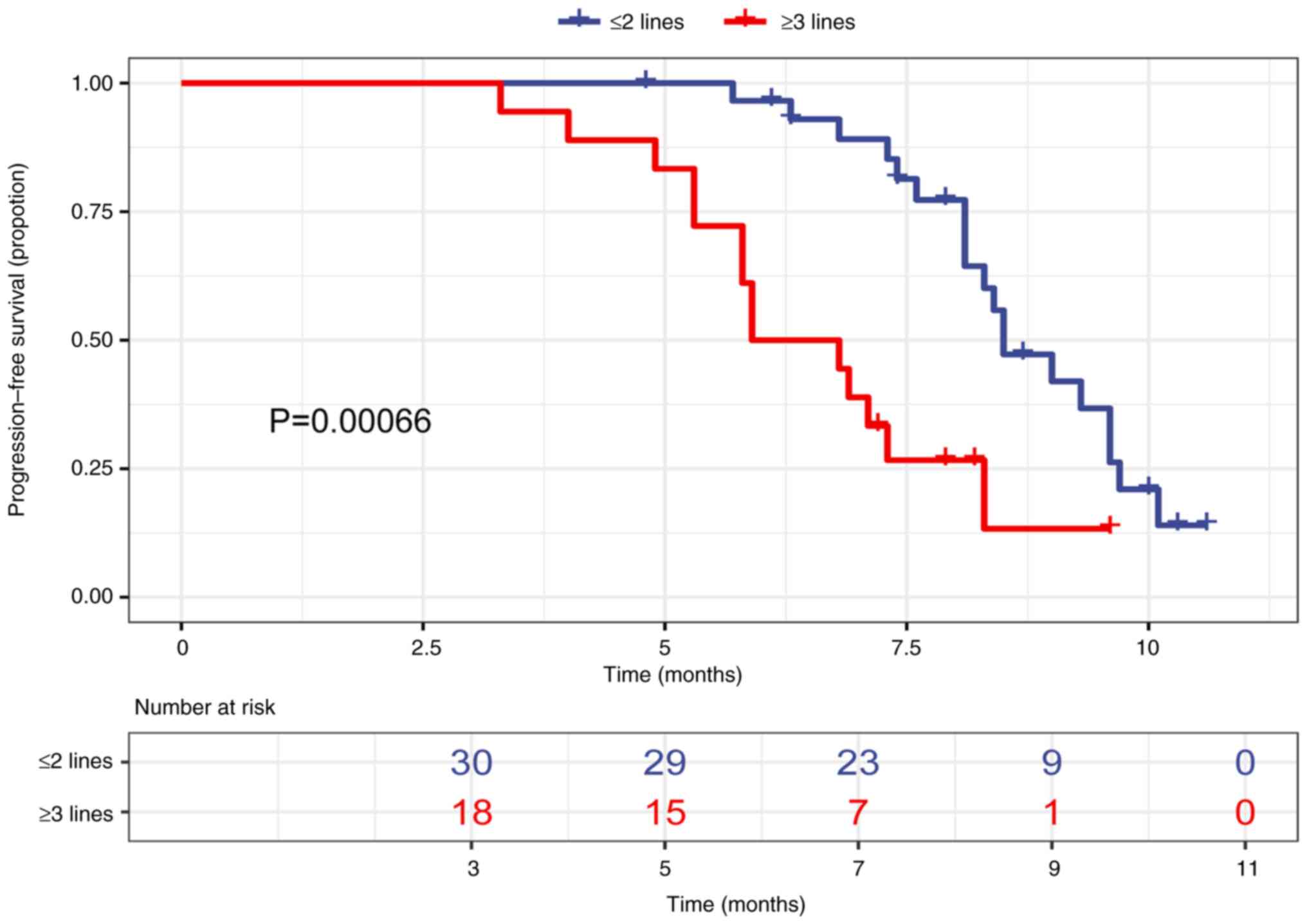
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blows FM, Driver KE, Schmidt MK, Broeks A,
van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO,
Blomqvist C, et al: Subtyping of breast cancer by
immunohistochemistry to investigate a relationship between subtype
and short and long term survival: A collaborative analysis of data
for 10,159 cases from 12 studies. PLoS Med. 7:e10002792010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swain SM, Baselga J, Miles D, Im YH, Quah
C, Lee LF and Cortés J: Incidence of central nervous system
metastases in patients with HER2-positive metastatic breast cancer
treated with pertuzumab, trastuzumab, and docetaxel: Results from
the randomized phase III study CLEOPATRA. Ann Oncol. 25:1116–1121.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verma S, Miles D, Gianni L, Krop IE,
Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, et al:
Trastuzumab emtansine for HER2-positive advanced breast cancer. N
Engl J Med. 367:1783–1791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Swain SM, Baselga J, Kim SB, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Heeson S, et al: Pertuzumab, trastuzumab, and docetaxel in
HER2-positive metastatic breast cancer. N Engl J Med. 372:724–734.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Awada A, Colomer R, Inoue K, Bondarenko I,
Badwe RA, Demetriou G, Lee SC, Mehta AO, Kim SB, Bachelot T, et al:
Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in
previously untreated metastatic ERBB2-positive breast cancer: The
NEfERT-T randomized clinical trial. JAMA Oncol. 2:1557–1564. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krop IE, Lin NU, Blackwell K, Guardino E,
Huober J, Lu M, Miles D, Samant M, Welslau M and Diéras V:
Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in
patients with HER2-positive metastatic breast cancer and central
nervous system metastases: A retrospective, exploratory analysis in
EMILIA. Ann Oncol. 26:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shitara K, Bang YJ, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive gastric
cancer. N Engl J Med. 382:2419–2430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pohlmann PR, Mayer IA and Mernaugh R:
Resistance to trastuzumab in breast cancer. Clin Cancer Res.
15:7479–7491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blair HA: Pyrotinib: First global
approval. Drugs. 78:1751–1755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu
Y, Li H, Yu S, Feng J, Wang S, et al: Pyrotinib or lapatinib
combined with capecitabine in HER2-positive metastatic breast
cancer with prior taxanes, anthracyclines, and/or trastuzumab: A
randomized, phase II study. J Clin Oncol. 37:2610–2619. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q,
Tong Z, Li H, Zhang Q, Sun T, et al: Pyrotinib plus capecitabine
versus lapatinib plus capecitabine for the treatment of
HER2-positive metastatic breast cancer (PHOEBE): A multicentre,
open-label, randomised, controlled, phase 3 trial. Lancet Oncol.
22:351–360. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan M, Ouyang Q, Sun T, Niu L, Yang J, Li
L, Song Y, Hao C, Chen Z, Orlandi A, et al: Pyrotinib plus
capecitabine for patients with human epidermal growth factor
receptor 2-positive breast cancer and brain metastases (PERMEATE):
A multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol.
23:353–361. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J,
Luo Y, Xing P, Lan B, Li M, et al: Phase I study and biomarker
analysis of pyrotinib, a novel irreversible pan-ErbB receptor
tyrosine kinase inhibitor, in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer. J Clin Oncol.
35:3105–3112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan M, Bian L, Hu X, Zhang Q, Ouyang Q,
Feng J, Yin Y, Sun T, Tong Z, Wang X, et al: Pyrotinib plus
capecitabine for human epidermal growth factor receptor 2-positive
metastatic breast cancer after trastuzumab and taxanes (PHENIX): A
randomized, double-blind, placebo-controlled phase 3 study. Transl
Breast Cancer Res. 1:132020. View Article : Google Scholar
|
|
20
|
Saura C, Oliveira M, Feng YH, Dai MS, Chen
SW, Hurvitz SA, Kim SB, Moy B, Delaloge S, Gradishar W, et al:
Neratinib plus capecitabine versus lapatinib plus capecitabine in
HER2-positive metastatic breast cancer previously treated with ≥2
HER2-directed regimens: Phase III NALA trial. J Clin Oncol.
38:3138–3149. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gradishar WJ, Krasnojon D, Cheporov S,
Makhson AN, Manikhas GM, Clawson A and Bhar P: Significantly longer
progression-free survival with nab-paclitaxel compared with
docetaxel as first-line therapy for metastatic breast cancer. J
Clin Oncol. 27:3611–3619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gradishar WJ, Krasnojon D, Cheporov S,
Makhson AN, Manikhas GM, Clawson A, Bhar P, McGuire JR and Iglesias
J: Phase II trial of nab-paclitaxel compared with docetaxel as
first-line chemotherapy in patients with metastatic breast cancer:
Final analysis of overall survival. Clin Breast Cancer. 12:313–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 5. US Department of Health and Human
Services National Institutes of Health, National Cancer Institute.
Published. November 27–2017
|
|
27
|
Wickham H: Ggplot2: Elegant graphics for
data analysis. Springer Publishing Company, Incorporated. 2009.
View Article : Google Scholar
|
|
28
|
Wong H, Leung R, Kwong A, Chiu J, Liang R,
Swanton C and Yau T: Integrating molecular mechanisms and clinical
evidence in the management of trastuzumab resistant or refractory
HER-2+ metastatic breast cancer. Oncologist. 16:1535–1546. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Urruticoechea A, Rizwanullah M, Im SA,
Ruiz ACS, Láng I, Tomasello G, Douthwaite H, Badovinac Crnjevic T,
Heeson S, Eng-Wong J and Muñoz M: Randomized phase III trial of
trastuzumab plus capecitabine with or without pertuzumab in
patients with human epidermal growth factor receptor 2-positive
metastatic breast cancer who experienced disease progression during
or after trastuzumab-based therapy. J Clin Oncol. 35:3030–3038.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olson EM, Najita JS, Sohl J, Arnaout A,
Burstein HJ, Winer EP and Lin NU: Clinical outcomes and treatment
practice patterns of patients with HER2-positive metastatic breast
cancer in the post-trastuzumab era. Breast. 22:525–531. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Y, Lin M, Zhang J, Wang B, Tao Z, Du
Y, Zhang S, Cao J, Wang L and Hu X: Real-world data of
pyrotinib-based therapy in metastatic HER2-positive breast cancer:
Promising efficacy in lapatinib-treated patients and in brain
metastasis. Cancer Res Treat. 52:1059–1066. 2020.PubMed/NCBI
|
|
32
|
Cortés J, Kim SB, Chung WP, Im SA, Park
YH, Hegg R, Kim MH, Tseng LM, Petry V, Chung CF, et al: Trastuzumab
deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J
Med. 386:1143–1154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Q, Ouyang D, Anwar M, Xie N, Wang S,
Fan P, Qian L, Chen G, Zhou E, Guo L, et al: Effectiveness and
safety of pyrotinib, and association of biomarker with
progression-free survival in patients with HER2-positive metastatic
breast cancer: A real-world, multicentre analysis. Front Oncol.
10:8112020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gori S, Rimondini S, De Angelis V, Colozza
M, Bisagni G, Moretti G, Sidoni A, Basurto C, Aristei C, Anastasi P
and Crinò L: Central nervous system metastases in HER-2 positive
metastatic breast cancer patients treated with trastuzumab:
Incidence, survival, and risk factors. Oncologist. 12:766–773.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garcia-Alvarez A, Papakonstantinou A and
Oliveira M: Brain metastases in HER2-positive breast cancer:
Current and novel treatment strategies. Cancers (Basel).
13:29272021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brosnan EM and Anders CK: Understanding
patterns of brain metastasis in breast cancer and designing
rational therapeutic strategies. Ann Transl Med. 6:1632018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brufsky AM, Mayer M, Rugo HS, Kaufman PA,
Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, et
al: Central nervous system metastases in patients with
HER2-positive metastatic breast cancer: Incidence, treatment, and
survival in patients from registHER. Clin Cancer Res. 17:4834–4843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petrelli F, Ghidini M, Lonati V, Tomasello
G, Borgonovo K, Ghilardi M, Cabiddu M and Barni S: The efficacy of
lapatinib and capecitabine in HER-2 positive breast cancer with
brain metastases: A systematic review and pooled analysis. Eur J
Cancer. 84:141–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Freedman RA, Gelman RS, Anders CK, Melisko
ME, Parsons HA, Cropp AM, Silvestri K, Cotter CM, Componeschi KP,
Marte JM, et al: TBCRC 022: A phase II trial of neratinib and
capecitabine for patients with human epidermal growth factor
receptor 2-positive breast cancer and brain metastases. J Clin
Oncol. 37:1081–1089. 2019. View Article : Google Scholar : PubMed/NCBI
|