Introduction
Breast cancer is the most prevalent malignancy in
women worldwide and poses a notable threat to human health
(1). Notably, 20–30% of patients
with breast cancer exhibit HER2 upregulation in the tumor tissue
(2,3). HER2-positive tumors are particularly
aggressive, and are associated with a poor prognosis due to reduced
overall survival (OS) and progression-free survival (PFS) (4). In addition, the results of a previous
study indicated that HER2-positive tumors are more likely to
metastasize to internal organs and the central nervous system (CNS)
(5). At present, there is no cure
for metastatic breast cancer (MBC); thus, treatment options that
extend survival, achieve remission and optimize quality of life are
required. The majority of anti-HER2-targeted therapies combined
with chemotherapy significantly improve the outcomes of patients
with HER2-positive breast cancer (6).
The development of anti-HER2 drugs has led to a
significant improvement in the survival of patients with
HER2-positive advanced breast cancer (ABC). Notably, anti-HER2
drugs, such as trastuzumab, pertuzumab, lapatinib, ado-trastuzumab
emtansine, neratinib and trastuzumab deruxtecan, have offered great
benefit to this group of patients (6–12).
Trastuzumab, also known as Herceptin, was the first targeted
anti-HER2 therapeutic drug to be used in clinical practice.
Notably, there are two types of trastuzumab resistance: Primary and
secondary trastuzumab resistance. Primary trastuzumab resistance
refers to progressive disease (PD) development during adjuvant
trastuzumab treatment after early breast cancer surgery, or PD
development within 1 year of the end of trastuzumab treatment.
Secondary trastuzumab resistance refers to PD development 1 year
after the end of adjuvant trastuzumab therapy following surgery for
early breast cancer, or re-emergence of PD after effective MBC
trastuzumab treatment (13). In
patients with HER2-positive ABC for whom trastuzumab therapy has
failed, the development of alternative targeted agents is
required.
In addition to targeting HER1, HER2 and HER4,
pyrotinib is an oral, irreversible, pan-erbB tyrosine kinase
inhibitor (TKI) (14). During phase
I, II and III studies, pyrotinib has been shown to exert notable
clinical benefits when combined with capecitabine, and it has been
reported to be well-tolerated by patients with HER2+ MBC (15–18).
The results of a randomized, open-label, multicenter, phase II
clinical trial demonstrated that treatment with pyrotinib and
capecitabine resulted in a notable improvement in objective
response rate (ORR) and a longer median PFS (mPFS), when compared
with lapatinib and capecitabine (15). Furthermore, the results of the phase
III, double-blind, multicenter, randomized PHENIX study
demonstrated that pyrotinib plus capecitabine significantly
prolonged mPFS (11.1 vs. 4.1 months) and improved ORR (68.6 vs.
16.0%) compared with capecitabine monotherapy (19). The results of the phase III PHOEBE
trial also revealed that pyrotinib plus capecitabine significantly
prolonged mPFS compared with lapatinib plus capecitabine (16). The results of previous studies have
demonstrated that treatment with pyrotinib and capecitabine may
lead to increased survival rates in patients; however, OS data are
lacking. Notably, earlier studies only included patients with
HER2-positive MBC without two or more prior systemic therapy
regimens (15,20). In August 2018, pyrotinib was
approved in China as a second-line targeted therapy for
HER2+ MBC. Notably, capecitabine is a commonly used
chemotherapy regimen in clinical practice, and numerous patients
may receive chemotherapy prior to receiving pyrotinib. In addition,
the therapeutic efficacy of pyrotinib in combination with other
chemotherapeutic agents is unclear; therefore, the choice of
chemotherapeutic agents for clinicians is limited at present.
Nanoparticulate albumin-bound paclitaxel is an
albumin-bound paclitaxel nanoparticle formulation suspended in
saline (21). This drug exhibits a
lower risk of allergy and an improved toxicity compared with
solvent-based paclitaxel, and does not require pre-treatment
(21). In patients with MBC, PFS
has been shown to be significantly longer following treatment with
albumin-bound paclitaxel compared with docetaxel (22). In 2005, the United States Food and
Drug Administration approved albumin-bound paclitaxel for the
treatment of MBC. This medication is specifically intended for
patients who have either failed combination chemotherapy or
experienced cancer recurrence within 6 months of completing
adjuvant chemotherapy (23).
Patients who have not responded to trastuzumab
treatment are provided with several treatment options. Patients may
choose to receive T-DM1, a small molecule TKI drug combined with
chemotherapy, or pertuzumab dual-target therapy combined with
trastuzumab. However, in China, T-DM1 is costly and not currently
covered by medical insurance reimbursement. Alternatively, Chinese
patients with HER2-positive breast cancer for whom trastuzumab
treatment has failed may receive pyrotinib in combination with
chemotherapy, or pertuzumab dual-target therapy combined with
trastuzumab. The present study aimed to explore the efficacy and
safety of pyrotinib in combination with albumin-bound paclitaxel
for the treatment of HER2-positive breast cancer. The cohort used
in the present study included both trastuzumab-sensitive and
-resistant patients.
Materials and methods
Patients and dose regimen
Pyrotinib combined with albumin-bound paclitaxel was
used to treat patients with HER2-positive ABC admitted to Xingtai
People's Hospital (Xingtai, China) between October 2018 and June
2020. All participants provided written informed consent and the
present study was approved by the institutional review board.
The inclusion criteria were as follows: i) Patients
with MBC with immunohistochemistry (IHC) class 3+ or HER2 gene
amplification confirmed using fluorescence in situ
hybridization. In cases where a re-biopsy of the metastatic site
was not possible, HER2 status was determined using the most recent
primary tumor specimen; ii) patients with at least one measurable
lesion according to the solid tumor efficacy evaluation criteria
version 1.1 (RECIST 1.1) (24). The
exclusion criteria were as follows: i) Patients who were involved
in pyrotinib-related clinical trials; ii) patients who refused to
provide written informed consent. In addition, patients who
discontinued pyrotinib treatment due to financial reasons were
excluded from the efficacy analysis. A total of 48 patients were
enrolled in the efficacy cohort.
A combination of chemotherapy and/or
anti-HER2-targeting agents was frequently used with pyrotinib in
routine clinical practice. Initial dosing, dose adjustment and
treatment discontinuation of pyrotinib were determined by the
associated physician based on clinical efficacy, AE class, physical
performance status and patient preference.
The treatment regimen was as follows: Intravenous
administration of albumin-bound paclitaxel (130 mg/m2)
on days 1, 8 and 15, every 21 days for 2–8 cycles, together with
oral administration of 400 or 320 mg pyrotinib once daily until
disease progression.
Efficacy assessments were performed every 8 weeks
and at the end of study treatment. Throughout the study, all
patients were monitored for survival and safety assessments were
conducted regularly. Assessments included physical examinations,
evaluations of Eastern Cooperative Oncology Group performance
status (25), vital signs, clinical
laboratory assessments and cardiac monitoring. The left ventricular
ejection fraction function was monitored at 12-week intervals in
all patients. In addition, AEs and serious AEs were documented.
Assessments
Data collection included demographic and baseline
information, including age, histological characteristics, treatment
history, metastatic site, prior therapies, medical history and any
concomitant conditions.
The present study aimed to determine PFS, defined as
the length of time from the administration of pyrotinib until
disease progression or death from any cause. In addition, the
secondary study endpoint was ORR, calculated as the percentage of
patients who achieved complete remission (CR) or partial remission
(PR).
Oncologic efficacy was assessed using RECIST 1.1,
along with physical examination and imaging. A retrospective
analysis of medical records and laboratory results was conducted to
identify the safety risks associated with pyrotinib treatment. AE
grade was assigned according to the Common Terminology Criteria 5.0
for AEs by the National Cancer Institute (26). Trastuzumab resistance was defined as
new recurrence within 12 months of surgery or diagnosed within 12
months, or disease progression while receiving first-line
trastuzumab therapy.
Statistical analysis
In the present study, mPFS was determined using the
Kaplan-Meier method and univariate analysis was conducted using the
log-rank test. The Cox proportional hazards model was used for
multivariate analysis. All statistical analyses were carried out
using SPSS (version 25.0; IBM Corp.) and ‘ggplot2’ package
(27) in R version 4.2.2
(http://www.r-project.org). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics
A total of 48 female patients with HER2+
MBC were admitted with pyrotinib plus albumin-bound paclitaxel at
Xingtai People's Hospital between October 2018 and June 2020.
Baseline characteristics are presented in Table I. All patients were female. The
median age of patients at diagnosis was 59 years (range, 32–74
years). In total, 37.5% of patients exhibited more than three sites
of metastases, and the most common sites of metastases included the
lung (54.2%) and liver (35.4%). A total of 77.1% of patients
experienced visceral metastases and 17 patients (35.4%) experienced
brain metastases. Almost all patients were treated with anti-HER2
therapy. Of these, 95.8% of patients were treated with trastuzumab
and 35.4% of patients were treated with lapatinib. In addition, 50%
of patients received three or more systemic treatments prior to
pyrotinib plus albumin-bound paclitaxel treatment.
 | Table I.Baseline patient characteristics
(n=48). |
Table I.
Baseline patient characteristics
(n=48).
| Characteristic | Value |
|---|
| Median age,
years | 59 |
| Lines of advanced
systemic therapy, n (%) |
|
| ≥3 | 24 (50%) |
| 2 | 23 (47.9%) |
| 1 | 1 (2.1%) |
| Brain metastases, n
(%) |
|
| No | 31 (64.6%) |
|
Yes | 17 (35.4%) |
| Hormone
receptorsa status, n
(%) |
|
|
Positive | 33 (68.8%) |
|
Negative | 15 (31.2%) |
| Trastuzumab
resistance status, n (%) |
|
|
Resistance | 25 (52.1%) |
|
Sensitive | 23 (47.9%) |
| Visceral
metastases, n (%) |
|
|
Yes | 37 (77.1%) |
| No | 11 (22.9%) |
| Number of
metastatic sites, n (%) |
|
| ≥3 | 18 (37.5%) |
| ≤2 | 30 (62.5%) |
Dose adjustment
A total of 95.8% of patients started treatment with
pyrotinib at the standard dose of 400 mg/day, while 4.2% started
treatment with pyrotinib at a dose of 320 mg/day (Table II). Moreover, a total of 8 (16.7%)
and 5 (10.4%) patients experienced pyrotinib dose reductions or
interruptions. A total of 10 (20.8%) patients experienced a dose
reduction of albumin-bound paclitaxel due to AEs and 4 (8.3%)
patients experienced interruptions to albumin-bound paclitaxel
treatment. No patients permanently discontinued treatment due to
AEs.
 | Table II.Treatment administration. |
Table II.
Treatment administration.
| Treatment | Number of patients
(%) |
|---|
| Pyrotinib |
|
|
Starting dosage |
|
|
400 mg/day | 46 (95.8) |
|
320 mg/day | 2 (4.2) |
| Dose
reduction |
|
|
400→320
mg/day | 7 (14.6) |
|
400→320→240
mg/day | 1 (2.1) |
|
Interruption | 5(10.4) |
|
Albumin-bound-paclitaxel |
|
| Dose
reduction due to AEs |
|
|
Yes | 10 (20.8) |
|
No | 38 (79.2) |
|
Interruption of
albumin-bound-paclitaxel due to AEs | 4 (8.3) |
Efficacy
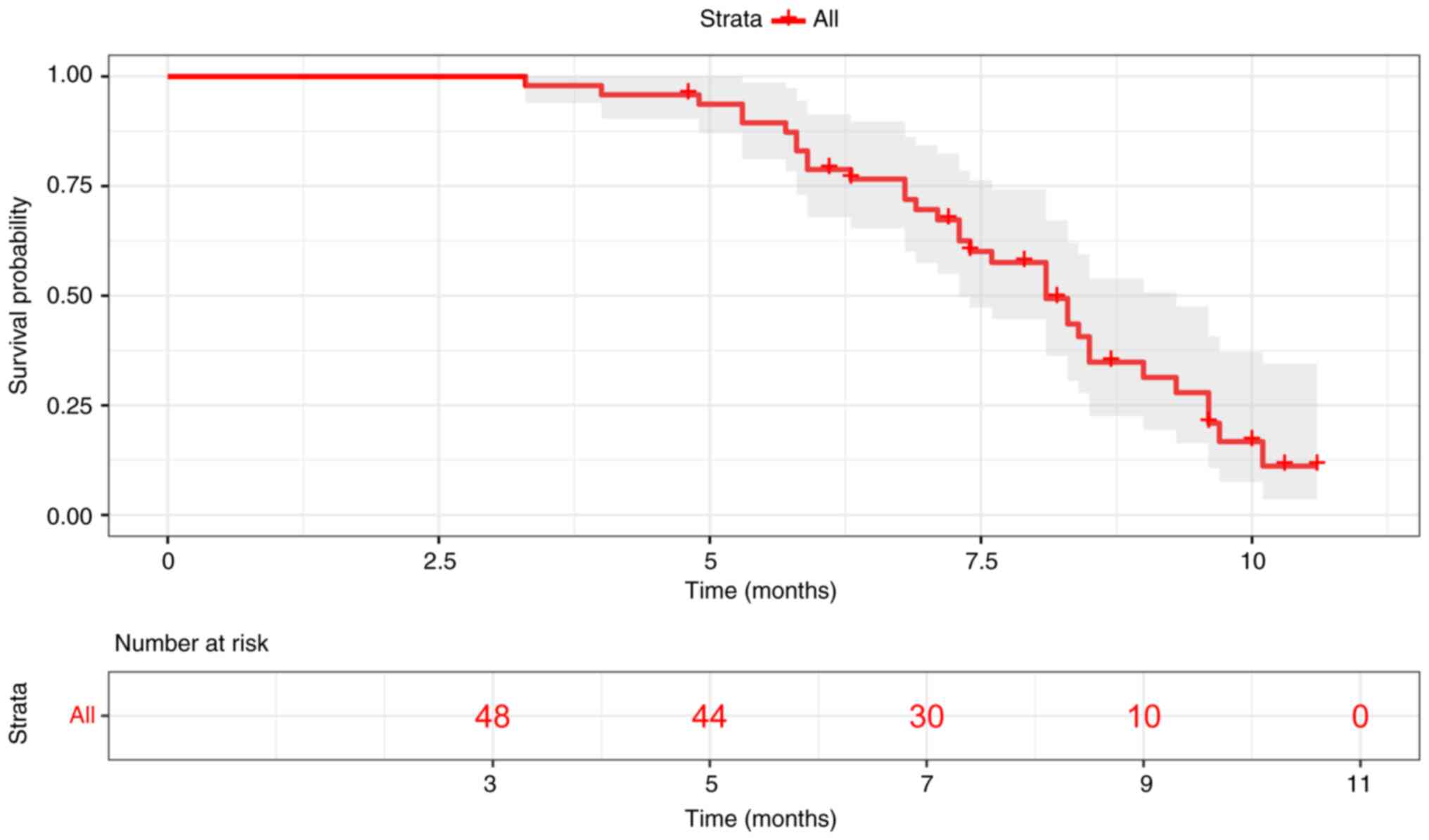
All patients were included in the PFS analysis with
a median follow-up of 8.7 months. The mPFS for 48 patients was 8.1
months (range, 3.3-10.6 months; Fig.
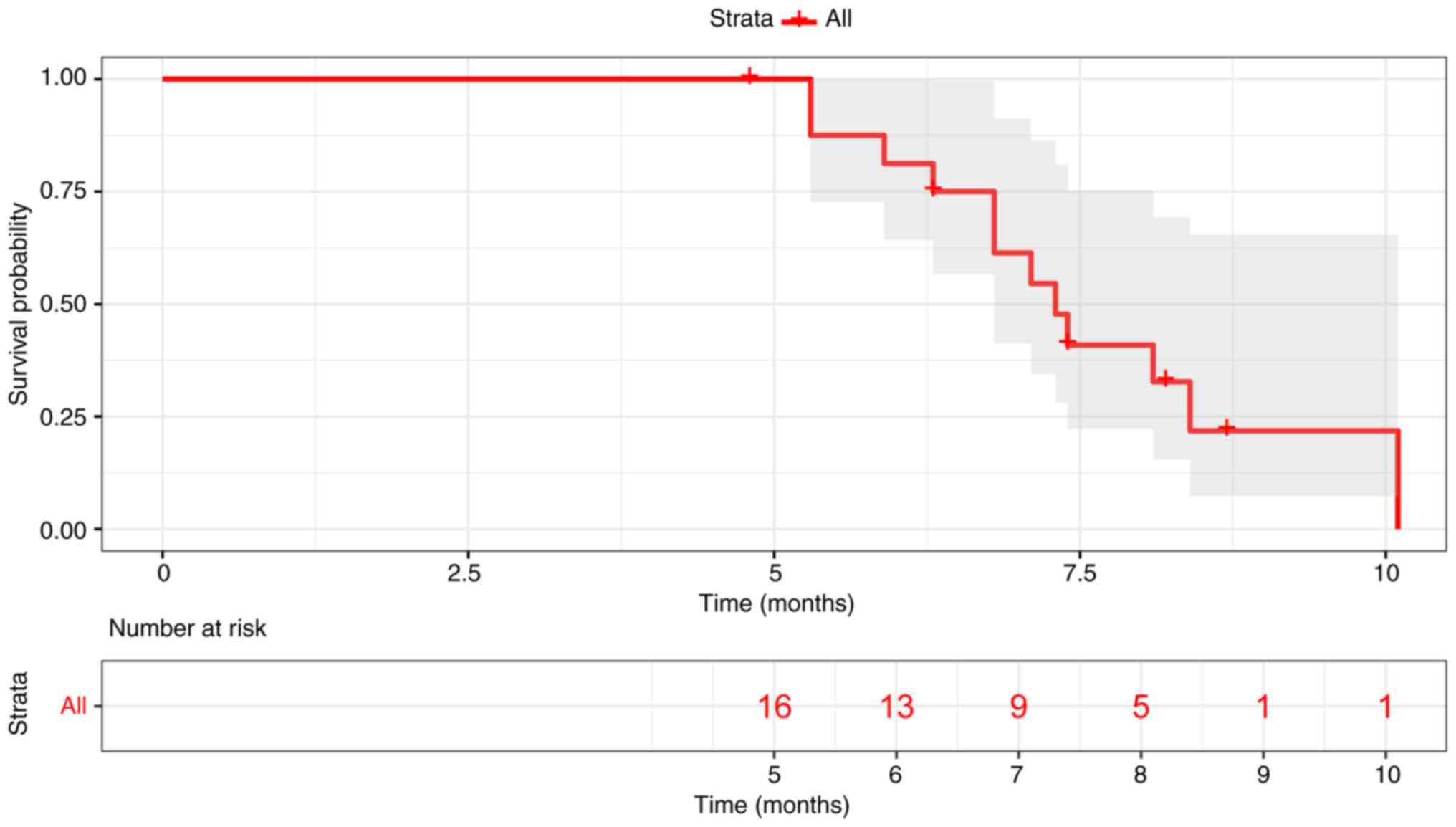
1). Patients who experienced metastases to the brain exhibited
a mPFS of 7.3 months (Fig. 2).
Notably, there was no significant difference in PFS between
patients who experienced metastases to the brain and those who did
not (7.3 months vs. 8.3 months; data not shown). In addition, 48
patients were included in the ORR analyses. The results of the
present study demonstrated that no patients experienced CR, 16
patients experienced PR and the ORR was 33.3% (Table III).
 | Table III.ORR in all patients (n=48). |
Table III.
ORR in all patients (n=48).
| Response | Number of patients
(%) |
|---|
| Complete
response | 0 (0) |
| Partial
response | 16 (33.3) |
| Stable disease | 24 (50.0) |
| Progressive
disease | 8 (16.7) |
| ORR | 16 (33.3) |
The results of the univariate analysis demonstrated
that age (<60 years vs. ≥60 years), hormone receptor status
(positive vs. negative), number of metastatic sites (≤2 vs. >2)
and whether lapatinib was previously applied (yes vs. no) were not
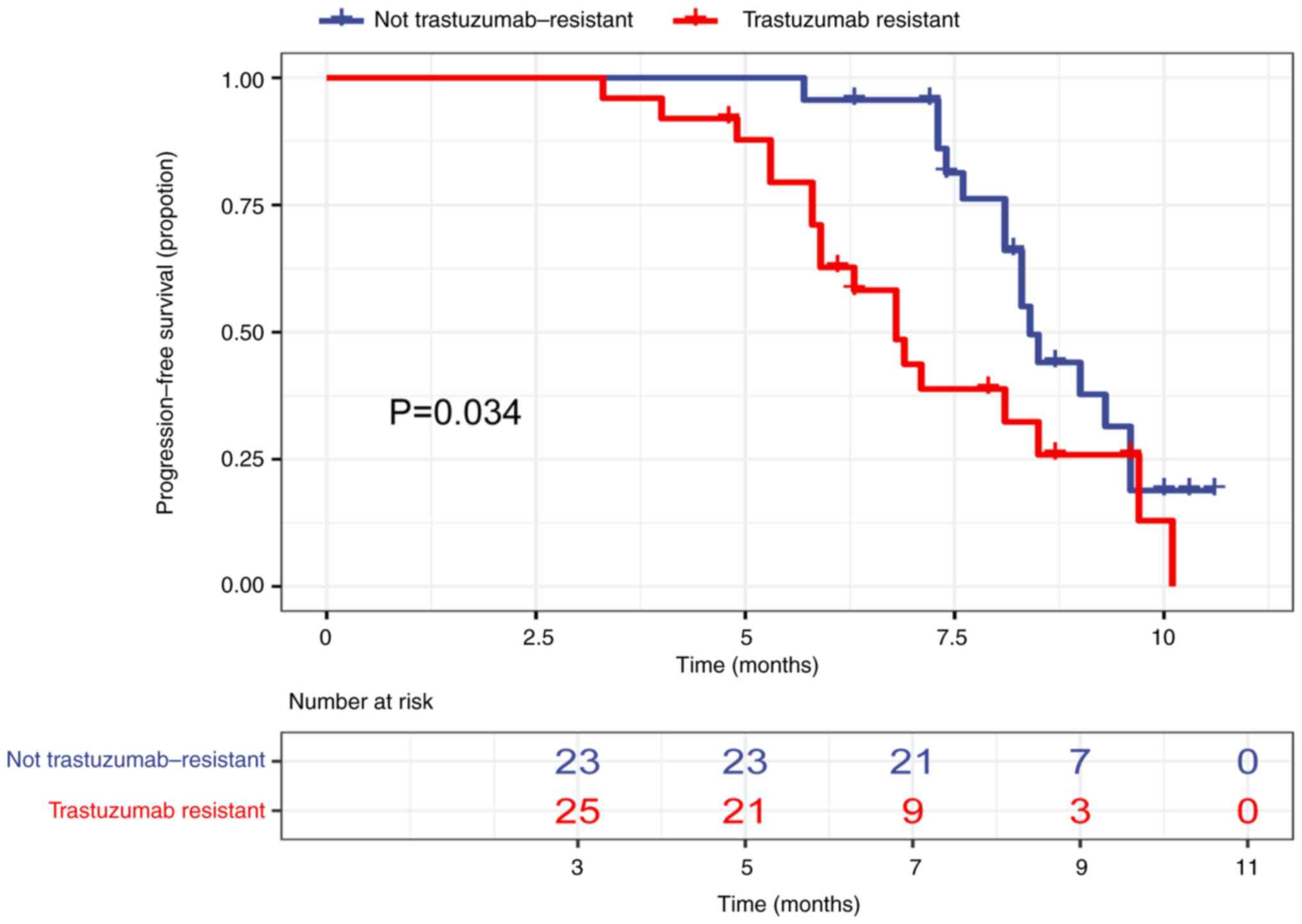
significantly associated with mPFS (Table IV). By contrast, trastuzumab
resistance status (resistant vs. not resistant) and number of
treatment lines (2 vs. ≥3) were significantly associated with mPFS
(Table IV). mPFS was 6.8 months in
patients with prior trastuzumab resistance and 8.4 months in
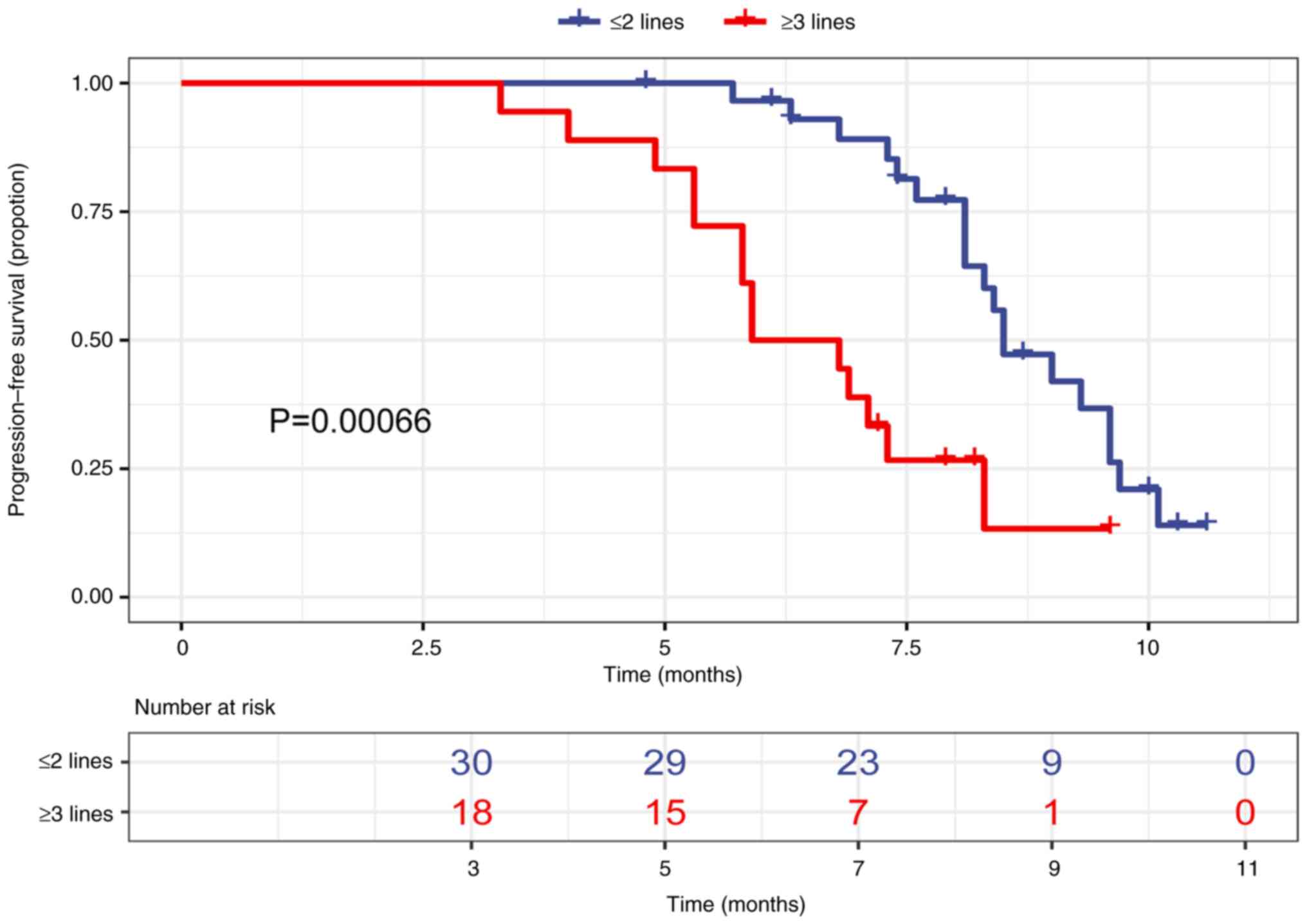
patients with no prior trastuzumab resistance (Fig. 3). Moreover, mPFS was 8.5 months in
patients with ≤2 lines of prior therapy and 6.35 months in patients
with ≥3 lines of prior therapy (Fig.
4). However, the results of the multivariate analysis
demonstrated that trastuzumab resistance was not an independent
predictor of mPFS, and the number of prior treatment lines was an
independent predictor of mPFS.
 | Table IV.Log-rank and Cox multivariate
analysis of factors associated with progression-free survival. |
Table IV.
Log-rank and Cox multivariate
analysis of factors associated with progression-free survival.
|
| Log-rank test | Cox multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | P-value | HR | P-value |
|---|
| Hormone receptor
status (positive vs. negative) | 1.074 | 0.424 |
|
|
| Trastuzumab
resistance status (resistance vs. refractoriness) | 2.023 | 0.034 | 1.577 | 0.265 |
| Age group (<60
years vs. ≥60 years) | 1.499 | 0.231 |
|
|
| Number of
metastatic sites (≤2 vs. >2) | 0.9491 | 0.878 |
|
|
| Prior exposure to
lapatinib (yes vs. no) | 1.209 | 0.588 |
|
|
| Lines of advanced
pyrotinib plus albumin-bound paclitaxel systematic therapy (2 vs.
≥3) | 0.3381 | 0.001 | 0.329 | 0.009 |
Safety indicators
Using laboratory findings and medical records of
patients, AEs were reported in the present study. The results of
the present study demonstrated that the most common grade 3–4 AEs
included diarrhea (22.9%), neutropenia (6.3%), anemia (4.2%) and
leukopenia (4.2%; Table V).
Notably, treatment with pyrotinib in combination with albumin-bound
paclitaxel did not result in any treatment-related deaths.
 | Table V.Grade 3–4 adverse events in all
patients (n=48). |
Table V.
Grade 3–4 adverse events in all
patients (n=48).
| Grade 3–4 adverse
events | Number of patients
(%) |
|---|
| Diarrhea | 11 (22.9) |
| Neutropenia | 3 (6.3) |
| Leukopenia | 2 (4.2) |
| Anemia | 2 (4.2) |
|
Thrombocytopenia | 1 (2.1) |
| Nausea and
vomiting | 1 (2.1) |
| Fatigue | 1 (2.1) |
| Weight loss | 1 (2.1) |
Discussion
HER2 upregulation is an independent indicator of
poor prognosis (4). Despite notable
improvements in the outcomes of patients with HER2-positive breast
cancer following treatment with anti-HER2-targeted therapy coupled
with chemotherapy, resistance often develops after 12 months
(6). In clinical practice, patients
with primary HER2-positive MBC and prior treatment with multiple
anti-HER2 therapies have demonstrated responses to pyrotinib-based
therapies. Various chemotherapy regimens include combinations such
as vinorelbine and paclitaxel (28). Pyrotinib is a newly approved
anti-HER2 TKI agent in China. The results of the present study
indicated that pyrotinib plus albumin-bound paclitaxel resulted in
an 8.1-month median PFS and a 33.3% ORR in patients with
HER2+ MBC.
The results of a previous study demonstrated that
trastuzumab, pertuzumab and capecitabine as a second-line
treatment, increased the mPFS to 11.1 months (29). In addition, a combination of
lapatinib and capecitabine has been shown to result in a PFS of 8.4
months (9). The results of the
present study revealed that the mPFS was 8.5 months in patients
with HER2+ MBC following treatment with second-line
pyrotinib plus albumin-bound paclitaxel, and was 6.3 months in
patients with HER2+ MBC treated with third- or
higher-line pyrotinib plus albumin-bound paclitaxel. Thus,
pyrotinib in combination with albumin-bound paclitaxel may be used
as a first-, second- or higher-line treatment option.
The results of a previous phase III study of
pyrotinib plus capecitabine demonstrated that mPFS was 11.1 months
and ORR was 68.6% (15). Variations
observed between the results obtained in the present study and
those of previous studies may be attributed to dissimilarities in
population sample sizes and cohorts, as well as differences in the
chemotherapeutic drugs used in conjunction with pyrotinib. The
cohort used in the present study consisted of patients who had
previously received trastuzumab, and 50% of patients previously
received three or more systemic therapies. By contrast, previous
clinical trials included patients who received a maximum of two
treatments, and some patients did not receive anti-HER2
therapy.
The cohort used in the present study included
patients with trastuzumab resistance. The general population of
patients with HER2-positive MBC typically receive aggressive
treatment with a variety of anti-HER2 medications. Therefore, the
results of the present study may offer valuable insights for
clinicians treating patients with HER2-positive MBC in non-clinical
trial settings. In addition, the present study exhibited a short
follow-up period of only 8.7 months, and >30% of patients were
still undergoing treatment at the time of study conclusion.
Notably, a previous phase III study did not include patients who
had previously received lapatinib (30), and 50.5% of patients included in the
present study received lapatinib prior to the study treatment. The
results of the phase III EMILIA trial demonstrated that T-DM1
enhanced the clinical outcomes in patients with metastatic
HER2-positive breast cancer; however, treatment with T-DM1 did not
exert an impact on the rate of CNS progression (6). Based on the results of the
DESTINY-Breast03 trial, T-DXd, a type of antibody-drug conjugate,
also known as DS8201, has been recommended as standard second-line
therapy following trastuzumab for patients with advanced
HER2-positive breast cancer. The T-DXd group demonstrated a highly
clinically meaningful and statistically significant improvement in
PFS, compared with patients with HER2-positive MBC treated with
T-DM1 (31). Pertuzumab, T-DM1
and/or T-DXd are widely used as first or second-line treatment
options in patients with HER2-positive breast cancer worldwide;
however, pertuzumab, T-DM1 and T-DXd are not yet included in
China's Medicare drug list. Thus, further investigations into the
use of pertuzumab, TDM1 and T-DXd are required.
A previous retrospective study evaluated the
efficacy and safety of pyrotinib-based treatments in a real-world
setting (32). Notably,
capecitabine was primarily used as the main component in the
combination chemotherapy regimen. The results of a single-center
retrospective study demonstrated that the mPFS of patients with
HER2-positive MBC treated with pyrotinib was 6.3 months and the ORR
was 29.5% (32). Moreover, the
results of a further multicenter analysis demonstrated that the
mPFS of patients treated with pyrotinib was 8.07 months and the ORR
was 40.7% (25). However, the
combination therapy was only administered to a small number of
patients in both studies. In addition, the results of the present
study demonstrated that the mPFS was 8.5 months in patients
following second-or-lower-line treatment, which was higher than the
8.1-month mPFS reported in a previous multicenter retrospective
study using pyrotinib-based regimens that included vincristine
(33). Despite these differences,
pyrotinib is considered an effective treatment for HER2+
MBC, particularly as a second-line therapy.
Patients with HER2-positive MBC exhibit an increased
risk of developing brain metastases, compared with patients who are
HER2-negative (34). However,
despite the use of anti-HER2 therapy, survival 34 in patients with
brain metastases remain low due to limited treatment options
(35). The macromolecular structure
of trastuzumab makes it difficult to penetrate the blood-brain
barrier; thus, the impact of this treatment on the brain of
patients with brain metastases remains to be fully elucidated
(36). Patients with cancer that
has metastasized to the brain are often treated with anti-HER2 TKIs
with a small molecular weight and a high permeability for
penetration through the blood-brain barrier (37). A meta-analysis of 12 studies
revealed that the combined use of lapatinib and capecitabine
resulted in a mPFS of 4 months in patients with HER2+
MBC with brain metastases (38).
The results of the TBCRC022 study demonstrated that the mPFS of
lapatinib-naïve patients with HER2-positive MBC and brain
metastases was 5.5 months following treatment with neratinib plus
capecitabine, and 3.1 months in lapatinib-treated patients with
HER2-positive MBC and brain metastases (39). The results of the present study
demonstrated that the mPFS of 17 patients with brain metastases was
7.3 months. This finding is consistent with the results of the
PHENIX study, which demonstrated a mPFS of 6.9 months in a cohort
of patients with brain metastases who were treated with pyrotinib
plus capecitabine (19).
The results of the present study demonstrated that a
combination of pyrotinib and albumin-bound paclitaxel was generally
well tolerated, despite diarrhea being the most common grade 3–4
AE, which is in line with the results of previous clinical studies
(16,19). Notably, all AEs were effectively
managed and did not result in the discontinuation of either
pyrotinib or albumin-bound paclitaxel during the study. The results
of the present study also demonstrated that the incidence of grade
3 or 4 neutropenia and leukopenia was higher than previous phase
III trials, at 6.3 and 4.2%, respectively (16,19).
This may be due to the concomitant use of albumin-bound paclitaxel
in the present study. Moreover, AEs may have been under reported in
the present study due its retrospective nature.
The present study exhibits a number of limitations.
Notably, the present study was conducted retrospectively and in an
observational manner, which may have resulted in missing data, and
potential recall and information bias. Moreover, the follow-up time
of the present study was short, leading to inconclusive OS data.
However, to the best of our knowledge, the present study is the
first to investigate the efficacy of the combined use of pyrotinib
and albumin-bound paclitaxel in a real-world setting. The results
of the present study provide key insights into the treatment
patterns and safety profiles of pyrotinib and albumin-bound
paclitaxel in a real-world clinical setting, which may aid in
guiding clinicians in decision-making processes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to restrictions
applied by Xingtai People's Hospital but are available from the
corresponding author on reasonable request.
Authors' contributions
LY and LZ conceived and designed the study. Material
preparation and data collection were performed by PP, FK, SZ and
XT. The draft of the manuscript was written by PP and revised by SZ
and LY. All authors read and approved the final manuscript. LY and
PP confirm the authenticity of all the raw data. Each of the
authors has sufficiently participated in the work to take public
responsibility for appropriate parts of the content, and agrees to
be accountable for all aspects of the work to ensure that questions
regarding the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were in accordance with The
Declaration of Helsinki (as revised in 2013). The study was
approved by the Institutional Ethics committee of Xingtai People's
Hospital [approval no. 2021(030)]. Written informed consent was
obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blows FM, Driver KE, Schmidt MK, Broeks A,
van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO,
Blomqvist C, et al: Subtyping of breast cancer by
immunohistochemistry to investigate a relationship between subtype
and short and long term survival: A collaborative analysis of data
for 10,159 cases from 12 studies. PLoS Med. 7:e10002792010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swain SM, Baselga J, Miles D, Im YH, Quah
C, Lee LF and Cortés J: Incidence of central nervous system
metastases in patients with HER2-positive metastatic breast cancer
treated with pertuzumab, trastuzumab, and docetaxel: Results from
the randomized phase III study CLEOPATRA. Ann Oncol. 25:1116–1121.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verma S, Miles D, Gianni L, Krop IE,
Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, et al:
Trastuzumab emtansine for HER2-positive advanced breast cancer. N
Engl J Med. 367:1783–1791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Swain SM, Baselga J, Kim SB, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Heeson S, et al: Pertuzumab, trastuzumab, and docetaxel in
HER2-positive metastatic breast cancer. N Engl J Med. 372:724–734.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Awada A, Colomer R, Inoue K, Bondarenko I,
Badwe RA, Demetriou G, Lee SC, Mehta AO, Kim SB, Bachelot T, et al:
Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in
previously untreated metastatic ERBB2-positive breast cancer: The
NEfERT-T randomized clinical trial. JAMA Oncol. 2:1557–1564. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krop IE, Lin NU, Blackwell K, Guardino E,
Huober J, Lu M, Miles D, Samant M, Welslau M and Diéras V:
Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in
patients with HER2-positive metastatic breast cancer and central
nervous system metastases: A retrospective, exploratory analysis in
EMILIA. Ann Oncol. 26:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shitara K, Bang YJ, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive gastric
cancer. N Engl J Med. 382:2419–2430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pohlmann PR, Mayer IA and Mernaugh R:
Resistance to trastuzumab in breast cancer. Clin Cancer Res.
15:7479–7491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blair HA: Pyrotinib: First global
approval. Drugs. 78:1751–1755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu
Y, Li H, Yu S, Feng J, Wang S, et al: Pyrotinib or lapatinib
combined with capecitabine in HER2-positive metastatic breast
cancer with prior taxanes, anthracyclines, and/or trastuzumab: A
randomized, phase II study. J Clin Oncol. 37:2610–2619. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q,
Tong Z, Li H, Zhang Q, Sun T, et al: Pyrotinib plus capecitabine
versus lapatinib plus capecitabine for the treatment of
HER2-positive metastatic breast cancer (PHOEBE): A multicentre,
open-label, randomised, controlled, phase 3 trial. Lancet Oncol.
22:351–360. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan M, Ouyang Q, Sun T, Niu L, Yang J, Li
L, Song Y, Hao C, Chen Z, Orlandi A, et al: Pyrotinib plus
capecitabine for patients with human epidermal growth factor
receptor 2-positive breast cancer and brain metastases (PERMEATE):
A multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol.
23:353–361. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J,
Luo Y, Xing P, Lan B, Li M, et al: Phase I study and biomarker
analysis of pyrotinib, a novel irreversible pan-ErbB receptor
tyrosine kinase inhibitor, in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer. J Clin Oncol.
35:3105–3112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan M, Bian L, Hu X, Zhang Q, Ouyang Q,
Feng J, Yin Y, Sun T, Tong Z, Wang X, et al: Pyrotinib plus
capecitabine for human epidermal growth factor receptor 2-positive
metastatic breast cancer after trastuzumab and taxanes (PHENIX): A
randomized, double-blind, placebo-controlled phase 3 study. Transl
Breast Cancer Res. 1:132020. View Article : Google Scholar
|
|
20
|
Saura C, Oliveira M, Feng YH, Dai MS, Chen
SW, Hurvitz SA, Kim SB, Moy B, Delaloge S, Gradishar W, et al:
Neratinib plus capecitabine versus lapatinib plus capecitabine in
HER2-positive metastatic breast cancer previously treated with ≥2
HER2-directed regimens: Phase III NALA trial. J Clin Oncol.
38:3138–3149. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gradishar WJ, Krasnojon D, Cheporov S,
Makhson AN, Manikhas GM, Clawson A and Bhar P: Significantly longer
progression-free survival with nab-paclitaxel compared with
docetaxel as first-line therapy for metastatic breast cancer. J
Clin Oncol. 27:3611–3619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gradishar WJ, Krasnojon D, Cheporov S,
Makhson AN, Manikhas GM, Clawson A, Bhar P, McGuire JR and Iglesias
J: Phase II trial of nab-paclitaxel compared with docetaxel as
first-line chemotherapy in patients with metastatic breast cancer:
Final analysis of overall survival. Clin Breast Cancer. 12:313–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 5. US Department of Health and Human
Services National Institutes of Health, National Cancer Institute.
Published. November 27–2017
|
|
27
|
Wickham H: Ggplot2: Elegant graphics for
data analysis. Springer Publishing Company, Incorporated. 2009.
View Article : Google Scholar
|
|
28
|
Wong H, Leung R, Kwong A, Chiu J, Liang R,
Swanton C and Yau T: Integrating molecular mechanisms and clinical
evidence in the management of trastuzumab resistant or refractory
HER-2+ metastatic breast cancer. Oncologist. 16:1535–1546. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Urruticoechea A, Rizwanullah M, Im SA,
Ruiz ACS, Láng I, Tomasello G, Douthwaite H, Badovinac Crnjevic T,
Heeson S, Eng-Wong J and Muñoz M: Randomized phase III trial of
trastuzumab plus capecitabine with or without pertuzumab in
patients with human epidermal growth factor receptor 2-positive
metastatic breast cancer who experienced disease progression during
or after trastuzumab-based therapy. J Clin Oncol. 35:3030–3038.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olson EM, Najita JS, Sohl J, Arnaout A,
Burstein HJ, Winer EP and Lin NU: Clinical outcomes and treatment
practice patterns of patients with HER2-positive metastatic breast
cancer in the post-trastuzumab era. Breast. 22:525–531. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Y, Lin M, Zhang J, Wang B, Tao Z, Du
Y, Zhang S, Cao J, Wang L and Hu X: Real-world data of
pyrotinib-based therapy in metastatic HER2-positive breast cancer:
Promising efficacy in lapatinib-treated patients and in brain
metastasis. Cancer Res Treat. 52:1059–1066. 2020.PubMed/NCBI
|
|
32
|
Cortés J, Kim SB, Chung WP, Im SA, Park
YH, Hegg R, Kim MH, Tseng LM, Petry V, Chung CF, et al: Trastuzumab
deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J
Med. 386:1143–1154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Q, Ouyang D, Anwar M, Xie N, Wang S,
Fan P, Qian L, Chen G, Zhou E, Guo L, et al: Effectiveness and
safety of pyrotinib, and association of biomarker with
progression-free survival in patients with HER2-positive metastatic
breast cancer: A real-world, multicentre analysis. Front Oncol.
10:8112020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gori S, Rimondini S, De Angelis V, Colozza
M, Bisagni G, Moretti G, Sidoni A, Basurto C, Aristei C, Anastasi P
and Crinò L: Central nervous system metastases in HER-2 positive
metastatic breast cancer patients treated with trastuzumab:
Incidence, survival, and risk factors. Oncologist. 12:766–773.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garcia-Alvarez A, Papakonstantinou A and
Oliveira M: Brain metastases in HER2-positive breast cancer:
Current and novel treatment strategies. Cancers (Basel).
13:29272021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brosnan EM and Anders CK: Understanding
patterns of brain metastasis in breast cancer and designing
rational therapeutic strategies. Ann Transl Med. 6:1632018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brufsky AM, Mayer M, Rugo HS, Kaufman PA,
Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, et
al: Central nervous system metastases in patients with
HER2-positive metastatic breast cancer: Incidence, treatment, and
survival in patients from registHER. Clin Cancer Res. 17:4834–4843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petrelli F, Ghidini M, Lonati V, Tomasello
G, Borgonovo K, Ghilardi M, Cabiddu M and Barni S: The efficacy of
lapatinib and capecitabine in HER-2 positive breast cancer with
brain metastases: A systematic review and pooled analysis. Eur J
Cancer. 84:141–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Freedman RA, Gelman RS, Anders CK, Melisko
ME, Parsons HA, Cropp AM, Silvestri K, Cotter CM, Componeschi KP,
Marte JM, et al: TBCRC 022: A phase II trial of neratinib and
capecitabine for patients with human epidermal growth factor
receptor 2-positive breast cancer and brain metastases. J Clin
Oncol. 37:1081–1089. 2019. View Article : Google Scholar : PubMed/NCBI
|