Introduction
Breast cancer is one of the most prevalent invasive
malignancies occurring in female patients, with an estimated
429,105 new cases and 124,002 related deaths in China in 2022
(1,2). Human epidermal growth factor receptor
(HER) 2-positive (HER2+) breast cancer is characterized
by the overexpression of HER2 (namely ErbB2) and is an aggressive
subtype of breast cancer, which accounts for 15–20% of all breast
cancer cases worldwide (3,4). Neoadjuvant treatment is recommended
for HER2+ breast cancer to reduce tumor load and
increase surgical feasibility; moreover, the development and
application of HER2-targeted agents, such as pertuzumab and
trastuzumab, improve disease-free survival of patients with
HER2+ breast cancer (5–7). The
KRISTINE and the BERENICE trials showed that neoadjuvant
trastuzumab/pertuzumab therapy combined with chemotherapy could
improve the total pathological complete response (tpCR) in patients
with HER2+ breast cancer (8–10).
Based on the aforementioned evidence, HER2-targeted agent-involved
neoadjuvant therapy is a reliable treatment selection for patients
with HER2+ breast cancer.
Pyrotinib, which was independently developed in
China, is a novel, irreversible dual pan-ErbB tyrosine kinase
inhibitor, targeting HER1, HER2 and HER4 (11,12).
Due to its potent efficacy and tolerable toxicity, pyrotinib
combined with chemotherapy was approved for advanced or metastatic
HER2+ breast cancer treatment in China (13,14).
However, the application of pyrotinib as neoadjuvant therapy in
treating HER2+ breast cancer was only reported in a
minority of studies (15,16). For example, one recent study
disclosed that the objective response rate (ORR) and tpCR reach
100.0 and 45.5%, respectively, following the completion of
neoadjuvant pyrotinib plus nab-paclitaxel, liposomal doxorubicin
and cyclophosphamide treatment in patients with HER2+
breast cancer (15). An additional
study showed that patients with HER2+ breast cancer
achieved an ORR of 100.0% and tpCR of 73.7% following neoadjuvant
pyrotinib plus epirubicin plus cyclophosphamide treatment and the
most common adverse events were diarrhea and leucopenia (16). Nevertheless, the sample size of the
aforementioned studies was relatively small (~20 patients), which
further limited the feasibility of the prognostic factor
analysis.
Therefore, the present study aimed to explore the
efficacy and safety profile of pyrotinib-associated neoadjuvant
therapy as well as the applications of its prognostic factors in
patients with HER2+ breast cancer.
Materials and methods
Patients
Between June 2020 and March 2022, the present study
retrospectively analyzed 49 HER2+ breast cancer patients
with pyrotinib-neoadjuvant therapy from Guangzhou Panyu Central
Hospital. Among them, 26 (53.1%) patients received pyrotinib plus
chemotherapy, and 23 (46.9%) patients were treated with pyrotinib
plus trastuzumab and chemotherapy. Patients who met the following
criteria were included: i) Those firstly diagnosed with breast
cancer; ii) those confirmed as HER2+, which was defined
using immunohistochemistry (IHC) (+++ or ++) and by gene
amplification via fluorescence in situ hybridization (FISH);
iii) those who were ≥18 years of age; iv) those who received
6-cycle pyrotinib-neoadjuvant therapy; v) those with a clinical
stage of T2-T3/N0-N2/M0; and vi) those with an eastern cooperative
oncology group performance status (ECOG PS) 0 or 1. Patients who
met the following criteria were excluded: i) Those with an
incomplete clinical and pathological response information; and ii)
pregnant or lactating patients. The detailed classification of
patients was assessed by the corresponding pathologists in the
Department of Pathology. The present study was approved by the
Ethics Committee of Guangzhou Panyu Central Hospital (approval no.
2021-SR-512). All patients provided written informed consent.
IHC
The tissues were fixed in 4% paraformaldehyde for 24
h at room temperature and embedded in paraffin. Sections were cut
to a 4-µm thickness. The sections were then deparaffizined in
xylene and rehydrated with serial ethanol. Antigen retrieval was
performed by heating in a microwave ovan and blocking with goat
serum (Beyotime Institute if Biotechnology) for 30 min at 37°C.
Next, the sections were cultivated with HER2 antibody (1:500; cat.
no. 18299-1-AP; Proteintech Group, Inc.) at 4°C overnight and
HRP-conjugated Goat Anti-Rabbit IgG (H+L) (1:1,000; cat. no.
SA00001-2; Proteintech Group, Inc.) at 37°C for 1 h. Finally, the
sections were stained with DAB and hematoxylin, and observed with a
light microscope (Nikon Corporation).
Recommended therapy regimens
All patients received 6-cycle pyrotinib-neoadjuvant
therapy with a 21-day cycle. The recommended therapy regimens
included i) pyrotinib plus chemotherapy and ii) pyrotinib plus
trastuzumab and chemotherapy. The details of the regimens were
reported in recent studies (17,18).
Data collection
The clinical characteristics of the patients were
obtained from the hospital's electronic medical records system that
was accessed between August 2022 and September 2022. The
characteristics specifically included in the present analysis were
age, menopausal status, ECOG PS, tumor size, clinical TNM stage,
HER2 status, hormone receptor (estrogen receptor and/or
progesterone receptor) status and Ki-67 levels. In addition, the
clinical TNM stage was assessed via the eighth TNM-based staging of
breast cancer (19). The follow-up
data were also acquired. The patients were followed up every two
treatment cycles since the treatment initiation. During the
follow-up, the results of lesion-relevant imaging examinations
(after 2-, 4- and 6-cycle therapy), such as chest and lymph node
CTs, or color ultrasound were obtained. Based on the aforementioned
imaging examination results, the clinical responses corresponding
to each time point were evaluated via the response evaluation
criteria in solid tumors following a 2-, 4- and 6-cycle therapy
(20,21). The clinical responses included
complete response (CR), partial response (PR), stable disease (SD)
and progression of disease (PD). The ORR and disease control rate
(DCR) were calculated according to those clinical responses. In
addition, the pathological evaluation of the surgical specimens was
retrieved, which was evaluated in the tpCR analysis. The tpCR was
determined by taking into account both pathological complete
response (pCR) in the breast [by Miller-Payne (MP) grading system]
and pCR in the lymph nodes (17).
In order to analyze the safety, the adverse events were counted.
Notably, the present study did not analyze the cancer tissue, while
all evaluations of hormone receptor status, ki-67 levels and
pathological evaluation in the surgical specimens were originally
completed during treatment and retrieved from the hospital's
electronic medical records system for analysis in the current
study.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for data description
and processing. GraphPad Prism 7.0 (GraphPad Software; Dotmatics)
was used to generate forest plots. Univariate and multivariate
logistic regression models were employed to analyze the associated
factors to tpCR and to assess the selection of the multivariate
model via a forward selection method. The c2 test was
used for comparing the tpCR rate between patients who received
pyrotinib plus trastuzumab and chemotherapy and patients who
received pyrotinib plus chemotherapy. P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline characteristics
A total of 49 patients (mean age, 55.2±9.1 years)
with HER2+ breast cancer was included in the present
study. Among them, 15 (30.6%) patients were premenopausal and the
other 34 (69.4%) patients were postmenopausal (Table I). The mean tumor size was 5.0±1.2
cm. With regard to the TNM stage, 28 (57.1%) patients were assessed
as stage IIB whereas the remaining 21 (42.9%) patients were
assessed as stage IIIA. In addition, 26 (53.1%) patients received
pyrotinib plus chemotherapy, while 23 (46.9%) patients were treated
with pyrotinib plus trastuzumab and chemotherapy. The detailed
baseline characteristics are shown in Table I. Moreover, the IHC examples in the
breast tumor tissue of patients with Ki-67 level <30 or ≥30%,
IHC++ and IHC+++ are shown in Fig.
S1.
 | Table I.Baseline characteristics of
HER2+ breast cancer patients. |
Table I.
Baseline characteristics of
HER2+ breast cancer patients.
| Characteristics | Patients (n=49) |
|---|
| Mean age ± SD,
years | 55.2±9.1 |
| Menopausal status, n
(%) |
|
|
Premenopausal | 15 (30.6) |
|
Postmenopausal | 34 (69.4) |
| ECOG PS, n (%) |
|
| 0 | 41 (83.7) |
| 1 | 8 (16.3) |
| Mean tumor size ± SD,
cm | 5.0±1.2 |
| Tumor stage, n
(%) |
|
| 2 | 32 (65.3) |
| 3 | 17 (34.7) |
| Node stage, n
(%) |
|
| 1 | 35 (71.4) |
| 2 | 14 (28.6) |
| Metastasis stage, n
(%) |
|
| 0 | 49 (100.0) |
| Detailed TNM stage,
n (%) |
|
|
T2N1M0 | 28 (57.1) |
|
T2N2M0 | 4 (8.2) |
|
T3N1M0 | 7 (14.3) |
|
T3N2M0 | 10 (20.4) |
| TNM stage, n
(%) |
|
|
IIB | 28 (57.1) |
|
IIIA | 21 (42.9) |
| HER2 status, n
(%) |
|
| IHC++
and amplification via fluorescence in situ
hybridization | 11 (22.4) |
|
IHC+++ | 38 (77.6) |
| Estrogen and/or
progesterone receptor-positive, n (%) | 31 (63.3) |
| Ki-67 level |
|
| Mean ±
SD, % | 40.8±16.1 |
| ≥30%, n
(%) | 32 (65.3) |
| Therapy regimens, n
(%) |
|
|
Pyrotinib plus
chemotherapy | 26 (53.1) |
|
Pyrotinib plus trastuzumab and
chemotherapy | 23 (46.9) |
Clinical response following different
therapy cycles
After a 2-cycle treatment, 23 (46.9%) and 26 (53.1%)
patients achieved PR and SD, while no patient was assessed as CR or
PD; the ORR and DCR were 46.9 and 100.0%, accordingly. Following a
4-cycle treatment, 2 (4.1%), 29 (59.2%) and 18 (36.7%) patients
achieved CR, PR and SD, respectively, while none of patients
underwent PD; moreover, the ORR and DCR were 63.3 and 100.0%,
respectively. Following a 6-cycle treatment, 4 (8.2%), 36 (73.4%)
and 9 (18.4%) patients achieved CR, PR and SD, respectively, and
none of the patients exhibited PD; the ORR and DCR reached 81.6 and
100.0%, respectively (Table
II).
 | Table II.Clinical response after 2, 4, and
6-cycle therapy in HER2+ breast cancer patients. |
Table II.
Clinical response after 2, 4, and
6-cycle therapy in HER2+ breast cancer patients.
| Items | After 2-cycle | After 4-cycle | After 6-cycle |
|---|
| Clinical response
by response evaluation criteria in solid tumors, n (%) |
|
|
|
|
Complete response | 0 (0.0) | 2 (4.1) | 4 (8.2) |
| Partial
response | 23 (46.9) | 29 (59.2) | 36 (73.4) |
| Stable
disease | 26 (53.1) | 18 (36.7) | 9 (18.4) |
|
Progressive disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Objective response
rate, n (%) | 23 (46.9) | 31 (63.3) | 40 (81.6) |
| Disease control
rate, n (%) | 49 (100.0) | 49 (100.0) | 49 (100.0) |
Pathological response
After the neoadjuvant treatment, 23 (46.9%), 12
(24.5%), 12 (24.5%) and 2 (4.1%) patients were evaluated as MP
grade 5, 4, 3 and 2, respectively. In addition, 23 (46.9%) and 40
(81.6%) patients achieved pCR in the breast tissue and the lymph
nodes, respectively. Consequently, a total of 22 (44.9%) patients
obtained tpCR (Table III). In
addition, pyrotinib plus trastuzumab and chemotherapy (compared
with that in the pyrotinib plus chemotherapy group) resulted in a
higher tpCR rate in patients with HER2+ breast cancer
(65.2 vs. 26.9%; P=0.007; Fig.
S2).
 | Table III.Pathological response in
HER2+ breast cancer patients who received
pyrotinib-involved neoadjuvant therapy. |
Table III.
Pathological response in
HER2+ breast cancer patients who received
pyrotinib-involved neoadjuvant therapy.
| Items | n (%) |
|---|
| Miller-Payne
grade |
|
| Grade
5 | 23 (46.9) |
| Grade
4 | 12 (24.5) |
| Grade
3 | 12 (24.5) |
| Grade
2 | 2 (4.1) |
| pCR in breast |
|
|
Yes | 23 (46.9) |
| No | 26 (53.1) |
| Pathological
complete response in lymph nodes |
|
|
Yes | 40 (81.6) |
| No | 9 (18.4) |
| Total pathological
complete response |
|
|
Yes | 22 (44.9) |
| No | 27 (55.1) |
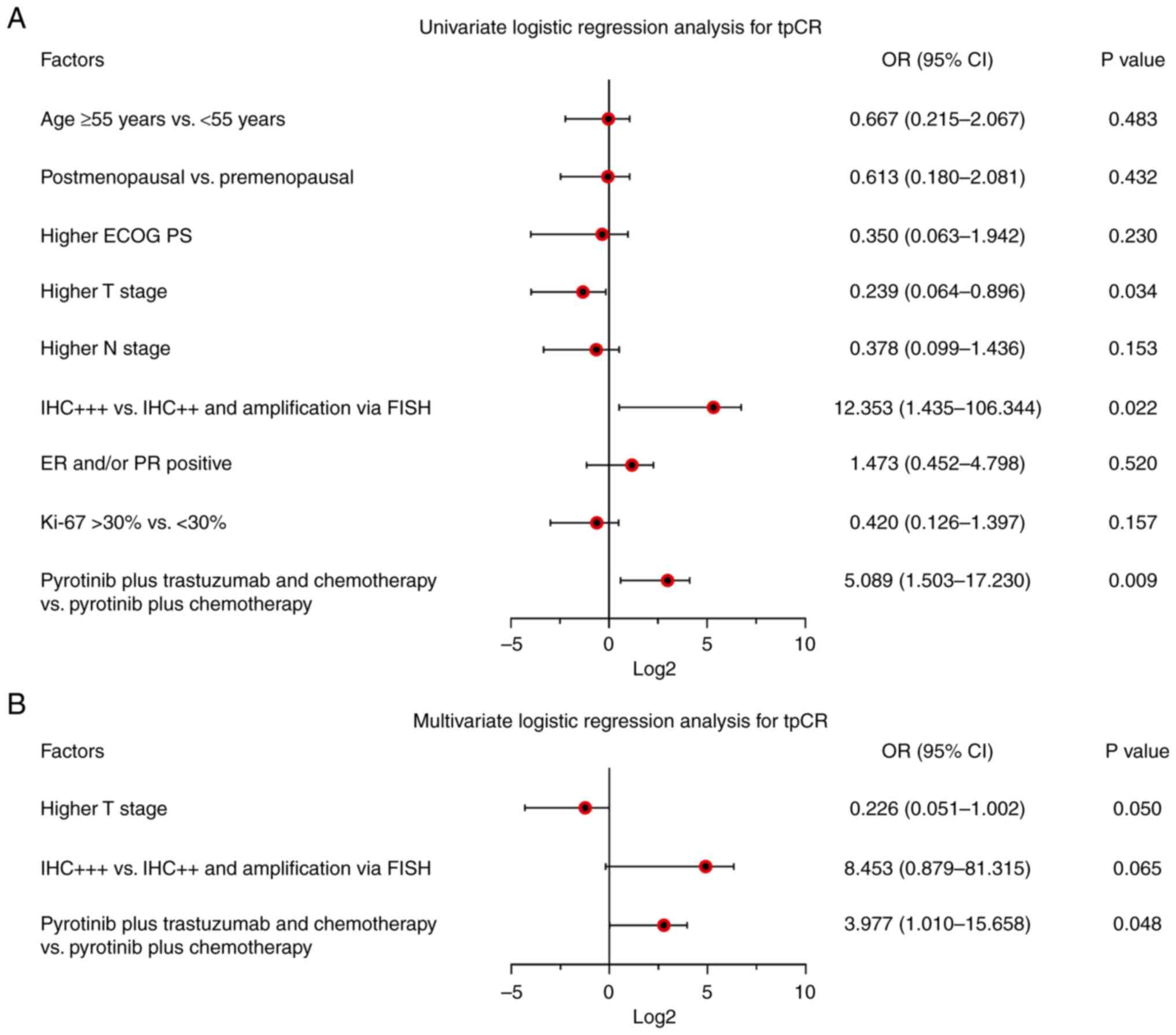
Prognostic factors
Higher T stage odds ratio (OR) (OR, 0.239 95% CI,
0.064-0.896; P=0.034) was related to declined tpCR; by contrast,
IHC+++ (vs. IHC++ and gene amplification via FISH; OR, 12.353; 95%
CI, 1.435-106.344; P=0.022) and pyrotinib plus trastuzumab and
chemotherapy (vs. pyrotinib plus chemotherapy; OR, 5.089; 95% CI,
1.503-17.230; P=0.009) were both associated with elevated tpCR in
patients with breast cancer (Fig.
1).
Moreover, subsequent multivariate logistic
regression analysis demonstrated that pyrotinib plus trastuzumab
and chemotherapy (vs. pyrotinib plus chemotherapy) was
independently correlated with increased tpCR (OR, 3.977; 95% CI,
1.010-15.658; P=0.048). By contrast, higher Tumor (T) stage (OR,
0.226; 95% CI, 0.051-1.002; P=0.050) and IHC+++ (vs. IHC++ and gene
amplification by FISH; O, 8.453; 95% CI, 0.879-81.315; P=0.065)
only showed a trend (without statistical significance) of
independently link with tpCR.
Adverse events
Generally, the majority of adverse events of
pyrotinib-neoadjuvant therapy were mild and controllable (Table IV). The most frequent adverse
events included diarrhea (81.6%), anemia (69.4%), nausea and
vomiting (63.3%), and fatigue (51.0%). In addition, the grade 3
adverse events included diarrhea (16.3%), anemia (12.3%), nausea
and vomiting (6.1%), fatigue (4.1%), thrombocytopenia (4.1%),
leukopenia (2.0%), hypomagnesemia (2.0%), elevated transaminase
(2.0%) and neutropenia (2.0%). No treatment-related death was
reported.
 | Table IV.Adverse events of pyrotinib-involved
neoadjuvant therapya. |
Table IV.
Adverse events of pyrotinib-involved
neoadjuvant therapya.
| Events, n (%) | Any grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|
| Diarrhea | 40 (81.6) | 14 (28.6) | 18 (36.7) | 8 (16.3) | 0 (0.0) |
| Anemia | 34 (69.4) | 18 (36.7) | 10 (20.4) | 6 (12.3) | 0 (0.0) |
| Nausea and
vomiting | 31 (63.3) | 14 (28.6) | 14 (28.6) | 3 (6.1) | 0 (0.0) |
| Fatigue | 25 (51.0) | 12 (24.5) | 11 (22.4) | 2 (4.1) | 0 (0.0) |
|
Thrombocytopenia | 21 (42.8) | 13 (26.5) | 6 (12.2) | 2 (4.1) | 0 (0.0) |
| Leukopenia | 19 (37.7) | 10 (20.4) | 8 (16.3) | 1 (2.0) | 0 (0.0) |
| Hypomagnesemia | 18 (36.7) | 10 (20.4) | 7 (14.3) | 1 (2.0) | 0 (0.0) |
| Elevated
transaminase | 18 (36.7) | 10 (20.4) | 7 (14.3) | 1 (2.0) | 0 (0.0) |
| Pruritus | 17 (34.6) | 11 (22.4) | 6 (12.2) | 0 (0.0) | 0 (0.0) |
| Neutropenia | 15 (30.6) | 9 (18.4) | 5 (10.2) | 1 (2.0) | 0 (0.0) |
| Anorexia | 14 (28.6) | 7 (14.3) | 7 (14.3) | 0 (0.0) | 0 (0.0) |
| Elevated
creatinine | 14 (28.6) | 9 (18.4) | 5 (10.2) | 0 (0.0) | 0 (0.0) |
| Hypokalemia | 9 (18.4) | 6 (12.2) | 3 (6.1) | 0 (0.0) | 0 (0.0) |
| Hyponatremia | 5 (10.2) | 3 (6.1) | 2 (4.1) | 0 (0.0) | 0 (0.0) |
| Oral
ulceration | 4 (8.2) | 2 (4.1) | 2 (4.1) | 0 (0.0) | 0 (0.0) |
Discussion
Recently, a small minority of studies supported the
treatment efficacy of pyrotinib-neoadjuvant therapy in patients
with HER2+ breast cancer (18,22).
For example, a recent study demonstrated that tpCR was 55.1% in
patients with HER2+ breast cancer who receive
neoadjuvant pyrotinib plus docetaxel, carboplatin and trastuzumab
treatment (18). An additional
study indicated that following treatment with neoadjuvant pyrotinib
plus albumin-bound paclitaxel, ORR and tpCR reached 100.0 and
57.1%, respectively, in patients with HER2+ breast
cancer (22). Similarly, the
current study demonstrated that ORR and DCR reached 81.6 and
100.0%, respectively, following treatment with 6-cycle
pyrotinib-neoadjuvant therapy in patients with HER2+
breast cancer; moreover, 46.9 and 81.6% of patients achieved pCR in
the breast tissue and in the lymph nodes, respectively, while 44.9%
achieved tpCR. The findings of the present study, together with
those reported in previous studies, implied the successful efficacy
of pyrotinib-neoadjuvant therapy in treating patients with
HER2+ breast cancer. The possible explanations are
listed as follows: i) Pyrotinib could durably restrain tumor
development by irreversibly inhibiting HER protein family
homologous or heterodimer formation and their auto-phosphorylation
(12,23); ii) a synergistic effect could be
present between pyrotinib and chemotherapy, whose combination
achieved improved treatment efficacy (24). Consequently, pyrotinib-neoadjuvant
therapy possessed optimal treatment efficacy in patients with
HER2+ breast cancer. In addition, although the
therapeutic regimen of the present study was similar to that used
in previous studies (17,18), one of the previous studies was a
phase II trial, which could not be extrapolated to the setting of
clinical practice (18), while the
other study had a relatively small sample size despite being a
retrospective study (17).
Consequently, the present study aimed to evaluate the efficacy and
safety of pyrotinib-neoadjuvant therapy in a clinical population
with a larger sample size.
With regard to the prognostic factors of patients
with HER2+ breast cancer, the present study demonstrated
that pyrotinib plus trastuzumab and chemotherapy (vs. pyrotinib
plus chemotherapy) was independently correlated with increased
tpCR, which could be explained by the following points: Dual-HER2
targeted treatment was shown to result in a stronger antitumor
effect; therefore, neoadjuvant pyrotinib plus trastuzumab and
chemotherapy (vs. pyrotinib plus chemotherapy) was independently
correlated with elevated tpCR in patients with HER2+
breast cancer (25,26). In addition, higher T stage and
IHC+++ (vs. IHC++ and amplification via FISH) could also predict
tpCR to some degree. The possible reason is the following: i)
Higher T stage, which represented larger tumor volume, resulting in
enhanced invasiveness and tumor malignancy; therefore, patients
with HER2+ breast cancer and higher T stage could not
readily achieve tpCR following pyrotinib-neoadjuvant treatment; ii)
HER2 protein levels were elevated in patients classified as IHC+++
compared with those noted in patients classified as IHC++ and those
who exhibited HER2 gene amplification via FISH; moreover, patients
with higher HER2 protein levels demonstrated improved treatment
response (27). Therefore, IHC+++
(vs. IHC++ and gene amplification via FISH) was associated with
elevated tpCR.
Previous studies showed that the most common adverse
events of pyrotinib-neoadjuvant therapy include diarrhea, dental
ulcer, leukopenia and hand-foot syndrome (16,17,28,29).
Consistent with the aforementioned studies, the present study
identified that the majority of these adverse events involved in
pyrotinib-neoadjuvant therapy were tolerable and controllable and
included mild or moderate diarrhea (81.6%), anemia (69.4%), nausea
and vomiting (63.3%). These findings supported the acceptable
toxicity profile of pyrotinib-neoadjuvant therapy in treating
patients with HER2+ breast cancer. Furthermore, diarrhea
was the most common side effect of pyrotinib and the possible
reason could be the following: Pyrotinib inhibited the downstream
signals of the epidermal growth factor receptor in the intestinal
epithelium, which led to the activation of the basolateral membrane
potassium channel and chloride secretory diarrhea (30,31).
Certain inevitable limitations existed in the
present study. Firstly, this was a single-arm study, while
randomized controlled trials would be necessary to compare the
efficacy and safety between pyrotinib and other HER2-targeted
agents used as neoadjuvant therapy in treating patients with
HER2+ breast cancer. Secondly, pyrotinib was developed
very recently and the general survival of patients with breast
cancer was relatively long; therefore, the follow-up duration
period was not adequate for the objective evaluation of patient
survival. Thirdly, the patients included in the present study were
all Chinese and the treatment efficacy and safety of
pyrotinib-neoadjuvant therapy should be evaluated in subsequent
studies in populations comprising other ethnicities.
In summary, pyrotinib-neoadjuvant therapy achieved a
tpCR of 44.9% and low incidence of grade 3–4 adverse events,
indicating that pyrotinib-neoadjuvant therapy presented a
relatively good efficacy and tolerance in treating patients with
HER2+ breast cancer. Nonetheless, the present findings
warrant further large-scale studies for verification.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW substantially contributed to the conception and
design of the study. HC and ZG were responsible for the
acquisition, analysis and interpretation of the data. All authors
contributed to manuscript drafting and critical revisions of the
intellectual content. HW and HC confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Guangzhou Panyu Central Hospital (approval no. 2021-SR-512) in
December 2021. All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Watkins EJ: Overview of breast cancer.
JAAPA. 32:13–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peddi PF and Slamon DJ: Frontiers in
HER2-positive breast cancer in 2020. Curr Opin Obstet Gynecol.
33:48–52. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goutsouliak K, Veeraraghavan J, Sethunath
V, De Angelis C, Osborne CK, Rimawi MF and Schiff R: Towards
personalized treatment for early stage HER2-positive breast cancer.
Nat Rev Clin Oncol. 17:233–250. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takada M and Toi M: Neoadjuvant treatment
for HER2-positive breast cancer. Chin Clin Oncol. 9:322020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cesca MG, Vian L, Cristóvão-Ferreira S,
Pondé N and de Azambuja E: HER2-positive advanced breast cancer
treatment in 2020. Cancer Treat Rev. 88:1020332020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Korde LA, Somerfield MR, Carey LA, Crews
JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A, et
al: Neoadjuvant chemotherapy, endocrine therapy, and targeted
therapy for breast cancer: ASCO guideline. J Clin Oncol.
39:1485–1505. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hurvitz SA, Martin M, Symmans WF, Jung KH,
Huang CS, Thompson AM, Harbeck N, Valero V, Stroyakovskiy D,
Wildiers H, et al: Neoadjuvant trastuzumab, pertuzumab, and
chemotherapy versus trastuzumab emtansine plus pertuzumab in
patients with HER2-positive breast cancer (KRISTINE): A randomised,
open-label, multicentre, phase 3 trial. Lancet Oncol. 19:115–126.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Li Y, Qi Y, Zhao E, Kong X, Yang
C, Yang Q, Zhang C, Liu Y and Song Z: Pegylated liposomal
doxorubicin, docetaxel, and trastuzumab as neoadjuvant treatment
for HER2-positive breast cancer patients: A phase II and biomarker
study. Front Oncol. 12:9094262022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dang C, Ewer MS, Delaloge S, Ferrero JM,
Colomer R, de la Cruz-Merino L, Werner TL, Dadswell K, Verrill M,
Eiger D, et al: BERENICE final analysis: Cardiac safety study of
neoadjuvant pertuzumab, trastuzumab, and chemotherapy followed by
adjuvant pertuzumab and trastuzumab in HER2-positive early breast
cancer. Cancers (Basel). 14:25962022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blair HA: Pyrotinib: First global
approval. Drugs. 78:1751–1755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xuhong JC, Qi XW, Zhang Y and Jiang J:
Mechanism, safety and efficacy of three tyrosine kinase inhibitors
lapatinib, neratinib and pyrotinib in HER2-positive breast cancer.
Am J Cancer Res. 9:2103–2119. 2019.PubMed/NCBI
|
|
13
|
Li C, Bian X, Liu Z, Wang X, Song X, Zhao
W, Liu Y and Yu Z: Effectiveness and safety of pyrotinib-based
therapy in patients with HER2-positive metastatic breast cancer: A
real-world retrospective study. Cancer Med. 10:8352–8364. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q,
Tong Z, Li H, Zhang Q, Sun T, et al: Pyrotinib plus capecitabine
versus lapatinib plus capecitabine for the treatment of
HER2-positive metastatic breast cancer (PHOEBE): A multicentre,
open-label, randomised, controlled, phase 3 trial. Lancet Oncol.
22:351–360. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao DS, Wang W, Chang JY, Zhang Y, Zhang
HW, Xu JX and Cai HF: Neoadjuvant pyrotinib plus nab-paclitaxel,
doxorubicin, and cyclophosphamide for HER2-positive locally
advanced breast cancer: A retrospective case-series study. Gland
Surg. 10:3362–3368. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xuhong J, Qi X, Tang P, Fan L, Chen L,
Zhang F, Tan X, Yan W, Zhong L, He C, et al: Neoadjuvant pyrotinib
plus trastuzumab and chemotherapy for stage I–III HER2-positive
breast cancer: A phase II clinical trial. oncologist.
25:e1909–e1920. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian C, Wang M, Liu H, Liu J, Xu M and Ma
L: Efficacy and safety of neoadjuvant pyrotinib plus
docetaxel/liposomal doxorubicin/cyclophosphamide for HER2-positive
breast cancer. Ir J Med Sci. Jul 13–2022.(Epub ahead of print).
|
|
18
|
Liu Z, Wang C, Chen X, Zhu J, Sun X, Xia
Q, Lu Z, Qiao J, Zhou Y, Wang H, et al: Pathological response and
predictive role of tumour-infiltrating lymphocytes in HER2-positive
early breast cancer treated with neoadjuvant pyrotinib plus
trastuzumab and chemotherapy (Panphila): A multicentre phase 2
trial. Eur J Cancer. 165:157–168. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cserni G, Chmielik E, Cserni B and Tot T:
The new TNM-based staging of breast cancer. Virchows Arch.
472:697–703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Litière S, Collette S, de Vries EG,
Seymour L and Bogaerts J: RECIST-learning from the past to build
the future. Nat Rev Clin Oncol. 14:187–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong X, He P, Chen J, Yan X, Wei B, Zhang
Z, Bu H, Li J, Tian T, Lv Q, et al: Neoadjuvant pyrotinib plus
trastuzumab and nab-paclitaxel for HER2-positive early or locally
advanced breast cancer: An exploratory phase II trial. Gland Surg.
11:216–225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Wu X, Zhou J, Zhu M, Yu H, Zhang
Y, Zhao Y, Han Z, Guo Y, Guan X, et al: Pyrotinib in the treatment
of women with HER2-positive advanced breast cancer: A multicenter,
prospective, real-world study. Front Oncol. 11:6993232021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C, Deng S, Chen J, Xu X, Hu X, Kong
D, Liang G, Yuan X, Li Y and Wang X: The synergistic effects of
pyrotinib combined with adriamycin on HER2-positive breast cancer.
Front Oncol. 11:6164432021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang J, Xu M, Wang C, Huang D, Zhang Z,
Chen Z, Zhu X and Li W: Dual HER2 targeted therapy with pyrotinib
and trastuzumab in refractory HER2 positive metastatic colorectal
cancer: A result from HER2-FUSCC-G study. Clin Colorectal Cancer.
21:347–353. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pop L, Suciu ID, Ionescu O, Ionescu P and
Toader OD: The dual blockade in the neoadjuvant setting of HER-2
positive early-stage breast cancer. J Med Life. 12:329–331. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahn S, Woo JW, Lee K and Park SY: HER2
status in breast cancer: Changes in guidelines and complicating
factors for interpretation. J Pathol Transl Med. 54:34–44. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mao X, Lv P, Gong Y, Wu X, Tang P, Wang S,
Zhang D, You W, Wang O, Zhou J, et al: Pyrotinib-containing
neoadjuvant therapy in patients with HER2-positive breast cancer: A
multicenter retrospective analysis. Front Oncol. 12:8555122022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Wang Y, Zhu M, Gu Y and Tang Y:
Clinical observation of neoadjuvant chemotherapy with pyrotinib
plus trastuzumab in HER2-positive breast cancer: A cohort study.
Gland Surg. 10:3389–3402. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bowen JM: Mechanisms of TKI-induced
diarrhea in cancer patients. Curr Opin Support Palliat Care.
7:162–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duan T, Cil O, Thiagarajah JR and Verkman
AS: Intestinal epithelial potassium channels and CFTR chloride
channels activated in ErbB tyrosine kinase inhibitor diarrhea. JCI
Insight. 4:e1264442019. View Article : Google Scholar : PubMed/NCBI
|