Introduction
Colorectal cancer (CRC) is the third most frequent
cancer and it causes a huge medical burden globally (1,2).
According to the recent Global Cancer Statistics, the number of
CRC-associated deaths reached ~1 million worldwide in 2020
(2). Meanwhile, there exists ~25%
of CRC patients diagnosed with metastatic CRC (mCRC), which is the
major cause for the CRC high mortality observed (3). At present, the survival time of mCRC
is still poor and the management choices for mCRC are limited
(4,5).
Programmed cell death in CRC can be regulated by
several factors, including radiation and chemotherapeutic drug
treatments. Immune surveillance also modulates the induction of
programmed cell death of CRC cells; for example, CD8+ T
cells modulate the programmed cell death of CRC cells via perforin
or the Fas ligand pathway (6–8).
Meanwhile, programmed cell death protein 1 (PD-1) can induce immune
escape and decrease antitumor immunity in CRC (9). Over the decades, PD-1 inhibitor-based
treatment has been widely applied for cancer treatment, where it
plays an antitumor role by inhibiting immune escape through
accelerating the antitumor immune response and promoting
sensitivity to radio-chemotherapy (10–12).
Certain studies have also reported that PD-1 inhibitor-based
treatment provides survival benefits in patients with mCRC
(13–15). However, >50% of patients with
mCRC fail to respond to PD-1 inhibitor-based treatment, which
consequently impairs their prognosis (16,17).
Thus, the exploration of potential biomarkers to reflect the
treatment response and survival of patients receiving PD-1
inhibitor-based treatment is imperative to promote the management
of mCRC.
Mucosa-associated lymphoid tissue lymphoma
translocation protein 1 (MALT1) is involved in the progression of
several tumors by regulating the malignant behaviors of tumor cells
(18,19). For instance, downregulation of MALT1
suppresses proliferation and promotes apoptosis of prostate cancer
cells in vivo and in vitro (20). Furthermore, knockdown of MALT1
inhibits the proliferation and migration of CRC cells (21). In addition, it has also been
reported that MALT1 is able to regulate the tumor immune escape and
inactivation of CD8+ T cells, which are viewed as
crucial processes for the antitumor activity of PD-1 inhibitors
(22). Overall, it can be deduced
that MALT1 may have the potential to influence the PD-1 inhibitor
response in patients with mCRC; however, related data are
scarce.
Thus, the present study aimed to explore the
association of blood MALT1 levels with the efficacy of PD-1
inhibitor-based treatment among patients with mCRC.
Patients and methods
Patients
A total of 75 patients with unresectable mCRC who
received PD-1 inhibitor treatment at Wuhan No. 8 Hospital (Wuhan,
China) between January 2019 and March 2022 were consecutively
enrolled in this prospective, cohort study. The major criteria for
inclusion were as follows: i) Confirmed as CRC by pathological
results; ii) diagnosis of unresectable mCRC; iii) >18 years old;
iv) Eastern Cooperative Oncology Group performance status (ECOG PS)
score within the scope of 0–2 (23); v) at least one assessable lesion;
and vi) planned to receive PD-1 inhibitor alone or combined with
other treatments. Meanwhile, the major criteria for exclusion were
as follows: i) Pregnancy or lactation; ii) autoimmune or
immunodeficiency diseases; and iii) other malignancies. In
addition, 20 healthy subjects were also included in the present
study as health controls (HCs). All individuals provided written
informed consent, and the study was approved by the Ethics
Committee of Wuhan No. 8 Hospital.
Clinical data and sample
collection
Peripheral blood and other baseline characteristics
were collected from all patients with mCRC before the initiation of
PD-1 inhibitor-based treatment. After 2 cycles (6 weeks) of
treatment (treatment regimen described below), peripheral blood was
again collected from the patients. Additionally, peripheral blood
was collected from the HCs after enrollment. Following the sample
collections, peripheral blood mononuclear cells (PBMCs) were
extracted from the peripheral blood with gradient density
centrifugation using Ficoll PM400 (Cytiva), and then the PBMCs were
used to detect MALT1 expression by reverse
transcription-quantitative PCR (RT-qPCR).
RT-qPCR
RT-qPCR was conducted for the quantitative analysis
of MALT1 mRNA expression. In brief, total RNA was extracted by
QIAamp RNA Blood Mini Kit (Qiagen GmbH) and reverse transcribed
using a QuantiNova Reverse Transcription Kit (Qiagen GmbH)
according to the manufacturer's protocol. Meanwhile, qPCR was
performed with a QuantiNova SYBR Green PCR Kit (Qiagen GmbH). The
thermocycling conditions were as follows: 1 cycle of 95°C for 60
sec, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec.
The relative expression was calculated using the 2−ΔΔCq
method and GAPDH was used as the internal reference (24). The primers used were as follows:
MALT1 foward, 5′-GATGCGTAATGCTGTGGATG-3′ and reverse,
5′-GGTATCATCGTAGTCATTTCTTTTCC-3′; and GAPDH forward,
5′-GCCAAGGTCATCCATGACAACTTTGG-3′ and reverse,
5′-GCCTGCTTCACCACCTTCTTGATGTC-3′.
Treatment
All patients with mCRC received PD-1 inhibitor-based
treatment, which mainly included three regimens: i) PD-1 inhibitor
(camrelizumab or sintilimab) plus chemotherapy (CapeOx or FOLFOX6);
ii) PD-1 inhibitor plus bevacizumab/apatinib; or iii) PD-1
inhibitor plus bevacizumab/apatinib and chemotherapy (CapeOx or
FOLFOX6). To be specific, camrelizumab or sintilimab were
administrated at 200 mg once for every 3-week cycle. Bevacizumab
was administered at 7.5 mg/kg once for every 2 or 3-week cycle
[combined with CapeOx once for every 3-week cycle or FOLFOX6 once
for every 2-week cycle; the detailed administration of CapeOx and
FOLFOX6 took a preceding study for reference (25)]. Apatinib was administered at 375 or
500 mg/day (the administration could be adjusted to 250 mg/day if
patients were not tolerant of the original regimen).
Assessment
Patients in the present study were followed up
continuously, with radiographic progression assessed every 4–6
weeks for the first 3 months and then every 2 months until disease
progression or death. Treatment response at the third month was
obtained. The progression-free survival (PFS) and overall survival
(OS) were calculated accordingly based on the follow-up data. PFS
was defined as the time from treatment initiation to disease
progression or death. OS was defined as the time from treatment
initiation to death.
Statistics
Statistical analysis and graph making were conducted
using SPSS 24.0 (IBM Corp.) and GraphPad Prism 9.0 (Dotmatics).
Wilcoxon's rank sum test was used for the comparison analyses of
MALT1 expression between patients with mCRC and HCs. The capability
of MALT1 expression level in discriminating patients with mCRC from
HCs was measured via the receiver operating characteristic (ROC)
curve. Wilcoxon's rank sum test was used for the comparison
analyses of MALT1 expression in patients with mCRC with different
characteristics (such as diagnosis, number of metastatic sites,
lung metastasis, liver metastasis, peritoneal metastasis, other
metastases and KRAS expression). Spearman's rank correlation test
was utilized to evaluate the correlation of MALT1 expression with
ECOG PS score and tumor differentiation. Wilcoxon's rank sum test
was also used for the comparison analyses of MALT1 expression
between objective response rate (ORR) patients [patients who
achieved complete response (CR) and partial response (PR)] and
non-ORR patients, as well as between disease control rate (DCR)
patients (patients who achieved CR, PR and stable disease) and
non-DCR patients. Beyond that, Wilcoxon's signed rank test was
applied to analyze the high and low of MALT1 expression levels
before and after PD-1 inhibitor-based treatment. The high and low
MALT1 expression levels were determined according to the median
value (2.529) of MALT1 expression in all patients with mCRC.
Meanwhile, if MALT1 expression declined >30% after 2 cycles of
treatment [(MALT1 expression at baseline-MALT1 expression after 2
cycles of treatment)/MALT1 expression at baseline >30%], this
was defined as MALT1 decline >30%. Kaplan-Meier curves and the
log-rank test were also utilized to evaluate the PFS and OS between
patients with mCRC with different MALT1 expression levels. Stepwise
forward regression method was used for multiple regression without
artificial selection of variables. Specifically, the stepwise
regression method introduced the independent variables into the
model successively according to the probability of the score test,
and then the likelihood ratio probability test was conducted based
on the assumed parameters to eliminate the independent variables
that were no longer statistically significant in the model. Such
steps were repeated until the end, when there were no more
variables outside the model that had a significant impact on the
dependent variable, and there were no more variables in the model
that could be eliminated that were not significant on the dependent
variable. Forward stepwise multivariate Cox's proportional hazards
regression analyses were used to screen the independent prognostic
factors for PFS and OS. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients with
mCRC
Among the 75 patients with mCRC (mean age 58.0±7.7
years; age range, 40–79 years) there were 44 (58.7%) patients
<60 years and 31 (41.3%) patients ≥60 years. There were 25
(33.3%) females and 50 (66.7%) males. With respect to the ECOG PS
score, 34 (45.3%), 39 (52.0%) and 2 (2.7%) patients had a score of
0, 1 and 2, respectively. Furthermore, there were 8 (10.7%), 28
(37.3%) and 39 (52.0%) patients with well, moderately and poorly
differentiated tumors, respectively. Moreover, 47 (62.7%), 42
(56.0%), 21 (28.0%) and 27 (36.0%) patients possessed lung, liver,
peritoneal and other metastases, respectively. As for
microsatellite instability (MSI) status, 68 (90.7%) patients were
MSI-low/microsatellite stable and 7 (9.3%) patients were MSI-high.
Regarding treatments, there were 25 (33.3%), 31 (41.3%) and 19
(25.3%) patients who received PD-1 inhibitor plus chemotherapy,
PD-1 inhibitor plus bevacizumab/apatinib and PD-1 inhibitor plus
bevacizumab/apatinib and chemotherapy, respectively (Table I).
 | Table I.Characteristics of the 75 patients
with metastatic colorectal cancer. |
Table I.
Characteristics of the 75 patients
with metastatic colorectal cancer.
| Patient
characteristics | Value |
|---|
| Age, years |
|
| Mean ±
SD | 58.0±7.7 |
| <60,
n (%) | 44 (58.7) |
| ≥60, n
(%) | 31 (41.3) |
| Sex, n (%) |
|
|
Female | 25 (33.3) |
|
Male | 50 (66.7) |
| ECOG PS score, n
(%) |
|
| 0 | 34 (45.3) |
| 1 | 39 (52.0) |
| 2 | 2 (2.7) |
| Diagnosis, n
(%) |
|
|
Rectum | 19 (25.3) |
|
Colon | 56 (74.7) |
| Differentiation, n
(%) |
|
|
Well | 8 (10.7) |
|
Moderate | 28 (37.3) |
|
Poor | 39 (52.0) |
| Metastatic sites, n
(%) |
|
|
Single | 29 (38.7) |
|
Multiple | 46 (61.3) |
| Lung metastasis, n
(%) | 47 (62.7) |
| Liver metastasis, n
(%) | 42 (56.0) |
| Peritoneal
metastasis, n (%) | 21 (28.0) |
| Other metastases, n
(%) | 27 (36.0) |
| KRAS, n (%) |
|
|
Wide-type | 54 (72.0) |
|
Mutation | 21 (28.0) |
| MSI status, n
(%) |
|
|
MSI-L/MSS | 68 (90.7) |
|
MSI-H | 7 (9.3) |
| Treatments, n
(%) |
|
| PD-1
inhibitor plus chemotherapy | 25 (33.3) |
| PD-1
inhibitor plus bevacizumab/apatinib | 31 (41.3) |
| PD-1
inhibitor plus bevacizumab/apatinib and chemotherapy | 19 (25.3) |
| Treatment lines, n
(%) |
|
|
1st | 0 (0.0) |
|
2nd | 37 (49.3) |
|
3rd | 27 (36.0) |
|
≥4th | 11 (14.7) |
Comparison of MALT1 expression levels
between patients with mCRC and HCs
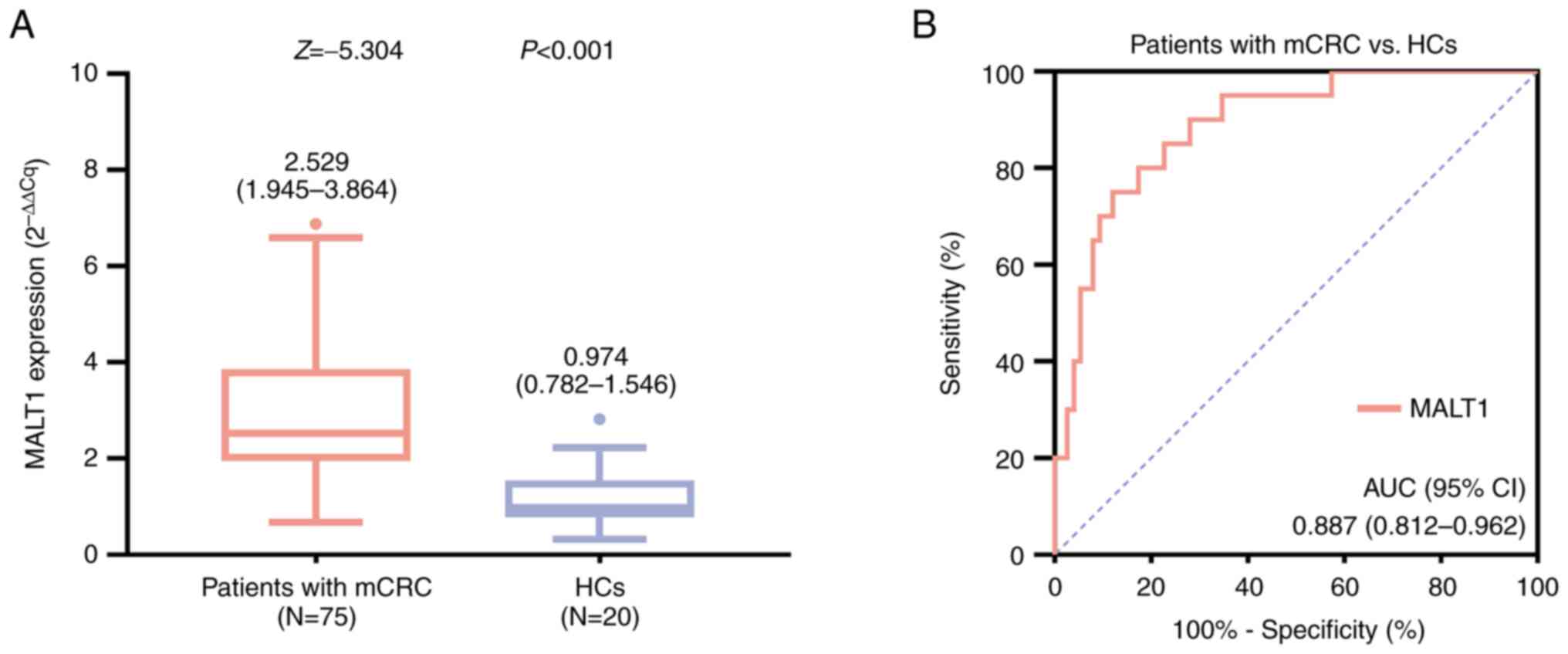
MALT1 expression was elevated in patients with mCRC
compared with that in HCs [median (interquartile range (IQR)),
2.529 (1.945-3.864) vs. 0.974 (0.782-1.546), respectively;
P<0.001; Fig. 1A]. In addition,
the ROC curve demonstrated that MALT1 expression level had a good
capability of distinguishing patients with mCRC from HCs, with an
area under the curve and 95% confidence interval (CI) of 0.887 and
0.812-0.962, respectively (Fig.
1B).
Correlation of MALT1 expression with
clinical characteristics of patients with mCRC
Elevated MALT1 expression was correlated with
multiple metastatic sites (P=0.032) and peritoneal metastasis
(P=0.029), while it was not correlated with other clinical and
pathological characteristics, including ECOG PS score, diagnosis,
differentiation, lung metastasis, liver metastasis, other
metastases, KRAS mutation or MSI status in patients with mCRC (all
P>0.05) (Table II).
 | Table II.Correlation of MALT1 expression level
with the clinical and pathological characteristics of patients with
metastatic colorectal cancer. |
Table II.
Correlation of MALT1 expression level
with the clinical and pathological characteristics of patients with
metastatic colorectal cancer.
| Patient
characteristics | Median MALT1
expression level before treatment (2−ΔΔCt) (IQR) | Z/ρ-value | P-value |
|---|
| ECOG PS score |
| −0.003a | 0.979 |
| 0 | 2.577
(1.998-3.794) |
|
|
| 1 | 2.505
(1.795-3.864) |
|
|
| 2 | 4.020
(2.210-NA) |
|
|
| Diagnosis |
| −0.280b | 0.779 |
| Rectal
cancer | 2.578
(1.501-4.300) |
|
|
| Colon
cancer | 2.519
(2.118-3.571) |
|
|
|
Differentiation |
| 0.143a | 0.221 |
|
Well | 2.577
(1.703-3.528) |
|
|
|
Moderate | 2.484
(1.630-3.530) |
|
|
|
Poor | 2.768
(2.034-4.300) |
|
|
| Metastatic
sites |
| −2.143b | 0.032 |
|
Single | 2.344
(1.527-3.117) |
|
|
|
Multiple | 2.554
(2.197-4.197) |
|
|
| Lung
metastasis |
| −0.088b | 0.930 |
| No | 2.556
(2.195-3.687) |
|
|
|
Yes | 2.508
(1.795-3.864) |
|
|
| Liver
metastasis |
| −0.096b | 0.923 |
| No | 2.823
(1.610-3.872) |
|
|
|
Yes | 2.501
(2.086-3.872) |
|
|
| Peritoneal
metastasis |
| −2.183b | 0.029 |
| No | 2.486
(1.721-3.498) |
|
|
|
Yes | 2.828
(2.445-4.666) |
|
|
| Other
metastases |
| −0.806b | 0.420 |
| No | 2.507
(1.820-3.861) |
|
|
|
Yes | 2.578
(2.103-3.864) |
|
|
| KRAS |
| −0.873b | 0.383 |
|
Wide-type | 2.579
(1.932-3.907) |
|
|
|
Mutation | 2.407
(1.873-3.146) |
|
|
| MSI status |
| −1.220b | 0.222 |
|
MSI-L/MSS | 2.556
(1.963-3.887) |
|
|
|
MSI-H | 2.290
(1.501-2.828) |
|
|
Association of MALT1 level before
treatment with treatment response in patients with mCRC
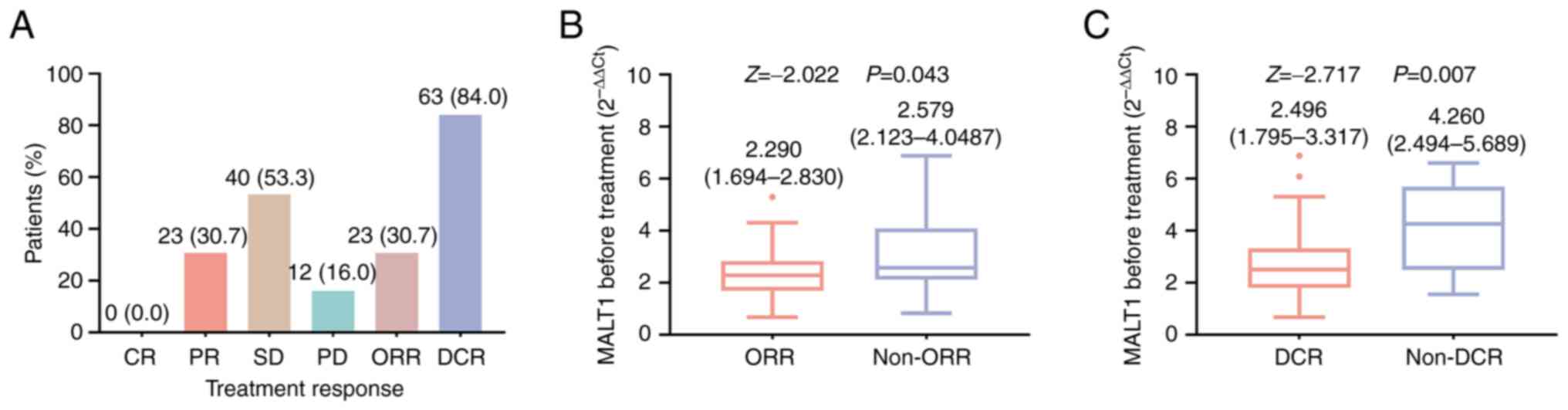
No patients achieved a complete response. Meanwhile,
the rates of partial response, stable disease and progressive
disease were 30.7, 53.3 and 16.0%, respectively, which led to an
ORR and DCR of 30.7 and 84.0%, respectively (Fig. 2A). In addition, MALT1 expression
before treatment was lower in ORR patients compared with that in
non-ORR patients [median (IQR), 2.290 (1.694-2.830) vs. 2.579
(2.123-4.087); P=0.043; Fig. 2B],
and it was also lower in DCR patients compared with that in non-DCR
patients [median (IQR), 2.496 (1.795-3.317) vs. 4.260
(2.494-5.689); P=0.007; Fig.
2C].
MALT1 expression level after
treatment, and its association with treatment response in patients
with mCRC
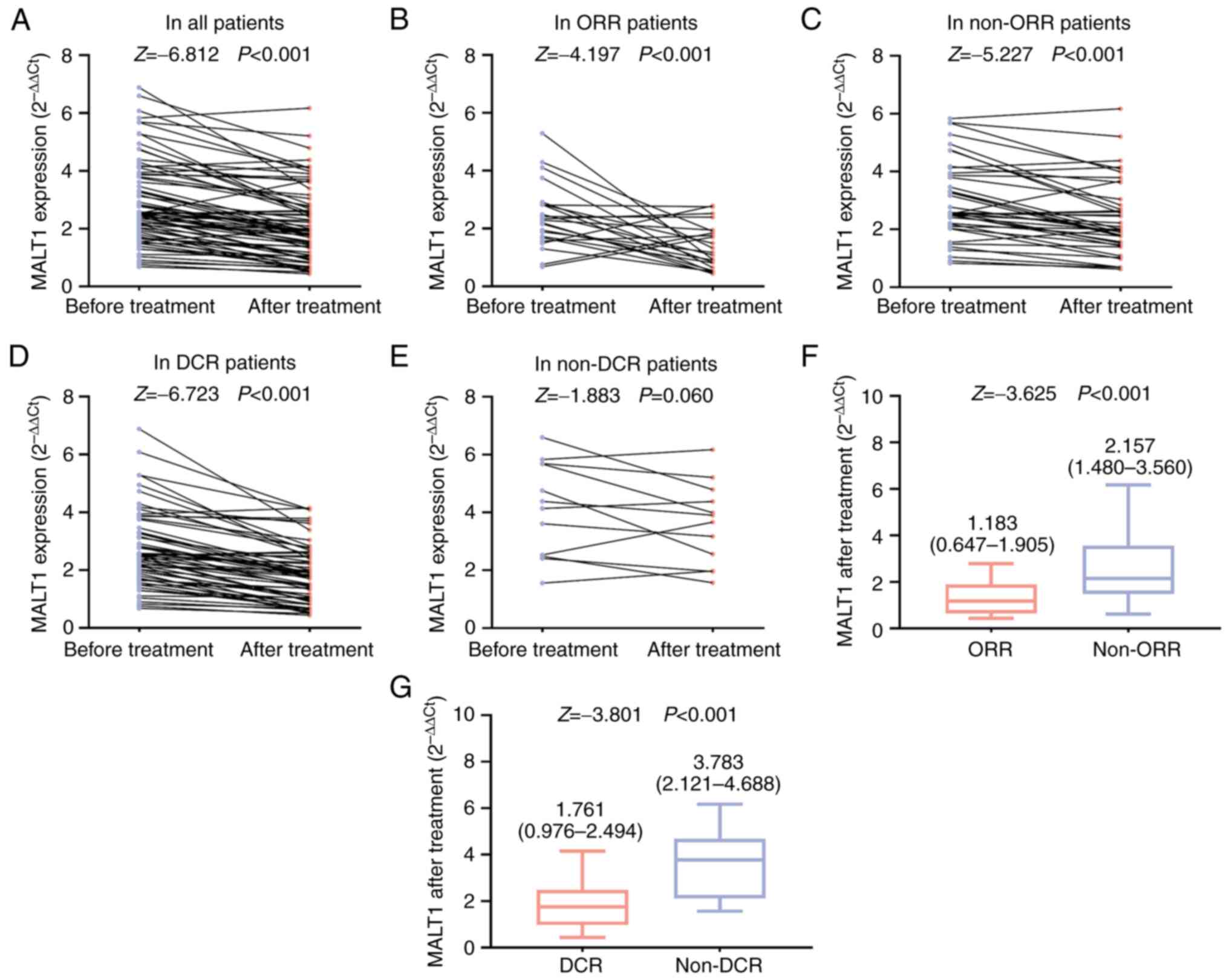
In all patients, the MALT1 expression level after
treatment was lower compared with that before treatment
(P<0.001; Fig. 3A). In ORR
patients (P<0.001; Fig. 3B),
non-ORR patients (P<0.001; Fig.
3C) and DCR patients (P<0.001; Fig. 3D), but not in non-DCR patients
(P=0.060; Fig. 3E), the MALT1
expression level after treatment was lower compared with that
before treatment. Additionally, the MALT1 expression level after
treatment was lower in ORR patients compared with that in non-ORR
patients [median (IQR), 1.183 (0.647-1.905) vs. 2.157
(1.480-3.560); P<0.001; Fig. 3F]
and was also lower in DCR patients compared with that in non-DCR
patients [median (IQR), 1.761 (0.976-2.494) vs. 3.783
(2.121-4.688); P<0.001; Fig.
3G].
Association of MALT1 with survival of
patients with mCRC
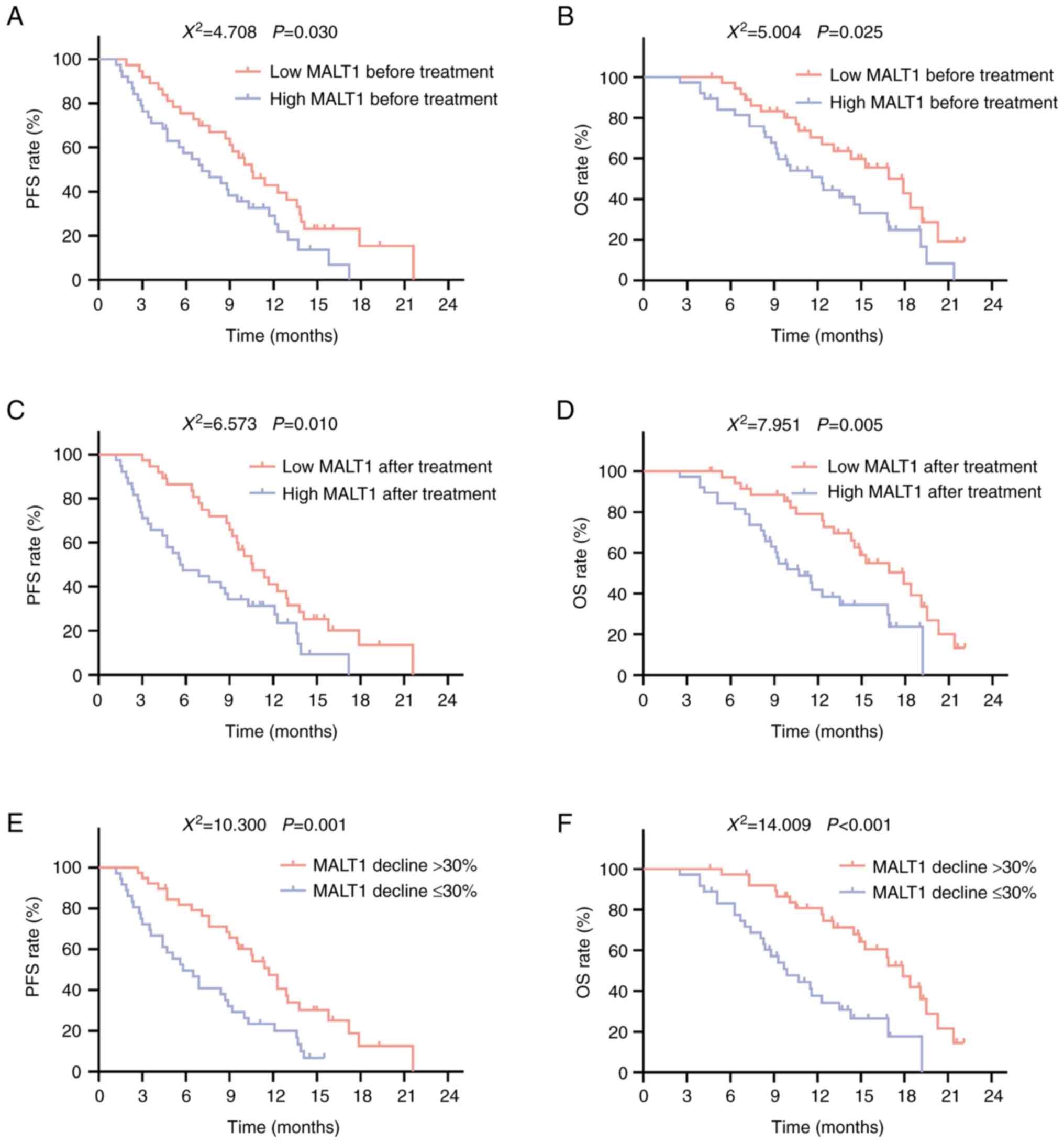
Low MALT1 expression before treatment was associated
with longer PFS (P=0.030; Fig. 4A)
and OS (P=0.025; Fig. 4B) times.
Meanwhile, low MALT1 expression after treatment was significantly
associated with prolonged PFS (P=0.010; Fig. 4C) and OS (P=0.005; Fig. 4D) times. In addition, a MALT1
expression decline of >30% was significantly associated with
longer PFS (P=0.001; Fig. 4E) and
OS (P<0.001; Fig. 4F) times.
Factors associated with PFS in
patients with mCRC
Univariate Cox's proportional hazards regression
analysis demonstrated that MALT1 expression decline (>30 vs.
≤30%) was associated with prolonged PFS time [hazard ratio (HR),
0.429; P=0.002], while MALT1 expression before treatment (high vs.
low), MALT1 expression after treatment (high vs. low), higher ECOG
PS score, worse differentiation, multiple (vs. single) metastatic,
lung metastasis (yes vs. no), peritoneal metastasis (yes vs. no)
and higher treatment lines (all P<0.05) were associated with
declined PFS. Forward stepwise multivariate Cox's proportional
hazards regression analysis demonstrated that MALT1 expression
decline (>30 vs. ≤30%; HR, 0.410; P=0.001) independently
predicted prolonged PFS time, while MALT1 expression before
treatment (high vs. low), worse differentiation and higher
treatment lines were independently associated with shorter PFS
times (all P<0.05) (Table
III).
 | Table III.Cox's proportional hazards regression
analysis for PFS. |
Table III.
Cox's proportional hazards regression
analysis for PFS.
| A, Univariate Cox's
proportional hazards regression analysis for PFS |
|---|
|
|---|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Patient
characteristics | P-value | HR | Lower | Upper |
|---|
| MALT1 decline
(>30 vs. ≤30%) | 0.002 | 0.429 | 0.252 | 0.731 |
| MALT1 expression
before treatment (high vs. low) | 0.033 | 1.769 | 1.049 | 2.983 |
| MALT1 expression
after treatment (high vs. low) | 0.012 | 1.958 | 1.160 | 3.306 |
| Age (≥60 vs. <60
years) | 0.229 | 1.374 | 0.818 | 2.308 |
| Sex (male vs.
female) | 0.236 | 1.407 | 0.800 | 2.473 |
| Higher ECOG PS
score | 0.016 | 1.849 | 1.119 | 3.055 |
| Diagnosis (colon
cancer vs. rectal cancer) | 0.898 | 1.043 | 0.549 | 1.981 |
| Worse
differentiation | 0.007 | 1.813 | 1.181 | 2.782 |
| Metastatic sites
(multiple vs. single) | 0.044 | 1.736 | 1.014 | 2.971 |
| Lung metastasis
(yes vs. no) | 0.019 | 1.955 | 1.117 | 3.422 |
| Liver metastasis
(yes vs. no) | 0.423 | 1.236 | 0.736 | 2.075 |
| Peritoneum
metastasis (yes vs. no) | <0.001 | 5.890 | 3.131 | 11.078 |
| Other metastases
(yes vs. no) | 0.150 | 0.670 | 0.389 | 1.155 |
| KRAS (mutation vs.
wild-type) | 0.287 | 1.384 | 0.760 | 2.520 |
| MSI status (MSI-H
vs. MSI-L/MSS) | 0.188 | 0.500 | 0.178 | 1.403 |
| Treatments |
|
|
|
|
| PD-1
inhibitor plus chemotherapy | Reference |
|
|
|
| PD-1
inhibitor plus bevacizumab/apatinib | 0.293 | 1.379 | 0.757 | 2.512 |
| PD-1
inhibitor plus bevacizumab/apatinib and chemotherapy | 0.332 | 0.714 | 0.362 | 1.410 |
| Higher
treatment lines | 0.002 | 1.834 | 1.255 | 2.678 |
|
| B, Forward
stepwise multivariate Cox's proportional hazards regression
analysis for PFS |
|
|
|
|
| 95% CI |
|
|
|
|
|
| Patient
characteristics | P-value | HR | Lower | Upper |
|
| MALT1 decline
(>30 vs. ≤30%) | 0.001 | 0.410 | 0.238 | 0.707 |
| MALT1 expression
before treatment (high vs. low) | 0.012 | 1.981 | 1.161 | 3.379 |
| Worse
differentiation | 0.004 | 1.883 | 1.226 | 2.891 |
| Higher treatment
lines | <0.001 | 2.205 | 1.484 | 3.277 |
Factors associated with OS in patients
with mCRC
Univariate Cox's proportional hazards regression
analysis demonstrated that MALT1 expression decline (>30 vs.
≤30%) was associated with prolonged OS time (HR, 0.321;
P<0.001), while MALT1 expression before treatment (high vs.
low), MALT1 expression after treatment (high vs. low), higher ECOG
PS score, worse differentiation, multiple (vs. single) metastatic
sites, peritoneal metastasis (yes vs. no) and higher treatment
lines were associated with shorter OS times (all P<0.05).
Forward stepwise multivariate Cox's proportional hazards regression
analysis demonstrated that MALT1 expression decline (>30 vs.
≤30%; HR, 0.276; P<0.001), PD-1 inhibitor plus
bevacizumab/apatinib (vs. PD-1 inhibitor plus chemotherapy;
HR=0.138; P=0.001) independently predicted prolonged OS time, while
age (≥60 vs. <60 years), worse differentiation and higher
treatment lines were independently associated with shorter OS times
(all P<0.05) (Table IV).
 | Table IV.Cox's proportional hazards regression
analysis for OS. |
Table IV.
Cox's proportional hazards regression
analysis for OS.
| A, Univariate Cox's
proportional hazards regression analysis for OS |
|---|
|
|---|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Patient
characteristics | P-value | HR | Lower | Upper |
|---|
| MALT1 decline
(>30 vs. ≤30%) | <0.001 | 0.321 | 0.172 | 0.600 |
| MALT1 expression
before treatment (high vs. low) | 0.028 | 1.927 | 1.072 | 3.463 |
| MALT1 expression
after treatment (high vs. low) | 0.006 | 2.370 | 1.277 | 4.397 |
| Age (≥60 vs. <60
years) | 0.121 | 1.589 | 0.885 | 2.853 |
| Sex (male vs..
female) | 0.170 | 1.597 | 0.818 | 3.116 |
| Higher ECOG PS
score | 0.002 | 2.714 | 1.443 | 5.106 |
| Diagnosis (colon
cancer vs. rectal cancer) | 0.632 | 0.833 | 0.395 | 1.758 |
| Worse
differentiation | 0.007 | 2.018 | 1.215 | 3.354 |
| Metastatic sites
(multiple vs. single) | 0.025 | 2.029 | 1.092 | 3.771 |
| Lung metastasis
(yes vs. no) | 0.078 | 1.744 | 0.939 | 3.236 |
| Liver metastasis
(yes vs. no) | 0.182 | 1.490 | 0.829 | 2.678 |
| Peritoneum
metastasis (yes vs. no) | <0.001 | 5.503 | 2.888 | 10.485 |
| Other metastases
(yes vs. no) | 0.283 | 0.713 | 0.385 | 1.323 |
| KRAS (mutation vs.
wide-type) | 0.171 | 1.641 | 0.808 | 3.335 |
| MSI status (MSI-H
vs. MSI-L/MSS) | 0.218 | 0.409 | 0.099 | 1.695 |
| Treatments |
|
|
|
|
| PD-1
inhibitor plus chemotherapy | Reference |
|
|
|
| PD-1
inhibitor plus bevacizumab/apatinib | 0.627 | 1.179 | 0.607 | 2.290 |
| PD-1
inhibitor plus bevacizumab/apatinib and chemotherapy | 0.154 | 0.561 | 0.253 | 1.242 |
| Higher
treatment lines | 0.003 | 1.884 | 1.232 | 2.881 |
|
| B, Forward
stepwise multivariate Cox's proportional hazards regression
analysis for OS |
|
|
|
|
| 95% CI |
|
|
|
|
|
| Patient
characteristics | P-value | HR | Lower | Upper |
|
| MALT1 decline
(>30 vs. ≤30%) | <0.001 | 0.276 | 0.142 | 0.534 |
| Age (≥60 vs. <60
years) | 0.026 | 2.397 | 1.112 | 5.167 |
| Worse
differentiation | <0.001 | 3.830 | 2.065 | 7.102 |
| Treatments |
|
|
|
|
| PD-1
inhibitor plus chemotherapy | Reference |
|
|
|
| PD-1
inhibitor plus bevacizumab/apatinib | 0.001 | 0.138 | 0.042 | 0.457 |
| PD-1
inhibitor plus bevacizumab/apatinib and chemotherapy | 0.101 | 0.497 | 0.216 | 1.145 |
| Higher treatment
lines | <0.001 | 5.222 | 2.342 | 11.646 |
Discussion
To date, the association between MALT1 expression
and disease risk in mCRC has been unclear. To the best of our
knowledge, only one published study compared the difference between
MALT1 expression in CRC tissue and adjacent tissue based on the
Gene Expression Omnibus database and immunohistochemistry staining,
which illustrated that MALT1 expression was markedly increased in
CRC tissue compared with adjacent tissue (21), suggesting the potential association
of MALT1 with CRC risk. In the present study, it was discovered
that blood MALT1 was elevated in patients with mCRC compared with
HCs, which also had the capability to discriminate patients with
mCRC from HCs. A potential explanation for this observation may be
that MALT1 could regulate several oncogenic signaling pathways of
mCRC (such as the NF-κB and extracellular signal-regulated
kinase/mitogen-activated protein kinase signal pathways) (21,26,27),
which might accelerate the occurrence of CRC. In addition, the
present study also demonstrated that MALT1 expression was
positively correlated with multiple metastases and peritoneal
metastasis. The possible reason for this observation may be that
MALT1 can accelerate CRC cell invasion and migration, which may
lead to multiple metastases and peritoneal metastases in mCRC
(20,21).
PD-1 inhibitor therapy combined with systematic
therapy and/or targeted therapy is widely applied in mCRC treatment
(15,17). However, the low response rate of
PD-1 inhibitor-based treatment is still a challenge for the
treatment of mCRC (16,17). Although previous studies have
discovered the predictors of treatment response of PD-1
inhibitor-based treatment among patients with mCRC (including
pan-immune-inflammation value and microsatellite instability-high),
the role of MALT1 in reflecting short-term efficacy in these
patients remains unclear (28,29).
The present study discovered that, before treatment, blood MALT1
levels were lower in patients with mCRC with a favorable treatment
response, while after treatment, blood MALT1 was notably decreased,
and its low post-treatment expression was significantly associated
with an improved treatment response in patients with mCRC receiving
PD-1 inhibitor-based treatment. This observation may be due to the
suggestions that: i) MALT1 could regulate the proliferation of CRC
cells, leading to CRC growth and, after treatment, the tumor growth
was decreased; therefore, MALT1 expression was reduced after
treatment (20,21); and ii) decrease in MALT1 before and
after treatment could inhibit tumor immune escape and promote
activation of CD8+ T cells, which may lead to the
elevated treatment response to PD-1 inhibitors (22,30).
The survival profile of patients with mCRC is dismal
(13–15). Thus, the exploration of potential
biomarkers to predict survival time among patients with mCRC is
imperative. In the present study, it was demonstrated that reduced
blood MALT1 levels before treatment were associated with longer PFS
and OS times. Meanwhile, lower blood MALT1 levels after treatment
and a >30% decline in MALT1 level were significantly associated
with prolonged PFS and OS times. The potential explanations for
these observations may be that: i) Lower MALT1 expression was
associated with better treatment response, thus lower MALT1
expression level was related to longer PFS and OS times among
patients with mCRC; and ii) decreased MALT1 expression may inhibit
the malignant behavior and immune escape of tumor cells, which may
lead to less disease burden and consequently result in longer PFS
and OS times (21,31).
The present study included two characteristics: i)
MALT1 level from PBMCs among patients with mCRC was detected in the
present study, which was convenient to acquire and, notably, the
detection of accessible blood MALT1 levels may help clinicians
promote the correct treatment management of patients with mCRC; and
ii) considering that MALT1 regulates tumor immune escape and
inactivation of CD8+ T cells (22), the present study explored the
association of blood MALT1 levels with the efficacy of PD-1
inhibitor-based treatment, while this association with PD-ligand 1
inhibitor-based treatment was not explored. Nevertheless, several
limitations exist in the present study: i) The sample size was
relatively small, which may lead to less generalization of
discoveries in the study; ii) the association of MALT1 expression
levels with treatment response of other immunotherapy methods among
patients with mCRC should be explored in a further study; and iii)
the included patients were patients with unresectable mCRC, so the
association of MALT1 expression level with the efficacy of PD-1
inhibitor-based treatment in patients with resectable CRC should be
explored in the future.
In conclusion, blood MALT1 levels were decreased
after PD-1 inhibitor-based treatment, and this decrease was
associated with low disease risk, better treatment response and
longer survival times of patients with mCRC.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX contributed to the conception and the design of
the study, project administration, resources and validation. CL and
FY were responsible for the acquisition and analysis of data,
methodology, interpretation of the data, and reviewing and editing
of the manuscript. WX and CL confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
Wuhan No.8 Hospital (Wuhan, China). All individuals included in the
study provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
mCRC
|
metastatic CRC
|
|
PD-1
|
programmed cell death protein-1
|
|
MALT1
|
mucosa-associated lymphoid tissue
lymphoma translocation protein 1
|
|
PBMC
|
peripheral blood mononuclear cells
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
ROC
|
receiver operating characteristic
|
|
IQR
|
interquartile range
|
|
ORR
|
objective response rate
|
|
DCR
|
disease control rate
|
References
|
1
|
You YN, Hardiman KM, Bafford A, Poylin V,
Francone TD, Davis K, Paquette IM, Steele SR and Feingold DL: On
Behalf of the Clinical Practice Guidelines Committee of the
American Society of Colon and Rectal Surgeons: The American Society
of Colon and rectal surgeons clinical practice guidelines for the
management of rectal cancer. Dis Colon Rectum. 63:1191–1222. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carlomagno C, De Stefano A, Rosanova M, De
Falco S, Attademo L, Fiore G and De Placido S: Multiple treatment
lines and prognosis in metastatic colorectal cancer patients.
Cancer Metastasis Rev. 38:307–313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engstrand J, Nilsson H, Stromberg C, Jonas
E and Freedman J: Colorectal cancer liver metastases-a
population-based study on incidence, management and survival. BMC
Cancer. 18:782018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu T, Jiang S, Teng X, Zhong L, Liu M,
Jin Y and Dong M: A comparison of panitumumab and cetuximab in the
treatment of KRAS wild-type metastatic colorectal cancer: A
systematic review and meta-analysis. Immunopharmacol Immunotoxicol.
45:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watson AJ: An overview of apoptosis and
the prevention of colorectal cancer. Crit Rev Oncol Hematol.
57:107–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Hong M, Li Y, Chen D, Wu Y and Hu
Y: Programmed cell death tunes tumor immunity. Front Immunol.
13:8473452022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Pei Y, Luo J, Huang Z, Yu J and
Meng X: Looking for the Optimal PD-1/PD-L1 Inhibitor in Cancer
Treatment: A comparison in basic structure, function, and clinical
practice. Front Immunol. 11:10882020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Zeng Q, Xu F, Jiang Y and Jiang Z:
Progress in programmed cell death-1/programmed cell death-ligand 1
pathway inhibitors and binding mode analysis. Mol Divers. Aug
10–2022.(Epub ahead of print). View Article : Google Scholar
|
|
11
|
Chen W, Huang Y, Pan W, Xu M and Chen L:
Strategies for developing PD-1 inhibitors and future directions.
Biochem Pharmacol. 202:1151132022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hao S, Zhang X, Han L, Ma X, Nie Y, Deng
J, Zhu H, Liu Q, Ai D, Chen Y, et al: PD-1 inhibitor enhanced
radiosensitivity by reactivating T cells and inducing G2/M phase
arrest in esophageal squamous cell carcinoma. Radiat Res.
198:458–466. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou H, Wang Y, Lin Y, Cai W, Li X and He
X: Preliminary efficacy and safety of camrelizumab in combination
with XELOX plus bevacizumab or regorafenib in patients with
metastatic colorectal cancer: A Retrospective study. Front Oncol.
11:7744452021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang FE, Zhang HJ, Yu CY and Liu AN:
Efficacy and safety of regorafenib or fruquintinib plus
camrelizumab in patients with microsatellite stable and/or
proficient mismatch repair metastatic colorectal cancer: An
observational pilot study. Neoplasma. 68:861–866. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oliveira AF, Bretes L and Furtado I:
Review of PD-1/PD-L1 Inhibitors in Metastatic dMMR/MSI-H Colorectal
Cancer. Front Oncol. 9:3962019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan A, Wang B, Wang X, Nie Y, Fan D, Zhao
X and Lu Y: Immunotherapy in colorectal cancer: Current
achievements and future perspective. Int J Biol Sci. 17:3837–3849.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang X, Cao Y, Li C, Yu H, Yang C and Liu
H: MALT1 as a promising target to treat lymphoma and other diseases
related to MALT1 anomalies. Med Res Rev. 41:2388–2422. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YY, Peng J and Luo XJ:
Post-translational modification of MALT1 and its role in B cell-
and T cell-related diseases. Biochem Pharmacol. 198:1149772022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan H, Xie Y, Zhang X, Wu S, Zhao H, Wu J,
Wang W and Lin C: Integrative analysis of MALT1 as a potential
therapeutic target for prostate cancer and its immunological role
in pan-cancer. Front Mol Biosci. 8:7149062021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian R, Niu X, Wang Y, Guo Z, Deng X, Ding
Z, Zhou M and Deng H: Targeting MALT1 suppresses the malignant
progression of colorectal cancer via miR-375/miR-365a-3p/NF-κB
Axis. Front Cell Dev Biol. 10:8450482022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang N, Ji F, Cheng L, Lu J, Sun X, Lin X
and Lan X: Knockout of immunotherapy prognostic marker genes
eliminates the effect of the anti-PD-1 treatment. NPJ Precis Oncol.
5:372021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hochster HS, Hart LL, Ramanathan RK,
Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr
Y, Saif MW, et al: Safety and efficacy of oxaliplatin and
fluoropyrimidine regimens with or without bevacizumab as first-line
treatment of metastatic colorectal cancer: Results of the TREE
Study. J Clin Oncol. 26:3523–3529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiba T, Soeno Y, Shirako Y, Sudo H,
Yagishita H, Taya Y, Kawashiri S, Okada Y and Imai K: MALT1
Inhibition of oral carcinoma cell invasion and ERK/MAPK Activation.
J Dent Res. 95:446–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giordano G, Febbraro A, Tomaselli E,
Sarnicola ML, Parcesepe P, Parente D, Forte N, Fabozzi A, Remo A,
Bonetti A, et al: Cancer-related CD15/FUT4 overexpression decreases
benefit to agents targeting EGFR or VEGF acting as a novel
RAF-MEK-ERK kinase downstream regulator in metastatic colorectal
cancer. J Exp Clin Cancer Res. 34:1082015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corti F, Lonardi S, Intini R, Salati M,
Fenocchio E, Belli C, Borelli B, Brambilla M, Prete AA, Quarà V, et
al: The Pan-Immune-Inflammation Value in microsatellite
instability-high metastatic colorectal cancer patients treated with
immune checkpoint inhibitors. Eur J Cancer. 150:155–167. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao P, Li L, Jiang X and Li Q: Mismatch
repair deficiency/microsatellite instability-high as a predictor
for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol.
12:542019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dumont C, Sivars U, Andreasson T, Odqvist
L, Mattsson J, DeMicco A, Pardali K, Johansson G, Yrlid L, Cox RJ,
et al: A MALT1 inhibitor suppresses human myeloid DC, effector
T-cell and B-cell responses and retains Th1/regulatory T-cell
homeostasis. PLoS One. 15:e02225482020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gomez Solsona B, Schmitt A,
Schulze-Osthoff K and Hailfinger S: The Paracaspase MALT1 in
Cancer. Biomedicines. 10:3442022. View Article : Google Scholar : PubMed/NCBI
|