Introduction
Sebaceous carcinoma (SC) is a rare skin carcinoma
characterized by sebocytic differentiation (1,2). SC is
classified according to the location of the tumor as ocular or
extraocular (1,2). While ocular SC comprises ~75% of all
SC cases, 10–30% of both ocular and extraocular SC patients have a
risk of tumor-related mortality (1,2).
Ocular SC is considered to arise from the meibomian glands,
modified sebaceous glands present in the tarsal plates of the upper
and lower eyelids and the glands of Zeis, which are also sebaceous
glands located in the eyelashes. In situ SC lesions are
frequently observed within the pre-existing sebaceous glands of the
meibomian glands or the glands of Zeis (3). However, the pathogenesis of
extraocular SC remains to be elucidated because no in situ
SC lesions within the pre-existing sebaceous glands of extraocular
skin tissues have been observed to date (4).
The origin of extraocular SC is an issue that needs
to be addressed in the field of dermatopathology. Several
hypotheses have been proposed about the histogenesis of extraocular
SC. Boecker et al (5,6)
hypothesized that progenitor cells (p63+/keratin
5+) present in the sebaceous ducts or the
interfollicular epidermis could be the cells of origin of some
parts of extraocular SC based on immunohistochemical analyses. The
authors also speculated that some extraocular SCs may originate
from intraepidermal pluripotent neoplastic cells because a
relatively large proportion of extraocular SC in their series had a
‘full-thickness intraepidermal neoplasia’ (5). In addition, the latter possibility is
supported by the presence of reported cases of SC in situ
and SC arising from squamous intraepidermal neoplasia (actinic
keratosis or Bowen's disease) (7–17).
These in situ neoplastic SCs in a few cases show positive
immunoreactivity for adipophilin (ADP) (7,11),
which is a useful sebaceous differentiation marker that shows
reactivity for intracellular lipid droplets. ADP expression has
also been reported in non-SCs in various organs (18–20).
Thus, sebaceous differentiation must be defined by considering both
typical histopathological features and ADP immunoreactivity.
However, the expression of ADP and androgen receptor (AR), which is
also a useful marker for sebaceous differentiation, has not been
reported in intraepidermal neoplastic cells present in extraocular
SC in most of the previously reported extraocular SCs.
In addition, although most SCs do not exhibit
follicular or apocrine differentiation, extremely rare cases of SC
with apocrine differentiation (seboapocrine carcinoma) have been
reported (21–25). This phenomenon is not unexpected
based on the common embryological origin of the
folliculosebaceous-apocrine unit (23). To date, only five cases (two ocular
and three extraocular) have been reported in English-language
literature (21–25).
The present study retrospectively reviewed the
clinicopathological features of ocular and extraocular SC
experienced in the Osaka Medical and Pharmaceutical University
(Takatsuki, Japan), with an emphasis on the presence of in
situ SC lesions and apocrine differentiation and discussed and
reviewed the histogenesis of extraocular SC.
Materials and methods
Patient selection
The present study selected consecutive patients with
SC who underwent biopsy and/or surgical resection at the Osaka
Medical and Pharmaceutical University Hospital between January 2001
and December 2020. Accordingly, 11 patients with ocular (eight
patients) and extraocular (three patients) SC were included in the
present study.
This retrospective, single-institution study was
conducted in accordance with the principles of the Declaration of
Helsinki and the study protocol was approved by the Institutional
Review Board of Osaka Medical and Pharmaceutical University
Hospital (approval nos. 2020-124 and 2022-212). All data were
anonymized. The Institutional Review Board waived the requirement
for informed consent because of the retrospective study design, as
medical records and archived samples were used with no risk to the
participants. In addition, the present study did not include
minors. Information regarding this study, such as the inclusion
criteria and opportunity to opt-out, was provided through the
institutional website (https://www.ompu.ac.jp/u-deps/path/img/file9.pdf).
Histopathological analysis
Biopsied and/or surgically resected specimens were
fixed in 10% formalin at room temperature for 24–48 h, sectioned
(3–5 mm), dehydrated in ethanol and xylene at room temperature,
embedded in paraffin (60°C), and stained with hematoxylin and eosin
for 5 min each at room temperature. A minimum of two researchers
independently evaluated histopathological features.
Immunohistochemical analyses
Tumor tissues were fixed in 10% formalin at room
temperature for 24–48 h, dehydrated in ethanol and xylene at room
temperature, and embedded in paraffin (60°C). The 4-µm tumor tissue
sections then underwent immunohistochemical staining using
autostainers (Discovery Ultra System; Roche Diagnostics; Leica
Bond-III; Leica Biosystems GmbH), according to the manufacturer's
instructions. Sections were incubated with mouse monoclonal
antibodies against ADP (cat. no. AP125; 1:100 dilution; Progen
Biotechnik GmbH), AR (cat. no. AR441; 1:100 dilution; Dako; Agilent
Technologies, Inc.), keratin 5/6 (cat. no. D5/16B4; 1:100 dilution;
Dako; Agilent Technologies, Inc.), Ki-67 (cat. no. MIB-I; 1:150
dilution; Dako; Agilent Technologies, Inc.), p53 (DO-7; 1:50
dilution; Dako; Agilent Technologies, Inc.) and p63 (cat. no. 4A4;
1:50 dilution; Dako; Agilent Technologies, Inc.) for 20 min at room
temperature. Secondary antibodies were pre-diluted and were used to
incubate the sections for 8 min at room temperature [Optivew DAB
Universal Kit (cat. no. 518-111427; Roche Diagnostics) and Novolink
Max Polymer Detection System (cat. no. RE7140-K; Leica Biosystems
GmbH)]. Then two researchers independently evaluated the results of
the immunohistochemical staining.
Results
Patient characteristics
Table I summarizes
the clinicopathological features of the study cohort. This study
included eight women and three men. The median patient age was 72
years (range: 50–90 years). The cohort comprised eight and three
patients with ocular and extraocular SC, respectively. The
extraocular SC was located on the cheek, fingers, or scalp.
 | Table I.Clinicopathological and
immunohistochemical characteristics of ocular and extraocular
sebaceous carcinomas. |
Table I.
Clinicopathological and
immunohistochemical characteristics of ocular and extraocular
sebaceous carcinomas.
| A, Ocular sebaceous
carcinoma |
|---|
|
|---|
| Characteristic | Age, years | Sex | Location | In situ
component | Apocrine
component | Adipophilin | Androgen
Receptor | p53 | Ki-67 labelling
index, % |
|---|
| Patient 1 | 50 | Female | Eyelid | - | - | + | + | + | 20 |
| Patient 2 | 83 | Male | Eyelid | - | - | + | + | - | 15 |
| Patient 3 | 79 | Female | Eyelid | + | + | + | + | + | 50 |
| Patient 4 | 82 | Female | Eyelid | - | - | + | + | + | 25 |
| Patient 5 | 71 | Female | Eyelid | + | - | + | + | + | 80 |
| Patient 6 | 72 | Female | Eyelid | - | - | + | + | + | 15 |
| Patient 7 | 80 | Female | Eyelid | + | - | + | + | - | 10 |
| Patient 8 | 66 | Female | Eyelid | + | - | + | + | - | 20 |
|
| B, Extraocular
sebaceous carcinoma |
|
|
Characteristic | Age,
years | Sex |
Location | In situ
component | Apocrine
component |
Adipophilin | Androgen
Receptor | p53 | Ki-67 labeling
index, % |
|
| Patient 9 | 90 | Female | Cheek | - | - | + | + | + | 35 |
| Patient 10 | 68 | Male | Finger | + | - | + | + | + | 10 |
| Patient 11 | 65 | Male | Scalp | - | - | + | - | + | 15 |
Histopathological features
Table I summarizes
the histopathological characteristics observed in this study. The
typical histopathological features of SC were as follows: an
infiltrative variable-sized nodular proliferation of basaloid
neoplastic cells with large round-to-oval nuclei and occasional
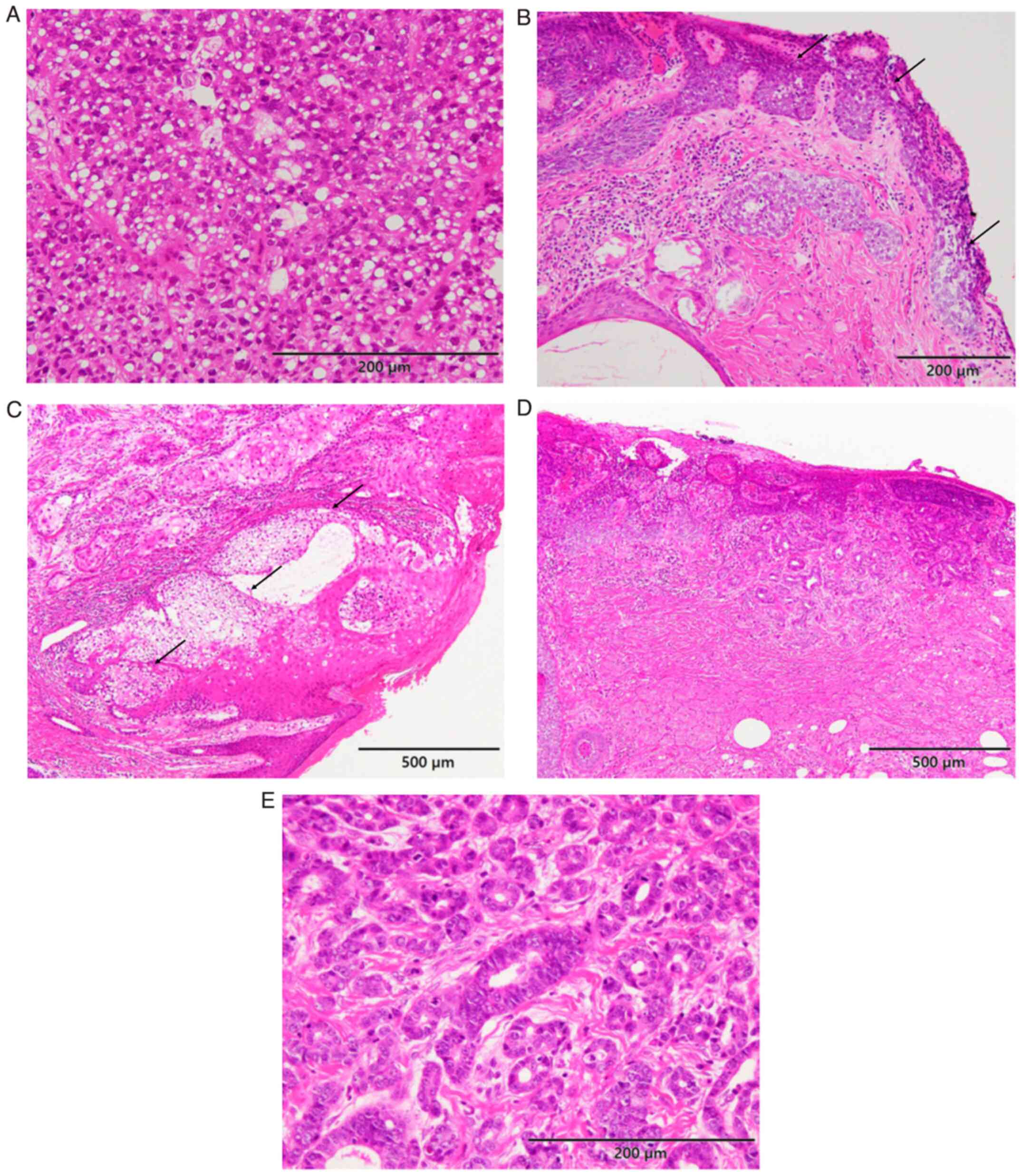
scalloped nuclei (Fig. 1A).
Occasional necrosis was observed within the nodules. Some
neoplastic cells had a clear cytoplasm and multi-vacuoles were
characteristically observed in the cytoplasm of the neoplastic
cells (Fig. 1A). In situ
(intraepithelial) lesions were noted in four of the eight ocular SC
cases and one of the three extraocular SC cases (the two remaining
extraocular SC cases showed no intraepithelial lesion; Fig. 1B for ocular SC and Fig. 1C for extraocular SC). Mild solar
elastosis was noted in the dermis around the tumor in an
extraocular SC case with in situ lesions. In addition, no
neoplastic cells were present within the pre-existing sebaceous
glands in any of the extraocular SC.
An apocrine component was observed in one patient
with ocular SC (patient 3). The tumor was composed of SC (~70% of
the tumor) and apocrine carcinoma components (~30%). The SC
component comprised variable-sized nodular proliferations of
basaloid cells (Fig. 1D). In
situ (intraepithelial) lesions in the SC were also observed. An
irregularly shaped glandular formation was observed with continuity
of the above-mentioned SC component (Fig. 1D). These neoplastic glandular cells
had large round-to-oval nuclei containing small nucleoli and
mitotic figures were frequently observed (Fig. 1E). Decapitation was also noted
(Fig. 1E). Accordingly, the latter
component was considered to be an apocrine carcinoma component;
thus, a diagnosis of seboapocrine carcinoma was made.
Immunohistochemical features
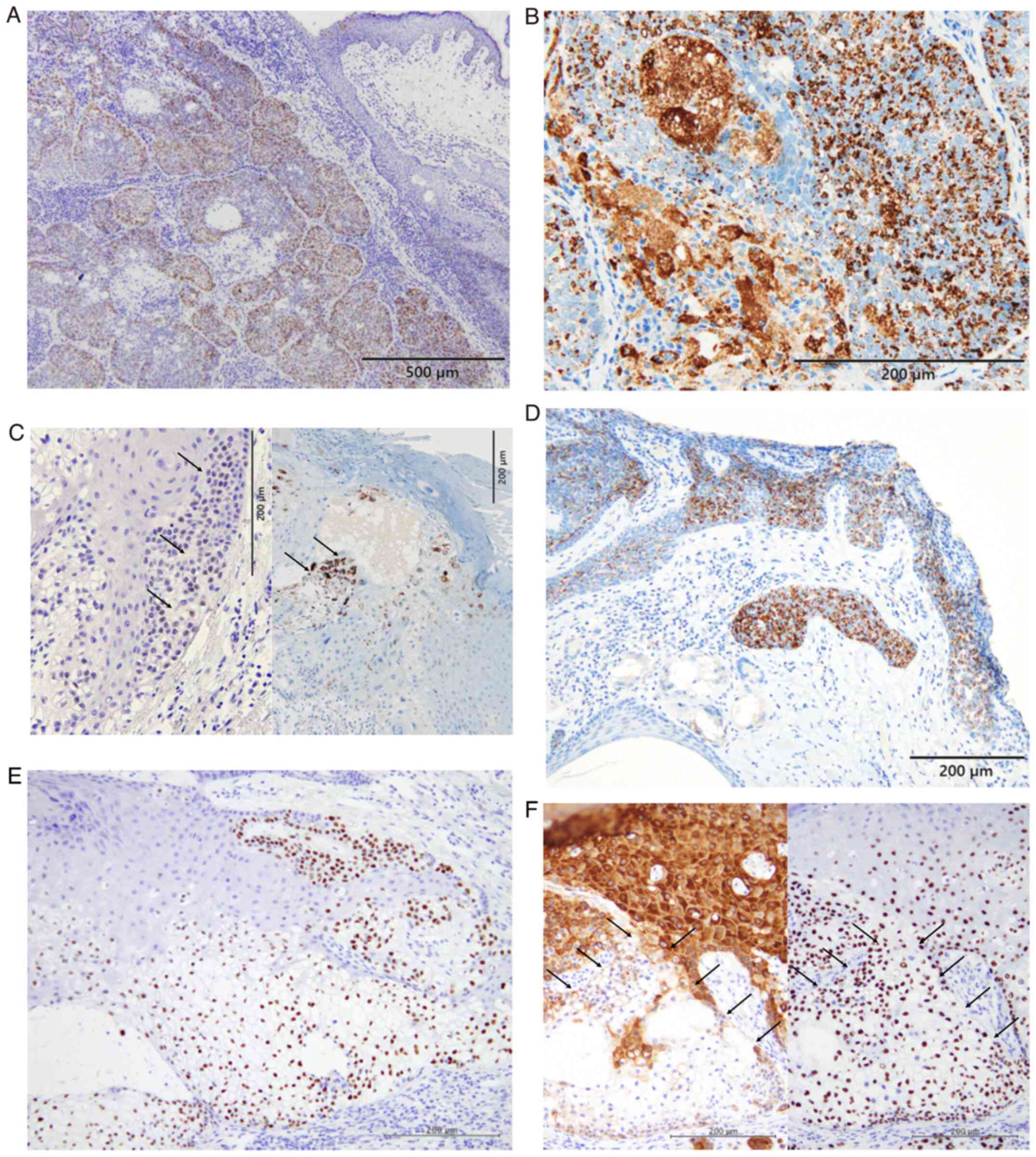
AR expression was observed in all eight patients
with ocular SC and two of the three patients with extraocular SC
(Fig. 2A). In addition. ADP
expression was observed in all patients with ocular and extraocular
SC (Fig. 2B). Fig. 2C shows positive immunoreactivity for
AR and ADP in an in situ (intraepidermal) lesion of
extraocular SC (Patient 10). In seboapocrine carcinoma, ADP
expression was present in both the invasive and in situ
(intraepithelial) components of the SC (Fig. 2D). p53 overexpression was noted in
all three extraocular SCs and five of the eight ocular SCs
(Fig. 2E) (Table I). Diffuse expression of p63 and
focal expression of keratin 5/6 were observed in an in situ
(intraepidermal) lesion of extraocular SC (Patient 10; Fig. 2F). The median Ki-67 labelling index
was 20% for ocular (range: 10–80%) and 15% for extraocular SC
(range: 10–35%).
Discussion
The present study comprehensively reviewed the
histopathological and immunohistochemical characteristics of eight
ocular and three extraocular SC cases. In situ
(intraepithelial) lesions were noted in four of eight ocular and
one of three extraocular SC cases and the neoplastic cells in these
in situ lesions, including extraocular SC, showed sebaceous
differentiation. It is recognized that ocular SC arises from the
meibomian glands or the glands of Zeis and the presence of in
situ lesions is not uncommon (1,3).
However, the origin of extraocular SC remains to be elucidated and
has been a controversial topic (4,5).
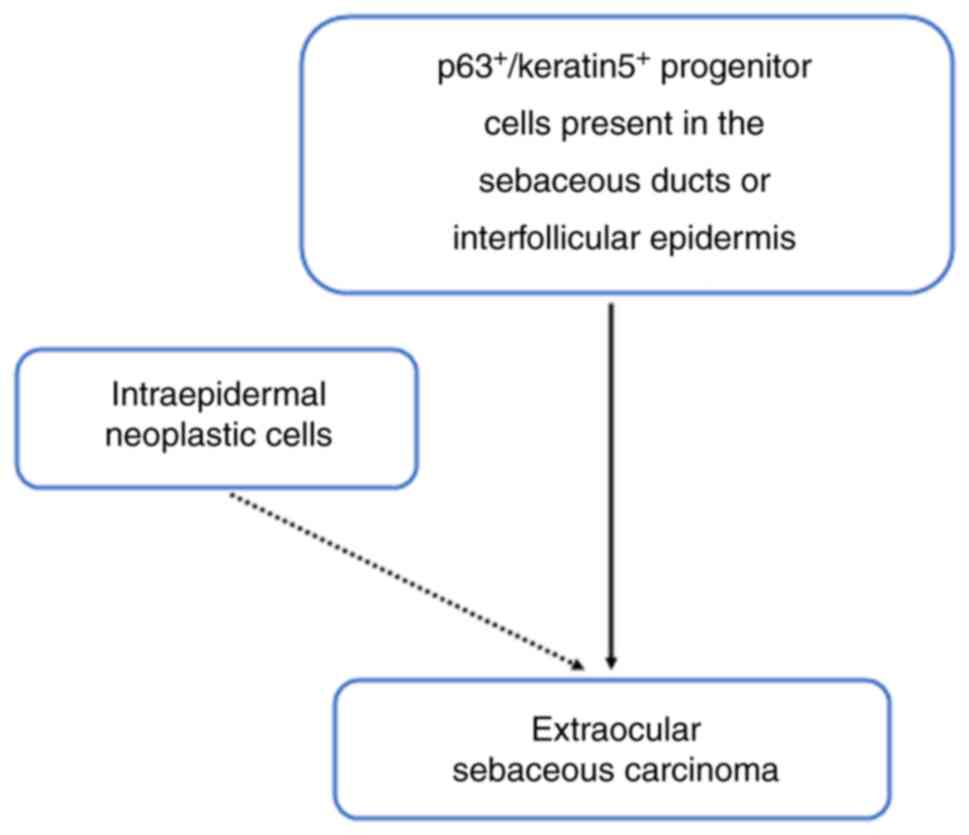
Boecker et al (5,6) proposed a model of sebaceous gland
development and the histogenesis of sebaceous gland neoplasms.
According to their model, mature sebocytes and ductal cells arise
from p63+/keratin 5+ progenitor cells and
extraocular sebaceous tumors may also arise from
p63+/keratin 5+ progenitor cells. Thus, these
progenitor cells present in the sebaceous ducts or the
interfollicular epidermis could be the cells of origin of some
parts of extraocular SC (Fig. 3)
(5,6). However, some cases of extraocular SC
might arise from the epidermis because, albeit rare, SC in
situ or SC (in situ or invasive) arising from squamous
intraepidermal neoplasm (actinic keratosis or Bowen's disease) have
been reported (7–17). Table
II summarizes the clinicopathological features of the
previously reported SC in situ and SC arising from a
squamous intraepidermal neoplasm of the extraocular sites. Of 15
patients, eight and seven had in situ and invasive SC
associated with squamous intraepidermal neoplasms, respectively,
and more than half of these lesions occurred in sun-exposed
regions.
 | Table II.Clinicopathological features of
extraocular sebaceous carcinomas in situ and sebaceous
carcinomas arising from squamous intraepidermal neoplasm. |
Table II.
Clinicopathological features of
extraocular sebaceous carcinomas in situ and sebaceous
carcinomas arising from squamous intraepidermal neoplasm.
| First author/s,
year | Patients | Age, years | Sex | Location | Associated squamous
lesion | Sebaceous
carcinoma | AR expression | ADP expresion | (Refs.) |
|---|
| Namiki et
al, 2018 | Patient 1 | 67 | Male | Abdomen | Bowen's
disease | in situ | ND | + | (7) |
| Misago et
al, 2015 | Patient 2 | 85 | Female | Cheek | Actinic
keratosis | invasive | ND | + | (8) |
| Misago et
al, 2015 | Patient 3 | 82 | Female | Cheek | Actinic keratosis
(bowenoid) | invasive | ND | + | (8) |
| Aung et al,
2014 | Patient 4 | 60 | Male | Forehead | Squamous cell
carcinoma in situ | in situ | ND | ND | (9) |
| Aung et al,
2014 | Patient 5 | 70 | Male | Neck | Actinic
keratosis | in situ | ND | ND | (9) |
| Aung et al,
2014 | Patient 6 | 85 | Female | Cheek | Actinic
keratosis | in situ | ND | ND | (9) |
| Ishida et
al, 2013 | Patient 7 | 67 | Female | Buttock | Bowen's
disease | invasive | + | + | (10) |
| Ishida et
al, 2012 | Patient 8 | 60 | Male | Neck | Actinic
keratosis | in situ | + | + | (11) |
| Nakashima et
al, 2010 | Patient 9 | 83 | Female | Cheek | Actinic
keratosis | invasive | ND | + | (12) |
| Ansai et al,
2000 | Patient 10 | 75 | Female | Temple | Actinic
keratosis | in situ | ND | ND | (13) |
| Ansai et al,
2000 | Patient 11 | 81 | Female | Cheek | Actinic
keratosis | invasive | ND | ND | (13) |
| Escalonilla et
al, 1999 | Patient 12 | 76 | Female | Vulva | Bowen's
disease | invasive | ND | ND | (14) |
| Oka et al,
1990 | Patient 13 | 81 | Female | Upper arm | Squamous cell
carcinoma in situ | in situ | ND | ND | (15) |
| Jacobs et
al, 1986 | Patient 14 | 89 | Female | Vulva | Bowen's
disease | invasive | ND | ND | (16) |
| Fulling et
al, 1981 | Patient 15 | 74 | Male | Cheek | Squamous cell
carcinoma in situ | in situ | ND | ND | (17) |
The frequency of the presence of in situ
(intraepidermal) lesions of extraocular SC remains to be
elucidated, although Boecker et al (5) reported that four of the six cases of
extraocular SC had full-thickness intraepidermal neoplasia and
three of the six cases also had actinic keratosis. However,
information on whether these intraepidermal lesions showed
sebaceous differentiation was not available. Notably, ADP
expression, for which >95% of extraocular SC showed positive
immunoreactivity (5), was observed
in two cases of SC in situ (7,11) and
AR expression, for which >80% of ocular and extraocular SC
showed positive immunoreactivity (5,26), was
also noted in one case of SC in situ (11). In the present study, one of the
three extraocular SC had intraepidermal neoplasia (in situ
lesion) overlying the invasive SC and this lesion showed sebaceous
differentiation (both AR and ADP expression). In addition, these
intraepidermal neoplastic cells showed diffuse positive
immunoreactivity for p63 and focal positive immunoreactivity for
keratin 5/6. Although this finding did not directly indicate that
intraepidermal neoplastic cells of extraocular SC arise from
p63+/keratin 5+ progenitor cells, these
intraepidermal neoplastic cells might have the characteristics of
these progenitor cells. Accordingly, these results suggest that a
proportion of extraocular SC may arise from intraepidermal
neoplasia. Therefore, additional analyses of extraocular SC,
especially the presence of intraepidermal neoplasia and sebaceous
differentiation in these intraepidermal lesions, are required to
clarify the histogenesis of extraocular SC.
Recently, the results of whole-exome sequencing have
shown that three distinct mutational patterns were present in SC;
ultraviolet (UV)-damaged signature, microsatellite instability
profiles and pauci-mutational signature (27). Ocular SC shows pauci-mutational
signature, whereas extraocular SC exhibits UV-damaged,
microsatellite instability and pauci-mutational signatures
(27). Accordingly, the genetic
backgrounds of ocular and extraocular SCs are different (27). In addition, SC showing UV-damaged
signatures occurs in the extraocular sites and its transcriptional
changes resemble those of cutaneous squamous cell carcinoma.
Therefore, it has been hypothesized that UV-damaged extraocular SC
arises from the subpopulation of intraepidermal keratinocytes or
the superficial portion of the folliculosebaceous unit, which is
vulnerable to UV damage, following the same mechanism of cutaneous
squamous cell carcinoma (27).
Although data on the genetic changes of SC accompanying
intraepidermal squamous neoplasia are not available, these tumors
may show UV-damaged signatures. Additionally, p53 overexpression
was noted in all extraocular SCs in the present cohort (by
contrast, it was observed in five of the eight ocular SCs, although
both extraocular and ocular SCs showed high proliferative
activities). Therefore, intraepidermal pluripotent neoplastic cells
may be the origin of a portion of extraocular SC. According to
these findings, the results of the present study and the reported
cases of SC in situ and SC arising from intraepidermal
squamous neoplasms, some parts of the extraocular SC may arise from
intraepidermal neoplastic cells.
In some rare cases, SC can show apocrine
differentiation, namely seboapocrine carcinoma (23,24).
However, only five cases of extraocular and ocular SCs with
sebaceous differentiation (three extraocular and two ocular SCs)
have been reported in the literature (21–25).
These findings are consistent with the common embryonic origin of
the folliculosebaceous-apocrine unit (23). The tumor in one patient with ocular
SC presented in the present study is the third reported case of
ocular seboapocrine carcinoma. Table
III summarizes the clinicopathological features of the
previously reported cases of this type of rare carcinoma. These
findings suggest that the frequency of apocrine differentiation in
SC may be higher at the extraocular site as ~75% of SCs occur in
the ocular region.
 | Table III.Clinicopathological features of
seboapocrine carcinoma. |
Table III.
Clinicopathological features of
seboapocrine carcinoma.
| First author/s,
year | Patients | Age, years | Sex | Location | Size, cm | Outcome | (Refs.) |
|---|
| Ishida and Okabe,
2012 | Patient 1 | 61 | Male | Eyelid | 2.1×1.5 | Alive with disease
38 months | (21) |
| Misago and | Patient 2 | 60 | Male | Eyelid | 3×2 | Not available | (22) |
| Narisawa,
2001 |
|
|
|
|
|
|
|
| Kazakov et al,
2007 | Patient 3 | 84 | Male | Shoulder | 4×3 | No evidence of
disease 6 months | (23) |
| Pinheiro and | Patient 4 | 76 | Female | Scalp | 3×1.7 | No evidence of
disease 29 months | (24) |
| Lopes,
2019 |
|
|
|
|
|
|
|
| Afroz et al,
2013 | Patient 5 | 54 | Male | Nose | 2.5×2 | No evidence of
disease 5 years | (25) |
The present study has some limitations. Although SC
is a rare carcinoma, this study was a retrospective,
single-institution analysis with a small sample size. Therefore,
the frequency of in situ (intraepithelial) lesions may be
biased and additional multi-institutional studies with larger
sample sizes are required to clarify the pathogenesis of
extraocular SC.
In conclusion, extraocular SC may arise from
progenitor cells present in the sebaceous duct or the
interfollicular epidermis and intraepidermal neoplastic cells with
pluripotency for sebaceous differentiation may also be the origin
of extraocular SC.
Acknowledgements
The authors would like to thank Ms. Shizuka Ono, Mr.
Yusuke Ohnishi and Mr. Naoto Kohno (all from Department of
Pathology, Osaka Medical and Pharmaceutical University) for their
technical assistance in this study.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated and analyzed in this study are
included in this published article.
Authors' contributions
DT and MI conceived and designed the present study,
performed histopathological and immunohistochemical analyses. DT,
MI, EY, KU and YH performed acquisition and analysis of data. DT
and MI drafted of the manuscript; tables and figures. DT and MI
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and the study protocol was approved by
the Institutional Review Board of Osaka Medical and Pharmaceutical
University (approval nos. 2020-124 and no. 2022-212). All data were
anonymized. The Institutional Review Board waived the requirement
for informed consent because of the retrospective design of the
study with no risk of identity exposure for the patients. This
study did not include minors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SC
|
sebaceous carcinoma
|
|
ADP
|
adipophilin
|
|
AR
|
androgen receptor
|
References
|
1
|
Dubovy S, Eberhart CG and Vemuganti G:
Sebaceous carcinoma of the conjunctiva and caruncle. WHO
Classification of Tumours of the Eye. 4th Edition. IARC; Lyon: pp.
25–26. 2018
|
|
2
|
Wick MR, Cree IA, Kazakov DV, Lazar AJ,
Michal M, Sangüeza OP, Singh R, Wood BA and Zembowicz A: Sebaceous
carcinoma. WHO Classification of Skin Tumours. 4th Edition. IARC;
Lyon: pp. 211–212. 2018
|
|
3
|
Rao NA, Hidayat AA, McLean IW and
Zimmerman LE: Sebaceous carcinomas of the ocular adnexa: A
clinicopathologic study of 104 cases, with five-year follow-up
data. Hum Pathol. 13:113–122. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kazakov DV, Kutzner H, Spagnolo DV, Rütten
A, Mukensnabl P and Michal M: What is extraocular cutaneous
sebaceous carcinoma in situ? Am J Dermatopathol. 32:857–858. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boecker W, Reusch M, Mielke V, Reusch U,
Hallermann C, Loening T, Tiemann M and Buchwalow I: Twenty-eight
cases of extraocular sebaceous carcinoma: A correlative
clinicopathological and immunohistochemical analysis of extraocular
sebaceous carcinomas and benign sebaceous gland tumors. Am J
Dermatopathol. 43:93–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boecker W, Reusch M, Mielke V, Reusch U,
Loening T, Tiemann M and Buchwalow I: Spatial analysis of p63, K5
and K7 defines two groups of progenitor cells that differentially
contribute to the maintenance of normal sebaceous glands,
extraocular sebaceous carcinoma and benign sebaceous tumors. J
Dermatol. 46:249–258. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Namiki T, Miura K, Yokozeki H and Ansai
SI: Bowen disease with sebaceous differentiation: A case report and
immunohistochemical analysis of adipophilin and cytokeratin 1. Am J
Dermatopathol. 40:841–845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Misago N, Kuwashiro M, Tsuruta N and
Narisawa Y: Sebaceous carcinoma in association with actinic
keratosis: A report of two cases with an immunohistochemical study.
J Dermatol. 42:616–620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aung PP, Batrani M, Mirzabeigi M and
Goldberg LJ: Extraocular sebaceous carcinoma in situ: Report of
three cases and review of the literature. J Cutan Pathol.
41:592–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishida M, Iwai M, Yoshida K, Kagotani A
and Okabe H: Sebaceous carcinoma associated with Bowen's disease: A
case report with emphasis on the pathogenesis of sebaceous
carcinoma. Int J Clin Exp Pathol. 6:3029–3032. 2013.PubMed/NCBI
|
|
11
|
Ishida M and Okabe H: Intraepidermal
sebaceous carcinoma occurring concurrently with actinic keratosis.
J Cutan Pathol. 39:731–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakashima K, Adachi K, Yamasaki A, Yamada
N, Yoshida Y and Yamamoto O: Sebaceous carcinoma with actinic
keratosis. Acta Derm Venereol. 90:196–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ansai S and Mihara I: Sebaceous carcinoma
arising on actinic keratosis. Eur J Dermatol. 10:385–388.
2000.PubMed/NCBI
|
|
14
|
Escalonilla P, Grilli R, Cañamero M,
Soriano ML, Fariña MC, Manzarbeitia F, Sáinz R, Matsukura T and
Requena L: Sebaceous carcinoma of the vulva. Am J Dermatopathol.
21:468–472. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oka K and Katsumata M: Intraepidermal
sebaceous carcinoma: Case report. Dermatologica. 180:181–185. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacobs DM, Sandles LG and Leboit PE:
Sebaceous carcinoma arising from Bowen's disease of the vulva. Arch
Dermatol. 122:1191–1193. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fulling KH, Strayer DS and Santa Cruz DJ:
Adnexal metaplasia in carcinoma in situ of the skin. J Cutan
Pathol. 8:79–88. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshikawa K, Ishida M, Yanai H, Tsuta K,
Sekimoto M and Sugie T: Prognostic significance of adipophilin
expression in biopsy specimens of patients with triple-negative
breast cancer. Oncol Lett. 23:1272022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashimoto Y, Ishida M, Ryota H, Yamamoto
T, Kosaka H, Hirooka S, Yamaki S, Kotsuka M, Matsui Y, Yanagimoto
H, et al: Adipophilin expression is an indicator of poor prognosis
in patients with pancreatic ductal adenocarcinoma: An
immunohistochemical analysis. Pancreatology. 19:443–448. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujimoto M, Matsuzaki I, Yamamoto Y,
Yoshizawa A, Warigaya K, Iwahashi Y, Kojima F, Furukawa F and
Murata SI: Adipophilin expression in cutaneous malignant melanoma.
J Cutan Pathol. 44:228–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishida M and Okabe H: Sebaceous carcinoma
with apocrine differentiation (seboapocrine carcinoma). Pathol Int.
62:438–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Misago N and Narisawa Y: Sebaceous
carcinoma with apocrine differentiation. Am J Dermatopathol.
23:50–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kazakov DV, Calonje E, Rütten A, Glatz K
and Michal M: Cutaneous sebaceous neoplasms with a focal glandular
pattern (seboapocrine lesions): A clinicopathological study of
three cases. Am J Dermatopathol. 29:359–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinheiro JAF and Lopes JMPB: Periocular
sebaceous carcinoma with apocrine differentiation: A case report
and review of the literature. Int J Surg Pathol. 27:432–436. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Afroz N, Zaidi N and Rizvi SAR: Sebaceous
carcinoma with apocrine differentiation: A rare entity with
aggressive behavior. Indian J Pathol Microbiol. 56:408–410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Na HY, Choe JY, Shin SA, Choung HK, Oh S,
Chung JH, Park M and Kim JE: Proposal of a provisional
classification of sebaceous carcinoma based on hormone receptor
expression and HER2 status. Am J Surg Pathol. 40:1622–1630. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
North JP, Golovato J, Vaske CJ, Sanborn
JZ, Nguyen A, Wu W, Goode B, Stevers M, McMullen K, Perez White BE,
et al: Cell of origin and mutation pattern define three clinically
distinct classes of sebaceous carcinoma. Nat Commun. 9:18942018.
View Article : Google Scholar : PubMed/NCBI
|