Introduction
Glioblastoma (GBM) is the most prevalent malignant
brain tumor in adults, primarily affecting the elderly population
with a median age at diagnosis of 65 years (1,2). The
recognition of glial tumors in the brain dates back to a report by
Rudolph Virchow in 1865 (3). In
1926, Harvard Cushing and Percival Bailey introduced the concept of
GBM and improved the grading system for gliomas (3). Despite more than a century of
research, the advancements in the treatment and prognosis of GBM
have been modest. The challenges of achieving complete resection
while preserving normal brain tissue during surgery, along with
chemotherapy resistance, contribute to a short median survival time
of 12–15 months (1), thus posing a
threat to the lives of patients.
GBM with a primitive neuronal component (GBM-PNC) is
a distinct morphological variant of GBM recognized by the World
Health Organization (WHO) in 2016 (4). This rare subtype accounts for only
0.5% of GBM cases and is histologically characterized by the
coexistence of malignant glioma cells with small round blue cells
(PNC) expressing neuronal cell surface markers (3,5).
GBM-PNC commonly manifests in the temporal lobe and exhibits an
aggressive nature with a poor survival rate (6). The present study describes a case of
GBM-PNC, and provides an analysis of the clinicopathological
characteristics, immunohistochemistry and gene detection findings
associated with GBM-PNC.
Case report
Case presentation
A 57-year-old woman was admitted to Sunshine Union
Hospital (Weifang, China) in October 2022 due to a sudden onset of
headache with nausea and vomiting for 5 days. The patient did not
experience loss of consciousness, limb inflexibility, slurred
speech, coughing or memory impairment. Their medical history
included type 2 diabetes mellitus and a previous episode of
significant bleeding requiring transfusion during the delivery of
their second child. Laboratory examination (Table SI) revealed elevated lactate
dehydrogenase (LDH) (277.61 U/l) levels. Magnetic resonance imaging
(MRI) of their brain revealed lumpy T1-weighted hypointense and
T2-weighted hyperintense signals in the junction area of the left
parietal-occipital-temporal lobe, exhibiting an irregular shape
with a clear boundary, measuring ~6.3×5.5 cm. Annular enhancement
of the marginal zone was visible after an enhanced scan (Fig. 1). After their blood sugar levels
were controlled with insulin, the patient underwent surgical
intervention. During surgery, the mass was located in the left
temporal lobe, extending towards the paraventricular region. The
mass within the mesial temporal lobe appeared solid, whereas the
mass near the paraventricular region exhibited cystic features with
the presence of yellowish purulent fluid. The tumors from the
temporal lobe and paraventricular region were separately excised
and sent for pathological examination to establish a definitive
diagnosis. The patient's blood pressure and temperature remained
within normal ranges after the operation. The patient exhibited
intact mental functioning and normal muscle tension in their limbs;
however, they experienced difficulties with speech clarity.
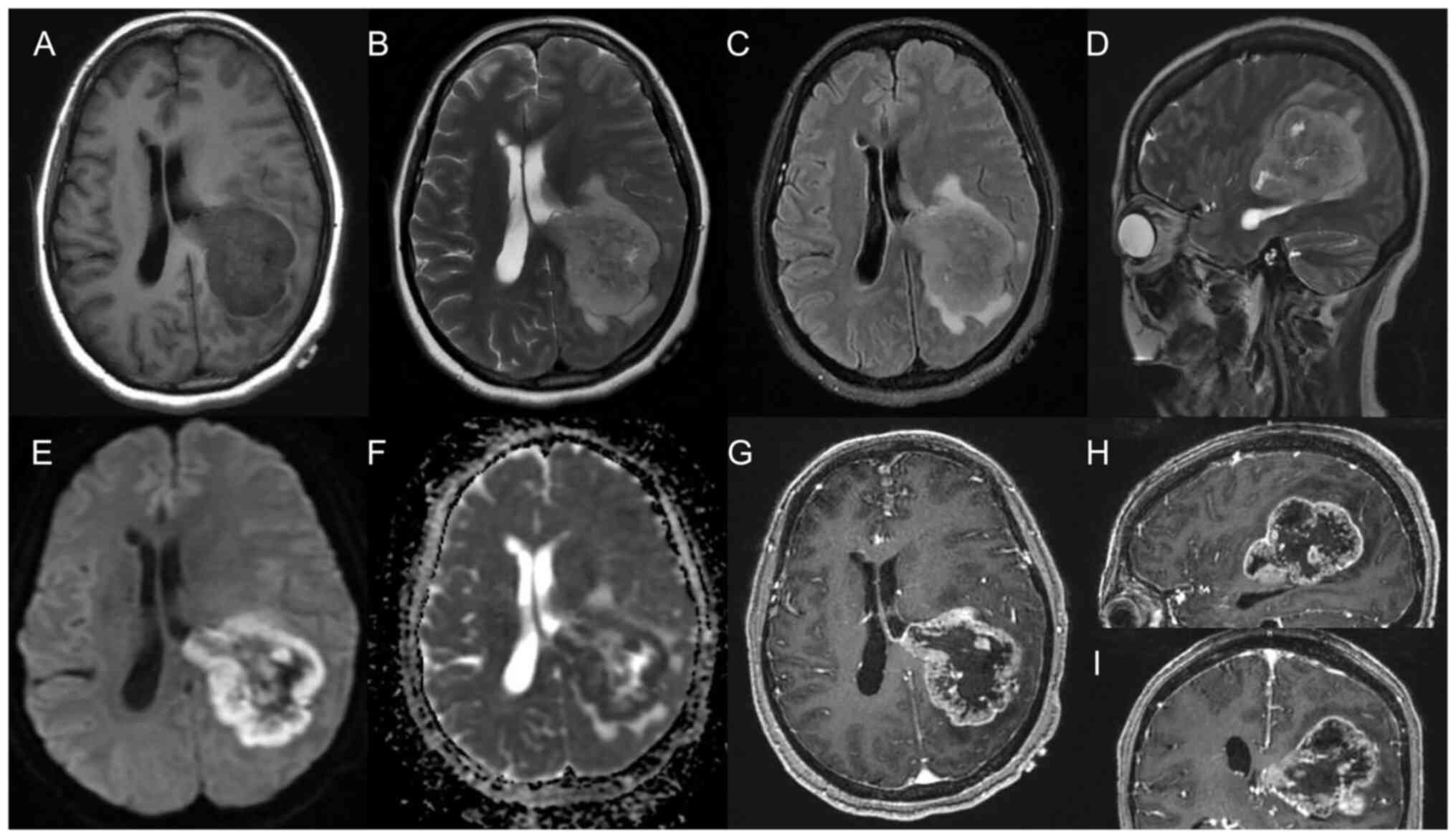 | Figure 1.Magnetic resonance imaging of the
tumor. (A) T1WI sequence at the junction of the left
parietal-occipital-temporal lobe was isointense/hypointense, with
an uneven internal signal and unclear edge. In addition, there was
a patchy obviously low signal area in the front and the edge was
still clear. (B) The lesion showed hyperintensity on the T2WI
sequence, an uneven internal signal and a patchy obviously
hyperintense area in the front. (C) The fat-suppressed T2 sequence
of the lesion showed high signal intensity, and a patchy higher
signal region was seen around it with an unclear edge. (D) The
sagittal position of the T2WI sequence showed clear margin of the
lesion and surrounding edema zone. (E) The lesion showed an annular
DWI sequence with high signal intensity. (F) ADC diagram of the
lesion showed annular low signal. (G) Annular enhancement of the
marginal zone was visible after an enhanced scan, involving the
posterior horn of the left lateral ventricle, and the midline was
deviated to the right. (H) Sagittal and (I) coronal images showed
the edge of the lesion annular enhancement, and the left lateral
ventricle was compressed and invaded. T1WI, T1-weighted imaging;
T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging; ADC,
apparent diffusion coefficient. |
Pathological findings
Macro-examination
The excised brain parenchyma (mesial temporal lobe)
revealed a gray mass measuring 2×2×1 cm. Similarly, a gray mass
measuring 2×2×1 cm was obtained from the paraventricular region.
The tissue specimens were fixed in 4% neutral formalin at room
temperature for 48 h, followed by dehydration with alcohol and
xylene. Subsequently, the specimens were embedded in paraffin at
62°C and cooled. Serial sections (4 µm) were then prepared and
stained with hematoxylin (~5%) for 5 min, followed by eosin (~1%)
staining for 2 min at room temperature. Immunohistochemical
staining and gene detection were also performed using the
aforementioned paraffin-embedded tissue.
Microscopic observation
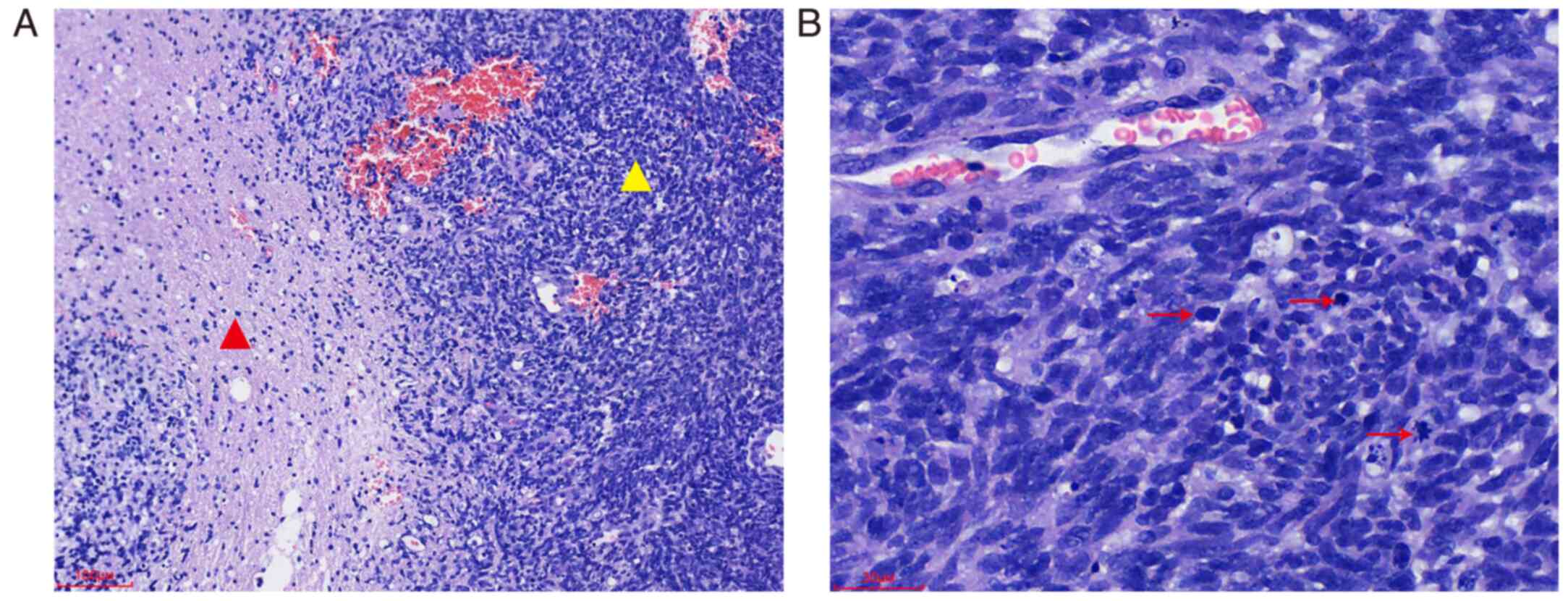
Hematoxylin and eosin (H&E) and
immunohistochemical staining were examined using an Olympus BX53
light microscope (Olympus Corporation). H&E staining showed a
slight to moderate increase in the density of glial cells compared
with normal tissue. The nuclei exhibited similar size and
morphology, displaying moderate atypia. They appeared round,
quasi-round or short spindle-shaped, with fine chromatin and either
absent or small nucleoli. Mitotic figures were infrequent. The PNC
consisted of closely arranged small round or short spindle-shaped
cells, exhibiting a flowing water-like pattern and abundant blood
vessels. The cells surrounding the necrotic areas were arranged in
a palisade manner, characterized by strongly stained nuclei and
increased mitotic figures (Fig.
2).
Immunohistochemical staining (Fig. 3) was performed overnight at 4°C
using the following primary antibodies (prediluted by the
manufacturer, Guangzhou Anbiping Pharmaceutical Technology Co.,
Ltd.): Anti-glial fibrillary acidic protein (GFAP; cat. no. IR086),
anti-p53 (cat. no. IM123), anti-CD56 (cat. no. IR040),
anti-synaptin (Syn; cat. no. IM136), anti-neuron-specific enolase
(NSE; cat. no. IM347), anti-vimentin (cat. no. IM142), anti-Ki-67
(cat. no. IR098), anti-thyroid transcription factor 1 (TTF-1; cat.
no. IM301), anti-pan-cytokeratin (pan-CK; cat. no. IM067) and
anti-chromogranin A (CgA; cat. no. IM053). For
immunohistochemistry, tissue sections (3 µm) were fixed in 4%
formalin at room temperature for 48 h before being in embedded in
paraffin. These sections were then rehydrated in a descending
alcohol series (xylene, 100% ethanol, 95% ethanol, 85% ethanol,
ethanol-free water) and underwent antigen retrieval using EDTA
antigen retrieval treatment (EnVision FLEX Target Retrieval
Solution, High pH; cat. no. K8000; Agilent Technologies, Inc.) in a
microwave on high heat for 2 min, followed by incubation at room
temperature for 8 min. Endogenous peroxidase activity was quenched
with 3% hydrogen peroxide in methanol before incubation with
primary antibodies. The secondary antibody was obtained from the
EnVision FLEX/HRP (prediluted by the manufacturer; cat. no. K8000;
Agilent Technologies, Inc.) and was used to treat sections at room
temperature for 25 min. Subsequently, a chromogen detection reagent
was applied (EnVision FLEX DAB+ Chromogen; cat. no. K8000, Agilent
Technologies, Inc.). The immunohistochemical staining showed strong
expression of GFAP, and expression of p53, vimentin and Ki-67 (15%)
in the glial component. Strong expression of Syn, p53, NSE, Ki-67
(90%) and TTF-1 was observed in the PNC. Additionally, CD56 was
observed in both components, whereas pan-CK and CgA were not
expressed.
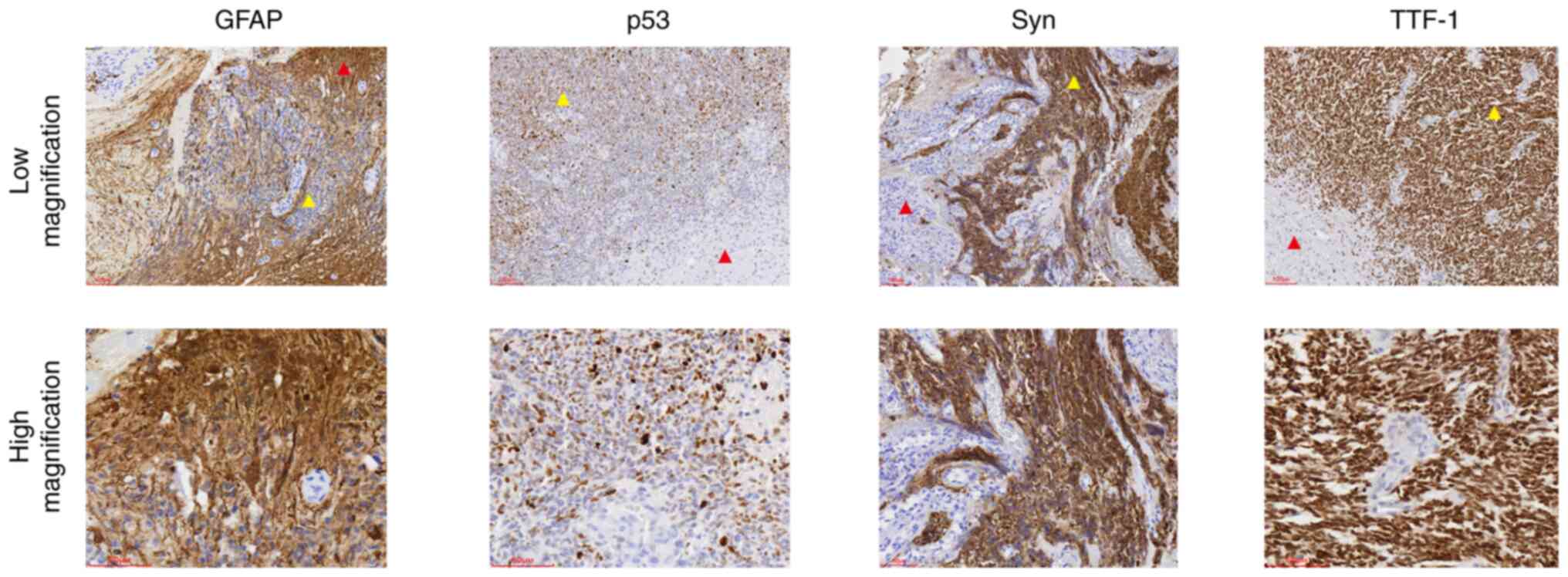 | Figure 3.Immunohistochemical staining of
glioblastoma with a PNC. The glial components exhibited staining
for GFAP, weak expression of p53, and negative staining for Syn and
TTF-1. The PNCs component exhibited negative staining for GFAP, and
strong staining of p53, Syn and TTF-1; red triangles indicate the
glial component, yellow triangles indicate the PNC component (low
magnification, ×100; scale bar, 100 µm; high magnification, ×200;
scale bar, 60 µm). GFAP, glial fibrillary acidic protein; PNC,
primary neuronal component; Syn, synaptin; TTF-1, thyroid
transcription factor 1. |
Pathological diagnosis
The patient was diagnosed with GBM-PNC in both the
brain parenchyma and paraventricular regions.
Gene detection
Gene detection (Sanger sequencing) was conducted on
selected paraffin-embedded tissue sections at Di'An Diagnostics
Group Co., Ltd. The results indicated the absence of mutations in
the isocitrate dehydrogenase (IDH)1 and IDH2 genes. Additionally,
no mutations were detected in the neurotrophic tyrosine kinase
receptor-1 (NTRK1), NTRK2 and NTRK3 genes using fluorescence in
situ hybridization, as determined by Di'An Diagnostics Group
Co., Ltd (Fig. S1).
Follow-up
Following discharge, the patient opted to continue
chemoradiotherapy treatment at a local hospital and declined our
request for further follow-up.
Discussion
In the WHO classification of tumors of the central
nervous system (2016), GBM can be divided into IDH-wildtype,
IDH-mutant, not otherwise specified GBM and GBM-PNC (6,7).
GBM-PNC is a very rare type of GBM, characterized by the presence
of a PNC within a glioma. The origin of GBM-PNC remains
controversial, and possible explanations include: i) The
development of a differentiated glial tumor from preexisting
neuronal cells, ii) the phenomenon of neuronal metaplasia or
dedifferentiation of astrocytic components into neuronal cells,
iii) collision tumors with two different clonal expansions, and iv)
the development of two components from normal stem cells (8). Donabedian et al (3) suggested that, due to the complexity of
GBM-PNC pathogenesis, there may be multiple pathways leading to the
occurrence of this disease.
The etiology of GBM-PNC remains unclear. Vollmer
et al (9) reported a case
with a previous history of leukemia, suggesting a potentially
increased risk of brain cancer due to therapeutic ionizing brain
radiation exposure. Among the patients reported by Donabedian et
al (3), one patient had a
sister who died from an unknown type of brain cancer; however, it
is unclear if there was a hereditary connection in that case. In
the present study, the patient had a history of massive bleeding
and diabetes; however, any associations with the development of
GBM-PNC are yet to be determined.
GBM-PNC typically presents with common clinical
manifestations observed in brain tumors, such as limb numbness,
headache, nausea and vomiting. Additionally, it may result in other
symptoms, such as hypoesthesia, transient amnesia, hemiplegia and
epilepsy. Limited information is available regarding the imaging
features of GBM-PNC. Valbuena et al (4) reported that the MRI radiological
features of this tumor typically show a heterogeneous T2-weighted
mass and a well-circumscribed lesion with gadolinium enhancement on
T1-weighted imaging. These features are often accompanied by
vasogenic edema. Heterogeneous enhancement associated with central
necrosis, cyst formation and tumoral hemorrhage may also be
observed. Other imaging findings are nonspecific, mainly showing a
cystic and solid intracranial mass compressing surrounding normal
brain tissue. Intra-cystic hemorrhage, edema, calcification and
necrosis may occur. In the present case report, the cystic portion
of the mass contained yellow purulent fluid, which, to the best of
our knowledge, is the first reported case of purulent fluid instead
of blood or hemorrhagic fluid, which could be mistaken for a brain
abscess.
Pathological examination of the PNC is recommended
in patients with intratumoral hemorrhage in GBM (10). In the present case report, a
laboratory examination showed an increase in LDH levels. Tan et
al (8) also reported elevated
LDH levels in their patient. Valvona et al (11) suggested that the interaction between
LDHA and IDH may influence patient prognosis and has been
associated with glioma. Therefore, it was hypothesized that LDH
detection may be a potential diagnostic indicator for GBM-PNC.
Histologically, GBM-PNC is characterized by two
distinct components. The glial component consists of astrocytes
with abundant eosinophilic cytoplasm, large vacuolar nuclei with
mitotic figures, necrosis and microvascular proliferation. The PNC
exhibits high cell density, reduced cytoplasm, hyperchromatic
nuclei and the formation of Homer-Wright chrysanthemum clusters.
There is a clear transition between the two components.
In line with the present findings, a review of the
current literature (Table SII)
(3–6,8–10,12–31)
indicated that the immunohistochemical profile of GBM-PNC is
diverse but generally consistent. The glial component typically
shows expression of GFAP, S-100, oligodendrocyte lineage
transcription factor 2 and vimentin. The Ki-67 proliferation index
ranges from 10 to 20%, and the expression rate of p53 is often
lower than that detected in the PNC. The expression of Syn,
neurofilament protein and NSE in the PNC suggests neural
differentiation, with a Ki-67 proliferation index of up to 90% and
high expression of p53. In both components, CD56 shows positive
staining. These findings demonstrate that immunohistochemistry may
have an accurate and feasible role in the diagnosis of GBM-PNC.
In the present case report, TTF-1 showed
immunopositivity in the PNC and negative staining in the glial
component. Previous studies have suggested that not all TTF-1
clones are equally applicable for diagnosing GBM-PNC (32,33).
Since TTF-1 is commonly used to identify metastatic tumors
originating from the lung or thyroid, different clones of TTF-1 may
lead to incorrect diagnoses. Therefore, if GBM-PNC is suspected, it
is recommended that the aforementioned panel of immunohistochemical
markers is used.
GBM-PNC requires a differential diagnosis from
papillary glioneuronal tumors (34)
and glioneuronal tumors with neuropil-like islands (35). Papillary glioneuronal tumors have
neuropil-like islands and are characterized as low-grade biphasic
tumors with astrocytic and neural differentiation. These tumors
commonly occur in young individuals. The glial component forms a
pseudopapillary structure surrounding the blood vessels, whereas
the neuronal component is located between the glial regions and
consists of nerve cells in an oligodendrocyte-like morphology with
very rare necrosis and mitotic figures. In the case of glioneuronal
tumors with neuropil-like islands, the histological morphology is
characterized by a background of infiltrating astrocytomas with
islands of neuropil-like tissue surrounded by oligodendrocyte-like
or atypical neuron-like cells.
There is currently no standardized treatment
approach for GBM-PNC. The most common treatment method involves
surgery followed by postoperative radiotherapy and chemotherapy.
Temozolomide (TMZ) is commonly used for GBM treatment, whereas
platinum-based chemotherapy is effective against peripheral
neuroectodermal tumors (6). GBM-PNC
tends to metastasize through the cerebrospinal fluid, with the
metastasizing component typically being the PNC, highlighting the
heterogeneity of the tumor (3,9).
Although metastases of GBM-PNC are rare, they can occur in the
spine (9), lungs (13) and throughout the skeletal system
(10). GBM-PNC metastases may be
more common than currently reported as the identification of
metastases may be rare due to the poor prognosis and short survival
time of patients. The short survival time may often prevent
metastatic tumors from causing noticeable symptoms before the death
of the patient. Therefore, patients with metastatic symptoms may
have a relatively better prognosis as their metastatic tumors have
had time to grow to a noticeable size (9).
In the present case report, the paraffin-embedded
tumor sample underwent IDH and NTRK gene detection. IDH1/2
mutations are a defining factor in the diagnosis of adult-type
diffuse glioma. There are three isoforms of IDH: IDH1, IDH2 and
IDH3. The IDH1/2 mutation is common in secondary GBM, accounting
for 73% of clinical cases, whereas it is rare in primary GBM,
occurring in only 3.7% of cases (36). It is generally considered that only
IDH1 and IDH2 can mutate in GBM, typically at arginine 132 in IDH1
(p.R132H) and arginine 172 in IDH2 (p.R172K). Mutations in either
IDH1 or IDH2 provide a growth advantage for mutant cells, and only
a single mutation is needed to mediate this advantage. Notably, few
studies have suggested a role for IDH3 in the occurrence and
development of GBM (36–40).
According to the 2021 WHO classification of central
nervous system tumors, the grading of diffuse gliomas relies not
only on the histological appearance but also on genetic parameters
(41). The IDH mutation is a key
factor in diagnosis, and is used to guide eligibility for glioma
therapy and clinical trials (42).
IDH1/2-mutant GBM generally has a better prognosis than wildtype
GBM, although the exact reason for this is unclear. The extent and
targets of IDH mutations that lead to genomic hypermethylation vary
greatly depending on the cellular context. This variability may
explain why IDH mutations are only favorable prognostic markers in
certain gliomas (43).
The NTRK gene family, which includes NTRK1, NTRK2
and NTRK3, encodes the tropomyosin receptor kinase (TRK) protein
family (TRKA, TRKB and TRKC). The TRK pathway has been implicated
in the pathogenesis of a number of types of cancer. Chromosomal
rearrangements resulting in oncogenic gene fusion, protein
overexpression and single nucleotide variants are the most commonly
described alterations associated with the NTRK gene family
(44). In the present case report,
NTRK gene detection was detected on the tumor tissue due to the
involvement of the NTRK gene family in neuronal development,
maintenance and protection, and its tendency to undergo fusions in
rare diseases such as GBM (45).
However, no fusion was detected in the NTRK1, NTRK2 or NTRK3 genes.
To the best of our knowledge, no study has linked NTRK to the
prognosis of GBM-PNC. However, Pekova et al (46) demonstrated that NTRK1
fusion-positive carcinomas are significantly associated with a
higher incidence of tumor multifocality and distant metastases in
thyroid carcinoma. Therefore, further studies on the relationship
between NTRK and GBM-PNC are warranted.
With the current emphasis on genetic testing in
disease research, there is an increasing interest in exploring the
genetic aspects of GBM-PNC. Recent case reports on GBM-PNC were
compiled and several genes that have been relatively well-studied
were identified (Tables I and
SII). The findings indicated that
85.7% of patients had wildtype IDH, 20.0% had wildtype tumor
protein 53 (TP53), 75% had wildtype ATRX chromatin remodeler, 25.0%
had wildtype epidermal growth factor receptor and 75.0% had
wildtype B-Raf proto-oncogene, serine/threonine kinase. Further
investigation into these genes may provide insights into the
characteristics of GBM-PNC. Moreover, Xu et al (47) reported that the mutation frequencies
of TP53, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic
subunit α, phosphoinositide-3-kinase regulatory subunit 1 and
phosphatase and tensin homolog in GBM-PNC were significantly higher
compared with those in GBM, suggesting that GBM-PNC represents a
distinct and rare variant of GBM.
 | Table I.Literature review of glioblastoma
with a primitive neuronal component (3–6,8–10,12–31). |
Table I.
Literature review of glioblastoma
with a primitive neuronal component (3–6,8–10,12–31).
| Feature | Value |
|---|
| Age (n=30) |
|
|
Range | 3 months-81
years |
|
Mean | 42.7 years |
|
Median | 47 years |
| Sex (n=30) |
|
|
Male | 17 (56.7%) |
|
Female | 13 (43.3%) |
| IDH status
(n=21) |
|
|
Mutant | 3 (14.3%) |
|
Wildtype | 18 (85.7%) |
| TP53 status
(n=15) |
|
|
Mutant | 12 (80.0%) |
|
Wildtype | 3 (20.0%) |
| ATRX status
(n=8) |
|
|
Mutant | 2 (25.0%) |
|
Wildtype | 6 (75.0%) |
| EGFR status
(n=4) |
|
|
Mutant | 3 (75.0%) |
|
Wildtype | 1 (25.0%) |
| BRAF status
(n=4) |
|
|
Mutant | 1 (25.0%) |
|
Wildtype | 3 (75.0%) |
It has been reported that epigenetics can influence
the survival and prognosis of patients with GBM (48). One commonly used method in GBM
diagnosis is the detection of methylation of the
O6-methylguanine-DNA methyltransferase (MGMT) promoter.
Hypermethylation of the MGMT promoter is associated with an
improved response to TMZ, leading to better patient outcomes
(49). Suwala et al
(32) suggested that GBM-PNC has a
unique methylation profile that is similar to IDH-wildtype GBM.
Therefore, treatment with TMZ and prognosis may also follow a
similar approach as IDH-wildtype GBM. However, due to the limited
number of patients, further extensive studies are still
required.
Separately evaluating the glioma component and the
PNC is important, in order to further study the biological
characteristics of PNC without being affected by the glioma
component; however, certain limitations hindered us from conducting
these tests. Firstly, the paraffin-embedded tissue samples
contained numerous blood clots, leaving limited tissue available
for additional immunohistochemistry and gene testing. Sufficient
tissue needed to be preserved for potential future tests that the
patient may require. Secondly, visually distinguishing the glioma
component from the PNC is challenging and the two components are
intricately intertwined under the microscope. This makes it
difficult to ensure complete separation and individual detection,
thereby compromising the accuracy of the results. Therefore, we
decided not to conduct other gene tests or MGMT methylation
tests.
In addition to the rarity and diverse clinical
pathological features of GBM-PNC, the present study discussed the
differential diagnosis from other similar tumor types, such as
small cell GBM and papillary glioneuronal tumors. Genetic and
epigenetic factors are important in GBM-PNC, including mutations in
genes such as IDH1/2 and NTRK1/2/3 and the potential influence of
epigenetics on patient prognosis. The current treatment approach
involves surgery, radiotherapy and chemotherapy; however, its
effectiveness is limited. Therefore, further research and a better
understanding of GBM-PNC are crucial for improving patient survival
and developing more effective treatment strategies.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mr. Ning Meng
(Department of Neurosurgery, Sunshine Union Hospital, Weifang,
China) for their meticulous operation on the patient and for
providing the tissue, and Mr. Xinxing Zhang (Imaging Center,
Sunshine Union Hospital) for the MRI images.
Funding
The present study was supported by the Research Projects of
Weifang Municipal Health Committee (grant no. WFWSJK-2022-239) and
the Sunshine Union Hospital Research Project (grant no.
2022YGRH043).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QM and XY drafted the manuscript and conceived the
study. QM, LML, NS, YC, LL, LG and WG performed the research and
analyzed the data. QM wrote the manuscript. XY and NS revised the
manuscript. XY and WG confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Sunshine Union Hospital (approval no. 2023-01-0003).
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thakur A, Faujdar C, Sharma R, Sharma S,
Malik B, Nepali K and Liou JP: Glioblastoma: Current status,
emerging targets, and recent advances. J Med Chem. 65:8596–8685.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gritsch S, Batchelor TT and Castro LN:
Diagnostic, therapeutic, and prognostic implications of the 2021
World Health Organization classification of tumors of the central
nervous system. Cancer. 128:47–58. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donabedian P, Tuna I, Rahman M, Gregory J,
Kresak J and Rees JH: Glioblastoma with a primitive neuroectodermal
component: Two cases with implications for glioblastoma
cell-of-origin. Clin Imaging. 73:139–145. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valbuena S, Ortega A, Centeno M and Rimbau
JM: Glioblastoma multiform with primitive neuronal component,
radiological and histology features: A case report. Egypt J
Neurosurg. 36:372021. View Article : Google Scholar
|
|
5
|
Poyuran R, Chandrasekharan K, Easwer HV
and Narasimhaiah D: Glioblastoma with primitive neuronal component:
An immunohistochemical study and review of literature. J Clin
Neurosci. 93:130–136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shayganfar A, Ebrahimian S, Mahzouni P,
Shirani F and Aalinezhad M: A review of glioblastoma tumors with
primitive neuronal component and a case report of a patient with
this uncommon tumor at a rare location. Clin Case Rep. 8:2600–2604.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banan R and Hartmann C: The new WHO 2016
classification of brain tumors-what neurosurgeons need to know.
Acta Neurochir (Wien). 159:403–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan CH, Phung TB and Xenos C: Glioblastoma
with primitive neuronal pattern in a girl aged 3 months: A rare
diagnosis at an unusual age. BMJ Case Rep. 2017:bcr20162186172017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vollmer K, Pantazis G, Añon J, Roelcke U
and Schwyzer L: Spinal metastases of supratentorial glioblastoma
with primitive neuronal component. World Neurosurg X. 2:1000192019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rong T, Zou W, Qiu X, Cui W, Zhang D, Wu
B, Kang Z, Li W and Liu B: A rare manifestation of a presumed
non-osteophilic brain neoplasm: Extensive axial skeletal metastases
from glioblastoma with primitive neuronal components. Front Oncol.
11:7606972021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valvona CJ, Fillmore HL, Nunn PB and
Pilkington GJ: The regulation and function of lactate dehydrogenase
a: Therapeutic potential in brain tumor. Brain Pathol. 26:3–17.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaidya MM, Parikh RC, Dhake RD, Vaidya SU
and Mahajan SD: Secondary glioblastoma with primitive neuronal
component. Neurol India. 70:459–461. 2022.PubMed/NCBI
|
|
13
|
Tamai S, Kinoshita M, Sabit H, Furuta T,
Miyashita K, Yoshimura K, Homma T, Harada K and Nakada M: Case of
metastatic glioblastoma with primitive neuronal component to the
lung. Neuropathology. 39:218–223. 2019.PubMed/NCBI
|
|
14
|
Sánchez-Ortega JF, Aguas-Valiente J,
Sota-Ochoa P and Calatayud-Pérez J: Glioblastoma with primitive
neuronal component: A case report and considerations of
fluorescence-guided surgery. Surg Neurol Int. 11:1782020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kay MD, Pariury HE, Perry A, Winegar BA
and Kuo PH: Extracranial metastases from glioblastoma with
primitive neuronal components on FDG PET/CT. Clin Nucl Med.
45:e162–e164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Georgiu C, MihuŢ E, Raus I, Mirescu ŞC,
Szabo L and Şovrea AS: Pediatric glioblastoma with giant cells and
‘supratentorial’ primitive neuroectodermal component-case report
and review of the literature. Rom J Morphol Embryol. 56:1165–1171.
2015.PubMed/NCBI
|
|
17
|
Chen YT, Hsu SS, Yip CM, Lai PH and Lee
HP: Glioblastoma with both oligodendroglioma and primitive
neuroectodermal tumor-like components in a case with 9-year
survival. Case Rep Surg. 2018:13826802018.PubMed/NCBI
|
|
18
|
Hendrych M, Solar P, Hermanova M, Slaby O,
Valekova H, Vecera M, Kopkova A, Mackerle Z, Kazda T, Pospisil P,
et al: Spinal metastasis in a patient with supratentorial
glioblastoma with primitive neuronal component: A case report with
clinical and molecular evaluation. Diagnostics (Basel). 13:1812023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piña-Oviedo S, De León-Bojorge B,
Cuesta-Mejías T, White MK, Ortiz-Hidalgo C, Khalili K and Valle LD:
Glioblastoma multiforme with small cell neuronal-like component:
Association with human neurotropic JC virus. Acta Neuropathol.
111:388–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maekawa K, Tokumitsu T, Noguchi H,
Nakamura E, Gi T, Horinouchi S, Yamashita S, Takeshima H, Asada Y
and Sato Y: Glioblastoma mimicking metastatic small cell carcinoma:
A case report with ultrastructural findings. Diagn Cytopathol.
49:E291–E296. 2021. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le BH and Sandusky M: 81 year-old male
with confusion and weakness. Brain Pathol. 20:867–870. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kandemir NO, Bahadir B, Gül S, Karadayi N
and Ozdamar SO: Glioblastoma with primitive neuroectodermal
tumor-like features: Case report. Turk Neurosurg. 19:260–264.
2009.PubMed/NCBI
|
|
23
|
Forbes V and Vredenburgh J: Primitive
neuroectodermal tumor with glioblastoma multiforme components in an
adult: A collision tumor. Cureus. 8:e4562016.PubMed/NCBI
|
|
24
|
Prelaj A, Rebuzzi SE, Caffarena G, Berrìos
JR, Pecorari S, Fusto C, Caporlingua A, Caporlingua F, Di Palma A,
Magliocca FM, et al: Therapeutic approach in glioblastoma
multiforme with primitive neuroectodermal tumor components: Case
report and review of the literature. Oncol Lett. 15:6641–6647.
2018.PubMed/NCBI
|
|
25
|
Karina A, Jonker BP, Morey A, Selinger C,
Gupta R and Buckland ME: Glioblastoma with primitive
neuroectodermal tumour-like components. Pathology. 44:270–273.
2012.PubMed/NCBI
|
|
26
|
Konar SK, Bir SC, Maiti TK, Patra DP,
Brahmbhatt AC, Jacobsohn JA and Nanda A: Early dural metastasis
from a case of glioblastoma with primitive neuroectodermal
differentiation: A case report and literature review. J Clin
Neurosci. 35:78–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ricard JA, Cramer SW, Charles R, Tommee
CG, Le A, Bell WR, Chen CC and Flanagan ME: Infratentorial
glioblastoma metastasis to bone. World Neurosurg. 131:90–94. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alvarado AM, Salacz ME and Chamoun RB:
Malignant glioma-primitive neuroectodermal tumor recurring as
PNET-like only subdural collection: Case report. Surg Neurol Int.
8:2432017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuhn SA, Hanisch UK, Ebmeier K, Beetz C,
Brodhun M, Reichart R, Ewald C, Deufel T and Kalff R: A paediatric
supratentorial primitive neuroectodermal tumour associated with
malignant astrocytic transformation and a clonal origin of both
components. Neurosurg Rev. 30:143–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wan KR, King NK, Low SY, Sitoh YY, Lee HY,
Wong CF and Ng WH: Synchronous multicentric glioblastoma with PNET
and O subtypes: Possible pathogenesis. Surg Neurol Int. 5:312014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Keisling MP, Samkari A, Halligan G,
Pascasio JM and Katsetos CD: Malignant glioma with primitive
neuroectodermal tumor-like component (MG-PNET): Novel microarray
findings in a pediatric patient. Clin Neuropathol. 35:353–367.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suwala AK, Stichel D, Schrimpf D, Maas
SLN, Sill M, Dohmen H, Banan R, Reinhardt A, Sievers P, Hinz F, et
al: Glioblastomas with primitive neuronal component harbor a
distinct methylation and copy-number profile with inactivation of
TP53, PTEN, and RB1. Acta Neuropathol. 142:179–189. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galloway M and Sim R: TTF-1 staining in
glioblastoma multiforme. Virchows Arch. 451:109–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Puzyrenko A, Cochran E, Giorgadze T and
Nomani L: Papillary glioneuronal tumors: Distinctive cytological
characteristics and cyto-histologic correlation. Ann Diagn Pathol.
53:1517572021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duan ZJ, Yao K and Qi XL: Glioneuronal
tumor with neuropil-like island: Report of four cases and review of
literature. Zhonghua Bing Li Xue Za Zhi. 45:324–328. 2016.(In
Chinese). PubMed/NCBI
|
|
36
|
Han S, Liu Y, Cai SJ, Qian M, Ding J,
Larion M, Gilbert MR and Yang C: IDH mutation in glioma: Molecular
mechanisms and potential therapeutic targets. Br J Cancer.
122:1580–1589. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller JJ: Targeting IDH-Mutant Glioma.
Neurotherapeutics. 19:1724–1732. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hartmann C, Meyer J, Balss J, Capper D,
Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, et
al: Type and frequency of IDH1 and IDH2 mutations are related to
astrocytic and oligodendroglial differentiation and age: A study of
1,010 diffuse gliomas. Acta Neuropathol. 118:469–474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yao J, Yi Y, Xia L and Wei L: Research
progres on that relationship between IDH1/2 gene mutation and
glioma. Chin J Neurosurg Dis. 16:81–83. 2017.
|
|
40
|
May JL, Kouri FM, Hurley LA, Liu J,
Tommasini-Ghelfi S, Ji Y, Gao P, Calvert AE, Lee A, Chandel NS, et
al: IDH3α regulates one-carbon metabolism in glioblastoma. Sci Adv.
5:eaat04562019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Chen H, Li G, Zhang J, Yao K, Wu C,
Li S and Qiu X: Radiotherapy delays malignant transformation and
prolongs survival in patients with IDH-mutant gliomas. Cancer Biol
Med. 19:1477–1486. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Unruh D, Zewde M, Buss A, Drumm MR, Tran
AN, Scholtens DM and Horbinski C: Methylation and transcription
patterns are distinct in IDH mutant gliomas compared to other IDH
mutant cancers. Sci Rep. 9:89462019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khotskaya YB, Holla VR, Farago AF, Shaw
KRM, Meric-Bernstam F and Hong DS: Targeting TRK family proteins in
cancer. Pharmacol Ther. 173:58–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gatalica Z, Xiu J, Swensen J and Vranic S:
Molecular characterization of cancers with NTRK gene fusions. Mod
Pathol. 32:147–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pekova B, Sykorova V, Mastnikova K,
Vaclavikova E, Moravcova J, Vlcek P, Lastuvka P, Taudy M, Katra R,
Bavor P, et al: NTRK fusion genes in thyroid carcinomas:
Clinicopathological characteristics and their impacts on prognosis.
Cancers (Basel). 13:19322021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu G, Zheng H and Li JY: Next-generation
whole exome sequencing of glioblastoma with a primitive neuronal
component. Brain Tumor Pathol. 36:129–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Verdugo E, Puerto I and Medina MÁ: An
update on the molecular biology of glioblastoma, with clinical
implications and progress in its treatment. Cancer Commun (Lond).
42:1083–1111. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cardia A, Epistolio S, Zaed I, Sahnane N,
Cerutti R, Cipriani D, Barizzi J, Spina P, Stefanini FM, Cerati M,
et al: Identification of MGMT downregulation induced by miRNA in
glioblastoma and possible effect on temozolomide sensitivity. J
Clin Med. 12:20612023. View Article : Google Scholar : PubMed/NCBI
|