Introduction
Lung cancer is a leading cause of cancer-related
death worldwide (1,2). Of note, ~85% of lung cancer cases have
been classified as non-small-cell lung cancer (NSCLC) (3), for which surgery and chemotherapy are
the primary treatment strategies. 5-Fluorouracil (5-FU) is one of
the most commonly used chemotherapeutic agents for patients with
NSCLC (4). Therapies affecting
cancer behaviors are important for patient outcomes, since the
5-year relative survival rate is as low as 6% for patients with
metastatic NSCLC (5). Furthermore,
the development of chemoresistance represents a major challenge for
the treatment of NSCLC (6,7).
Previous studies have suggested that local
anesthetics administered perioperatively can affect the outcome of
oncological surgeries (8,9). Lidocaine is a common local anesthetic
for regional nerve block, which has been reported to block
exogenous tumor necrosis factor (TNF)-α-induced increases in lung
cancer cell invasion (10,11) because of its anti-inflammatory
properties. In addition to its usage as a conventional
antiarrhythmic agent (12),
intravenous lidocaine can also be used to treat various types of
chronic pain (13) and other
specific conditions (14,15), such as refractory chronic daily
headaches (16). Furthermore,
lidocaine has been recommended as one of the main modalities in
enhanced recovery after surgery (ERAS) protocols, as it can block
the priming of polymorphonuclear granulocytes (17). However, at the serum concentrations
achieved by intravenous infusion, whether lidocaine can affect
epithelial-mesenchymal transition (EMT) and its accompanying
phenomena remain unclear.
Although high-dose lidocaine (2–8 mM) has been
validated to enhance cancer cell apoptosis, and to inhibit the
mitogen-activated protein pathway in the growth, migration and
invasion of lung cancer (18,19),
different phenomena can be observed in response to different scales
of lidocaine concentrations. Contrary to previous reports, our
preliminary data showed that lidocaine concentrations corresponding
to intravenous infusion in clinical scenarios (1–20 µM) did not
affect the proliferation of lung cancer cells. These render the
clinical effects of lidocaine on lung cancer questionable. For
lidocaine to be infused either intravenously or epidurally, the
concentrations to which tumors are directly exposed must be below
the toxic concentration [21 µM (5 µg/ml)] to be significant in
translational medicine.
The present study used the A549 cell line as a
common model of NSCLC (20,21) to investigate whether clinically
relevant concentrations of lidocaine could influence EMT and any
associated phenomena, including its impact on the effect of 5-FU in
lung cancer cells.
Materials and methods
Cell culture and drug treatment
Human NSCLC A549 cells (CCL-185) were purchased from
the American Type Culture Collection (ATCC). Cells were grown in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% heat-inactivated fetal
bovine serum (Corning, Inc.), and 1% penicillin and streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). Cells were cultured at
37°C in a humidified atmosphere containing 5% CO2. For
experimental purposes, cells cultured to the exponential growth
phase (~70% confluence) were used without serum starvation. With
the same culture conditions, the mouse lung cancer cell line LLC.LG
was obtained from the Agriculture Biotechnology Research Center
(Academia Sinica). A combination of 5-FU (MilliporeSigma) and
lidocaine (AstraZeneca) was used to determine whether these drugs
have synergistic or additive effects on the cells. The
concentration of 5-FU was the same as the one used to exert a 30%
inhibitory effect in the pilot study.
Cell viability and colony formation
assays
A sulforhodamine B (SRB) assay was used to measure
drug-induced cytotoxicity (22) and
cell proliferation (23). In brief,
A549 and LCC.LG cells were seeded in 96-well plates
(7×103/well) and incubated for 24 h. Subsequently, 5-FU
and lidocaine were each diluted in DMEM and cells were incubated at
37°C for 24 h. Following treatment with SRB, cell viability was
evaluated by measuring the absorbance at 570 nm in a 96-well
flat-bottomed plate reader. By comparing the absorbance in the
experimental wells with that in the control well, the percentage of
viable cells was determined.
For the colony formation assay, A549 cells were
seeded in 6-well plates (4×102/well) at 37°C in an
atmosphere containing 5% CO2. The cells were treated
with lidocaine (final concentrations: 10 and 20 µM), 5-FU (3.125
µM) or with their combinations. The colony-forming potential of
A549 cells after treatment was assessed on day 15. The cells were
fixed with 4% paraformaldehyde at room temperate for 30 min and
then stained with crystal violet (0.005%) for 30 min at room
temperate, followed by manually counting the number of
colonies.
Migration assay
A migration assay was performed in a Transwell
chamber with a pore size of 8.0 µm in a 24-well plate (Corning,
Inc.). A549 and LLC.LG cell density was adjusted to
1×106 cells/ml. With 100 µl cell suspension in
serum-free medium, cells were treated with different concentrations
of lidocaine (0, 1, 5 and 10 µM) with or without 5FU (0.0375, 3.125
µM) and placed in the upper chamber of the Transwell inserts.
Medium containing 10% FBS (500 µl) was added to the lower chamber.
After incubation at 37°C for 16 h, cell migration was evaluated by
counting the number of cells that penetrated the membrane. The
cells on the lower surface of the chamber membrane were fixed in
95% ethanol for 10 min at room temperature and the cells in the
internal compartment were removed with a cotton swab. After the
chamber was air-dried, the cells were stained with DAPI (1 µg/ml)
for 10 min at room temperature and five randomly selected fields of
view (magnification, ×100) were captured using a fluorescence
microscope for cell counting.
Anoikis-resistant cell aggregation
assay
A549 cells (2×103 cells/well) were
exposed to 10 µM lidocaine or an equal volume of DMEM on 6-well
plates coated with poly-2-hydroxyethyl methacrylate (poly-HEMA;
MilliporeSigma). Images of cell clusters growing in 6-well
poly-HEMA plates were captured on day 5. Thereafter, the medium was
removed from each well and the cells were harvested 1 day later (on
day 6). After anchoring the cell clusters on the 6-well plate
without poly-HEMA layer, the detached cells were removed. Before
being fixed with 4% formalin solution for 30 sec at room
temperature for counting, the cell clusters were stained with 0.01%
(w/v) crystal violet at room temperature for 60 min. The result of
the crystal violet staining for determining the viability of
cultured cells was used to estimate cell survival (24). The cells were visualized and counted
using an inverted microscope (CKX31; Olympus Corporation). The
number of cell clusters was scored using ImageJ software (version
1.48v; National Institutes of Health).
Western blot analysis
A549 cells were seeded in a 10 cm2 dish
and incubated in culture medium for 24 h. Thereafter, the cells
were treated with lidocaine (10 µM) or incubated with 20 ng/ml
TGF-β (R&D Systems, Inc.) at 37°C for 48 h to induce EMT. The
use of human TGF-β to elicit EMT in a mouse lung cancer cell line
is also feasible as previously described (25). Subsequently, cells were cultured to
logarithmic growth phase, and were collected and lysed in RIPA
Lysis and Extraction Buffer (cat. no. R0278-50ML; Sigma-Aldrich;
Merck KGaA). The protein concentration of total cell lysates was
accessed using the bicinchoninic acid method. A total of 30 µg
total proteins/lane were separated by SDS-PAGE on 10% gels (Bio-Rad
Laboratories, Inc.), then transferred to a PVDF membrane. The
membrane was blocked for 1 h at room temperature in TBST (10 mM
Tris, pH 7.5; 150 mM NaCl; 0.1% Tween 20) with 5% skim milk. After
incubation with the following primary antibodies for 1 h at room
temperature: E-cadherin (1:1,000 dilution; cat. no. 3195), vimentin
(1:1,000; cat. no. 5741), Slug (1:1,000; cat. no. 9585) and GAPDH
(1:1,000; cat. no. 2118) (all from Cell Signaling Technology,
Inc.), the membrane was washed three times in TBST (10 min/wash)
and then incubated with secondary antibodies, including anti-rabbit
(1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.) and
anti-mouse (1:5,000; cat no. 7076; Cell Signaling Technology,
Inc.,) in TBST at room temperature for 1 h. The membrane was
subsequently washed three times with TBST (10 min/wash) and the
bands were visualized using enhanced electrochemiluminescence (ECL
pierce kit; cat. no. 32109; Thermo Fisher Scientific, Inc.) to
analyze the expression of target proteins. ImageJ software (version
1.48v) was used for semi-quantification of the western blots and
all measurements were normalized against the GAPDH loading
control.
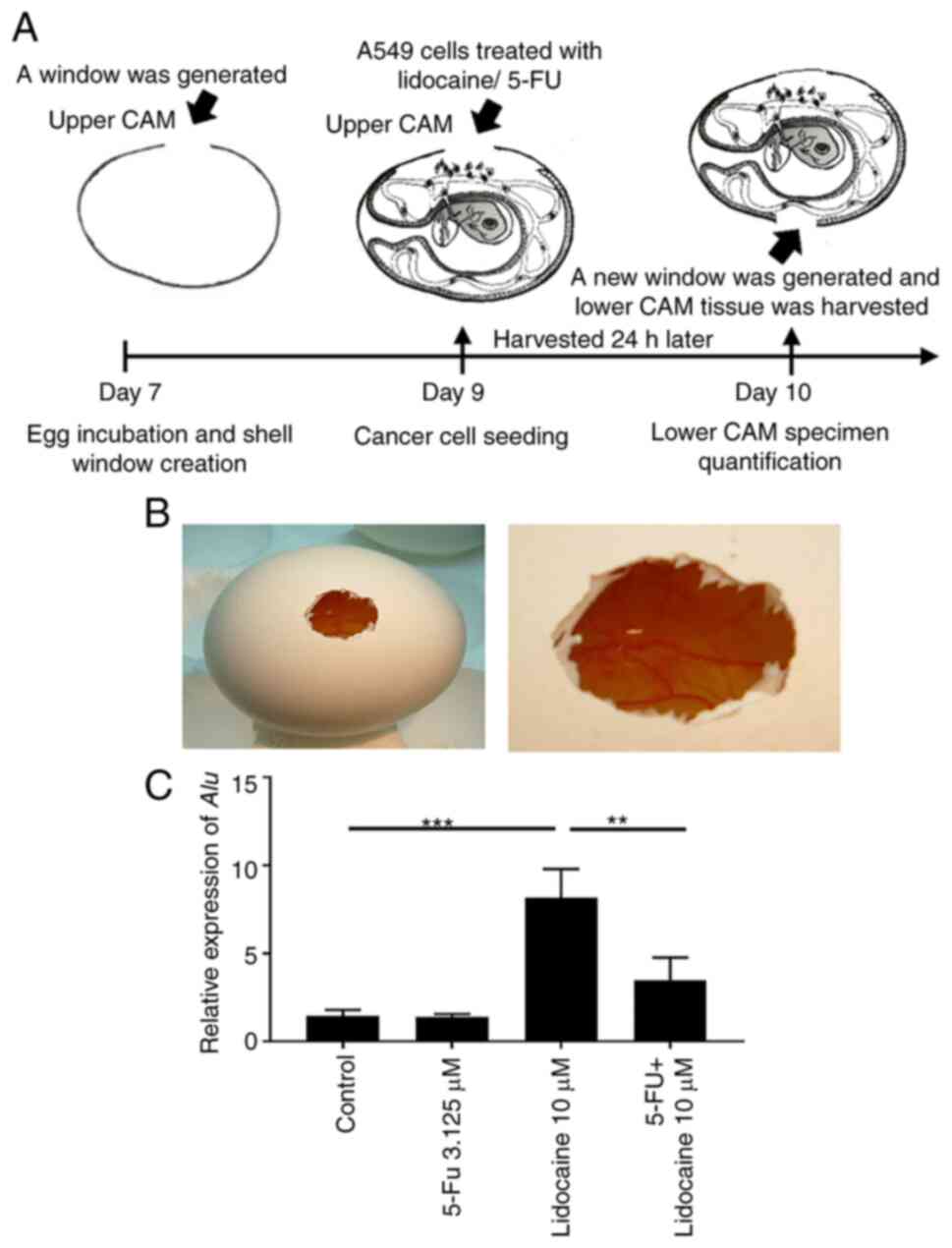
Chick chorioallantoic membrane (CAM)
assay
A total of 9 fertilized chicken eggs were obtained
from the Animal Health Research Institute, Council of Agriculture,
Executive Yuan, and were incubated at 37°C in an atmosphere
containing 80% relative humidity (26). All methods were carried out in
accordance with the relevant guidelines and regulations (The AVMA
Guidelines for the Euthanasia of Animals: 2013 Edition-September
19, 2013). A small window was made in the shell on day 7 of chick
embryo development under aseptic conditions. The eggs were returned
to the incubator immediately after resealing the window on day 7.
After 2 days, the eggs in the incubator were taken out for A549
administration into the upper CAM. Briefly, A549 suspensions
(1×106) were mixed with hydrogel (10 mg/ml) at a total
volume of 20 µl. Lidocaine (10 µM), 5-FU (3.125 µM) or both were
mixed together with the A549 cells and hydrogel. Hydrogel grafts
were placed on top of the CAM and eggs were resealed and returned
to the incubator for 24 h until day 10 (3 chicken embryos per
group). On day 10, the eggshell was cut and the lower CAM tissue
was harvested for DNA extraction (DNA extraction kit; cat. no.
TX-CD001; TOOLS) and human Alu sequences were quantified
using quantitative PCR (qPCR). Total RNA was extracted using an
RNeasy Mini Kit and treated with RNase-free DNase I set (Qiagen
GmbH) according to the manufacturer's protocol. Total RNA (1 µg)
was reverse-transcribed using oligo (dT) primers and a reverse
transcription system (Promega Corp.). Reactions were carried out
using Fast SYBR® Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) on the Step One Plus
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) by denaturation at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and 60°C for 40 sec. Melting curve analyses were
performed to verify the amplification specificity. Relative
quantification of gene expression was performed according to the
2−ΔΔCq method using StepOne Software 2.0 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) (27). The detection of human tumor cells is
based on the quantitative detection of human Alu sequences
present in chick DNA extracts, and is a modification of the method
developed by Kim et al (28). The design of the Alu primers
was performed as described in a previous study (29). To detect human cells, primers
specific for the human Alu sequences (sense:
5′-ACGCCTGTAATCCCAGCACTT-3′; and antisense:
5′-TCGCCCAGGCTGGAGTGCA-3′) were used to amplify the human
Alu repeats present in genomic DNA (28). A quantitative measure of amplifiable
chick DNA was obtained through amplification of the chick GAPDH
(chGAPDH) fragment with chGAPDH primers (sense:
5′-GAGGAAAGGTCGCCTGGTGGATCG-3′; antisense:
5′-GGTGAGGACAAGCAGTGAGGAACG-3′) using the same PCR conditions as
described for Alu. On day 10, freezing of the whole egg was
applied to end the experiments.
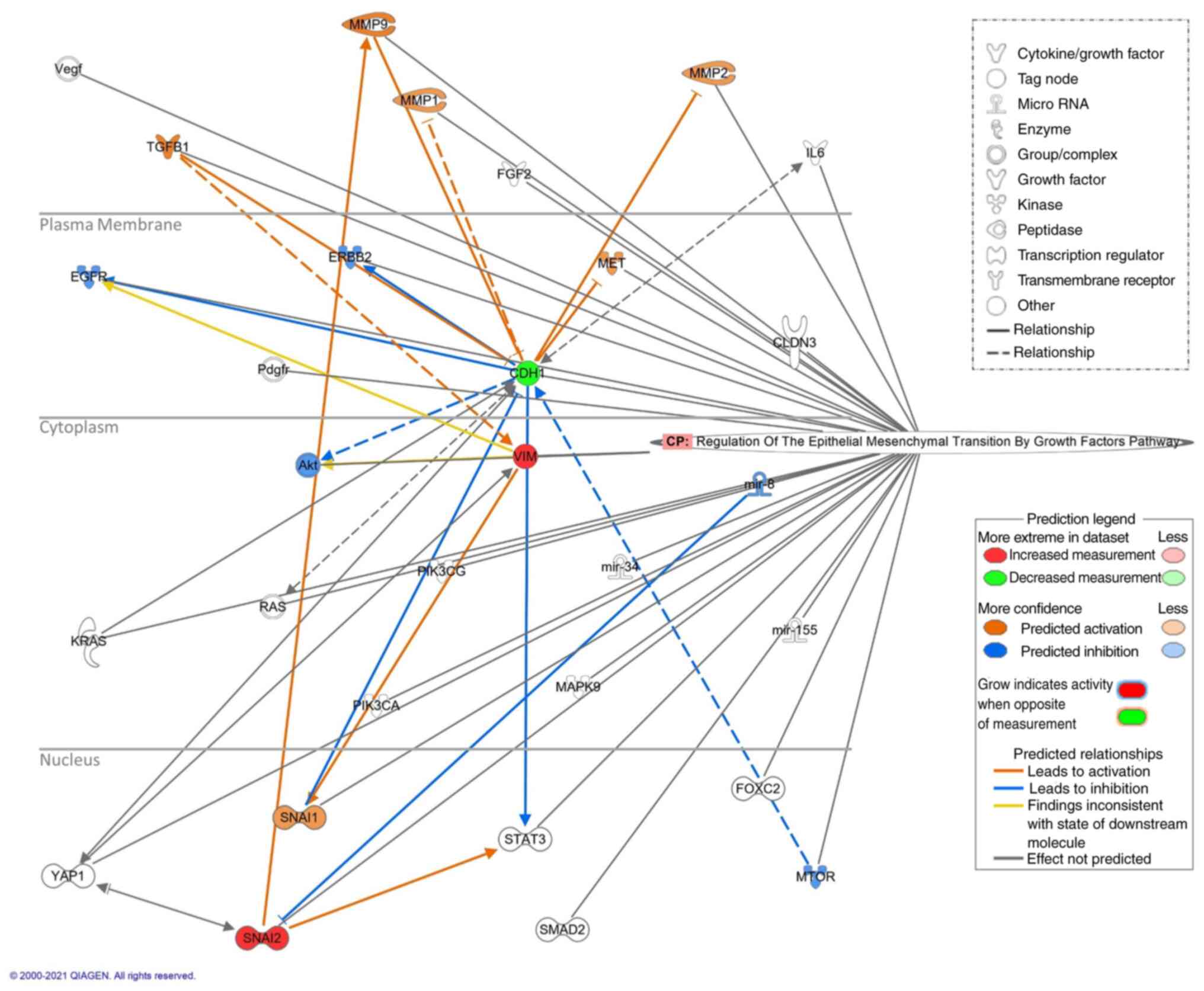
Ingenuity pathway analysis (IPA)
To build the conditioned metastasis pathway and to
systematically investigate the impact of lidocaine-altered EMT
proteins on all other related genes, the relevant networks were
generated using IPA (version 68752261; Qiagen GmbH). Focusing on
the most extensive pathway regarding metastasis in the ‘Diseases
and Functions’ of the IPA system, the pathway ‘Metastasis’ with
3,521 associated molecules was then restricted to ‘Human’, ‘Genes,
RNAs and Proteins’, ‘downregulation and upregulation’, and filtered
on ‘non-small cell lung carcinoma’ by inclusion (‘AND’) using the
BioProfiler function. The IPA overlay function within this
conditioned metastasis pathway further allowed the selection of
‘Regulation of the Epithelial Mesenchymal Transition by Growth
Factors Pathway’ as the canonical pathway to be displayed. A total
of 30 molecules were revealed to be involved in the final
conditioned pathway. To discover possible gene alterations involved
in NSCLC metastasis in terms of EMT, the path explorer tool of the
IPA system was used to identify all possible relationships between
the measured proteins (vimentin, Slug and E-cadherin) and the
remaining 27 molecules in this conditioned metastasis pathway. The
molecule activity predictor tool was then utilized to predict the
impact of the three measured proteins altered by lidocaine.
To further investigate the impact of measured
prototypical EMT markers (vimentin and E-cadherin) and their
molecular switch (Slug) on EMT (30), NSCLC and apoptosis of NSCLC, the
path explorer tool of the IPA system was used to discover the
impact of each molecule by limiting the relationships to
‘activation’, ‘causation’ and ‘inhibition’ via the filter function.
Based on the derived shortest paths and one more path beyond the
shortest path, the impact of the individual measured molecule was
predicted via the molecule activity predictor tool.
Statistical analysis
Data are presented as the mean ± SD. All data were
analyzed using GraphPad Prism version 7.0 software (Dotmatics).
Differences between multiple groups were analyzed using one-way
ANOVA for single variable analysis, followed by Tukey's
multiple-comparisons post-hoc test. Differences between two groups
were analyzed using Student's t-test after passing the Shapiro-Wilk
normality test. Mann-Whitney U-test was applied if the data failed
the normality test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of lidocaine and 5-FU on cell
survival
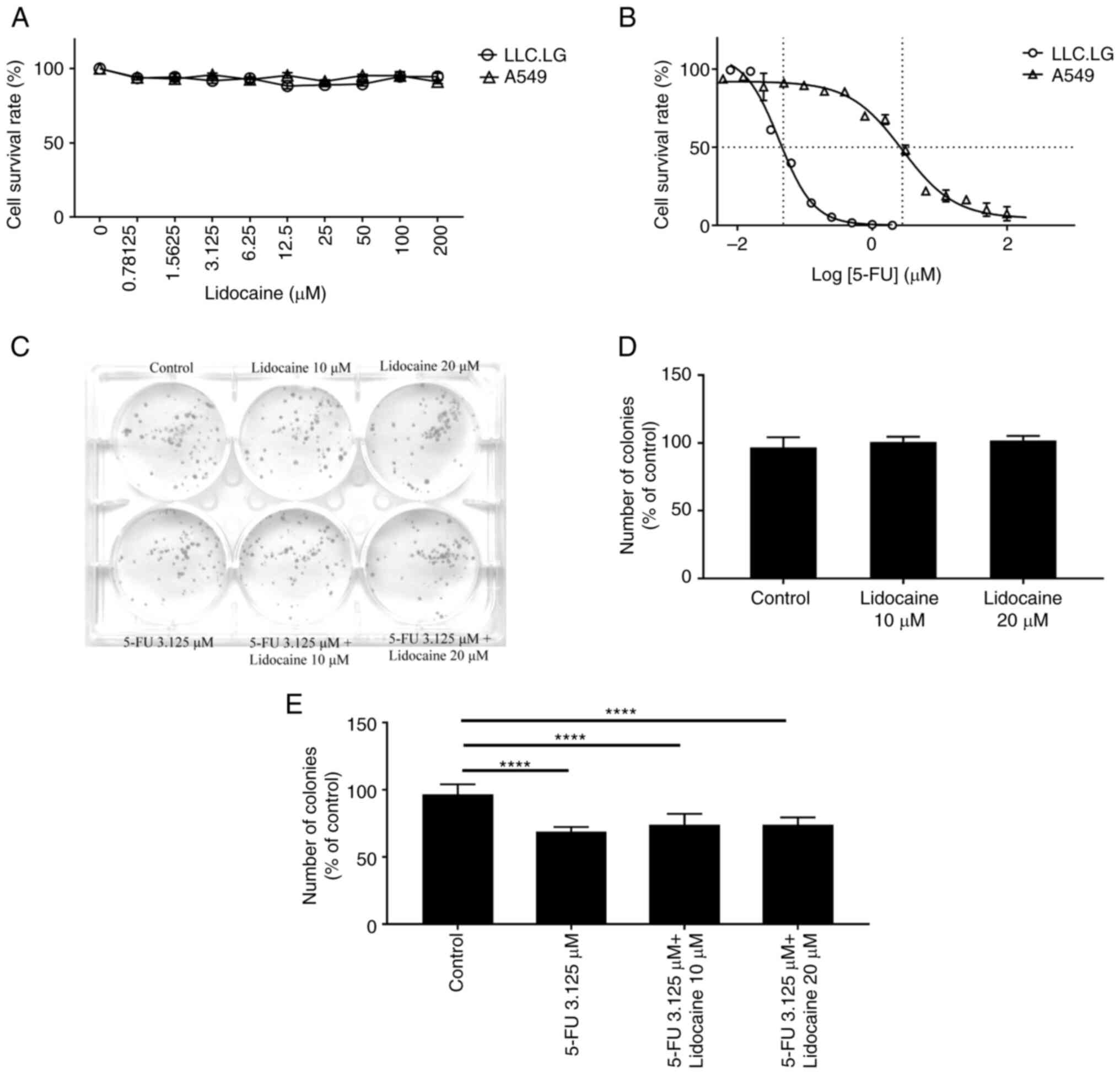
A549 and LLC.LG cells were treated with lidocaine
(0–200 µM) for 24 h. Lidocaine at various concentrations did not
reduce cell viability (Fig. 1A).
According to the SRB assay, the half-maximal inhibitory
concentration (IC50) of 5-FU for A549 and LLC.LG cells
was 2.808 and 0.042 µM, respectively (Fig. 1B). The 5-FU concentration resulting
in ~30% A549 and LLC.LG cell viability inhibition in the SRB assay
was 3.125 and 0.0375 µM (Fig. S1),
respectively. The number of colonies formed by A549 cells did not
differ significantly between cells treated with lidocaine and
untreated cells; however, it significantly differed between cells
treated with 5-FU and untreated cells (Fig. 1C-E). To more easily detect the
lidocaine's additive or synergistic effect on 5-FU's inhibition, we
chose a concentration of 5-FU that has a 30% inhibitory effect.
Although a majority of the range (~70%) was left in the 5-FU-based
inhibitory model to observe the expected synergistic or additive
effect exerted by lidocaine at moderate (10 µM) or high normal
(approaching clinically toxic level; 20 µM) concentrations, it was
revealed that lidocaine did not aggravate the cytotoxic effects of
5-FU.
Lidocaine does not influence the
effect of 5-FU on cell survival at clinically relevant
concentrations
According to the results of the colony formation
assay, lidocaine at a high normal concentration (approaching
clinically toxic level; 20 µM) did not aggravate the cytotoxic
effects of 5-FU. In the subsequent experiment, the present study
aimed to verify whether lidocaine at a range of clinically safe
concentrations (<20 µM) could influence the effect of 5-FU on
cell survival and to assess whether it had a dose-dependent effect.
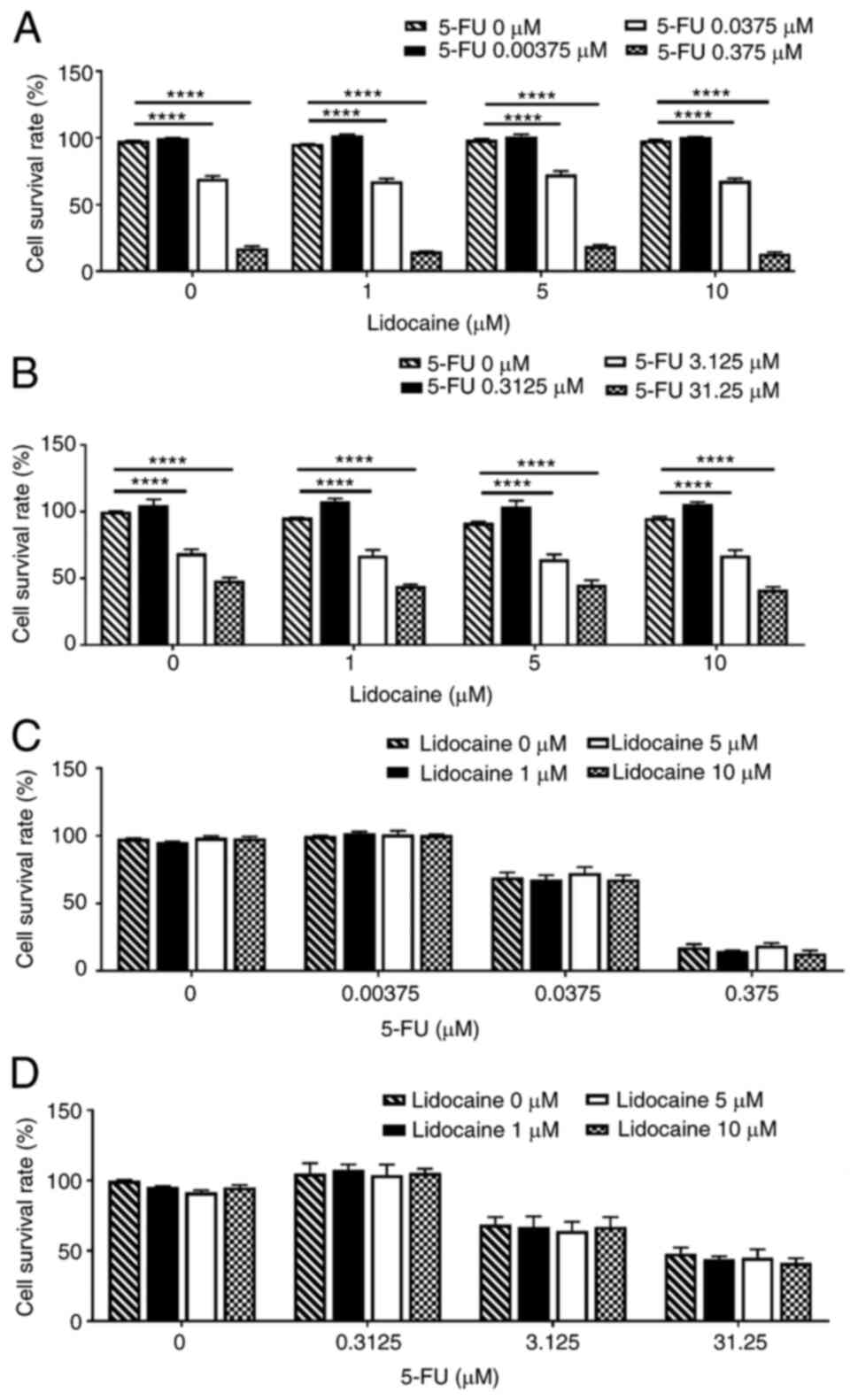
Therefore, lidocaine concentrations at 1, 5 and 10 µM were
selected. The LLC.LG cells exposed to 5-FU at 0–0.375 µM for 24 h
showed a dose-dependent reduction in viability (Fig. 2A). There was a positive association
between the increase in 5-FU dosage and decrease in LLC.LG cell
viability; however, the addition of lidocaine did not further
affect cell viability. Treatment of A549 cells with 5-FU at 0–31.25
µM for 24 h also resulted in a dose-dependent reduction in cell
viability (Fig. 2B). No
dose-dependency was found regarding the effect of lidocaine
(Fig. 2C and D). These findings
indicated that 5-FU directly reduced LLC.LG and A549 cell
viability, whereas treatment with lidocaine at clinically relevant
concentrations did not alter the effect of 5-FU on the viability of
either of the cell lines.
Lidocaine attenuates the 5-FU-induced
inhibitory effect on cell migration and promotes EMT at clinically
relevant concentrations
The results of the cell migration assay showed that
the number of migrated LLC.LG cells treated with 5-FU was lower
than that in the control group. However, 5-FU combined with 1 µM
lidocaine resulted in cell migration similar to that in the control
group (Fig. 3A), indicating that
lidocaine reversed the inhibitory effect exerted by 5-FU on cell
migration. Similar results were obtained in A549 cells (Fig. 3B). To further study the effects of
lidocaine on A549 cell migration, the epithelial marker E-cadherin
and the mesenchymal marker vimentin were assessed using western
blotting. Lidocaine and TGF-β (positive control) significantly
upregulated the expression levels of vimentin, and downregulated
the expression levels of E-cadherin, indicating that EMT was
induced (Fig. 3C and D). The
absence of an additive or synergistic effect from the combination
of lidocaine and TGF-β indicated that there may be a negative
feedback loop triggered by the combination of lidocaine and TGF-β,
or a negative interaction between their downstream effector
pathways. The expression of Slug, the molecular switch immediately
upstream of EMT in lung cancer (30), was also revealed to be upregulated
by lidocaine (Fig. 3E). The
aforementioned alterations in A549 cells indicated that EMT was
induced, at least in part, by lidocaine at clinically relevant
concentrations.
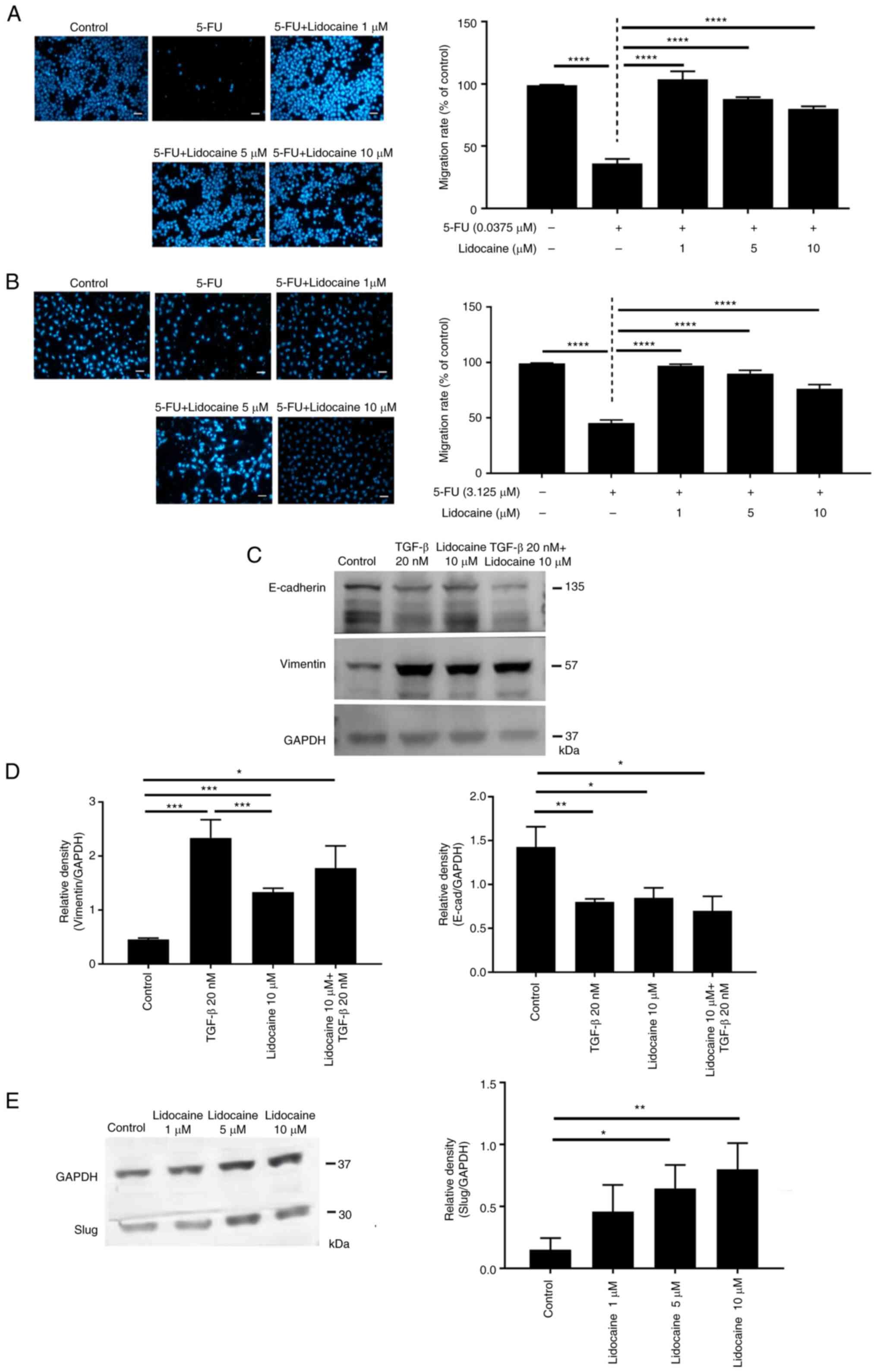 | Figure 3.Attenuation of the inhibitory effects
of 5-FU on cell migration by lidocaine. Combination effect in (A)
LLC.LG (n=6 for 5-FU 0.0375 µM group, n=7 for other groups) and (B)
A549 (n=4) cells. Cell migration is indicated by the number of
DAPI-stained nuclei. Medium group served as the control. Values are
expressed as the mean ± SD. ****P<0.0001. Scale bar, 100 µm. (C)
E-cadherin and vimentin expression. A549 cells were treated with 20
nM TGF-β and lidocaine for 48 h, and the expression levels of
epithelial and mesenchymal markers were determined using western
blotting. Naive group served as the control. (D)
Semi-quantification of vimentin and E-cadherin (n=3). Naive group
served as the control. (E) Upregulation of Slug expression with
lidocaine treatment (n=4). A549 cells were treated with different
concentrations of lidocaine for 48 h and the expression levels of
Slug, the molecular switch upstream of prototypical
epithelial-mesenchymal transition markers, were determined using
western blot analysis. The cropped blots for E-cadherin, vimentin,
Slug and GAPDH were grouped from different parts of the same gel
and the grouping was made explicit by using (C) the white spaces or
(E) cutting line. Values are expressed as the mean ± SD.
*P<0.05, **P<0.01, ***P<0.001. 5-FU, 5-fluorouracil. |
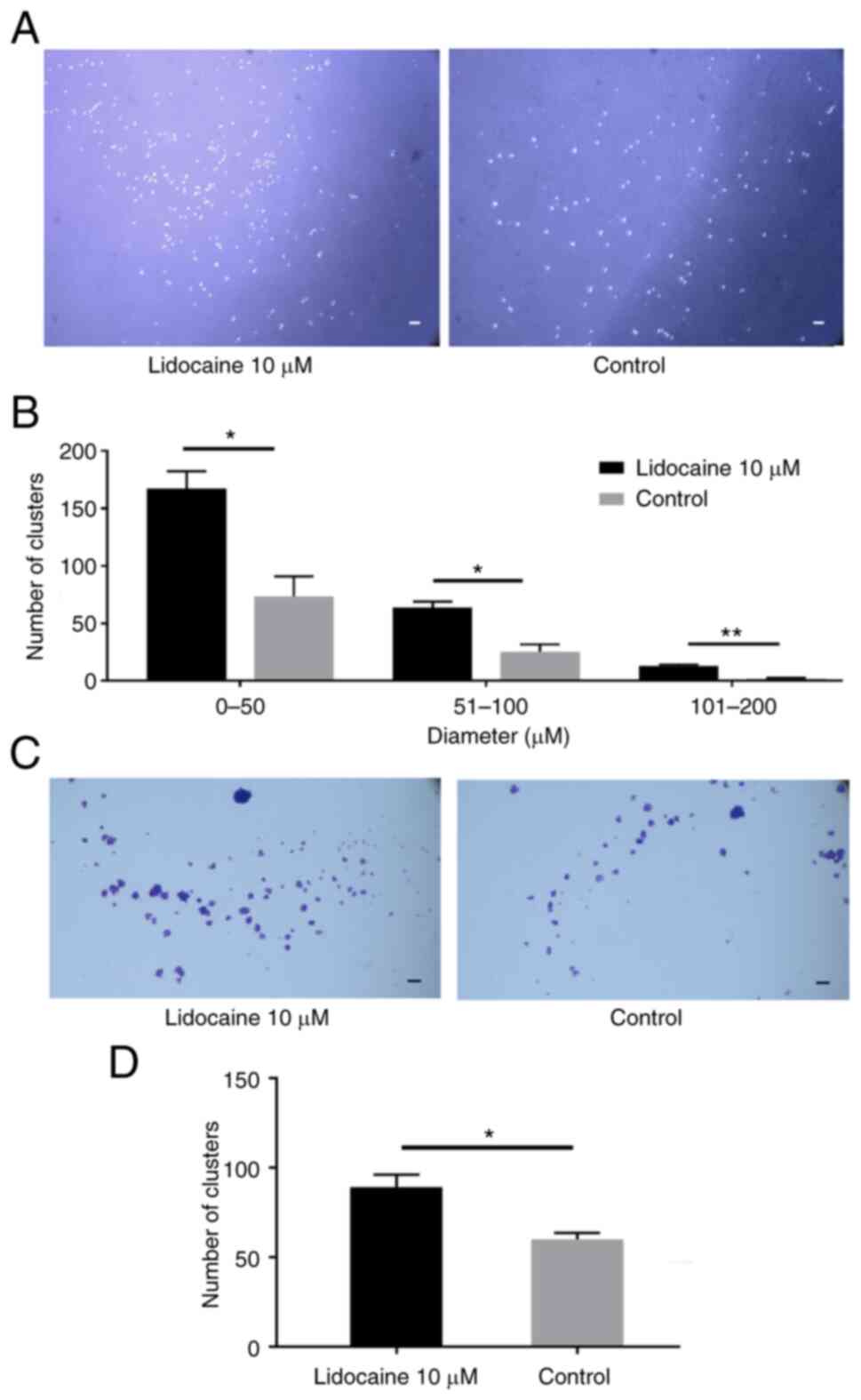
Lidocaine induces anoikis resistance
by forming cell clusters at clinically relevant concentrations
Besides being associated with cell migration, EMT is
also a characteristic of anoikis resistance (31). Therefore, the present study
performed an anoikis-resistant cell aggregation assay and revealed
that the number of cell clusters was significantly increased with
lidocaine administration (Fig. 4).
Thus, we hypothesized that A549 cells had the potential to progress
towards EMT, a hallmark of cancer stemness. This was, at least in
part, supported by the results of western blotting (Fig. 3C-E). Furthermore, the anchoring of
the cell clusters on the 6-well plate without poly-HEMA layer
confirmed that the number of clusters formed by aggregation
associated with the cell survival status determined by the crystal
violet assay (Fig. 4). That led to
two important assumptions. First, lidocaine at clinically relevant
concentrations is not likely a cause for lung cancer cell death.
Second, the lung cancer cells did not dissolve into a single cell
state when placed on the plate again after lidocaine treatment,
indicating a trend towards sphere formation, which is
characteristic of stemness.
Effect of lidocaine on cancer
metastasis
Compared with the control group, Alu
expression was significantly increased upon treatment with 10 µM
lidocaine (Fig. 5), which
corresponds with the finding that lidocaine reduces the
5-FU-induced inhibitory effects on cell migration, induces EMT and
increases anoikis-resistant cell aggregation. Notably, Alu
expression was increased by lidocaine treatment when compared with
that induced by 5-FU alone and the control. The addition of 5-FU to
lidocaine reduced Alu expression when compared with lidocaine
alone, while there was no statistically significant difference
compared with the control (Fig.
5C).
Impact of the measured EMT proteins on
the conditioned metastasis pathway and NSCLC
Effect of measured EMT proteins on the
conditioned metastasis pathway
Regarding the relationship between the measured
molecules (increased expression of Slug and vimentin, and decreased
expression of E-cadherin) and the remaining 27 molecules in this
conditioned metastasis pathway, 24 relationships were observed. Of
the affected molecules, those that were predicted to be activated
included SNAI1, MMP1, MMP2, MMP9, TGFB1 and MET, and those that
were predicted to be inhibited included Akt, ERBB2, EGFR, MTOR and
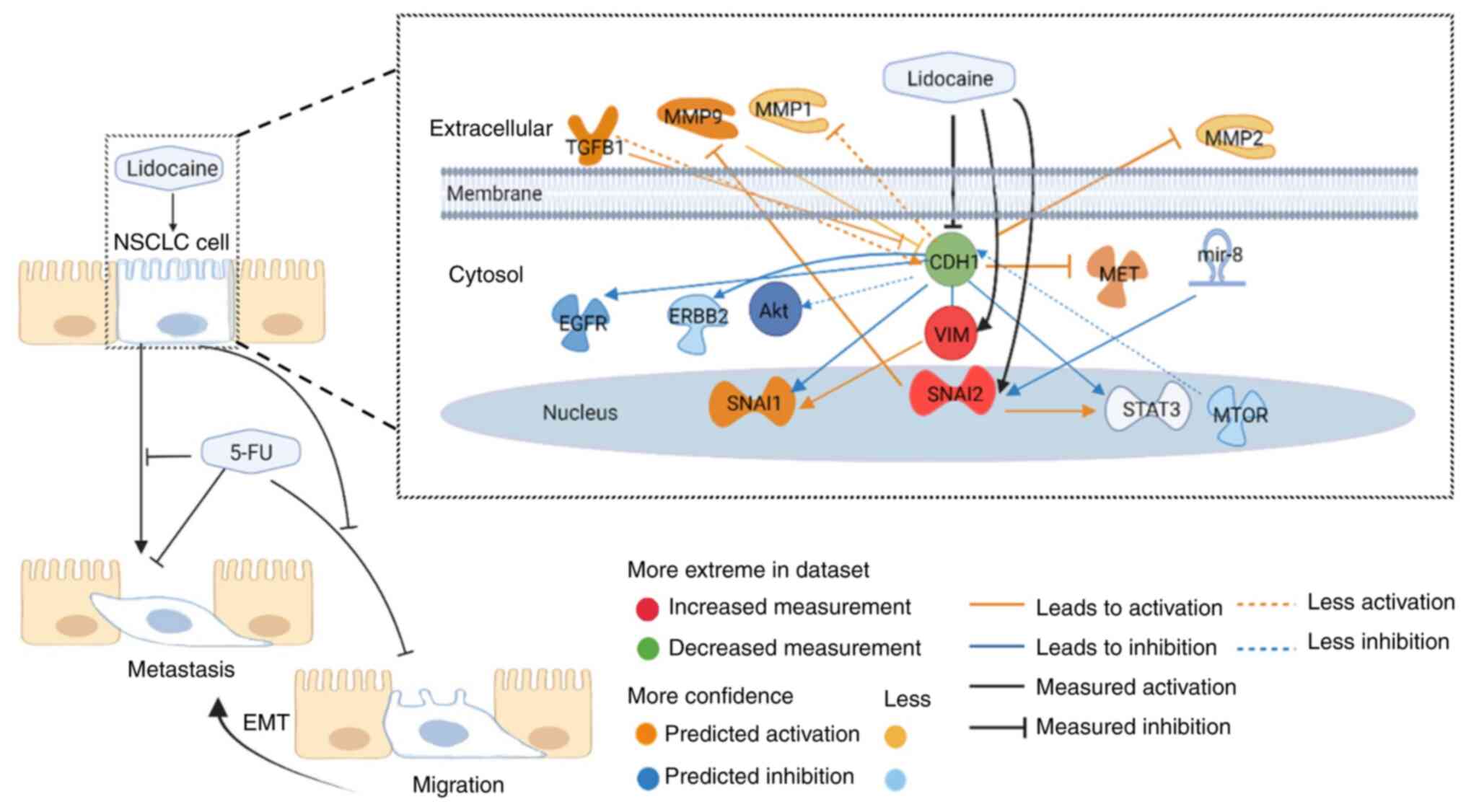
miR-8 (Fig. 6). The main results of
the present study are schematically presented in Fig. 7, which integrated the results of
lung cancer behaviors and prediction of associated gene expressions
according to measured EMT protein alterations in response to
lidocaine and 5-FU treatment in A549 cells.
Effect of individual measured proteins
on NSCLC, EMT and apoptosis of NSCLC
Upregulation of Slug expression strongly promoted
EMT but did not affect NSCLC and its apoptosis (Fig. S2). In addition, the upregulation of
vimentin expression strongly promoted NSCLC and EMT, but did not
affect the apoptosis of NSCLC (Fig.
S3). Downregulation of E-cadherin expression also strongly
promoted NSCLC and EMT, despite possible activation of NSCLC
apoptosis (Fig. S4).
Discussion
The present study revealed that lidocaine, at
clinically relevant concentrations (1–10 µM), could reduce the
inhibitory effect induced by 5-FU on the migration of lung cancer
cells, whereas the survival of lung cancer cells was not affected.
In addition to inducing EMT by altering migration-related EMT
markers (slug, vimentin and E-cadherin), anoikis-resistant cell
aggregation characteristic of EMT was also increased with lidocaine
treatment in this dosing range. Furthermore, the potential of
lidocaine-induced lung cancer metastasis was evidenced using a CAM
model. IPA analysis based on the results of measured prototypical
EMT proteins and their molecular switch yielded the predicted gene
expression map. This predicted gene expression map revealed the
effect of measured EMT proteins on the conditioned metastasis
pathway. The possible relationships between the measured proteins
(vimentin, E-cadherin and Slug) affected by lidocaine and the
molecules in this conditioned metastasis pathway showed those that
were predicted to be activated included SNAI1, MMP1, MMP2, MMP9,
TGFB1 and MET, and those that were predicted to be inhibited
included Akt, ERBB2, EGFR, MTOR and miR-8. Based on these
phenomena, it was proposed that at clinically relevant
concentrations, lidocaine may cause potential negative therapeutic
effects on lung cancer.
To the best of our knowledge, the present study is
the first to report the effect of clinically relevant
concentrations of lidocaine on EMT, EMT-related cancer behaviors
and chemoresistance in NSCLC. Although the involvement of EMT has
been studied in epithelial cancer stem cells (CSCs) in various
tumors, data are currently limited for NSCLC (32). Furthermore, EMT has long been linked
to drug resistance in NSCLC; however, but the mechanisms underlying
EMT-related resistance are still far from being fully explored
(33). The benefit of the present
study lies in the discovery of the effect of clinically relevant
concentrations of lidocaine on EMT and various cancer behaviors in
NSCLC, both in vivo and in vitro. In a recent review
that focused on the impact of EMT on NSCLC, vimentin and E-cadherin
were regarded as the most relevant EMT markers to be examined in
routine practice. This was mainly because higher vimentin
expression in tumor cells has been proposed as a predictor of
metastasis and both markers have been shown to be independent
predictors of cancer mortality (34). Tumor cells utilize EMT as a strategy
to acquire CSC-like properties and achieve resistance to antitumor
drugs (33). The results of the
present study validated that lidocaine attenuated the inhibitory
effect of 5-FU on cell migration, while promoting EMT and the
associated anoikis resistance, indicating that clinically relevant
concentrations of lidocaine may contribute to antitumor drug
resistance. Furthermore, as a key molecule in EMT-induced cell
migration and an invariably expressed protein on EMT-transformed
CSCs, vimentin is central to EMT-mediated metastasis (31). Therefore, lidocaine-induced vimentin
upregulation has the potential, at least in part, to be associated
with attenuation of 5-FU-induced migratory inhibition, metastasis
and cancer stemness (such as anoikis resistance) as indicated in
the present results.
Because the EMT-governing mechanisms are complex
non-linear networks (35), the
IPA-based network analyses applied in the present study may be a
practical tool to systematically predict gene alteration based on
web bench results, such as altered EMT markers. The predicted gene
expression profile could foster further research. Being the only
local anesthetic agent allowed for intravenous administration,
lidocaine has been recommended as part of the protocol for ERAS
during the perioperative period to facilitate postoperative
recovery in some surgical procedures with variable evidence levels,
including thoracic surgery with a moderate evidence level (17). However, it has been confirmed that
even when continuous intravenous infusion is performed at a 2
mg/min or 1.33 mg/kg/h, which is a dosage higher than that
recommended by the ERAS protocol (17) (0.5–1 mg/min) for prolonged periods
(4 or 24 h) after colorectal resection, the concentration does not
exceed the generally recognized toxic concentration of ~20 µM (5.0
µg/ml) (36,37). The safety of prolonged lidocaine
infusion at this higher rate (2 mg/min) was further evidenced by
the fact that the first clinical signs of toxicity were not
achieved when lidocaine was administered intravenously over 14 days
in cases of severe migraine (16).
As for regional blocks, the plasma lidocaine concentrations
achieved during epidural administration was ~1 µM. Therefore, it is
reasonable to assume that clinically relevant concentrations of
lidocaine achieved through either the epidural or intravenous route
during the perioperative period or using higher doses for longer
duration during migraine treatment were <20 µM in terms of
clinical signs of toxicity.
Numerous studies have indicated that lidocaine can
inhibit tumor invasion and metastasis (38,39).
There is also evidence that tumor cell proliferation causes
increased activity of voltage-gated sodium channels (VGSCs) and
blocking these channels with local anesthetics may potentially
inhibit tumor progression (40,41).
However, in the present study, the clinical concentrations of
lidocaine did not affect cell viability but they did promote
migration, thus indicating the importance of pathways other than
VGSCs. Data from previous research have shown that the effect of
lidocaine on lung cancer cell behavior and associated gene
expression depends on the range of concentrations that the cells
are exposed to and varies with the investigation protocols. Some
studies have demonstrated that 8 mM lidocaine can inhibit the
viability, migration and invasion of the A549 lung cancer cells,
and can induce apoptosis (18,42).
However, patients have not been exposed to 8 mM lidocaine in
clinical practice. Although lidocaine at high concentrations can
result in inhibition in a number of aspects, clinically relevant
low concentrations have seldom been explored in clinical setups. As
a biphasic effect of drugs is not uncommon in clinical practice
from our previous experience (43),
it may be premature to deny the possibility that clinically
relevant concentrations of lidocaine (<20 µM) could exert
opposite effects when compared with high concentrations in the
millimolar scale.
Previous studies have reported that exposure to 8 µM
lidocaine can reduce the barrier property of A549 cells using
electric cell-substrate impedance sensing (ECIS) technology
(44). However, to the best of our
knowledge, the effect of 8 µM lidocaine on cell migration has yet
to be clarified. The ECIS findings indirectly support the current
result that clinically relevant concentrations of lidocaine can
suppress the inhibitory effect of 5-FU on migration, because the
attenuation of barrier function involves the first step in cancer
cell dissemination, including migration. Another related study
reported that 10 µM lidocaine inhibits the invasion and migration
of lung cancer cells induced by TNFα (10). However, the living environment of
the cells, as stimulated by TNFα, is no longer physiological while
the cells are exposed to a clinical concentration of lidocaine. The
present results varied from these previous findings due to a
different protocol setting that was without potent inflammatory
stimuli, which proved the negative effects of clinically relevant
concentrations of lidocaine through both in vitro and in
vivo studies. The negative therapeutic effects of lidocaine on
lung cancer cell behavior were further exemplified by analyzing
anoikis-resistant cell aggregation. Furthermore, the status of
crystal violet staining to confirm cell survival after reattachment
was similar to the status when the attached cells were forced to be
suspended from the plate to show how lidocaine induces the
anoikis-resistant ability in lung cancer. Cell reattachment also
occurred irreversibly in the form of cell clusters because
reattached cell clumps did not disperse into single cells,
indicating the progression towards sphere formation. As a stem cell
characteristic, the tendency of sphere formation hints at the
possibility of stemness transformation induced by clinically
relevant concentrations of lidocaine in A549 cells, a phenomenon
that requires further verification.
When translating the findings to clinical practice
for patients with lung cancer, especially those using 5-FU as an
adjuvant therapy, considering the possible negative effects of
intravenous lidocaine infusion should not be neglected. Analgesic
methods other than intravenous lidocaine infusion should be chosen
to treat refractory headaches in such patients. When an epidural
block is required, local anesthetics other than lidocaine should be
considered. Based on the present findings, before implementing
intravenous lidocaine infusion as a part of the ERAS protocol for
lung cancer surgery, the risk-benefit ratio should be re-calculated
because the ERAS lidocaine concentration (<20 µM) tends to
increase lung cancer migration and metastasis according to the
findings of this study. Additionally, the CAM model was applied to
simulate and evaluate in vivo tumor cell growth
(intravasation) and metastasis (45). The CAM model is a well-established
in vivo system used to study the cancer behaviors of various
tumors. It has also been proven to be a highly efficient in
vivo approach to evaluate compounds with cancer-modulating
activities (46). The tumor cells
can break down the extracellular matrix in tissues and then
penetrate, migrate and infiltrate into chick embryo blood vessels
for circulation. By assessing whether the lower CAM contains DNA
components of the injected tumor cells, it is possible to determine
whether lidocaine at such low concentrations enhances the overall
ability of tumor cells in terms of moving into chick embryo blood
vessels. Additionally, the effect of lidocaine on the metastatic
tendency of lung cancer can be explored (26). Notably, CAM is a relatively simple,
fast and low-cost model, and the method has been widely used to
study the effects of different drugs on cell behaviors (47,48).
The present study elucidated the effects of clinically relevant
concentrations of lidocaine on the metastatic behavior of human
lung cancer cells. However, the number of cells inoculated in these
in vivo experiments is considerably higher than that of the
circulating tumor cells in patients with lung cancer.
During the invasion-metastasis cascade, tumor cells
exit their primary sites of growth (local invasion, intravasation),
translocate systemically (survival in the circulation, arrest at a
distant organ site, extravasation), and adapt to survive and thrive
in the foreign microenvironments of distant tissues
(micrometastasis formation, metastatic colonization). Angiogenesis
is a part of the invasion process (49). Like any of the mechanisms during
metastasis, angiogenesis is considered one of the critical steps to
support cancer metastasis. However, the cancer-causing or
cancer-promoting substance does not necessarily potentiate every
step related to tumor metastasis, and recent evidence has shown
that tumors can grow without angiogenesis (50). Furthermore, considering the fact
that non-angiogenic tumors have also been described in
histopathology studies of NSCLC and carcinoma metastases in the
lung (50), the notion that NSCLC
is not necessarily angiogenesis-dependent may not be biased.
Therefore, although the CAM model can be used to assess
angiogenesis, the most appropriate endpoint to be measured should
be the metastasis itself, rather than any other step during
metastasis. For this reason, metastasis was examined in the CAM
model in the present study.
The reason why a higher concentration (3.125 µM) of
5-FU only reduced cell survival by 30% when compared with its
predicted IC50 value (2.808 µM) may be described as
follows. The IC50 value was merely first approximated by
GraphPad Prism software, and a dose-response curve was depicted
with IC50 predicted from the non-linear correlation
equation. With the predicted value of IC50 (2.808 µM)
narrowed down from a broader range of concentrations, the serially
diluted concentrations (2.34-4.68 µM) near the predicted
IC50 (2.808 µM) were validated in a further SRB assay.
Because the difference in biological response between
concentrations of 3.125 and 2.34 µM was not significant, 3.125 µM
was used as the experimental concentration in the subsequent study.
To summarize, 3.125 µM was the concentration that was chosen near
the ‘predicted’ IC50 value (2.808 µM). Therefore, 30%
inhibition by 3.125 µM is possible because 2.808 µM is just a
preliminary predicted value from a non-linear equation for
IC50 performed using GraphPad.
The present study has a few limitations. First, the
simulated laboratory conditions on lidocaine are limited, and
ultimate studies involving human trials with clinically relevant
lidocaine concentrations are recommended. Second, physiological
mechanisms are very complex, especially regarding cancer
physiology. Therefore, surgical techniques and anesthesia methods
need to be considered more comprehensively in clinical practice.
Third, only two cancer cell lines were studied whose cell
characteristics are not completely representative of cancer cells
that are directly cultured from clinical tissues. Thus, there is a
need to establish a model for studying the effects of lidocaine on
lung cancer primary culture. The present study directly stimulated
lung cancer cell lines with lidocaine using a range of clinical
concentrations without the support of the tumor microenvironment.
Besides, there is always a high level of circulating inflammatory
cytokines in cancer patients undergoing surgeries. However, the
present study used the CAM model that simulated an in vivo
tumor environment wherein residual tumor cells are ready to
metastasize to the circulatory system for distant metastasis.
Fourth, as for the effects of lidocaine on metastasis-related gene
expression, it would not be possible to measure the expression of
all genes responsible for metastasis. Nonetheless, to address this
issue and systemically discover the essential genes responsible for
metastasis in our study setting, the present study generated
relevant networks using IPA, and performed prediction analysis
according to the results of the measured prototypical EMT markers
(vimentin and E-cadherin) and their molecular switch (Slug).
Unfortunately, the results from IPA predictions were not
experimentally validated. The trend that cell migration decreased
with an increase in lidocaine concentration when combined with 5-FU
suggested that complex interactions between these two drugs may
exist in terms of migration. It would not be possible to explain
this trend with the current data and further investigation is
warranted in this regard. To reveal the versatility of lidocaine,
the effects of lidocaine were evaluated in both mouse and human
cell lines (LLC.LG and A549) only in the first half of the study.
In the future, we hope to perform mouse experiments based on the
results of the mouse cell lines (LLC.LG) to develop drugs against
lung cancer.
In conclusion, the present findings revealed that
clinically relevant concentrations of lidocaine may lead to
enhanced migratory and metastatic effects in human lung cancer
cells. The phenomena accompanying lidocaine-aggravated migration
and metastasis included the altered expression of prototypical EMT
markers and their molecular switch, anoikis-resistant cell
aggregation characteristic of EMT, and attenuation of the
5-FU-induced inhibitory effect on cell migration. The findings also
indicated that at clinically relevant concentrations, lidocaine may
contribute to resistance toward antitumor drugs. Based on these
findings, caution should be exercised before administering
intravenous/epidural lidocaine to reach clinically relevant
concentrations (1–20 µM), either as a part of the ERAS protocol or
as a treatment option for patients with lung cancer that have
migraines. Additionally, relevant samples should be collected from
ongoing clinical studies to establish the association between
lidocaine and the clinical outcomes of lung cancer surgery using
primary cultures produced under the ERAS protocol or epidural
infusion, elucidate relevant mechanisms, and validate the impact of
intravenous lidocaine on tumor progression and cancer stemness.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Hualien Armed Forces
General Hospital, Taiwan (grant no. HAFGH-A_112001), the Chi Mei
Medical Center, Taiwan (grant no. 108CM-TMU-13), and the Ministry
of Science and Technology, Taiwan (grant no. MOST
108-2314-B-038-066-).
Availability of data and materials
The datasets used and/or analyzed during the current
study available from the corresponding author on reasonable
request.
Authors' contributions
JAL conceptualized the study. JAL, BYH and WHH
designed the study. WHH, SWL, SMC and JDH performed the main
experiments and prepared the first draft of the manuscript. WHH
acquired and analyzed the data. WHH and SYW interpreted the data.
BYH, KYC and YTT reviewed the draft of the manuscript. SWL, HWF,
BYH, WHH and CYF performed the CAM model. SMC, JAL, SYC, KYC and
YTT performed IPA analysis and produced the figure. JDH and WHH
carried out the Transwell migration study. JDH and JAL revised the
manuscript and acquired the funding. WHH and JAL confirmed the
authenticity of all the raw data and performed the statistical
analysis. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mountain CF: The international system for
staging lung cancer. Semin Surg Oncol. 18:106–115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen R, Manochakian R, James L, Azzouqa
AG, Shi H, Zhang Y, Zhao Y, Zhou K and Lou Y: Emerging therapeutic
agents for advanced non-small cell lung cancer. J Hematol Oncol.
13:582020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noro R, Miyanaga A, Minegishi Y, Okano T,
Seike M, Soeno C, Kataoka K, Matsuda K, Yoshimura A and Gemma A:
Histone deacetylase inhibitor enhances sensitivity of
non-small-cell lung cancer cells to 5-FU/S-1 via down-regulation of
thymidylate synthase expression and up-regulation of p21(waf1/cip1)
expression. Cancer Sci. 101:1424–1430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ettinger DS, Wood DE, Aggarwal C, Aisner
DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac
LR, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 1.2020. J Natl Compr Canc Netw. 17:1464–1472. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan X, Zhang C, Gao W, Sun B, Jiang B and
Song P: Overexpression of microRNA-124-5p sensitizes non-small cell
lung cancer cells to treatment with 5-fluorouracil via AEG-1
regulation. Oncol Lett. 21:52021.PubMed/NCBI
|
|
7
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tedore T: Regional anaesthesia and
analgesia: Relationship to cancer recurrence and survival. Br J
Anaesth. 115 (Suppl 2):ii34–ii45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xuan W, Hankin J, Zhao H, Yao S and Ma D:
The potential benefits of the use of regional anesthesia in cancer
patients. Int J Cancer. 137:2774–2784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piegeler T, Schläpfer M, Dull RO, Schwartz
DE, Borgeat A, Minshall RD and Beck-Schimmer B: Clinically relevant
concentrations of lidocaine and ropivacaine inhibit TNFα-induced
invasion of lung adenocarcinoma cells in vitro by blocking the
activation of Akt and focal adhesion kinase. Br J Anaesth.
115:784–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piegeler T, Votta-Velis EG, Liu G, Place
AT, Schwartz DE, Beck-Schimmer B, Minshall RD and Borgeat A:
Antimetastatic potential of amide-linked local anesthetics:
Inhibition of lung adenocarcinoma cell migration and inflammatory
Src signaling independent of sodium channel blockade.
Anesthesiology. 117:548–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Samarin MJ, Mohrien KM and Oliphant CS:
Continuous intravenous antiarrhythmic agents in the intensive care
unit: Strategies for safe and effective use of amiodarone,
lidocaine, and procainamide. Crit Care Nurs Q. 38:329–344. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kosharskyy B, Almonte W, Shaparin N,
Pappagallo M and Smith H: Intravenous infusions in chronic pain
management. Pain Physician. 16:231–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keller D, Seamon J and Jones JS: BET 2:
Usefulness of IV lidocaine in the treatment of renal colic. Emerg
Med J. 33:825–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen NL, Kome AM, Lowe DK, Coyne P and
Hawks KG: Intravenous lidocaine as an adjuvant for pain associated
with sickle cell disease. J Pain Palliat Care Pharmacother.
29:359–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williams DR and Stark RJ: Intravenous
lignocaine (lidocaine) infusion for the treatment of chronic daily
headache with substantial medication overuse. Cephalalgia.
23:963–971. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dunn LK and Durieux ME: Perioperative use
of intravenous lidocaine. Anesthesiology. 126:729–737. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun H and Sun Y: Lidocaine inhibits
proliferation and metastasis of lung cancer cell via regulation of
miR-539/EGFR axis. Artif Cells Nanomed Biotechnol. 47:2866–2874.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang HW, Wang LY, Jiang L, Tian SM, Zhong
TD and Fang XM: Amide-linked local anesthetics induce apoptosis in
human non-small cell lung cancer. J Thorac Dis. 8:2748–2757. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Du Q, Zhao Q, Zhang M, Qin X,
Jiang Y and Luan Y: A heme-regulatable chemodynamic nanodrug
harnessing transcription factor Bach1 against lung cancer
metastasis. J Colloid Interface Sci. 610:698–708. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ning J, Cui X, Li N, Li N, Zhao B, Miao J
and Lin Z: Activation of GRP78 ATPase suppresses A549 lung cancer
cell migration by promoting ITGB4 degradation. Cell Adh Migr.
16:107–114. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ediriweera MK, Tennekoon KH, Samarakoon
SR, Thabrew I and De Silva ED: Induction of apoptosis in MCF-7
breast cancer cells by sri lankan endemic mango (Mangifera
zeylanica) fruit peel through oxidative stress and analysis of
its phytochemical constituents. J Food Biochem. 41:e122942017.
View Article : Google Scholar
|
|
23
|
Orellana EA and Kasinski AL:
Sulforhodamine B (SRB) assay in cell culture to investigate cell
proliferation. Bio Protoc. 6:e19842016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feoktistova M, Geserick P and Leverkus M:
Crystal violet assay for determining viability of cultured cells.
Cold Spring Harb Protoc. 2016.pdb.prot087379, 2016. View Article : Google Scholar
|
|
25
|
Miyashita N, Enokido T, Horie M, Fukuda K,
Urushiyama H, Strell C, Brunnström H, Micke P, Saito A and Nagase
T: TGF-β-mediated epithelial-mesenchymal transition and
tumor-promoting effects in CMT64 cells are reflected in the
transcriptomic signature of human lung adenocarcinoma. Sci Rep.
11:223802021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ho BY, Wu YM, Hsu YW, Hsu LC, Kuo YH,
Chang KJ and Pan TM: Effects of Monascus-fermented rice extract on
malignant cell-associated neovascularization and intravasation
determined using the chicken embryo chorioallantoic membrane model.
Integr Cancer Ther. 9:204–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim J, Yu W, Kovalski K and Ossowski L:
Requirement for specific proteases in cancer cell intravasation as
revealed by a novel semiquantitative PCR-based assay. Cell.
94:353–362. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kariya Y, Kato K, Hayashizaki Y, Himeno S,
Tarui S and Matsubara K: Revision of consensus sequence of human
Alu repeats-a review. Gene. 53:1–10. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Unahabhokha T, Chanvorachote P, Sritularak
B, Kitsongsermthon J and Pongrakhananon V: Gigantol inhibits
epithelial to mesenchymal process in human lung cancer cells. Evid
Based Complement Alternat Med. 2016:45616742016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Usman S, Waseem NH, Nguyen TKN, Mohsin S,
Jamal A, Teh MT and Waseem A: Vimentin is at the heart of
epithelial mesenchymal transition (EMT) mediated metastasis.
Cancers (Basel). 13:49852021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mahmood MQ, Shukla SD, Ward C and Walters
EH: The underappreciated role of epithelial mesenchymal transition
in chronic obstructive pulmonary disease and its strong link to
lung cancer. Biomolecules. 11:13942021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu X, Chen L, Liu L and Niu X:
EMT-mediated acquired EGFR-TKI resistance in NSCLC: Mechanisms and
strategies. Front Oncol. 9:10442019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ancel J, Dewolf M, Deslée G, Nawrocky-Raby
B, Dalstein V, Gilles C and Polette M: Clinical impact of the
epithelial-mesenchymal transition in lung cancer as a biomarker
assisting in therapeutic decisions. Cells Tissues Organs.
211:91–109. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herroeder S, Pecher S, Schönherr ME,
Kaulitz G, Hahnenkamp K, Friess H, Böttiger BW, Bauer H, Dijkgraaf
MG, Durieux ME and Hollmann MW: Systemic lidocaine shortens length
of hospital stay after colorectal surgery: A double-blinded,
randomized, placebo-controlled trial. Ann Surg. 246:192–200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaba A, Laurent SR, Detroz BJ, Sessler DI,
Durieux ME, Lamy ML and Joris JL: Intravenous lidocaine infusion
facilitates acute rehabilitation after laparoscopic colectomy.
Anesthesiology. 106:11–18. 5–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mammoto T, Higashiyama S, Mukai M, Mammoto
A, Ayaki M, Mashimo T, Hayashi Y, Kishi Y, Nakamura H and Akedo H:
Infiltration anesthetic lidocaine inhibits cancer cell invasion by
modulating ectodomain shedding of heparin-binding epidermal growth
factor-like growth factor (HB-EGF). J Cell Physiol. 192:351–358.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mao L, Lin S and Lin J: The effects of
anesthetics on tumor progression. Int J Physiol Pathophysiol
Pharmacol. 5:1–10. 2013.PubMed/NCBI
|
|
40
|
Koltai T: Voltage-gated sodium channel as
a target for metastatic risk reduction with re-purposed drugs.
F1000Res. 4:2972015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Elajnaf T, Baptista-Hon DT and Hales TG:
Potent inactivation-dependent inhibition of adult and neonatal
NaV1.5 channels by lidocaine and levobupivacaine. Anesth Analg.
127:650–660. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang L, Hu R, Cheng Y, Wu X, Xi S, Sun Y
and Jiang H: Lidocaine inhibits the proliferation of lung cancer by
regulating the expression of GOLT1A. Cell Prolif. 50:e123642017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin JA, Chen JH, Lee YW, Lin CS, Hsieh MH,
Chang CC, Wong CS, Chen JJ, Yeh GC, Lin FY and Chen TL: Biphasic
effect of curcumin on morphine tolerance: A preliminary evidence
from cytokine/chemokine protein array analysis. Evid Based
Complement Alternat Med. 2011:4521532011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chan SM, Lin BF, Wong CS, Chuang WT, Chou
YT and Wu ZF: Levobuipivacaine-induced dissemination of A549 lung
cancer cells. Sci Rep. 7:86462017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lokman NA, Elder ASF, Ricciardelli C and
Oehler MK: Chick chorioallantoic membrane (CAM) assay as an in vivo
model to study the effect of newly identified molecules on ovarian
cancer invasion and metastasis. Int J Mol Sci. 13:9959–9970. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Merlos Rodrigo MA, Casar B, Michalkova H,
Jimenez Jimenez AM, Heger Z and Adam V: Extending the applicability
of in ovo and ex ovo chicken chorioallantoic membrane assays to
study cytostatic activity in neuroblastoma cells. Front Oncol.
11:7073662021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Agrawal S, Chaugule S, More S, Rane G and
Indap M: Methanolic extract of Euchelus asper exhibits in-ovo
anti-angiogenic and in vitro anti-proliferative activities. Biol
Res. 50:412017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ribatti D: The chick embryo
chorioallantoic membrane (CAM). A multifaceted experimental model.
Mech Dev. 141:70–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pezzella F and Gatter KC: Evidence showing
that tumors can grow without angiogenesis and can switch between
angiogenic and nonangiogenic phenotypes. J Natl Cancer Inst.
108:djw0322016. View Article : Google Scholar : PubMed/NCBI
|