Introduction
Oesophageal cancer is the seventh most common cancer
globally, responsible for 1 in 18 cancer-related deaths and over
500,000 deaths every year (1). The
two most common histological subtypes are squamous cell carcinoma
and adenocarcinoma (2). Squamous
cell carcinoma is the predominant type worldwide but the incidence
of oesophageal adenocarcinoma (EAC) exceeds that of squamous cell
carcinoma in higher-income countries (3). The 5-year survival rate of EAC is
approximately 20% (4,5).
Curative treatment intents can only be performed for
operable patients. For inoperable patients chemo-(radio)-therapy
alone is the preferred treatment option (6). All operable EAC patients should be
treated with neoadjuvant chemotherapy or chemoradiotherapy, before
surgery if possible since it improves survival significantly
(7,8). Therapy with a combination of cisplatin
and fluorouracil the anti-HER2 monoclonal antibody trastuzumab has
also been shown to be efficient in HER2-positive advanced disease
(9). In addition, patients with
PD-L1-positive oesophageal cancer who were treated with
pembrolizumab or nivolumab had promising results. The prognosis of
our patients could be improved by the introduction of neoadjuvant
therapies. Today, about 20–25% of operable patients are still alive
five years after primary diagnosis (10,11).
However, the majority of patients die as a result of their tumour
disease due to (late) relapses. The chemotherapies then available
are only therapeutically effective to a very limited extent.
Primarily inoperable patients or (palliative) patients with
hematogenous metastases show a disastrous prognosis, only very few
patients survive five years. According to national guidelines,
patients who have received neoadjuvant CROSS and have no vital
tumour postoperatively do not receive further adjuvant therapy.
Despite improvements described above there is still a great need
for therapies in the recurrent/metastatic situation. Syndecan-1
(CD138) is cell-surface heparan sulphate (12), one of four members of the syndecan
family, and includes 288 amino acids which make it the second
largest among (13). Syndecans play
a role in adjusting cell-cell and cell-matrix interactions
(14). They also play roles such as
modulation of cell proliferation and invasive growth (15,16).
CD138 is physiologically expressed on plasma cells and various
epithelial cells (17). CD138
expression is best known to be highly specific for multiple myeloma
(18). With that said, CD138
overexpression is also reported in various carcinomas such as
breast, pancreatic, gallbladder, endometrial, ovarian, prostate and
urinary bladder cancers (14).
Specific drugs targeting CD138 have recently been
assessed in various tumours. For instance, indatuximab ravtansine,
a monoclonal antibody-linked cytotoxic agent that targets CD138, is
reported to be very efficient on multiple myeloma (19,20).
This antibody-drug conjugate (ADC) uses the CD138 binding site on
the cancer cell to hijack the cell and place the actual therapeutic
agent in the tumour cell in a targeted and highly concentrated
manner. The same principle of ADC is successfully used with
trastuzumab deruxtecan (target protein Her2/neu) and also with
sacituzumab govitecan (target protein TROP2) in breast carcinoma
(21,22).
Our group recently demonstrated that sacituzumab
govitecan is also effective in EAC and that the efficacy is
dependent on the level of expression of TROP2 on the tumour cell
(23). The same relationship was
shown for CD138 in breast carcinoma. Indatuximab ravtansine led to
an increased complete response in xenografts of triple-negative
breast carcinomas that strongly expressed CD138 in
immunohistochemistry (24,25). This makes CD138 an ideal predictive
therapy-relevant biomarker. Another recently identified agent,
VIS823, showed promising results in a preclinical study on multiple
myeloma cell lines (26).
Almost nothing is known about the significance and
expression level of CD138 in EAC. We aim to answer the following
questions with this work: i) how many EACs express CD138? ii) what
are the histomorphological, molecular and clinical characteristics
of CD138-positive EAC? iii) is CD138 suitable as a relevant
prognostic marker in EAC? iv) does neoadjuvant therapy (after CROSS
or FLOT) have an impact on the expression level of CD138 in
EAC?
Materials and methods
Patient cohort
Patients were included in this analysis if they had
undergone primary surgery with curative intent for primary EAC at
the Department of General Surgery Department, University of Cologne
between 1996 and 2020 and if sufficient formalin-fixed
paraffin-embedded material of the primary tumour was available.
Demographic, histopathological and survival data were retrieved
from clinical records and histopathological reports with respect to
tumour characteristics including the stage of disease at the time
of diagnosis according to the AJCC TNM staging system (8th edition,
2020) (27). In case of missing
data on follow-up, patients were phoned to follow up in terms of
the current tumour status. The study was performed according to the
regulations of the Ethics Committee of the University of Cologne
(approval nos. 20-1583 and 10-242).
TMA preparation and
immunohistochemical assessment of CD138 expression
One tissue cylinder (diameter 1.2 mm) per case was
punched out from one tumour-bearing formalin-fixed,
paraffin-embedded (FFPE) block using a semiautomated precision
instrument. The cylinders were then transferred to an empty
paraffin block. Tissue slides were stained with antibodies against
CD138 (clone EP85, rabbit, 1 : 500 pretreatment with EDTA buffer,
Epitomics, Burlingame, CA, USA). All immunohistochemical staining
was carried out with a Leica BOND-MAX stainer (Leica Biosystems,
Wetzlar, Germany) in accordance with the manufacturer's protocol.
Counterstaining was done using haematoxylin and bluing reagent.
Two pathologists with special expertise in the field
of EAC (DA, AQ) assessed the membranous expression of CD138 on
tumour cells. A tumour cell was counted positive if ≥50% of the
membrane showed CD138 expression. The percentage of tumour cells
with CD138 expression in relation to all tumour cells was
calculated. The categorization of our staining analyses was based
on the previously published data on the subject (28). The staining intensity was determined
semi-quantitatively and divided into four intensity levels (0, 1+,
2+, 3+). Four groups were then created to perform statistical
analysis: tumours without expression of CD138 on their tumour cell
membrane were classified as negative, tumours with a low expression
intensity in ≤70% of tumour cells and moderate intensity in ≤30% of
tumour cells were scored as weakly positive (1+), tumours with low
staining intensity in >70% of tumour cells, moderate intensity
in <30 to 70% or strong intensity in ≤30% of tumour cells were
classified as moderately positive (2+) and tumours with moderate
intensity in >70% of tumour cells or strong intensity in >30%
were classified as strongly positive (3+). As also mentioned in the
same paper, this classification corresponds to a standard
categorization that has also been used by other research groups in
various immunohistochemical studies (29).
Fluorescence in situ
hybridization
Fluorescence in situ hybridization (FISH) to
evaluate the ERBB2 gene amplification status was performed
with a Zytolight SPEC ERBB2/CEN 17 Dual Probe Kit (Zytomed Systems
GmbH, Germany) according to the manufacturer's protocol. Sample
processing was performed as previously described (30).
Statistical analysis
The expression of CD138 in tumour cells was
dichotomized in two ways: first, negative (0) tumours were compared
to positive tumours (1–3+). In the second step, tumours with strong
expression of CD138 were analysed in comparison to the other
tumours (0, 1+, 2+).
The expression levels were correlated to patients'
sex, age and histopathological parameters including tumour stage
(pT) and lymph node status (pN0, pN+). Additionally, CD138
expression data were correlated with ERBB2-amplification
status (Her2/neu) and MET status.
For the statistical comparisons, chi-square test,
Fisher's exact test and one-way ANOVA were used with a Bonferroni
correction for multiple comparisons. P<0.05 was considered
statistically significant, P<0.1 as a statistical trend.
For survival analysis, Kaplan-Meier curves were
generated and overall survival between the CD138 expression
subgroups was compared using a log-rank test, or, in the case of
crossing curves, a Breslow test. Additionally, multivariate Cox
proportional hazard analysis was performed including prognostic
factors like age, sex, tumour stage, lymph node status and the
application of neoadjuvant therapy.
For all analysis and data visualization, Python v.
3.9 was used on PyCharm Community Edition 2022.2 including commonly
free available packages (numpy, pandas, scipy.stats, matplotlib,
pingouin, lifelines).
Results
Characteristics of the patient
cohort
The total cohort included 723 patients; 632 patients
were male (87.4%) and 91 were female (12.6%); 453 patients (62.7%)
received neoadjuvant treatment [CROSS regime, FLOT or not further
specified (NOS)]. Table I contains
detailed data for the total cohort as well as patient subgroups,
respectively.
 | Table I.Histopathological parameters of the
patient cohort (n=723). |
Table I.
Histopathological parameters of the
patient cohort (n=723).
|
|
| Neoadjuvant
treatment |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Primary
surgery | CROSS | FLOT/NOS | Total |
|---|
| Sex, n (%) |
|
|
|
|
|
Male | 234 (86.7) | 199 (88.1) | 199 (87.7) | 632 (87.4) |
|
Female | 36 (13.3) | 27 (11.9) | 28 (12.3) | 91 (12.6) |
| Median age at
surgery, years (range) | 68 (30–91) | 59 (27–83) | 62 (34–85) | 64 (27–91) |
| pT/ypT, n (%) |
|
|
|
|
|
Submucosa-limited (pT1) | 84 (31.1) | 26 (11.5) | 21 (9.3) | 131 (18.1) |
|
Extensive (pT2+) | 186 (68.9) | 200 (88.5) | 206 (90.7) | 592 (81.9) |
| pN/ypN, n (%) |
|
|
|
|
|
pN0 | 109 (40.4) | 92 (41.1) | 76 (33.5) | 277 (38.4) |
|
pN1+ | 161 (59.6) | 132 (58.9) | 151 (66.5) | 444 (61.6) |
| UICC stage, n
(%) |
|
|
|
|
| I | 64 (23.8) | 18 (7.9) | 14 (6.2) | 96 (13.3) |
| II | 34 (12.6) | 27 (11.9) | 28 (12.3) | 89 (12.3) |
|
III | 102 (37.9) | 112 (49.6) | 111 (48.9) | 325 (45.0) |
| IV | 69 (25.7) | 69 (30.5) | 74 (32.6) | 212 (29.4) |
| ERBB2
status, n (%) |
|
|
|
|
| Wild
type | 222 (88.1) | 181 (91.0) | 193 (93.2) | 596 (90.6) |
|
Amplified | 30 (11.9) | 18 (9.0) | 14 (6.8) | 62 (9.4) |
| MET status,
n (%) |
|
|
|
|
| Wild
type | 257 (95.2) | 203 (89.9) | 207 (91.2) | 667 (92.3) |
|
Amplified | 13 (4.8) | 23 (10.1) | 20 (8.8) | 56 (7.7) |
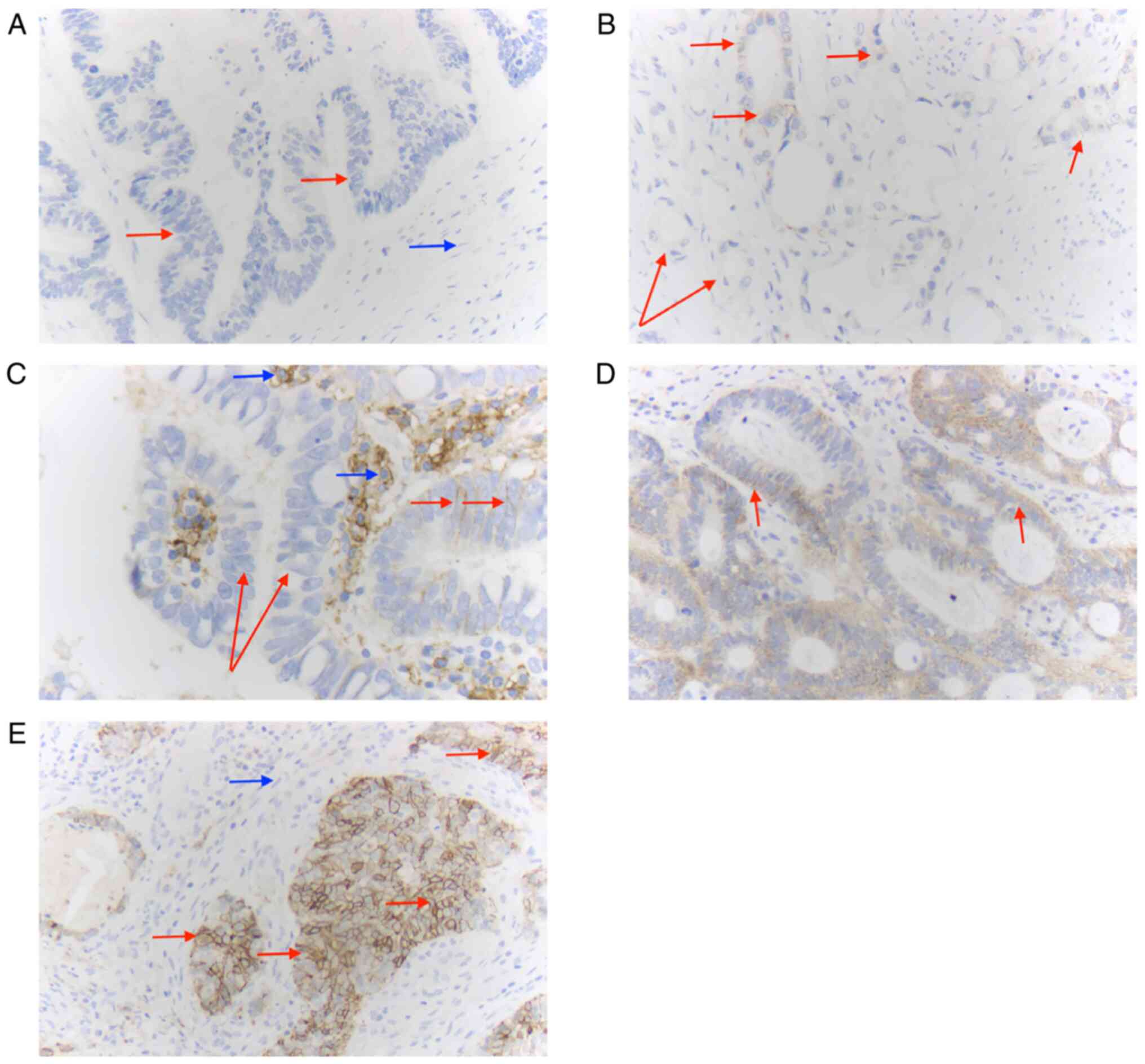
Extent of CD138 expression in
adenocarcinoma of the oesophagus (EAC)
When dichotomized into negative and positive
tumours, the majority of EACs did not express CD138 [491 (67.9%)
vs. 232 (32.1%)]. Ninety-six tumours displayed strong expression of
CD138 (13.3%) (Fig. 1). These
proportions were observed more or less in all treatment groups,
with tumours slightly more often expressing strong levels of CD138
after neoadjuvant CROSS treatment (Tables II and III).
 | Table II.Histopathological parameters in
CD138-expression subgroups. |
Table II.
Histopathological parameters in
CD138-expression subgroups.
| Characteristic | CD138 negative | CD138 positive | CD138 strong
expression |
|---|
| Sex, n (%) |
|
|
|
|
Male | 426 (86.8) | 206 (88.8) | 84 (87.5) |
|
Female | 65 (13.2) | 26 (11.2) | 12 (12.5) |
| Median age at
surgery, years (range) | 64 (27–91) | 63 (30–85) | 62.5 (39–84) |
| pT/ypT, n (%) |
|
|
|
|
Submucosa-limited (pT1) | 72 (14.7) | 59 (25.4) | 30 (31.3) |
|
Extensive (pT2+) | 419 (85.3) | 173 (74.6) | 66 (68.8) |
| pN/ypN, n (%) |
|
|
|
|
pN0 | 168 (34.2) | 109 (47.0) | 57 (59.4) |
|
pN1+ | 322 (65.6) | 122 (52.6) | 39 (40.6) |
| UICC stage, n
(%) |
|
|
|
| I | 55 (11.2) | 42 (18.1) | 21 (21.9) |
| II | 52 (10.5) | 37 (15.9) | 17 (17.7) |
|
III | 226 (46.0) | 99 (42.7) | 39 (40.6) |
| IV | 158 (32.2) | 54 (23.3) | 19 (19.8) |
| ERBB2
status, n (%) |
|
|
|
| NA | 35 (7.1) | 30 (12.9) | 15 (15.6) |
| Wild
type | 430 (87.6) | 166 (71.6) | 64 (66.7) |
|
Amplified | 26 (5.3) | 36 (15.5) | 17 (17.7) |
| MET status,
n (%) |
|
|
|
| Wild
type | 448 (91.2) | 219 (94.4) | 90 (93.8) |
|
Amplified | 43 (8.8) | 13 (5.6) | 6 (6.2) |
 | Table III.Extent of CD138 expression in
treatment cohorts. |
Table III.
Extent of CD138 expression in
treatment cohorts.
|
|
| Neoadjuvant
treatment |
|
|---|
|
|
|
|
|
|---|
| Intensity | Primary surgery, n
(%) | CROSS, n (%) | FLOT/NOS, n
(%) | Total, n (%) |
|---|
| Negative (0) | 195 (72.2) | 137 (60.6) | 159 (70.0) | 491 (67.9) |
| Weak (1+) | 5 (1.9) | 8 (3.5) | 1 (0.4) | 14 (1.9) |
| Moderate (2+) | 38 (14.1) | 44 (19.5) | 40 (17.6) | 122 (16.9) |
| Strong (3+) | 32 (11.9) | 37 (16.4) | 27 (11.9) | 96 (13.3) |
| Positive
(1–3+) | 75 (27.8) | 89 (39.4) | 68 (30.0) | 232 (32.1) |
CD138 expression is correlated with a
less extensive tumour and lower lymph node stage
When CD138-positive and CD138-negative tumours were
compared, CD138-positive tumours were more frequently limited to
the mucosa and submucosa (pT1), while CD138-negative tumours more
often showed extensive infiltration (pT2+) (P=0.008, chi-square
test). Additionally, tumours expressing CD138 less often
metastasized to the lymph nodes (pN0 vs. pN+) than CD138-negative
tumours (P=0.014, chi-square test).
This was also observed separately for strong
expression levels of CD138 compared to no, weak or moderate CD138
expression: patients with high levels of CD138 in tumour tissue had
a lower tumour stage and more often no lymph node metastasis
(P=0.007, P<0.001, chi-square test).
No significant association between CD138 expression
and age or sex of the patients was observed (ANOVA, chi-square
test; data not shown). Furthermore, there was no interdependence
observed between CD138 levels and the presence or absence of
neoadjuvant treatment (chi-square test, Fisher's exact test, ANOVA;
data not shown).
CD138 expression is correlated to
ERBB2 amplification
While CD138-negative tumours only had an
ERBB2 (Her2/neu) amplification in 27.9% of cases, the
majority of CD138-positive tumours were Her2/neu-amplified (58.1%,
P<0.001, chi-square test).
Tumour tissue with strong CD138 expression more
often displayed an Her2/neu amplification than tumours with no or
weaker expression (27.4% vs. 10.7%, P=0.004, chi-square test).
Tumours treated with primary surgery and
CD138-positive tumours were more frequently Her2/neu-amplified as
well compared to negative tumours (53.3% vs. 23.7%, P=0.014,
chi-square test). The same was observed for patients who received
CROSS neoadjuvant therapy (83.3% vs. 34.8%, P<0.001, chi-square
test).
There was no correlation observed between MET
status and CD138 expression (data not shown).
Strong CD138 expression is an
independent favourable prognostic marker
In the next step, CD138 expression was correlated
with overall survival (OS). Survival data were available for 639
patients (88.4%); the minimal follow-up period included was 1
month. During the clinical follow-up, 360 patients died (56.3%).
The median time of follow-up was 22.3 months (range 1–233
months).
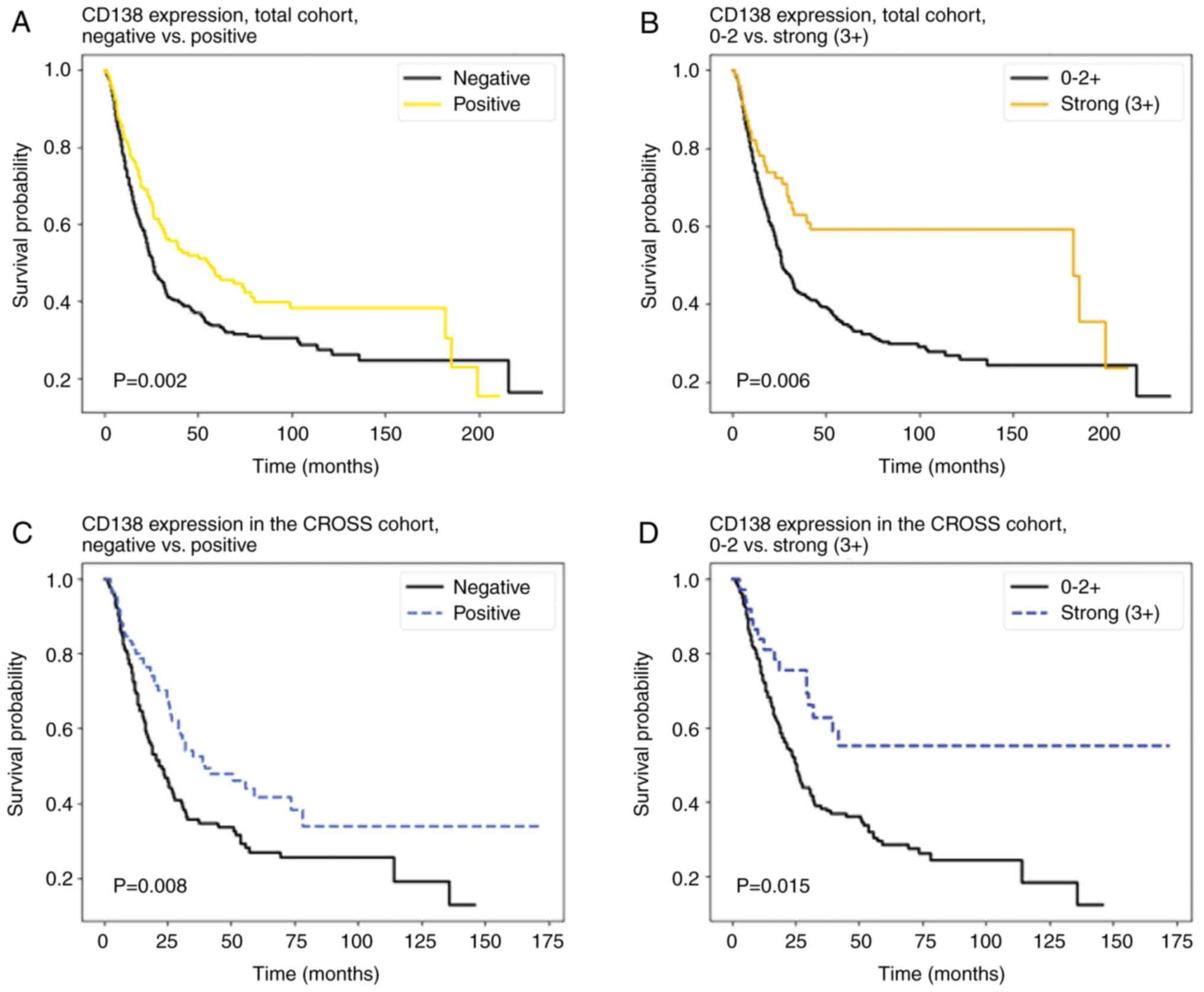
Patients with CD138-positive tumours had a
significantly longer OS than patients without CD138 expression
(26.2 vs. 20.2 months, P=0.002) (Fig.
2A).
In univariate analysis, an expression of CD138 was a
favourable prognostic factor (HR 0.73, 95% CI 0.59–0.92, P=0.007).
When other covariates were included (age, pT, pN, ERBB2
status, MET status), CD138 expression did not remain a
significant prognostic factor (HR 0.87, 95% CI 0.69–1.11,
P=0.28).
When tumours with a strong CD138 expression were
compared to tumours with no or weaker CD138 expression, a
significant survival advantage was seen in tumours with strong
CD138 expression (31.7 vs. 21.5 months, P=0.006), (Fig. 2B). In univariate analysis, strong
CD138 expression was a prognostic favourable factor (HR 0.56, 95%
CI 0.39–0.80, P=0.001). In multivariate Cox analysis, strong levels
of CD138 were an independent favourable prognostic factor as well
(HR 0.63, 95% CI 0.43–0.94, P=0.02) (Table IV).
 | Table IV.Multivariate analysis for CD138
expression in patient cohorts. |
Table IV.
Multivariate analysis for CD138
expression in patient cohorts.
| A, Total
cohort |
|---|
|
|---|
|
| CD138 positive vs.
negative | CD138 strong vs.
negative/weak |
|---|
|
|
|
|
|---|
| Covariate | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.02
(1.01–1.03) | 0.001 | 1.01 (1.01–02) | 0.001 |
| pT | 1.27
(1.08–1.48) | 0.003 | 1.23
(1.08–1.48) | 0.003 |
| pN | 1.49
(1.34–1.65) | <0.001 | 1.48
(1.34–1.65) | <0.001 |
| ERBB2
status | 0.81
(0.53–1.23) | 0.32 | 0.82
(0.54–1.24) | 0.35 |
| MET
status | 1.16
(0.80–1.67) | 0.44 | 1.20
(0.83–1.72) | 0.34 |
| CD138 | 0.87
(0.68–1.11) | 0.27 | 0.63
(0.43–0.94) | 0.02 |
|
| B, CROSS
cohort |
|
|
| CD138 positive
vs. negative | CD138 strong vs.
negative/weak |
|
|
|
|
|
Covariate | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| Age | 1.01
(0.98–1.03) | 0.48 | 1.01
(0.99–1.03) | 0.47 |
| pT | 1.26
(0.95–1.68) | 0.1 | 1.29
(0.97–1.71) | 0.08 |
| pN | 1.57
(1.33–1.84) | <0.001 | 1.54
(1.31–1.81) | <0.001 |
| ERBB2
status | 0.92
(0.43–1.96) | 0.82 | 0.91
(0.43–1.93) | 0.81 |
| MET
status | 1.01
(0.58–1.76) | 0.98 | 1.04
(0.60–1.81) | 0.88 |
| CD138 | 0.66
(0.44–0.97) | 0.04 | 0.43
(0.24–0.79) | 0.007 |
|
| C, (y)pT2+
cohort |
|
|
| CD138 positive
vs. negative | CD138 strong vs.
negative/weak |
|
|
|
|
|
Covariate | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| Age | 1.01
(1.00–1.02) | 0.02 | 1.01
(1.00–1.02) | 0.02 |
| pN | 1.45
(1.31–1.61) | <0.001 | 1.45
(1.31–1.61) | <0.001 |
| ERBB2
status | 0.65
(0.39–1.09) | 0.1 | 0.67
(0.40–1.11) | 0.12 |
| MET
status | 1.15
(0.79–1.68) | 0.46 | 1.19
(0.82–1.74) | 0.36 |
| CD138 | 0.85
(0.66–1.11) | 0.24 | 0.61
(0.39–0.95) | 0.03 |
|
| D, (y)pN0
cohort |
|
|
| CD138 positive
vs. negative | CD138 strong vs.
negative/weak |
|
|
|
|
|
Covariate | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| Age | 1.01
(1.00–1.02) | 0.02 | 1.01
(1.00–1.02) | 0.02 |
| pT | 1.43
(1.13–1.82) | 0.003 | 1.44
(1.14–1.82) | 0.002 |
| ERBB2
status | 0.68
(0.35–1.33) | 0.26 | 0.71
(0.37–1.38) | 0.31 |
| MET
status | 2.34
(1.06–5.16) | 0.04 | 2.33
(1.06–5.14) | 0.04 |
| CD138 | 0.93
(0.60–1.45) | 0.75 | 0.56
(0.29–1.09) | 0.09 |
|
| E,
ERBB2-not amplified |
|
|
| CD138 positive
vs. negative | CD138 strong vs.
negative/weak |
|
|
|
|
|
Covariate | HR (95%
CI) | P-value | HR (95%
CI) | P-value |
|
| Age | 1.02
(1.01–1.03) | <0.001 | 1.02
(1.01–1.03) | <0.001 |
| pT | 1.25
(1.08–1.45) | 0.003 | 1.25
(1.08–1.45) | 0.003 |
| pN | 1.55
(1.41–1.71) | <0.001 | 1.55
(1.40–1.71) | <0.001 |
| MET
status | 1.15
(0.81–1.63) | 0.45 | 1.18
(0.83–1.68) | 0.36 |
| CD138 | 0.85
(0.68–1.07) | 0.16 | 0.66
(0.46–0.94) | 0.02 |
When sub-divided by treatment, no significant
advantage in OS was observed in the cohort with primary surgery or
the FLOT/NOS cohort (data not shown). However, patients who
received neoadjuvant CROSS therapy and had CD138-positive tumours
lived significantly longer (30.75 vs. 20.75 months, P=0.008)
(Fig. 2C). Patients with strong
CD138 expression lived longer compared to patients with weaker
intratumoural CD138 expression (33.2 vs. 23.1 months, P=0.015)
(Fig. 2D). Positivity for CD138 was
a favourable prognostic factor in this subgroup (HR 0.64, 95% CI
0.45–0.91, P=0.02), as well as strong expression (3+) of CD138
compared to weaker expression (HR 0.49, 95% CI 0.29–0.84,
P=0.01).
CD138 is a favourable prognostic
marker in tumours with higher tumour stage
In the next step, the patients were sub-divided into
a cohort with mucosa/submucosa-limited tumours [(y)pT1] and
patients with deeper infiltrating tumours [(y)pT2+]. For (y)pT2+
tumours, a statistical trend towards prolonged OS was observed for
CD138-positive tumours and tumours with strong CD138 expression
(median OS positive tumours: 21.8 months vs. 17.5 months, P=0.07;
median OS tumours with strong CD138 expression: 29.1 months vs.
18.8 months, P=0.08). In multivariate analysis, strong CD138
expression was a significant favourable prognostic factor (HR 0.65,
95% CI 0.43–0.99, P=0.04).
CD138 is a favourable prognostic
marker in tumours without lymph node involvement
The same effect was observed in patients without
lymph node metastasis: patients with (y)pN0 tumours displayed a
statistical trend for longer OS when the tumour was positive for
CD138 (45.3 months vs. 38.7 months, P=0.07). When tumours had
strong CD138 expression, a longer OS was observed as well (45.9
months vs. 39.9 months, P=0.08). In univariate analysis, a strong
CD138 expression (3+) was a favourable prognostic factor (HR 0.52,
95% CI 0.28–0.95, P=0.03). In multivariate analysis, however, a
statistical favourable trend was shown for strong expression of
CD138 (HR 0.56, 95% CI 0.29–1.09, P=0.09) (Table IV).
CD138 is a favourable prognostic
marker in tumours without ERBB2 amplification
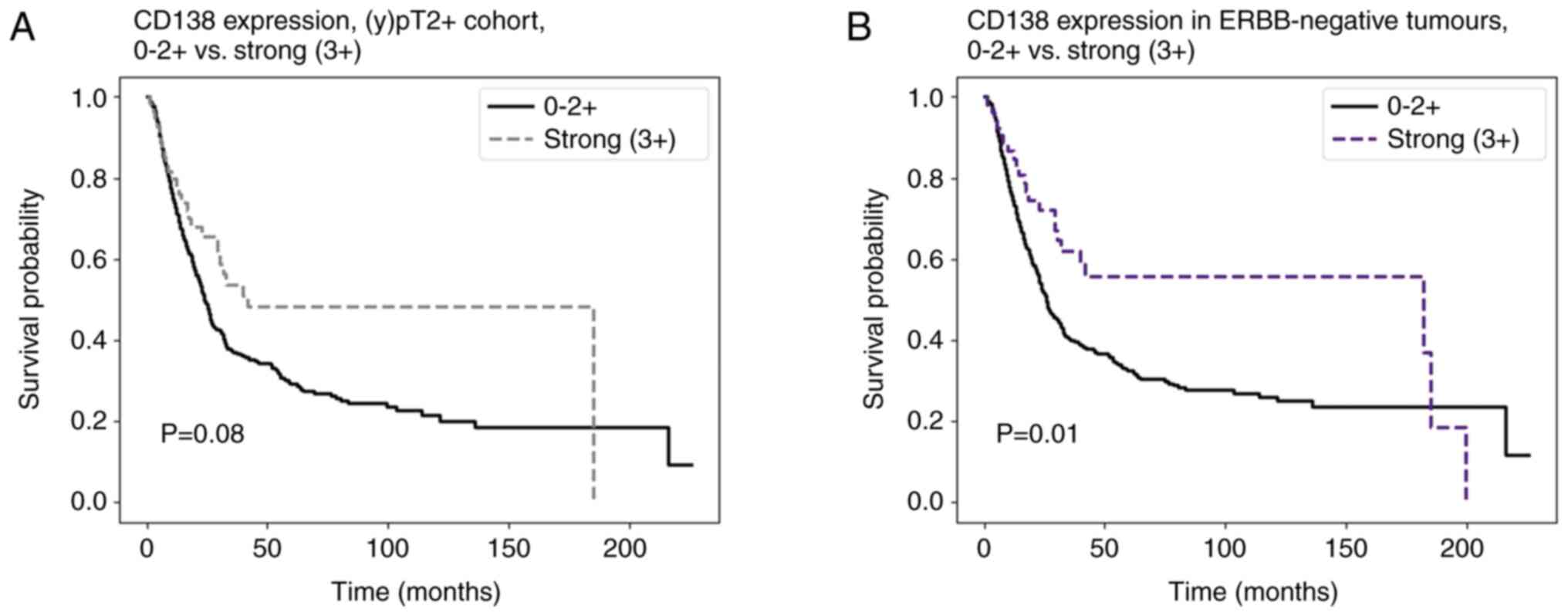
In tumours without Her2/neu amplification, positive
expression of CD138 was significantly correlated to a longer OS
(23.2 months vs. 18.9 months, P=0.03). The same was observed for
strong expression of CD138 compared to weaker or no expression
(29.7 vs. 19.6 months, P=0.01) (Fig.
3). In multivariate analysis, strong expression of CD138
remained an independent favourable prognostic marker (HR 0.61, 95%
CI 0.40–0.93, P=0.02) (Table
IV).
Discussion
In this study, the protein expression of CD138 and
its clinical and molecular as well as prognostic significance were
assessed in the largest cohort of EAC so far. We observed that
CD138 expression was strong in 13.3% of the included cases. These
tumours with high CD138 expression levels may be particularly
amenable to targeted therapy with ADC such as indatuximab
ravtansine. Whilst CD138 is best known for staining plasma cells
and therefore is mainly used in the diagnostic assessment of plasma
cell tumours (31), CD138
expression has been shown in up to 87% of different tumour
entities, in which 71% had strong positive staining in at least one
case (28). To the best of our
knowledge, just one study before has evaluated the expression of
CD138 in a large number of human tumours, but that included only a
limited number of EACs (n=33). Based on this study, 30% of EACs
(n=11) strongly expressed CD138 (28). There are several studies on CD138
expression in tumours and related prognosis: cell-surface CD138
expression has been demonstrated as favourable in mesothelioma,
gastric cancer, hepatocellular carcinoma, cervical cancer and
bladder cancer (32). Kusumoto
et al (33) demonstrated
that in ovarian cancers, epithelial CD138 expression is
significantly lower in advanced disease. Additionally, in prostate
adenocarcinomas, CD138 overexpression predicts early recurrence and
is associated with a higher Gleason grade (34). Lendorf et al (35) found similar results in breast cancer
cases, in which CD138 expression is associated with tumours of
higher grade. There are several studies on CD138 expression and its
prognostic significance in oesophageal squamous cell carcinomas
showing CD138 expression to be higher in less invasive tumours
(lower T stage) and in better differentiated tumours (36,37).
This study demonstrates for the first time that
CD138 is also a favourable prognostic marker in specific patient
subgroups (e.g., those with tumours resected after neoadjuvant
therapy using the CROSS protocol). Since more than 90% of operable
patients receive neoadjuvant therapy and the CROSS protocol is a
very commonly used therapeutic regime in Europe, these findings
have high clinical and therapeutical relevance. However, it must be
noted that, according to the results of the Checkmate 577 study,
all patients with vital tumour after surgery, who received
neoadjuvant CROSS treatment, additionally receive 1 year of
nivolumab; concluding prognostic statements on this specific group
are not yet possible, since this therapy has only been used for a
very short time (38).
Apart from CD138 being a possible prognostic
indicator, therapeutical agents which specifically target CD138
could be a new treatment approach. Several of these agents have
already shown promising results in some cancer types: indatuximab
ravtansine (BT062) is the ADC comprising the anti-CD138 monoclonal
antibody (nBT062) and the microtubule-binding cytotoxic agent
maytansinoid DM4 (39); when used
in multiple myeloma patients, 75% achieved a state of stable
disease (40). In addition, it has
been observed that in CD138-positive, triple-negative breast cell
carcinoma cell lines, indatuximab ravtansine therapy is highly
effective showing complete remission (41). This efficacy of indatuximab
ravtansine in breast carcinoma correlated with the expression
levels of CD138 on tumour cells, assessed with
immunohistochemistry. This emphasizes the potential of CD138 as a
predictive, therapeutically relevant biomarker for EAC as well.
The easy applicability and broad availability of a
prognostic and predictive biomarker is a great advantage in
practical and clinical everyday routine, as the implementation of
PD-L1 and its immense therapeutical implications have demonstrated.
Especially the assessment of a biomarker by immunohistochemistry, a
routine procedure available in almost all pathology institutes,
fulfils these requirements.
Due to the fact that CD138 is expressed in a variety
of normal epithelial cells and plasma cells (28), treatment side effects could be
potentially problematic; indatuximab ravtansine, however, as
demonstrated by first trials, is tolerated well, with the most
common adverse side effects being grade 1 or 2 (diarrhoea and
fatigue) (40). Grade 3–4 adverse
effects are neutropaenia, anaemia and thrombocytopaenia (42). Another newly developed agent is
VIS832, a humanized IgG1-κ monoclonal antibody targeting human
CD138, inducing immune cell-mediated cytotoxicity (26). The preclinical trial demonstrated a
promising efficacy of VIS832 in vitro as well as in
vivo (26).
Our study has some limitations. These include the
retrospective nature of the analyses and the carcinomas were from a
large single-tumour centre. We only studied operable patients.
Future clinical studies should also determine the expression level
of CD138 in endoscopic biopsies from the EAC in nonoperable or
primarily hematogenously metastasized (palliative) patients. As an
advantage, and this can also be understood as a prospect for future
studies: We used a long-established, commercially available and
well-established immunohistochemical antibody to determine CD138 on
tumour cells. Analyses are thus readily reproducible. Also,
clinical trials testing the efficacy of the ADC indatuximab
ravtansine in EAC need to correlate therapeutic response with the
expression level of CD138 on tumour cells. For this, a technique
that is easy to use and also widely available in pathology
institutes is helpful.
In conclusion, this is the largest and most
comprehensive study on the significance of CD138 (syndecan-1)
expression in EAC. We demonstrated that a significant proportion of
EAC is strongly CD138-positive (13.3%). CD138 is already utilized
by ADCs such as indatuximab ravtansine, whose effectiveness depends
on the extent of CD138 on tumour cells. This makes CD138 an ideal
predictive, therapeutically relevant biomarker. Future clinical
trials now need to show how effective the corresponding ADCs are in
CD138-positive EACs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated during and/or analysed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
DA, AS, RB and AQ made substantial contributions to
conception and design. DA, AS, CB and FG were responsible for
analysis and interpretation of data. DA, AS, TZ and AQ wrote the
main manuscript. CB, WS and TZ were responsible for the data
collection. TZ, WS and RB have reviewed the text. All authors were
involved in drafting the manuscript or revising it critically for
important intellectual content. All authors read and approved the
final manuscript. AQ, DA and AS confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The objective of the project is primarily in the
field of diagnostics and quality assurance; approval was obtained
from the University of Cologne Ethics Committee (approval nos.
20-1583 and 10-242). All authors confirm that methods used were
carried out in accordance with relevant guidelines and regulations.
The experimental protocols were approved by the licensing
committees. We confirm that written informed consent was obtained
from all subjects and/or their legal guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lepage C, Rachet B, Jooste V, Faivre J and
Coleman MP: Continuing rapid increase in esophageal adenocarcinoma
in England and Wales. Am J Gastroenterol. 103:2694–2699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tustumi F, Kimura CM, Takeda FR, Uema RH,
Salum RA, Ribeiro-Junior U and Cecconello I: Prognostic factors and
survival analysis in esophageal carcinoma. Arq Bras Cir Dig.
29:138–141. 2016.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He H, Chen N, Hou Y, Wang Z, Zhang Y,
Zhang G and Fu J: Trends in the incidence and survival of patients
with esophageal cancer: A SEER database analysis. Thorac Cancer.
11:1121–1128. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lagergren J and Lagergren P: Oesophageal
cancer. BMJ. 341:c62802010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Surgical resection with or without
preoperative chemotherapy in oesophageal cancer, . A randomised
controlled trial. Lancet. 359:1727–1733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gebski V, Burmeister B, Smithers BM, Foo
K, Zalcberg J and Simes J; Australasian Gastro-Intestinal Trials
Group, : Survival benefits from neoadjuvant chemoradiotherapy or
chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet
Oncol. 8:226–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doi T, Piha–Paul SA, Jalal SI, Mai–Dang H,
Saraf S, Csiki MK and Bennouna J: Updated results for the advanced
esophageal carcinoma cohort of the phase 1b KEYNOTE-028 study of
pembrolizumab. J Clin Oncol. 34 (Suppl 15):S4046. 2016. View Article : Google Scholar
|
|
11
|
Piro G, Carbone C, Santoro R, Tortora G
and Melisi D: Predictive biomarkers for the treatment of resectable
esophageal and esophago-gastric junction adenocarcinoma: From
hypothesis generation to clinical validation. Expert Rev Mol Diagn.
18:357–370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bernfield M, Götte M, Park PW, Reizes O,
Fitzgerald ML, Lincecum J and Zako M: Functions of cell surface
heparan sulfate proteoglycans. Annu Rev Biochem. 68:729–777. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Czarnowski D: Syndecans in cancer: A
review of function, expression, prognostic value, and therapeutic
significance. Cancer Treat Res Commun. 27:1003122021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palaiologou M, Delladetsima I and Tiniakos
D: CD138 (syndecan-1) expression in health and disease. Histol
Histopathol. 29:177–189. 2014.PubMed/NCBI
|
|
15
|
Nikolova V, Koo CY, Ibrahim SA, Wang Z,
Spillmann D, Dreier R, Kelsch R, Fischgräbe J, Smollich M, Rossi
LH, et al: Differential roles for membrane-bound and soluble
syndecan-1 (CD138) in breast cancer progression. Carcinogenesis.
30:397–407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hassan H, Greve B, Pavao MS, Kiesel L,
Ibrahim SA and Götte M: Syndecan-1 modulates β-integrin-dependent
and interleukin-6-dependent functions in breast cancer cell
adhesion, migration, and resistance to irradiation. FEBS J.
280:2216–2227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fears CY and Woods A: The role of
syndecans in disease and wound healing. Matrix Biol. 25:443–456.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhodapkar MV, Abe E, Theus A, Lacy M,
Langford JK, Barlogie B and Sanderson RD: Syndecan-1 is a
multifunctional regulator of myeloma pathobiology: Control of tumor
cell survival, growth, and bone cell differentiation. Blood.
91:2679–2688. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schönfeld K, Zuber C, Pinkas J, Häder T,
Bernöster K and Uherek C: Indatuximab ravtansine (BT062)
combination treatment in multiple myeloma: Pre-clinical studies. J
Hematol Oncol. 10:132017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly KR, Chanan–Khan A, Heffner LT, Somlo
G, Siegel DS, Zimmerman T, Karnad A, Munshi NC, Jagannath S,
Greenberg AL, et al: Indatuximab ravtansine (BT062) in combination
with lenalidomide and low-dose dexamethasone in patients with
relapsed and/or refractory multiple myeloma: Clinical activity in
patients already exposed to lenalidomide and bortezomib. Blood.
124:47362014. View Article : Google Scholar
|
|
21
|
Koster KL, Huober J and Joerger M: New
antibody-drug conjugates (ADCs) in breast cancer-an overview of
ADCs recently approved and in later stages of development. Explor
Target Antitumor Ther. 3:27–36. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rugo HS, Tolaney SM, Loirat D, Punie K,
Bardia A, Hurvitz SA, O'Shaughnessy J, Cortés J, Diéras V, Carey
LA, et al: Safety analyses from the phase 3 ASCENT trial of
sacituzumab govitecan in metastatic triple-negative breast cancer.
NPJ Breast Cancer. 8:982022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoppe S, Meder L, Gebauer F, Ullrich RT,
Zander T, Hillmer AM, Buettner R, Plum P, Puppe J, Malter W and
Quaas A: Trophoblast cell surface Antigen 2 (TROP2) as a predictive
bio-marker for the therapeutic efficacy of sacituzumab govitecan in
adenocarcinoma of the esophagus. Cancers (Basel). 14:47892022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ibrahim SA, Hassan H, Vilardo L, Kumar SK,
Kumar AV, Kelsch R, Schneider C, Kiesel L, Eich HT, Zucchi I, et
al: Syndecan-1 (CD138) modulates triple-negative breast cancer stem
cell properties via regulation of LRP-6 and IL-6-mediated STAT3
signaling. PLoS One. 8:e857372013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schönfeld K, Herbener P, Zuber C, Häder T,
Bernöster K, Uherek C and Schüttrumpf J: Activity of indatuximab
ravtansine against triple-negative breast cancer in preclinical
tumor models. Pharm Res. 35:1182018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu T, Chaganty B, Lin L, Xing L,
Ramakrishnan B, Wen K, Hsieh PA, Wollacott A, Viswanathan K, Adari
H, et al: VIS832, a novel CD138-targeting monoclonal antibody,
potently induces killing of human multiple myeloma and further
synergizes with IMiDs or bortezomib in vitro and in vivo. Blood
Cancer J. 10:1102020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. John Wiley
& Sons; 2017
|
|
28
|
Kind S, Merenkow C, Büscheck F, Möller K,
Dum D, Chirico V, Luebke AM, Höflmayer D, Hinsch A, Jacobsen F, et
al: Prevalence of Syndecan-1 (CD138) expression in different kinds
of human tumors and normal tissues. Dis Markers. 2019:49283152019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Simon R, Mirlacher M and Sauter G:
Immunohistochemical analysis of tissue microarrays. Methods Mol
Biol. 664:113–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plum PS, Gebauer F, Krämer M, Alakus H,
Berlth F, Chon SH, Schiffmann L, Zander T, Büttner R, Hölscher AH,
et al: HER2/neu (ERBB2) expression and gene amplification
correlates with better survival in esophageal adenocarcinoma. BMC
Cancer. 19:382019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chilosi M, Adami F, Lestani M, Montagna L,
Cimarosto L, Semenzato G, Pizzolo G and Menestrina F:
CD138/syndecan-1: A useful immunohistochemical marker of normal and
neoplastic plasma cells on routine trephine bone marrow biopsies.
Mod Pathol. 12:1101–1106. 1999.PubMed/NCBI
|
|
32
|
Szatmári T, Ötvös R, Hjerpe A and Dobra K:
Syndecan-1 in cancer: Implications for cell signaling,
differentiation, and prognostication. Dis Markers. 2015:7960522015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kusumoto T, Kodama J, Seki N, Nakamura K,
Hongo A and Hiramatsu Y: Clinical significance of syndecan-1 and
versican expression in human epithelial ovarian cancer. Oncol Rep.
23:917–925. 2010.PubMed/NCBI
|
|
34
|
Zellweger T, Ninck C, Mirlacher M,
Annefeld M, Glass AG, Gasser TC, Mihatsch MJ, Gelmann EP and
Bubendorf L: Tissue microarray analysis reveals prognostic
significance of syndecan-1 expression in prostate cancer. Prostate.
55:20–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lendorf ME, Manon-Jensen T, Kronqvist P,
Multhaupt HA and Couchman JR: Syndecan-1 and syndecan-4 are
independent indicators in breast carcinoma. J Histochem Cytochem.
59:615–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mikami S, Ohashi K, Usui Y, Nemoto T,
Katsube K, Yanagishita M, Nakajima M, Nakamura K and Koike M: Loss
of syndecan-1 and increased expression of heparanase in invasive
esophageal carcinomas. Jpn J Cancer Res. 92:1062–1073. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szumilo J, Burdan F, Zinkiewicz K, Dudka
J, Klepacz R, Dabrowski A and Korobowicz E: Expression of
syndecan-1 and cathepsins D and K in advanced esophageal squamous
cell carcinoma. Folia Histochem Cytobiol. 47:571–578.
2009.PubMed/NCBI
|
|
38
|
Horiba MN, Casak SJ, Mishra-Kalyani PS,
Roy P, Beaver JA, Pazdur R, Kluetz PG, Lemery SJ and Fashoyin-Aje
LA: FDA approval summary: Nivolumab for the adjuvant treatment of
adults with completely resected esophageal/gastroesophageal
junction cancer and residual pathologic disease. Clin Cancer Res.
28:5244–5248. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ikeda H, Hideshima T, Fulciniti M, Lutz
RJ, Yasui H, Okawa Y, Kiziltepe T, Vallet S, Pozzi S, Santo L, et
al: The monoclonal antibody nBT062 conjugated to cytotoxic
Maytansinoids has selective cytotoxicity against CD138-positive
multiple myeloma cells in vitro and in vivo. Clin Cancer Res.
15:4028–4037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jagannath S, Heffner LT Jr, Ailawadhi S,
Munshi NC, Zimmerman TM, Rosenblatt J, Lonial S, Chanan-Khan A,
Ruehle M, Rharbaoui F, et al: Indatuximab ravtansine (BT062)
monotherapy in patients with relapsed and/or refractory multiple
myeloma. Clin Lymphoma Myeloma Leuk. 19:372–380. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schönfeld K, Herbener P, Zuber C, Häder T,
Bernöster K, Uherek C and Schüttrumpf J: Activity of indatuximab
ravtansine against triple-negative breast cancer in preclinical
tumor models. Pharm Res. 35:1182018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kelly KR, Ailawadhi S, Siegel DS, Heffner
LT, Somlo G, Jagannath S, Zimmerman TM, Munshi NC, Madan S,
Chanan-Khan A, et al: Indatuximab ravtansine plus dexamethasone
with lenalidomide or pomalidomide in relapsed or refractory
multiple myeloma: A multicentre, phase 1/2a study. Lancet Haematol.
8:e794–e807. 2021. View Article : Google Scholar : PubMed/NCBI
|