The S100 protein family is the largest innate immune
protein family containing EF-hand structural domains in vertebrates
(1,2). As damage-associated molecular
patterns, the expression of S100 proteins is increased to exert
anti-infective effects following bacterial infection in epithelial
cells (3,4). However, the overexpression of S100
protein family members leads to the secretion of numerous
cytokines, chemokines or growth factors, such as tumor necrosis
factor-α, C-C motif chemokine ligand (CCL)-2, CCL-20, C-X-C motif
chemokine ligand 10, and interleukin (IL)-1, IL-6, IL-8, IL-10 and
IL-12 (5–8). These factors cause tissue damage
(9), and attract and activate
immunosuppressive cells to alter the tumor microenvironment,
promoting tumor growth and metastasis (10). The structure of S100 proteins is
highly conserved in vertebrates (11), and is comprised of three exons.
Thus, family members exhibit a number of similarities in both
sequence and conformation (5); they
all have hydrophobic amino acid domains in the N- and C-terminal
regions (11). Notably, the first
exon following the initiation codon of each member is unique, and
each protein possesses specific functions. To date, ~25 family
members have been identified (2,12–15).
Among these, the S100A subfamily (S100A1-S100A18) is
clustered in the epidermal differentiation complex region of 1q21;
S100B is located on 21q22, S100G is located on Xp22,
S100P is located on 4p16 and S100Z is located on 5q13
(2,5,12,16,17).
While S100S, S100T and S100U are all orthologs in
fish, no corresponding gene locus has been located in humans. The
majority of the aforementioned family members exist as low
molecular weight dimers of 9–14 kDa (5). In addition, some proteins form
tetramers, while S100G exists as a monomer (12,13).
The expression of S100 proteins is tissue-specific (12,16–18).
S100A1 and S100A3 are predominantly expressed in heart and hair
cuticle cells, respectively (12,16).
S100A6 is preferentially expressed in fibroblasts (16). S100A8 and S100A9 are constitutively
expressed in myeloid cells (16,17).
S100B is normally expressed in the brain and can be used as a
marker for brain injury (18).
However, tumor progression can alter their expression and disrupt
these specific expression patterns. The expression levels of
S100A2, S100A4, S100A6, S100A10, S100A14 and S100A16 are high in
pancreatic ductal adenocarcinoma (19). The overexpression of S100A8, S100A9,
S100A11 and S100P has been shown to be associated with a worse
prognosis of patients with breast cancer (16); however, the expression of S100A1,
S100A3, S100A5, S100A6, S100A13 and S100G is associated with
improved outcomes of patients with ovarian cancer (17); these findings suggest that the S100
proteins play complex roles in the development of cancer.
The most common types of head and neck squamous cell
carcinoma (HNSCC) are oral squamous cell carcinoma (OSCC),
hypopharyngeal squamous cell carcinoma (HSCC), nasopharyngeal
squamous cell carcinoma (NSCC) and papillary thyroid carcinoma
(PTC) (20). Unlike other tumors,
HNSCC has a complex anatomical structure at its site of origin,
where numerous muscles, bones, blood vessels and nerves congregate,
which protect the brain and participate in respiration, metabolism
and other physiological processes (21). Due to the unique nature of the head
and neck region, surgical resection may impede the ability of the
patient to speak and swallow, which would significantly decrease
their quality of life (22).
Oncolytic virus therapy (23),
chimeric antigen receptor T-cells (24), immune checkpoint inhibitor therapy
(25) and other cutting-edge
immunotherapy approaches, such as peptide vaccines, mRNA vaccines
and adoptive cell therapy using T-cell receptor-engineered T cells,
have made a significant impact in HNSCC recently (26–28).
However, the use of these immunotherapy approaches is associated
with high costs, and the treatment efficacy can be influenced by
multiple factors of the tumor microenvironment (25); thus, the disease still has a 5-year
overall survival rate of <50% (29). Currently, there is an urgent need
for HNSCC prevention, early diagnosis and therapy. The results of a
previous study demonstrated that HNSCC is closely associated with
hypercalcemia (30). However,
numerous members of the S100 protein family are calcium receptors,
and the structure and function of their encoded proteins are
regulated by Ca2+ (2).
When Ca2+ is injected through voltage-gated or
receptor-mediated channels, the conformation and hydrophobicity of
S100 proteins are changed via converting or binding the signal of
Ca2+ (2,14). This leads to interaction with the
hydrophobic structural domains of target proteins, resulting in
cell cycle dysregulation and apoptosis (2,14). In
addition, S100 proteins are involved in the formation of the
squamous epithelial keratinized envelope (31), which forms a keratinized layer to
protect the skin barrier. Notably, the head and neck are covered by
numerous squamous epithelial cells. Some studies have indicated
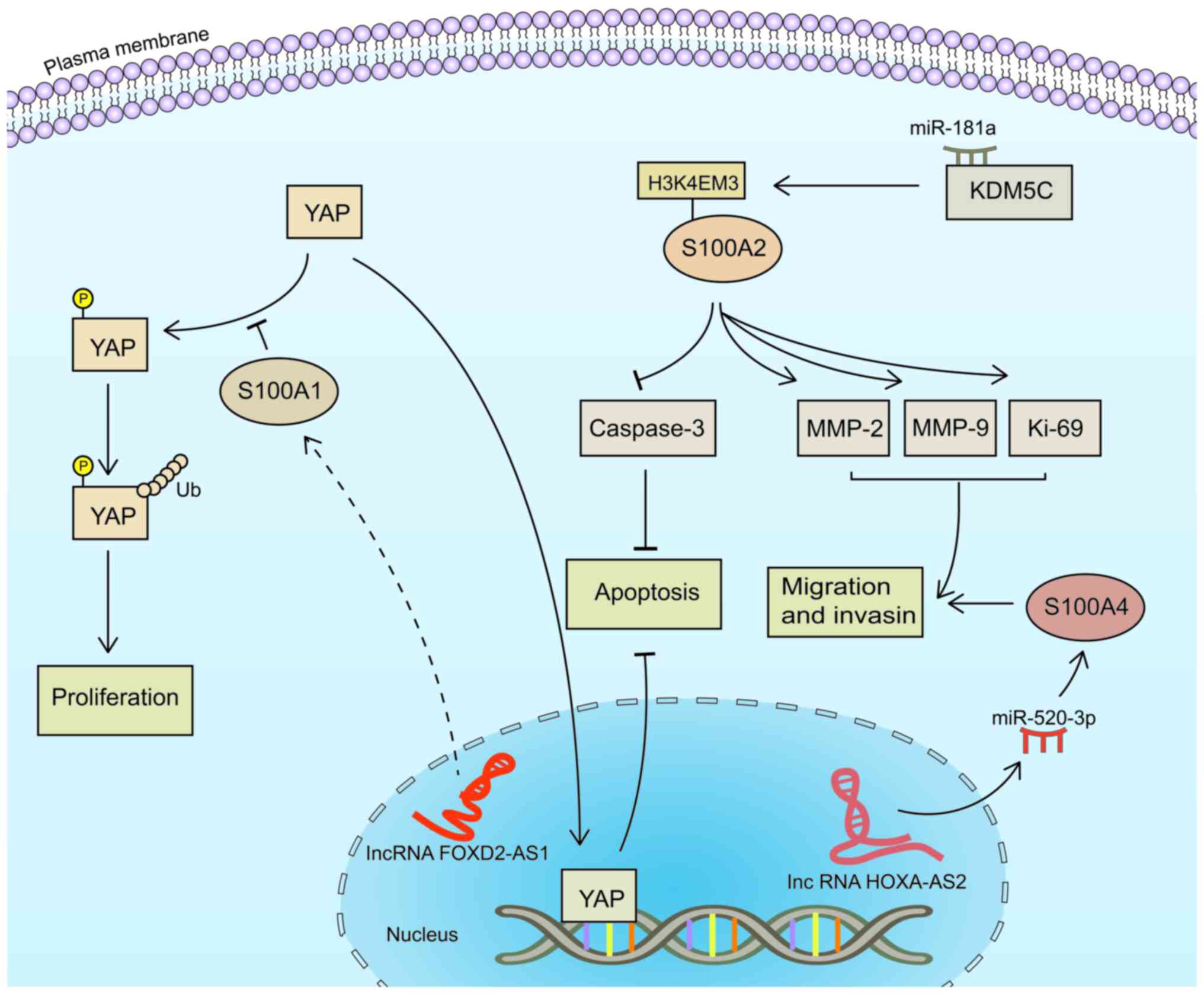
that S100 proteins and HNSCC may be closely associated (31–34).
An investigation of the association between S100 proteins and HNSCC
is thus necessary. Therefore, the expression and mechanisms
underlying S100 protein family members in the aforementioned
subtypes of HNSCC are discussed in the present review. The present
review also aimed to discuss whether S100 protein family members
exhibit potential as therapeutic targets of HNSCC, and whether
specific changes in S100 protein family members in HNSCC are caused
by human papillomavirus (HPV).
Changes in the expression of S100 protein members
differ in OSCC. The results of previous studies have demonstrated
that the expression levels of S100A1, S100A3, S100A6, S100A11,
S100A13, S100A14, S100A16 and S100Z are decreased in patients with
OSCC, while the expression levels of S100A2, S100A4, S100A7,
S100A8, S100A10, S100A12 and S100P are increased (15,35–46).
In a previous study, the transcriptome analysis of 93 specimens
from OSCC tissues and 87 specimens from adjacent tissues
demonstrated that the S100A1 and S100A4 expression
levels were significantly decreased in OSCC, while those of
S100A2, S100A3, S100A7 and S100A11 were increased,
compared with those in adjacent tissues (47). Moreover, it has been demonstrated
that S100A9 expression is increased in OSCC (15,40,48),
whereas the invasion of T1- and T2-stage OSCC mediated by S100A9
has been found to be reduced following treatment with an anti-CD147
antibody (49). However, The Cancer
Genome Atlas (TCGA) demonstrated that the S100A8 and
S100A9 expression levels are reduced in 90% of OSCC cases
(50), and this reduction was
associated with decreased tumor grading, a decreased expression of
epidermal growth factor receptor, epithelial differentiation, the
overexpression of apoptosis-related genes, and reduced tumor cell
migration and invasion (51). These
factors may be due to a wide range of sites involved in OSCC.
Therefore, different tissue sites have been selected and different
reagents have been used in different experiments. S100A7 expression
was previously examined by the immunohistochemical staining of 41
samples from OSCC tissues (>45 years old), 36 samples from OSCC
tissues (<45 years old), 40 samples from oral potentially
malignant disorders (OPMD) and 36 samples from oral inflammatory
lesions (45). Although S100A7
expression was significantly increased in the nuclear, cytoplasmic
and membrane staining of OSCC tissues, it was significantly
decreased in OPMD, and it was completely absent from oral
inflammatory lesions (45). S100A7
may thus serve as a marker to distinguish between oral inflammatory
lesions, OPMD and OSCC. The expression of S100A7 protein was not,
however, associated with age in that study (45). Another study found a negative
association between age and the overexpression of S100A7 mRNA in
OSCC (38). The age groups used in
the two studies differed, however; the first study (45) used 45 years as the grouping age,
while the second study (38) used
65 years. Additionally, the assessment methods used in the two
studies differed; immunohistochemical staining was used to detect
S100A7 protein expression in the first study (45), while qPCR was used to detect S100A7
mRNA expression in the second study (38).
It was previously discovered that the overexpression
of S100A16 not only inhibited the proliferation and invasion of
OSCC cells, but also significantly reduced their ability of sphere
formation (44). The sphere
formation potency, which was used as a surrogate to isolate cancer
stem cells (CSCs) from various tumors, can be used to evaluate the
capability for self-renewal in vitro (52). It was also found that the expression
of self-renewal markers, such as octamer-binding transcription
factor 4A or B-cell-specific moloney murine leukemia virus
integration site 1, was downregulated, confirming the results of
sphere formation (44).
Additionally, tumors with higher differentiation in mouse models
caused by S100A16 overexpression may be related to the upregulation
of the expression of involucrin, cytokeratin and other
differentiation markers (44). The
10-year survival rate is poorer in patients with OSCC, as OSCC has
lower levels of S100A16 protein than normal tissue, which promotes
tumor proliferation and metastasis (44). Sphere formation potency was also
associated with drug resistance (52,53).
Previously, the KB, SAS and DU145 cell lines with sphere formation
ability were demonstrated to exhibit resistance to lapatinib, which
was not favorable for the treatment of patients with OSCC (52,53);
however, S100A16 was involved in this process required further
investigation. OSCC recurrence is also closely linked to S100
proteins. In addition to downregulating E-cadherin expression
(54) to facilitate tumor
metastasis, the overexpression of S100A4 in OSCC can also regulate
stem cells or CSCs, leading to OSCC recurrence through the
phosphatase and tensin homolog (PTEN)/phosphorylated intracellular
phosphatidylinositol kinase (PI3K)/protein kinase B (AKT) signaling
pathway (42). In a previous study,
235 Indian patients with OSCC were divided into the high- and
low-risk groups based on the degree of staining of S100A2 in the
cytoplasm (43). Only 30% of
patients in the low-risk group relapsed, compared to 86% of
patients in the high-risk group who experienced recurrence or
mortality within 1 year. This finding was confirmed in a cohort of
Canadian patients with OSCC, where the rates of recurrence and
mortality within 1 year were 81% in the high-risk group and 29% in
the low-risk group, respectively (43). It is thus suggested that S100
proteins affect the prognosis of patients with OSCC.
DNA, RNA, proteins and other substances which are
associated with the initiation and progression of OSCC may be
secreted into saliva; thus, this is considered as a main source of
biomarkers, which can be collected in a non-invasive manner
(60). In a previous study, the
proteomics analysis of 60 saliva samples from healthy individuals,
and patients with OPMD and OSCC revealed that the level of S100A2
in saliva was significantly higher in patients with OSCC, which may
be related to the invasion of OSCC (61), indicating that the detection of
salivary S100 proteins may be helpful for diagnosis. The efficacy
of salivary S100A2 alone in predicting OSCC was poor, and when
combined with solute carrier family 3 member 2 and IL-1 receptor
antagonist protein, the sensitivity of diagnosis was increased to
83.33% (61). Additionally, it was
previously discovered that there was a high association between the
expression levels of S100A7 and S100A8 in the saliva of patients
with OSCC and the tumor clinical stage (41). The analysis of saliva samples from
100 patients with OSCC revealed that the S100A7 protein was
substantially elevated in both T1 and T2 stages (41). The area under receiver-operating
characteristic curves (AUROC) was used to rate the sensitivity and
specificity of S100A7 protein in predicting T1 stage OSCC [0.71
(95% CI, 0.88–1.04)] and T2 stage OSCC [0.68 (95% CI, 0.89–1.01)],
respectively (41). Therefore,
salivary S100A7 protein is a possible marker for OSCC at the T1 and
T2 stages. The salivary S100A8 protein may be used as a potential
marker of OSCC at the T3 and T4 stages. In patients with T3 and T4
stage OSCC, S100A8 protein was detected in 92.9 and 100% of
samples, respectively (41). In
patients with T3 stage OSCC, the AUROC was 0.99 (95% CI,
0.58–1.00), while in those with T4 stage disease, it was 0.98 (95%
CI, 0.63–1.06) (41). According to
the aforementioned studies, salivary S100 proteins may prove
helpful for the diagnosis of OSCC and even the clinical stage.
However, the collection time, processing manner, measurement method
and storage conditions of saliva remain to be standardized
(62).
The prognosis of patients with HSCC may be affected
by the expression of S100 proteins. Patients with HSCC with a low
S100A12 expression do not often respond to conventional
treatment; thus, the prognosis of patients with HSCC who have a
high expression of S100A12 is improved, compared with that
of those with low expression levels (51). However, the prognosis of patients
with HSCC with high levels of S100A4 expression differs. The
results of a previous study demonstrated that migration and
invasion were inhibited following the knockdown of S100A4 in
FaDu cells, and HSCC metastasis in Drosophila and mouse
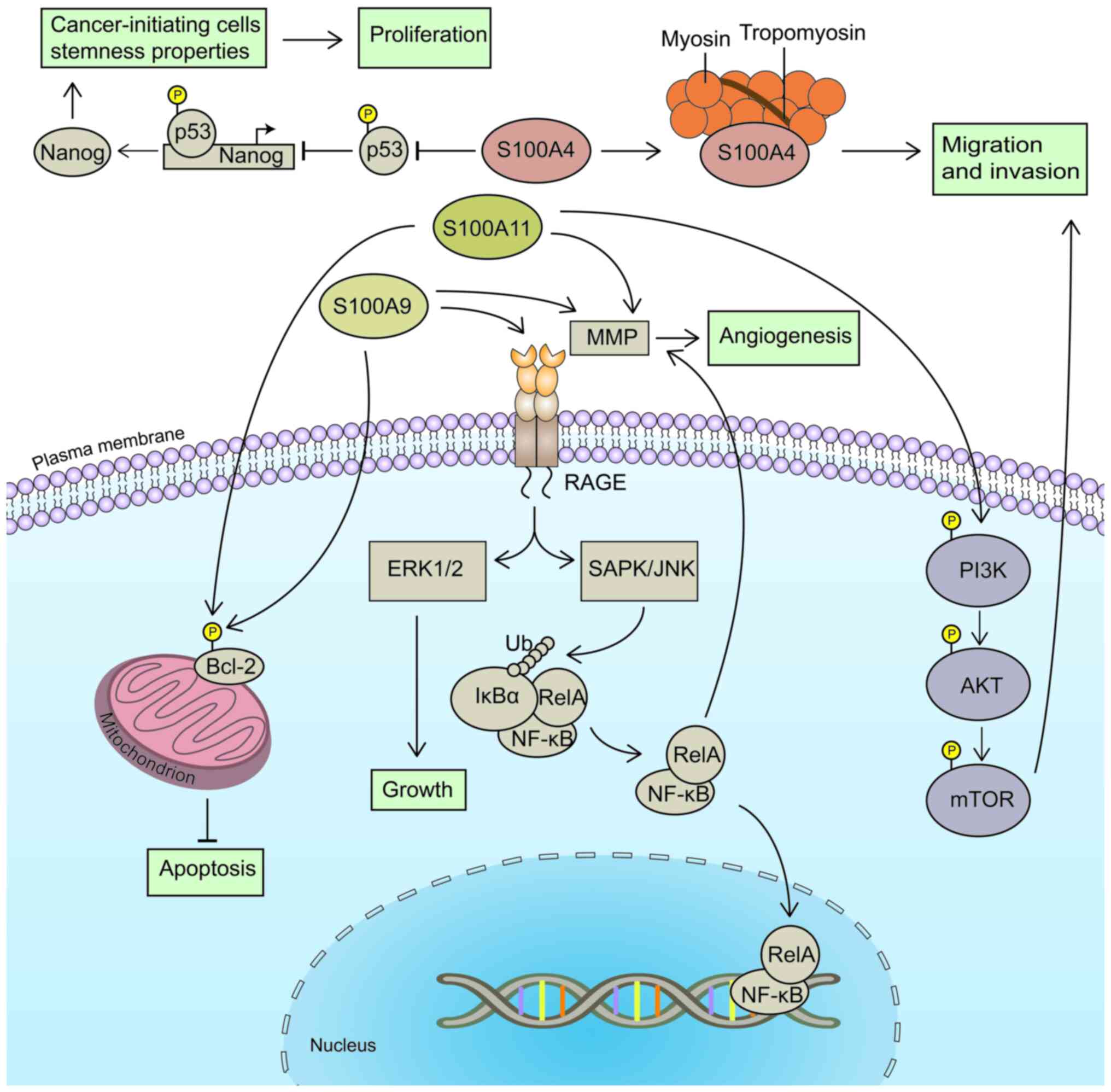
models was reduced (63). On the
one hand, S100A4 may inhibit the activation of p53 and reduce p53
binding to the promoter region of Nanog to upregulate its
transcriptional activity (64).
This may enhance the stem cell properties of cancer-initiating
cells (64), thus promoting tumor
proliferation (Fig. 2). On the
other hand, S100A4 may interact with actin and tropomyosin to cause
cell migration (12). Therefore,
patients with HSCC with an elevated expression of S100A4 often
exhibit a poor prognosis (Fig. 2).
The expression levels of S100A9 and S100A11 in HSCC are markedly
higher than those in adjacent healthy tissues (65,66).
Moreover, the expression of S100A9 increases with the severity of
clinical stages; its expression is higher in patients with stage
III and IV HSCC than in those with stage I and II disease (65). Compared with patients with a low
S100A9 expression, patients with high expression levels of S100A9
also exhibit a poor prognosis and a significantly reduced 5-year
survival rate (65).
The expression levels of matrix metalloprotein
(MMP)2, MMP7 and MMP9 have been found to be significantly decreased
following the downregulation of S100A9 and S100A11
expression (65,66) (Fig.
2). MMPs belong to the zinc finger protein family that degrade
almost all components of the extracellular matrix (67), and are thus considered essential
proteases in the progression of epithelial-mesenchymal transition.
MMPs also induce angiogenesis (68), to provide energy to support tumor
cell diffusion. In addition, the downregulation of S100A9
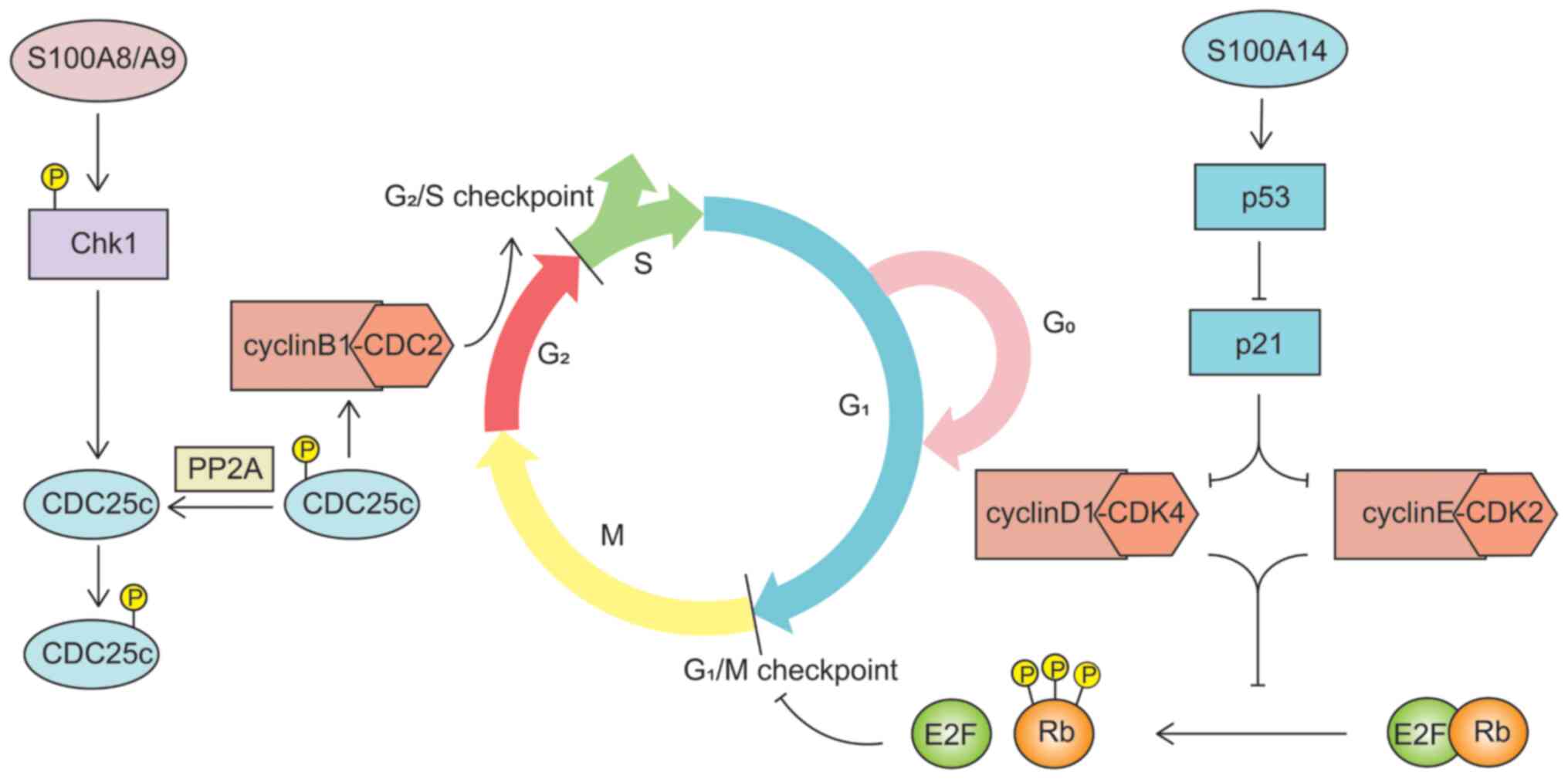
expression has been shown to significantly reduce the expression of
nuclear factor κB (NF-κB), the phosphorylation of NF-κB and B-cell
lymphoma 2 (Bcl-2) (65). Notably,
restoring the expression of NF-κB reverses the inhibitory effects
on cell proliferation and invasion (65). Bcl-2 is a well-established inhibitor
of apoptosis, regulating apoptosis by controlling the release of
mitochondrial cytochrome c (69). Apoptosis is also promoted via the
reduction of mitochondrial cytochrome c phosphorylation
(69). Thus, S100A9 may promote
HSCC proliferation in this manner (Fig.
2). In addition, S100A9 may bind the receptor of advanced
glycosylation end products (RAGE) to promote the phosphorylation of
extracellular regulated protein kinases 1/2 (ERK1/2),
stress-activated protein kinase/c-Jun amino-terminal kinase (JNK)
and inhibitor of NF-κBα (IκBα) (70). In turn, this activates the
mitogen-activated protein kinase (MAPK) signaling pathway and
causes the nuclear translocation of NF-κB (70) (Fig.
2). Moreover, the expression levels of PI3K, AKT, mammalian
target of rapamycin (mTOR) and Bcl-2 have been shown to be reduced
following the knockdown of S100A11 in FaDu cells, suggesting
that S100A11 may affect migration via mediating the PI3K/Akt/mTOR
signaling pathway (66) (Fig. 2). In conclusion, S100 proteins may
affect downstream genes in HSCC through NF-κB or PI3K/Akt/mTOR
signaling pathways, to promote the initiation and progression of
HSCC.
The sustained upregulation of S100A4 expression is
observed during dedifferentiation, using two-dimensional liquid
chromatography and tandem mass spectrometry, combined with isotopic
labeling, and relative and absolute quantification techniques
(71). These methods have been used
to analyze keratinizing NSCC, non-keratinizing NSCC and
undifferentiated NSCC, and the results have demonstrated that
S100A7, S100A8 and S100A9 expression levels are also significantly
reduced (71). In a previous study,
the S100A6 protein levels were higher in the poorly differentiated
cell line, CNE2, than in the well-differentiated cell line, NP96,
in NSCC, and S100A6 expression was also increased (72). The upregulation of S100A6 led to
cell proliferation, which was decreased by SB20358 disrupting the
p38/MAPK signaling pathway, which led to cell growth arrest and
apoptosis (72). These findings
suggest that S100A6 controls the proliferation of NSCC cells via
the p38/MAPK signaling pathway (72). The growth of NSCC, as well as its
differentiation are both enhanced by S100 proteins. S100A8
and S100A9 not only promote the activation of Thr308 and
Ser473 through the PI3K/AKT pathway (73), following the control of the
expression of effector targets in the nucleus to control the
expression of effector targets in the nucleus and promote cell
proliferation, but also increase the expression of MMP7, MMP9 and
MMP12, which are involved in the metastasis of NSCC (67). The proliferation or migration of
C666-1 cells is increased by S100P combined with RAGE, which also
activates the NF-κB signaling pathway and MAPK (74).
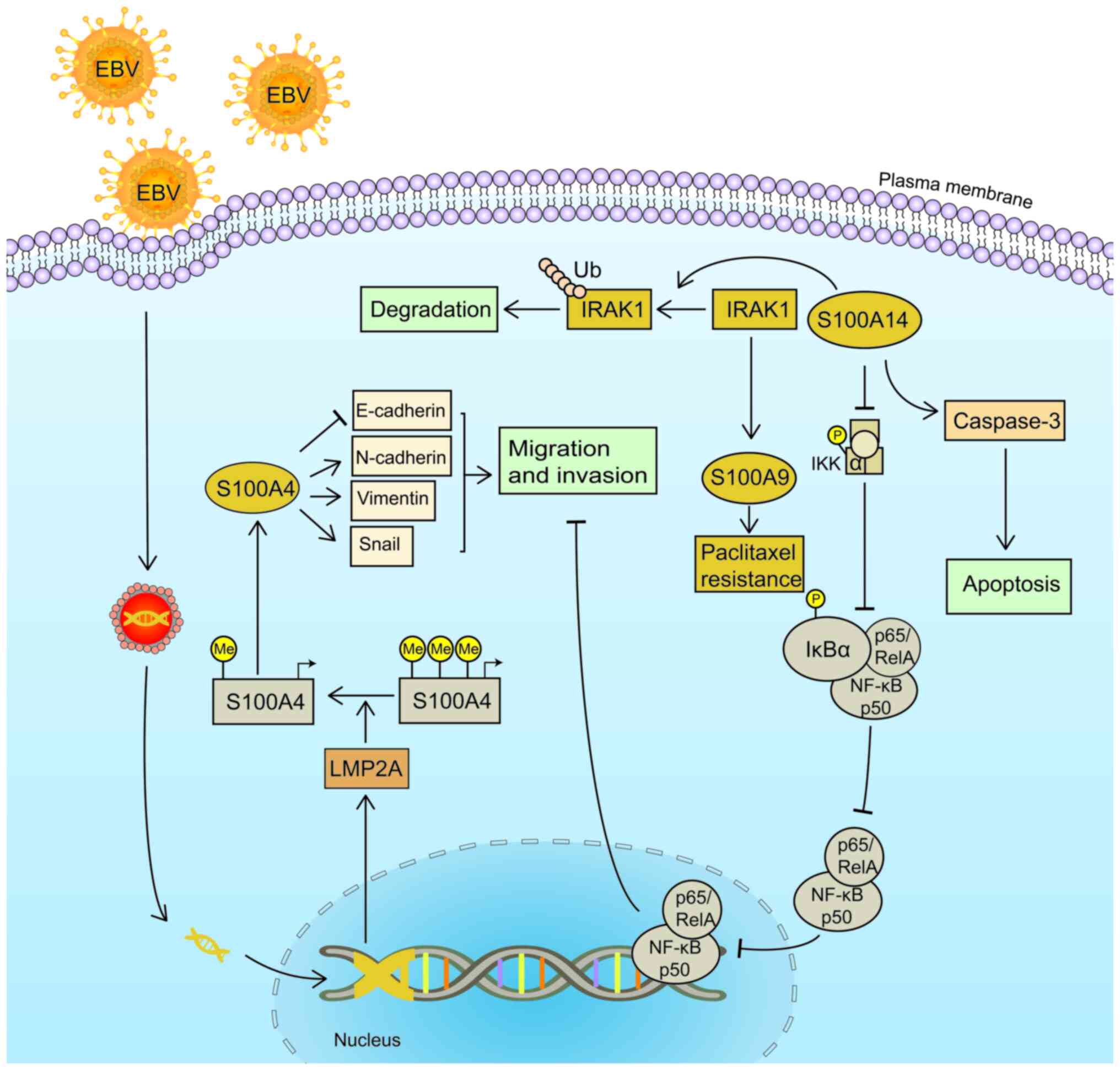
At present, paclitaxel is one of the conventional
treatment strategies used for NSCC (75); however, metastasis to the neck,
local recurrence or drug resistance may lead to a decreased
efficacy. The increased phosphorylation of IL-1 receptor associated
kinase (IRAK1) was found in NSCC samples following metastasis and
recurrence caused by paclitaxel resistance. Notably, the
IRAK1/S100A9 axis is closely associated with drug resistance in
NSCC (75) (Fig. 3). The sensitivity to paclitaxel was
found to be increased in S26 and CNE2 cell lines following
S100A9 knockdown; however, the resistance to paclitaxel was
restored following the addition of recombinant S100A9 (75). Notably, the deletion of IRAK1
also significantly reduced the expression of S100A9 (75). S100A14 promoted the
degradation of IRAK1, and the expression and phosphorylation of
IRAK1 were reduced following treatment with the IRAK1 inhibitor,
T2457, in CNE-1 and S26 cell lines (76). In addition, it was also demonstrated
that the increased expression of S100A14 and the inhibition
of cell migration were reversed following treatment with IL-1β to
induce IRAK1 phosphorylation (76).
Following the overexpression of S100A14 in S18 and 5–8F cell
lines, the expression of caspase-3 and apoptosis were increased,
and the phosphorylation of IκBα and inhibitor of kappa B kinase
were reduced. This inhibited the nuclear translocation of p50 and
p65, and impaired NSCC migration (76) (Fig.
3). Therefore, S100 proteins may improve chemosensitivity and
reduce drug resistance in NSCC.
Epstein-Barr virus (EBV)-related NSCC is extremely
common; there are ~78,100 new cases of EBV-related NSCC worldwide
each year (77). EBV encodes latent
membrane protein 2A (LMP2A) (78).
The expression of S100A4 in CNE-1 cells with or without ectopic
LMP2A expression was previously measured using a transfer gene
array, and the results demonstrated that S100A4 expression was
significantly upregulated in LMP2A-positive NSCC (78). Another previous study also reported
that LMP2A was involved in gene methylation regulation in
EBV-related gastric cancer, inducing signal transducer and
activator of transcription 3 phosphorylation and activating DNA
methyltransferase 1 (79). This led
to the inhibition of PTEN deleted on chromosome 10 promoter
methylation, reducing its transcription (79). Methylation levels of CNE-1 and
CNE-1-LMP2A cells were also previously detected, to verify whether
LMP2A exhibited a similar function in NSCC (78). The results demonstrated that LMP2A
was significantly demethylated in CNE-1 cells without the ectopic
expression of LMP2A (Fig. 3), and
S100A4 expression was increased following the inhibition of DNA
methyltransferase via 5-aza-2-deoxycytidine (78). This resulted in the increased
expression of N-cadherin, vimentin and Snail, and in the reduced
expression of E-cadherin (78)
(Fig. 3). These results suggested
that the expression of S100 proteins may be regulated by
methylation, and EBV-induced hypomethylation may reduce expression
in NSCC.
The clinical proteomic and immunohistochemical
analysis of HPV-18-positive and -negative OSCC has indicated that
S100A8 protein is upregulated >10-fold in HPV-18-positive OSCC
(98). S100A8 and S100A9
heterodimers induce the transfer of myeloid differentiation factor
adaptor proteins from the cytoplasm to the Toll-like receptor 4
(TLR4) receptor complex, activating extracellular signal-regulated
kinases, NF-κB, p38 and JNK, to induce pro-inflammatory signal
transduction, thus activating the innate immune system (99). In a previous study, the results
obtained from TCGA demonstrated that S100A8 and
S100A9 RNA expression levels in HPV-positive HNSCC were
lower than those in HPV-negative HNSCC; however, the difference was
not statistically significant (100). HPV-16 exerts no significant effect
on S100A8 mRNA expression levels; however, it has been shown
to reduce S100A8 promoter activity, suggesting that HPV may
regulate the expression of S100 proteins at the
post-transcriptional translation stage (101). In comparison to healthy skin,
S100A8 and S100A9 are hypomethylated in HPV-induced
warts (102). It is necessary to
determine whether HPV may influence S100A8 expression throughout
the post-transcriptional translation stage of HNSCC and how this
regulation relates to methylation. It is also necessary to learn
more about the S100 protein family members in HNSCC and how they
relate to HPV.
Numerous previous studies have verified that
members of the S100 protein family are closely associated with
HNSCC (Table I). Certain members of
the S100 protein family may block the cell cycle and induce YAP
phosphorylation to regulate cell proliferation (Table I). Moreover, these proteins mediate
NF-κB, PI3K/AKT/mTOR and other signaling pathways to participate in
HNSCC invasion, metastasis and recurrence (Table I). Thus, further investigations into
the role of S100 proteins in the initiation and progression of
HNSCC are required. Vertical flow microarrays based on gold
nanoparticles and surface-enhanced Raman spectroscopy have been
developed to reduce pain in patients, using detection in saliva,
and to differentiate OSCC from healthy groups and quantify
S100P mRNA expression levels (103). Thus, these methods may be useful
mainstream tools for HNSCC detection due to their non-invasive,
convenient and accurate nature. At present, there are no drugs
available which can directly target this family that are used in
clinical practice; however, numerous investigations are focused on
increasing the sensitivity of tumors to chemotherapeutic drugs that
regulate S100 proteins. Low expression levels of S100A11 in HSCC
improve the sensitivity of FaDu cells to the thymidylate synthase
inhibitor 5-fluorouracil (66), and
the inhibition of the IRAK1-S100A9 axis may reduce the resistance
of NSCC to paclitaxel (75).
Moreover, AKT and ERK1/2 signaling inactivation via S100A4
knockdown induces apoptosis, which reverses the resistance to
vemurafenib and inhibits thyroid cancer cell invasion and
proliferation (104). Blocking the
binding of S100 proteins to target proteins has been attempted in
order to cure tumors in clinical practice. Quinoline-3-carboxamide
derivatives suppress tumors by interfering with the interaction of
S100A8/S100A9 with TLR4 or RAGE (105). Amiloridezinol blocks the
interaction of S100A13 with fibroblast growth factor 1 (FGF1),
decreasing S100A13-FGF1 complex formation (106). In addition, the covalent
modification of S100A7, S100A10 and S100A11 through
transglutaminase, and the S-nitrosylation or phosphorylation of
S100A1, S100A8, S100A9 and S100B also indirectly affects disease
progression (2). Although the
association between other family members and HNSCC requires further
research, the present review provides an overview of the mechanisms
underlying S100 proteins in HNSCC, and may provide novel insight
into the prevention, diagnosis and treatment of HNSCC.
Not applicable.
The present study was supported by grants from the National
Natural Science Foundation of China (nos. 81760490 and 82160185),
the Natural Science Foundation of Guangxi (no. 2017GXNSFAA198239)
and the Innovation Project of Guangxi Graduate Education (no.
YCSW2023426).
Not applicable.
YHu and XZ conceived and designed the study. YHu
wrote the manuscript. YHa, MH and YZ participated in revising and
proofreading the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sapkota D, Costea DE, Blo M, Bruland O,
Lorens JB, Vasstrand EN and Ibrahim SO: S100A14 inhibits
proliferation of oral carcinoma derived cells through G1-arrest.
Oral Oncol. 48:219–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonzalez LL, Garrie K and Turner MD: Role
of S100 proteins in health and disease. Biochim Biophys Acta Mol
Cell Res. 1867:1186772020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zaia AA, Sappington KJ, Nisapakultorn K,
Chazin WJ, Dietrich EA, Ross KF and Herzberg MC: Subversion of
antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm
of TR146 epithelial cells after invasion by Listeria monocytogenes.
Mucosal Immunol. 2:43–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou X, Sorenson BS, Ross KF and Herzberg
MC: Augmentation of epithelial resistance to invading bacteria by
using mRNA transfections. Infect Immun. 81:3975–3983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh P and Ali SA: Multifunctional role
of S100 protein family in the immune system: An Update. Cells.
11:22742022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crowe LAN, McLean M, Kitson SM, Melchor
EG, Patommel K, Cao HM, Reilly JH, Leach WJ, Rooney BP, Spencer SJ,
et al: S100A8 & S100A9: Alarmin mediated inflammation in
tendinopathy. Sci Rep. 9:14632019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schenten V, Plançon S, Jung N, Hann J,
Bueb JL, Bréchard S, Tschirhart EJ and Tolle F: Secretion of the
phosphorylated form of S100A9 from neutrophils is essential for the
proinflammatory functions of extracellular S100A8/A9. Front
Immunol. 9:4472018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Veirman K, De Beule N, Maes K, Menu E,
De Bruyne E, De Raeve H, Fostier K, Moreaux J, Kassambara A, Hose
D, et al: Extracellular S100A9 protein in bone marrow supports
multiple myeloma survival by stimulating angiogenesis and cytokine
secretion. Cancer Immunol Res. 5:839–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Z, Jiang D, Huang X, Cai M, Yuan K and
Huang P: S100A8 as a promising biomarker and oncogenic immune
protein in the tumor microenvironment: An integrative pancancer
analysis. J Oncol. 2022:69476522022.PubMed/NCBI
|
|
10
|
Basso D, Fogar P and Plebani M: The
S100A8/A9 complex reduces CTLA4 expression by immature myeloid
cells: Implications for pancreatic cancer-driven immunosuppression.
Oncoimmunology. 2:e244412013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kligman D and Hilt DC: The S100 protein
family. Trends Biochem Sci. 13:437–443. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allgower C, Kretz AL, von Karstedt S,
Wittau M, Henne-Bruns D and Lemke J: Friend or Foe: S100 proteins
in cancer. Cancers (Basel). 12:20372020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delangre E, Oppliger E, Berkcan S,
Gjorgjieva M, Correia de Sousa M and Foti M: S100 proteins in fatty
liver disease and hepatocellular carcinoma. Int J Mol Sci.
23:110302022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Austermann J, Spiekermann C and Roth J:
S100 proteins in rheumatic diseases. Nat Rev Rheumatol. 14:528–541.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raffat MA, Hadi NI, Hosein M, Mirza S,
Ikram S and Akram Z: S100 proteins in oral squamous cell carcinoma.
Clin Chim Acta. 480:143–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang S, Wang Z, Liu W, Lei R, Shan J, Li
L and Wang X: Distinct prognostic values of S100 mRNA expression in
breast cancer. Sci Rep. 7:397862017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai Y, Li LD, Li J and Lu X: Prognostic
values of S100 family members in ovarian cancer patients. BMC
Cancer. 18:12562018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kleindienst A, Hesse F, Bullock MR and
Buchfelder M: The neurotrophic protein S100B: Value as a marker of
brain damage and possible therapeutic implications. Prog Brain Res.
161:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li HB, Wang JL, Jin XD, Zhao L, Ye HL,
Kuang YB, Ma Y, Jiang XY and Yu ZY: Comprehensive analysis of the
transcriptional expressions and prognostic value of S100A family in
pancreatic ductal adenocarcinoma. BMC Cancer. 21:10392021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arantes L, De Carvalho AC, Melendez ME and
Lopes Carvalho A: Serum, plasma and saliva biomarkers for head and
neck cancer. Expert Rev Mol Diagn. 18:85–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chi H, Xie X, Yan Y, Peng G, Strohmer DF,
Lai G, Zhao S, Xia Z and Tian G: Natural killer cell-related
prognosis signature characterizes immune landscape and predicts
prognosis of HNSCC. Front Immunol. 13:10186852022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J, Lai F, Liu Y, Tan Z, Zheng C, Wang
J, Guo H, Jiang L, Ge X, Lan X, et al: Novel computer-aided
reconstruction of soft tissue defects following resection of oral
and oropharyngeal squamous cell carcinoma. World J Surg Oncol.
20:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Runge A, Mayr M, Schwaiger T, Sprung S,
Chetta P, Gottfried T, Dudas J, Greier MC, Glatz MC, Haybaeck J, et
al: Patient-derived head and neck tumor slice cultures: A versatile
tool to study oncolytic virus action. Sci Rep. 12:153342022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mei Z, Zhang K, Lam AK, Huang J, Qiu F,
Qiao B and Zhang Y: MUC1 as a target for CAR-T therapy in head and
neck squamous cell carinoma. Cancer Med. 9:640–652. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Li ZY, Zhou GQ and Sun Y: An
Immune-related gene prognostic index for head and neck squamous
cell carcinoma. Clin Cancer Res. 27:330–341. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Jiang N, Chen M, Sui B and Liu X:
Identification of tumor antigens and immune subtypes in head and
neck squamous cell carcinoma for mRNA vaccine development. Front
Cell Dev Biol. 10:10647542022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei T, Leisegang M, Xia M, Kiyotani K, Li
N, Zeng C, Deng C, Jiang J, Harada M, Agrawal N, et al: Generation
of neoantigen-specific T cells for adoptive cell transfer for
treating head and neck squamous cell carcinoma. Oncoimmunology.
10:19297262021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Massarelli E, William W, Johnson F, Kies
M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, et al:
Combining immune checkpoint blockade and tumor-specific vaccine for
patients with incurable human papillomavirus 16-related cancer: A
phase 2 clinical trial. JAMA Oncol. 5:67–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasahira T, Kurihara-Shimomura M,
Shimojjukoku Y, Shima K and Kirita T: Searching for new molecular
targets for oral squamous cell carcinoma with a view to clinical
implementation of precision medicine. J Pers Med. 12:4132022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sundriyal D, Arya L, Saha R, Walia M and
Nayak PP: Hypercalcemia of malignancy: Time to pull the brakes.
Indian J Surg Oncol. 13:28–32. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roesch Ely M, Nees M, Karsai S, Magele I,
Bogumil R, Vorderwulbecke S, Ruess A, Dietz A, Schnolzer M and
Bosch FX: Transcript and proteome analysis reveals reduced
expression of calgranulins in head and neck squamous cell
carcinoma. Eur J Cell Biol. 84:431–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Argyris PP, Slama Z, Malz C, Koutlas IG,
Pakzad B, Patel K, Kademani D, Khammanivong A and Herzberg MC:
Intracellular calprotectin (S100A8/A9) controls epithelial
differentiation and caspase-mediated cleavage of EGFR in head and
neck squamous cell carcinoma. Oral Oncol. 95:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliveira MV, Fraga CA, Barros LO, Pereira
CS, Santos SH, Basile JR, Gomez RS, Guimaraes AL and De-Paula AM:
High expression of S100A4 and endoglin is associated with
metastatic disease in head and neck squamous cell carcinoma. Clin
Exp Metastasis. 31:639–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tripathi SC, Matta A, Kaur J, Grigull J,
Chauhan SS, Thakar A, Shukla NK, Duggal R, DattaGupta S, Ralhan R
and Siu KW: Nuclear S100A7 is associated with poor prognosis in
head and neck cancer. PLoS One. 5:e119392010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sapkota D, Bruland O, Bøe OE, Bakeer H,
Elgindi OA, Vasstrand EN and Ibrahim SO: Expression profile of the
S100 gene family members in oral squamous cell carcinomas. J Oral
Pathol Med. 37:607–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Driemel O, Murzik U, Escher N, Melle C,
Bleul A, Dahse R, Reichert TE, Ernst G and von Eggeling F: Protein
profiling of oral brush biopsies: S100A8 and S100A9 can
differentiate between normal, premalignant, and tumor cells.
Proteomics Clin Appl. 1:486–493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu S, Arellano M, Boontheung P, Wang J,
Zhou H, Jiang J, Elashoff D, Wei R, Loo JA and Wong DT: Salivary
proteomics for oral cancer biomarker discovery. Clin Cancer Res.
14:6246–6252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kesting MR, Sudhoff H, Hasler RJ,
Nieberler M, Pautke C, Wolff KD, Wagenpfeil S, Al-Benna S, Jacobsen
F and Steinstraesser L: Psoriasin (S100A7) up-regulation in oral
squamous cell carcinoma and its relation to clinicopathologic
features. Oral Oncol. 45:731–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sapkota D, Bruland O, Costea DE, Haugen H,
Vasstrand EN and Ibrahim SO: S100A14 regulates the invasive
potential of oral squamous cell carcinoma derived cell-lines in
vitro by modulating expression of matrix metalloproteinases, MMP1
and MMP9. Eur J Cancer. 47:600–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roman E, Lunde ML, Miron T,
Warnakulasauriya S, Johannessen AC, Vasstrand EN and Ibrahim SO:
Analysis of protein expression profile of oral squamous cell
carcinoma by MALDI-TOF-MS. Anticancer Res. 33:837–845.
2013.PubMed/NCBI
|
|
41
|
Jou YJ, Hua CH, Lin CD, Lai CH, Huang SH,
Tsai MH, Kao JY and Lin CW: S100A8 as potential salivary biomarker
of oral squamous cell carcinoma using nanoLC-MS/MS. Clin Chim Acta.
436:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Natarajan J, Hunter K, Mutalik VS and
Radhakrishnan R: Overexpression of S100A4 as a biomarker of
metastasis and recurrence in oral squamous cell carcinoma. J Appl
Oral Sci. 22:426–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumar M, Srivastava G, Kaur J, Assi J,
Alyass A, Leong I, MacMillan C, Witterick I, Shukla NK, Thakar A,
et al: Prognostic significance of cytoplasmic S100A2 overexpression
in oral cancer patients. J Transl Med. 13:82015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sapkota D, Bruland O, Parajuli H, Osman
TA, Teh MT, Johannessen AC and Costea DE: S100A16 promotes
differentiation and contributes to a less aggressive tumor
phenotype in oral squamous cell carcinoma. BMC Cancer. 15:6312015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sood A, Mishra D, Kharbanda OP, Chauhan
SS, Gupta SD, Deo SSV, Yadav R, Ralhan R, Kumawat R and Kaur H:
Role of S100 A7 as a diagnostic biomarker in oral potentially
malignant disorders and oral cancer. J Oral Maxillofac Pathol.
26:166–172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pandey S, Osman TA, Sharma S, Vallenari
EM, Shahdadfar A, Pun CB, Gautam DK, Uhlin-Hansen L, Rikardsen O,
Johannessen AC, et al: Loss of S100A14 expression at the
tumor-invading front correlates with poor differentiation and worse
prognosis in oral squamous cell carcinoma. Head Neck. 42:2088–2098.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tyszkiewicz T, Jarzab M, Szymczyk C, Kowal
M, Krajewska J, Jaworska M, Fraczek M, Krajewska A, Hadas E,
Swierniak M, et al: Epidermal differentiation complex (locus 1q21)
gene expression in head and neck cancer and normal mucosa. Folia
Histochem Cytobiol. 52:79–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qu ZF, Ma H, Duan XF, Wu R and Zou Y: The
expression and significance of S100A9 in oral squamous cell
carcinoma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
31:219–222. 2017.(In Chinese). PubMed/NCBI
|
|
49
|
Suzuki S, Honda K, Nanjo H, Iikawa N,
Tsuji T, Kawasaki Y, Yamazaki K, Sato T, Saito H, Shiina K and
Ishikawa K: CD147 expression correlates with lymph node metastasis
in T1-T2 squamous cell carcinoma of the tongue. Oncol Lett.
14:4670–4676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Argyris PP, Slama ZM, Ross KF,
Khammanivong A and Herzberg MC: Calprotectin and the initiation and
progression of head and neck cancer. J Dent Res. 97:674–682. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mints M, Landin D, Nasman A, Mirzaie L,
Ursu RG, Zupancic M, Marklund L, Dalianis T, Munck-Wikland E and
Ramqvist T: Tumour inflammation signature and expression of S100A12
and HLA class I improve survival in HPV-negative hypopharyngeal
cancer. Sci Rep. 11:17822021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ohnishi Y, Yasui H, Kakudo K and Nozaki M:
Lapatinib-resistant cancer cells possessing epithelial cancer stem
cell properties develop sensitivity during sphere formation by
activation of the ErbB/AKT/cyclin D2 pathway. Oncol Rep.
36:3058–3064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ohnishi Y, Yasui H, Nozaki M and Nakajima
M: Molecularly-targeted therapy for the oral cancer stem cells. Jpn
Dent Sci Rev. 54:88–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moriyama-Kita M, Endo Y, Yonemura Y,
Heizmann CW, Miyamori H, Sato H, Yamamoto E and Sasaki T: S100A4
regulates E-cadherin expression in oral squamous cell carcinoma.
Cancer Lett. 230:211–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun Q, Wang R, Wang Y, Luo J, Wang P and
Cheng B: Notch1 is a potential therapeutic target for the treatment
of human hepatitis B virus X protein-associated hepatocellular
carcinoma. Oncol Rep. 31:933–939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Khammanivong A, Wang C, Sorenson BS, Ross
KF and Herzberg MC: S100A8/A9 (calprotectin) negatively regulates
G2/M cell cycle progression and growth of squamous cell carcinoma.
PLoS One. 8:e693952013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cheng S, Zhang X, Huang N, Qiu Q, Jin Y
and Jiang D: Down-regulation of S100A9 inhibits osteosarcoma cell
growth through inactivating MAPK and NF-κB signaling pathways. BMC
Cancer. 16:2532016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li R, Li W, He F, Zhang M, Luo H and Tang
H: Systematic screening identifies a TEAD4-S100A13 axis modulating
cisplatin sensitivity of oral squamous cell carcinoma cells. J Oral
Pathol Med. 50:882–890. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Takeuchi S, Kasamatsu A, Yamatoji M,
Nakashima D, Endo-Sakamoto Y, Koide N, Takahara T, Shimizu T, Iyoda
M, Ogawara K, et al: TEAD4-YAP interaction regulates tumoral growth
by controlling cell-cycle arrest at the G1 phase. Biochem Biophys
Res Commun. 486:385–390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sivadasan P, Gupta MK, Sathe G, Sudheendra
HV, Sunny SP, Renu D, Hari PS, Gowda H, Suresh A, Kuriakose MA and
Sirdeshmukh R: Salivary proteins from dysplastic leukoplakia and
oral squamous cell carcinoma and their potential for early
detection. J Proteomics. 212:1035742020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shan J, Sun Z, Yang J, Xu J, Shi W, Wu Y,
Fan Y and Li H: Discovery and preclinical validation of proteomic
biomarkers in saliva for early detection of oral squamous cell
carcinomas. Oral Dis. 25:97–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cheng YS, Rees T and Wright J: A review of
research on salivary biomarkers for oral. Clin Transl Med. 3:32014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu J, Gross N, Zang Y, Cao S, Yang F, Yang
Z, Yu W, Lei D and Pan X: Overexpression of S100A4 predicts
migration, invasion, and poor prognosis of hypopharyngeal squamous
cell carcinoma. Mol Diagn Ther. 23:407–417. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cheng LH, Hung KF, Huang TF, Hsieh HP,
Wang SY, Huang CY and Lo JF: Attenuation of cancer-initiating cells
stemness properties by abrogating S100A4 calcium binding ability in
head and neck cancers. Oncotarget. 7:78946–78957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu P, Quan H, Kang J, He J, Luo S, Xie C,
Xu J, Tang Y and Zhao S: Downregulation of Calcium-Binding Protein
S100A9 Inhibits Hypopharyngeal Cancer Cell Proliferation and
Invasion Ability Through Inactivation of NF-kappaB Signaling. Oncol
Res. 25:1479–1488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang C, Lin C, Tao Q, Zhao S, Liu H and Li
L: Evaluation of calcium-binding protein A11 promotes the
carcinogenesis of hypopharygeal squamous cell carcinoma via the
PI3K/AKT signaling pathway. Am J Transl Res. 11:3472–3480.
2019.PubMed/NCBI
|
|
67
|
Hu W, Tao Z, Zhou Q, Zhao D, Gu L, Zhu S
and Chen J: Effects of S100 calcium-binding protein A8 (S100A8) and
S100 calcium-binding protein A9 (S100A9) on matrix
metalloproteinase (MMP) expression in nasopharyngeal carcinoma
CNE-2 cells. Transl Cancer Res. 10:1874–1884. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Isaksen B and Fagerhol MK: Calprotectin
inhibits matrix metalloproteinases by sequestration of zinc. Mol
Pathol. 54:289–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Carlsson H, Yhr M, Petersson S, Collins N,
Polyak K and Enerback C: Psoriasin (S100A7) and calgranulin-B
(S100A9) induction is dependent on reactive oxygen species and is
downregulated by Bcl-2 and antioxidants. Cancer Biol Ther.
4:998–1005. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ichikawa M, Williams R, Wang L, Vogl T and
Srikrishna G: S100A8/A9 activate key genes and pathways in colon
tumor progression. Mol Cancer Res. 9:133–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xiao Z, Li M, Li G, Fu Y, Peng F, Chen Y
and Chen Z: Proteomic characterization reveals a molecular portrait
of nasopharyngeal carcinoma differentiation. J Cancer. 8:570–577.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li A, Shi D, Xu B, Wang J, Tang YL, Xiao
W, Shen G, Deng W and Zhao C: S100A6 promotes cell proliferation in
human nasopharyngeal carcinoma via the p38/MAPK signaling pathway.
Mol Carcinog. 56:972–984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wen L, Ding Y, Chen X, Tian K, Li D, Liang
K and Yue B: Influences of S100A8 and S100A9 on proliferation of
nasopharyngeal carcinoma cells through PI3K/Akt signaling pathway.
Biomed Res Int. 2021:99173652021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang C, Wang X, Han A, Wang Y and Jiang H:
Proof-of-concept study investigating the role of S100P-RAGE in
nasopharyngeal carcinoma. Exp Ther Med. 21:4702021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu L, Liu S, Deng P, Liang Y, Xiao R,
Tang LQ, Chen J, Chen QY, Guan P, Yan SM, et al: Targeting the
IRAK1-S100A9 axis overcomes resistance to paclitaxel in
nasopharyngeal carcinoma. Cancer Res. 81:1413–1425. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Meng DF, Sun R, Liu GY, Peng LX, Zheng LS,
Xie P, Lin ST, Mei Y, Qiang YY, Li CZ, et al: S100A14 suppresses
metastasis of nasopharyngeal carcinoma by inhibition of NF-kB
signaling through degradation of IRAK1. Oncogene. 39:5307–5322.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Png YT, Yang AZY, Lee MY, Chua MJM and Lim
CM: The role of NK cells in EBV infection and EBV-associated NPC.
Viruses. 13:3002021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lin Z, Deng L, Ji J, Cheng C, Wan X, Jiang
R, Tang J, Zhuo H, Sun B and Chen Y: S100A4 hypomethylation affects
epithelial-mesenchymal transition partially induced by LMP2A in
nasopharyngeal carcinoma. Mol Carcinog. 55:1467–1476. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hino R, Uozaki H, Murakami N, Ushiku T,
Shinozaki A, Ishikawa S, Morikawa T, Nakaya T, Sakatani T, Takada K
and Fukayama M: Activation of DNA methyltransferase 1 by EBV latent
membrane protein 2A leads to promoter hypermethylation of PTEN gene
in gastric carcinoma. Cancer Res. 69:2766–2774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chakladar J, Li WT, Bouvet M, Chang EY,
Wang-Rodriguez J and Ongkeko WM: Papillary thyroid carcinoma
variants are characterized by Co-dysregulation of immune and cancer
associated genes. Cancers (Basel). 11:11792019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Martinez-Aguilar J, Clifton-Bligh R and
Molloy MP: A multiplexed, targeted mass spectrometry assay of the
S100 protein family uncovers the isoform-specific expression in
thyroid tumours. BMC Cancer. 15:1992015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nipp M, Elsner M, Balluff B, Meding S,
Sarioglu H, Ueffing M, Rauser S, Unger K, Höfler H, Walch A and
Zitzelsberger H: S100-A10, thioredoxin, and S100-A6 as biomarkers
of papillary thyroid carcinoma with lymph node metastasis
identified by MALDI imaging. J Mol Med (Berl). 90:163–174. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhao M, Wang KJ, Tan Z, Zheng CM, Liang Z
and Zhao JQ: Identification of potential therapeutic targets for
papillary thyroid carcinoma by bioinformatics analysis. Oncol Lett.
11:51–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang X, Sun Z, Tian W, Piao C, Xie X, Zang
J, Peng S, Yu X and Wang Y: S100A12 is a promising biomarker in
papillary thyroid cancer. Sci Rep. 10:17242020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang G, Li HN, Cui XQ, Xu T, Dong ML, Li
SY and Li XR: S100A1 is a potential biomarker for papillary thyroid
carcinoma diagnosis and prognosis. J Cancer. 12:5760–5771. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Huang P and Xue J: Long non-coding RNA
FOXD2-AS1 regulates the tumorigenesis and progression of breast
cancer via the S100 calcium binding protein A1/Hippo signaling
pathway. Int J Mol Med. 46:1477–1489. 2020.PubMed/NCBI
|
|
87
|
Tian J and Luo B: Identification of three
prognosis-related differentially expressed lncRNAs driven by copy
number variation in thyroid cancer. J Immunol Res.
2022:92037962022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xia F, Chen Y, Jiang B, Du X, Peng Y, Wang
W, Huang W, Feng T and Li X: Long Noncoding RNA HOXA-AS2 promotes
papillary thyroid cancer progression by regulating
miR-520c-3p/S100A4 pathway. Cell Physiol Biochem. 50:1659–1672.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cheon MG, Son YW, Lee JH, Jang HH and
Chung YS: Mts1 Up-regulation is associated with aggressive
pathological features in thyroid cancer. Cancer Genomics
Proteomics. 16:369–376. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wang Y, Ye H, Yang Y, Li J, Cen A and Zhao
L: microRNA-181a promotes the oncogene S100A2 and enhances
papillary thyroid carcinoma growth by mediating the expression of
histone demethylase KDM5C. J Endocrinol Invest. 45:17–28. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Economopoulou P, Kotsantis I and Psyrri A:
Special issue about head and neck cancers: HPV positive cancers.
Int J Mol Sci. 21:33882020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tokuzen N, Nakashiro KI, Tojo S, Goda H,
Kuribayashi N and Uchida D: Human papillomavirus-16 infection and
p16 expression in oral squamous cell carcinoma. Oncol Lett.
22:5282021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Mork J, Lie AK, Glattre E, Hallmans G,
Jellum E, Koskela P, Møller B, Pukkala E, Schiller JT, Youngman L,
et al: Human papillomavirus infection as a risk factor for
squamous-cell carcinoma of the head and neck. N Engl J Med.
344:1125–1131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wuerdemann N, Wittekindt C, Sharma SJ,
Prigge ES, Reuschenbach M, Gattenlöhner S, Klussmann JP and Wagner
S: Risk factors for overall survival outcome in surgically treated
human papillomavirus-negative and positive patients with
oropharyngeal cancer. Oncol Res Treat. 40:320–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Granata R, Miceli R, Orlandi E, Perrone F,
Cortelazzi B, Franceschini M, Locati LD, Bossi P, Bergamini C,
Mirabile A, et al: Tumor stage, human papillomavirus and smoking
status affect the survival of patients with oropharyngeal cancer:
An Italian validation study. Ann Oncol. 23:1832–1837. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Paver EC, Currie AM, Gupta R and Dahlstrom
JE: Human papilloma virus related squamous cell carcinomas of the
head and neck: Diagnosis, clinical implications and detection of
HPV. Pathology. 52:179–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lo WY, Lai CC, Hua CH, Tsai MH, Huang SY,
Tsai CH and Tsai FJ: S100A8 is identified as a biomarker of
HPV18-infected oral squamous cell carcinomas by suppression
subtraction hybridization, clinical proteomics analysis, and
immunohistochemistry staining. J Proteome Res. 6:2143–2151. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu S, Xie Y, Luo W, Dou Y, Xiong H, Xiao
Z and Zhang XL: PE_PGRS31-S100A9 interaction promotes mycobacterial
survival in macrophages through the regulation of NF-κB-TNF-α
signaling and arachidonic acid metabolism. Front Microbiol.
11:8452020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Khammanivong A, Sorenson BS, Ross KF,
Dickerson EB, Hasina R, Lingen MW and Herzberg MC: Involvement of
calprotectin (S100A8/A9) in molecular pathways associated with
HNSCC. Oncotarget. 7:14029–14047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gyöngyösi E, Szalmás A, Ferenczi A,
Póliska S, Kónya J and Veress G: Transcriptional regulation of
genes involved in keratinocyte differentiation by human
papillomavirus 16 oncoproteins. Arch Virol. 160:389–398. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Alghamdi MA, Al-Eitan LN, Tarkhan AH and
Al-Qarqaz FA: Global gene methylation profiling of common warts
caused by human papillomaviruses infection. Saudi J Biol Sci.
28:612–622. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Han S, Locke AK, Oaks LA, Cheng YL and
Coté GL: Nanoparticle-based assay for detection of S100P mRNA using
surface-enhanced Raman spectroscopy. J Biomed Opt. 24:1–9.
2019.
|
|
104
|
Jiao X, Zhang H, Xu X, Yu Y, Zhang H,
Zhang J, Ning L, Hao F, Liu X, Niu M, et al: S100A4 knockout
sensitizes anaplastic thyroid carcinoma cells harboring
BRAFV600E/Mt to Vemurafenib. Cell Physiol Biochem. 49:1143–1162.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Björk P, Björk A, Vogl T, Stenström M,
Liberg D, Olsson A, Roth J, Ivars F and Leanderson T:
Identification of human S100A9 as a novel target for treatment of
autoimmune disease via binding to quinoline-3-carboxamides. PLoS
Biol. 7:e972009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mouta Carreira C, LaVallee TM, Tarantini
F, Jackson A, Lathrop JT, Hampton B, Burgess WH and Maciag T:
S100A13 is involved in the regulation of fibroblast growth factor-1
and p40 synaptotagmin-1 release in vitro. J Biol Chem.
273:22224–22231. 1998. View Article : Google Scholar : PubMed/NCBI
|