Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide (1). Nivolumab is
an immune checkpoint inhibitor (ICI) that has shown significant
prognostic benefit in the treatment of non-small-cell lung cancer
(NSCLC) (2,3). Based on this benefit, other ICIs, such
as pembrolizumab and atezolizumab, have also been used in the
treatment of NSCLC, and they have shown prognostic benefits
(4,5). Nivolumab combined with ipilimumab has
been recently shown to improve NSCLC prognosis (6,7).
Although programmed death ligand-1 (PD-L1) expression in tumor
cells is mainly used as a biomarker for ICI treatment in patients
with NSCLC (8), its predictive
ability is inadequate. Tumor mutational burden and microsatellite
instability have also been used as biomarkers (9,10), but
they have limited predictive ability. Therefore, highly accurate
biomarkers are needed to predict the therapeutic effect of ICI
treatment in patients with NSCLC.
We have previously reported that PD-L1 SNPs
might predict the therapeutic effect of nivolumab in patients with
NSCLC (11,12). Particularly, the PD-L1 SNP
rs822339 predicted prolonged overall survival (OS) in patients with
NSCLC treated with nivolumab (13).
However, PD-L1 SNPs alone are still inadequate as predictive
biomarkers of nivolumab efficacy because of the lack of improvement
in the progressive disease rate in the first 3 months after
treatment initiation. PD-1 inhibitors such as nivolumab mainly
target cytotoxic T cells. However, some malignant tumors are
characterized by cold tumors that lack cytotoxic T cell
infiltration (14). In cold tumors,
macrophages play an important role in tumor growth and metastasis
(15). Macrophages have
antigen-presenting capacity and are involved in the immune response
(16). Nonetheless,
tumor-associated macrophages have reduced antigen-presenting
capacity (17).
CD47 was first reported in 1987 as a cell surface
antigen encoded by human chromosome 3 (18). It is expressed on red blood cells
(RBCs) and plays a role in RBC prevention of phagocytosis by
binding to signal regulatory protein α (SIRPα) expressed on
macrophages (19). Hence, CD47 is
called the ‘do not eat me’ signal (20). CD47 is also expressed on cancer
cells and plays a role in preventing phagocytosis by binding to
SIRPα expressed in macrophages (21,22).
It has been reported that CD47 expression is associated with the
prognosis of patients with advanced NSCLC (23). Therefore, CD47 could be a potential
target for cancer immunotherapy. For example, it has been reported
that blocking CD47 in lung cancer activates macrophage-mediated
phagocytosis and enhances the anti-tumor effect (24,25).
Notably, CD47 SNP rs3804639 has been reported
to be associated with the frequency of distant metastases in
colorectal cancer (26). The study
evaluated CD47 SNP rs3804639 in 613 patients with colorectal
cancer, and patients with the G/G genotype of the CD47 SNP
rs3804639 had a lower frequency of distant metastases than those
with the G/T or T/T genotypes of the CD47 SNP rs3804639.
This suggests that the CD47 function of macrophages is lower in the
G/G genotype of CD47 SNP rs3804639. Thus, CD47 SNP
rs3804639 may be a candidate predictive biomarker of nivolumab
efficacy in NSCLC.
In this study, we aimed to investigate the
association between CD47 SNP and the therapeutic effect of
nivolumab in patients with NSCLC. The study mainly focused on the
CD47 molecule associated with macrophages and tumor immunity. We
hypothesized that SNPs related to macrophages could also be a
predictive biomarker of the therapeutic effect of nivolumab in
patients with NSCLC. Further, we hypothesized that the CD47
SNPs reported in other cancers might be associated with lung
cancer. The CD47 SNP genotypes were measured in patients
with NSCLC treated with nivolumab, and the clinical outcomes were
analyzed retrospectively.
Patients and methods
Study design and patients
This retrospective study initially evaluated 181
consecutive patients who were pathologically diagnosed with NSCLC
and treated with nivolumab monotherapy at Kyoto University Hospital
(Kyoto, Japan) between January 2016 and July 2020. Among them, 17
patients who lacked DNA samples, those with active cancers other
than NSCLC, and those who died within 1 day after nivolumab
initiation were excluded. Finally, 164 patients were included in
the analysis. All patients were treated with nivolumab monotherapy
as second- or later-line treatment. Nivolumab was administered at 3
mg/kg or 240 mg every 2 weeks. The patient selection flow chart is
shown in Fig. 1.
The association between CD47 SNP genotypes
and progression-free survival (PFS) or OS was analyzed.
PD-L1 SNP was measured in the same patient cohort and
combined with the CD47 SNP for OS analysis. To determine
whether the predictive effect of the CD47 SNP is specific to
ICI treatment, 722 patients with advanced NSCLC who received a
diagnosis between January 2006 and December 2015 were evaluated.
Among them, 478, 139, and 7 patients who lacked DNA samples,
received no chemotherapy for NSCLC during data collection, and
received ICI during data collection, respectively, were excluded.
Finally, 98 patients with available DNA samples and those treated
without ICIs were included in the non-ICI cohort for the analysis
of the association between CD47 SNP and clinical outcomes.
Data including age, sex, driver mutation status, and survival
outcomes were collected from the medical records.
This study, which included both ICI and non-ICI
cohorts, was approved by the Ethics Review Board of Kyoto
University Hospital (certification number: G0788) and conducted in
accordance with the principles of the Declaration of Helsinki.
Written informed consent was obtained from all study participants
in the ICI and non-ICI cohorts.
Genotyping and SNP selection
SNP genotyping was performed as described in our
previous study in detail (12).
Briefly, genomic DNA was extracted from peripheral blood samples
using Gene Prep Star NA-480 (Kurabo, Osaka, Japan). Genotyping was
performed using the TaqMan genotyping assay (Applied Biosystems,
Foster City, CA, USA) and TaqMan genotyping master mix (Applied
Biosystems) and analyzed using an Applied Biosystems 7300 Real-Time
polymerase chain reaction System (Applied Biosystems). The
polymerase chain reaction (PCR) solution consisted of 1 µl of DNA
sample, 12.5 µl of TaqMan genotyping master mix, 0.3125 µl of
TaqMan genotyping assay primer probe mix, and 11.2 µl of
nuclease-free water. The baseline fluorescence measurements were
taken at 25°C, followed by the following PCR protocol: incubation
of samples at 95°C for 10 min, 40 cycles of denaturation at 92°C
for 15 sec, and annealing and extension at 60°C for 1 min, with a
final fluorescence measurement at 60°C. SNPs reported to be
associated with cancer were selected. Among them, SNPs with minor
allele frequencies greater than 0.2 were selected. The TaqMan
genotyping assay primer probe mix for the CD47 SNP rs3804639
(catalog number: 4351379) was purchased from Applied
Biosystems.
Evaluation of nivolumab efficacy and
clinical outcomes
Data on clinical characteristics and treatment
courses were extracted from the medical records. Radiographic
imaging was performed every 6 to 8 weeks. Treatment responses were
evaluated according to the Response Evaluation Criteria in Solid
Tumors (RECIST) version 1.1 (27).
PFS was measured from the initiation of nivolumab administration
until the date of disease progression or death. OS was measured
from the initiation of nivolumab administration until death or the
last follow-up date. Patients with no recorded disease progression
at the last follow-up were censored. The cut-off for data
collection was August 2021. In the non-ICI cohort, OS was measured
from the initiation of cytotoxic chemotherapy or tyrosine kinase
inhibitors until death or the last follow-up date. The cut-off for
data collection in the non-ICI cohort was December 2016.
Statistical analysis
Patient characteristics were compared by genotype
using the following statistical tests: Kruskal-Wallis test was used
for age, and the Fisher's exact test was used for all other
variables. PFS and OS survival curves were generated using the
Kaplan-Meier method and compared by genotype using the log-rank
test. Univariate and multivariate analyses were performed using the
Cox regression model to estimate hazard ratios (HRs) with 95%
confidence intervals (CIs). P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using the JMP Pro statistical software version 15.2.0
(SAS Institute, Cary, NC, USA).
Results
Patient characteristics and clinical
outcomes
The ICI cohort had a median age of 69 years (range,
30–85 years), and 105 patients (64.0%) were male. The patient
characteristics according to the CD47 SNP rs3804639 genotype
are summarized in Table I. A total
of 150 patients (91.5%) had an Eastern Cooperative Oncology Group
(ECOG) performance status (PS) of 0 or 1, and 116 (70.7%) patients
had adenocarcinoma. Further, 35 (21.3%) patients harbored epidermal
growth factor receptor (EGFR) mutations, and 34 (20.7%) patients
had liver metastases. Eighty two (50.0%) patients were administered
nivolumab as second-line treatment, while the remaining patients
received nivolumab as third- or later-line treatment. CD47
SNP rs3804639 genotypes were not associated with clinical factors.
With respect to clinical outcomes, the overall response rate was
14.6%, and the disease control rate was 48.2% in all patient
populations. The median PFS was 2.2 (95% CI, 1.9–2.8) months, and
the median OS was 16.1 (95% CI, 11.1–23.0) months in the patient
populations.
 | Table I.Patient characteristics according to
CD47 rs3804639 genotype |
Table I.
Patient characteristics according to
CD47 rs3804639 genotype
|
|
| CD47
rs3804639 |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Overall
(n=164) | G/G (n=87) | G/T (n=70) | T/T (n=7) |
P-valuea |
|---|
| Median age, years
(range) | 69 (30–85) | 69 (30–83) | 70 (33–85) | 70 (64–79) | 0.23 |
| Sex, n (%) |
|
|
|
| 0.75 |
|
Female | 59 (36.0%) | 30 (34.5%) | 27 (38.6%) | 2 (28.6%) |
|
|
Male | 105 (64.0%) | 57 (65.5%) | 43 (61.4%) | 5 (71.4%) |
|
| Smoking status, n
(%) |
|
|
|
| 0.32 |
|
Current/former | 113 (68.9%) | 63 (72.4%) | 45 (64.3%) | 5 (71.4%) |
|
|
Never | 51 (31.1%) | 24 (27.6%) | 25 (35.7%) | 2 (28.6%) |
|
| ECOG PS, n (%) |
|
|
|
|
|
|
0-1 | 150 (91.5%) | 82 (94.3%) | 61 (87.1%) | 7 (100%) | 0.26 |
|
2-3 | 14 (8.5%) | 5 (5.7%) | 9 (12.9%) | 0 (0%) |
|
| Histology, n
(%) |
|
|
|
| 0.37 |
|
Adenocarcinoma | 116 (70.7%) | 57 (65.5%) | 54 (77.1%) | 5 (71.4%) |
|
|
Squamous | 35 (21.3%) | 20 (23.0%) | 13 (18.6%) | 2 (28.6%) |
|
|
Others | 13 (7.9%) | 10 (11.5%) | 3 (4.3%) | 0 (0%) |
|
| EGFR mutation, n
(%) |
|
|
|
| 0.35 |
|
Positive | 35 (21.3%) | 16 (18.4%) | 19 (27.1%) | 0 (0%) |
|
|
Negative or unknown | 129 (78.7%) | 71 (81.6%) | 51 (72.9%) | 7 (100%) |
|
| PD-L1 status, n
(%) |
|
|
|
| 0.06 |
|
Positive | 42 (25.6%) | 27 (31.0%) | 14 (20.0%) | 1 (14.3%) |
|
|
Negative | 45 (27.4%) | 21 (24.1%) | 19 (27.1%) | 5 (71.4%) |
|
|
Unknown | 77 (47.0%) | 39 (44.8%) | 37 (52.9%) | 1 (14.3%) |
|
| Treatment line, n
(%) |
|
|
|
| 1.00 |
|
Second | 82 (50.0%) | 44 (50.6%) | 32 (45.7%) | 6 (85.7%) |
|
| Third
or later | 82 (50.0%) | 43 (49.4%) | 38 (54.3%) | 1 (14.3%) |
|
| Liver metastasis, n
(%) |
|
|
|
| 1.00 |
|
Positive | 34 (20.7%) | 18 (20.7%) | 13 (18.6%) | 3 (42.9%) |
|
|
Negative | 130 (79.3%) | 69 (79.3%) | 57 (81.4%) | 4 (57.1%) |
|
Association between clinical outcomes
and CD47 SNP rs3804639
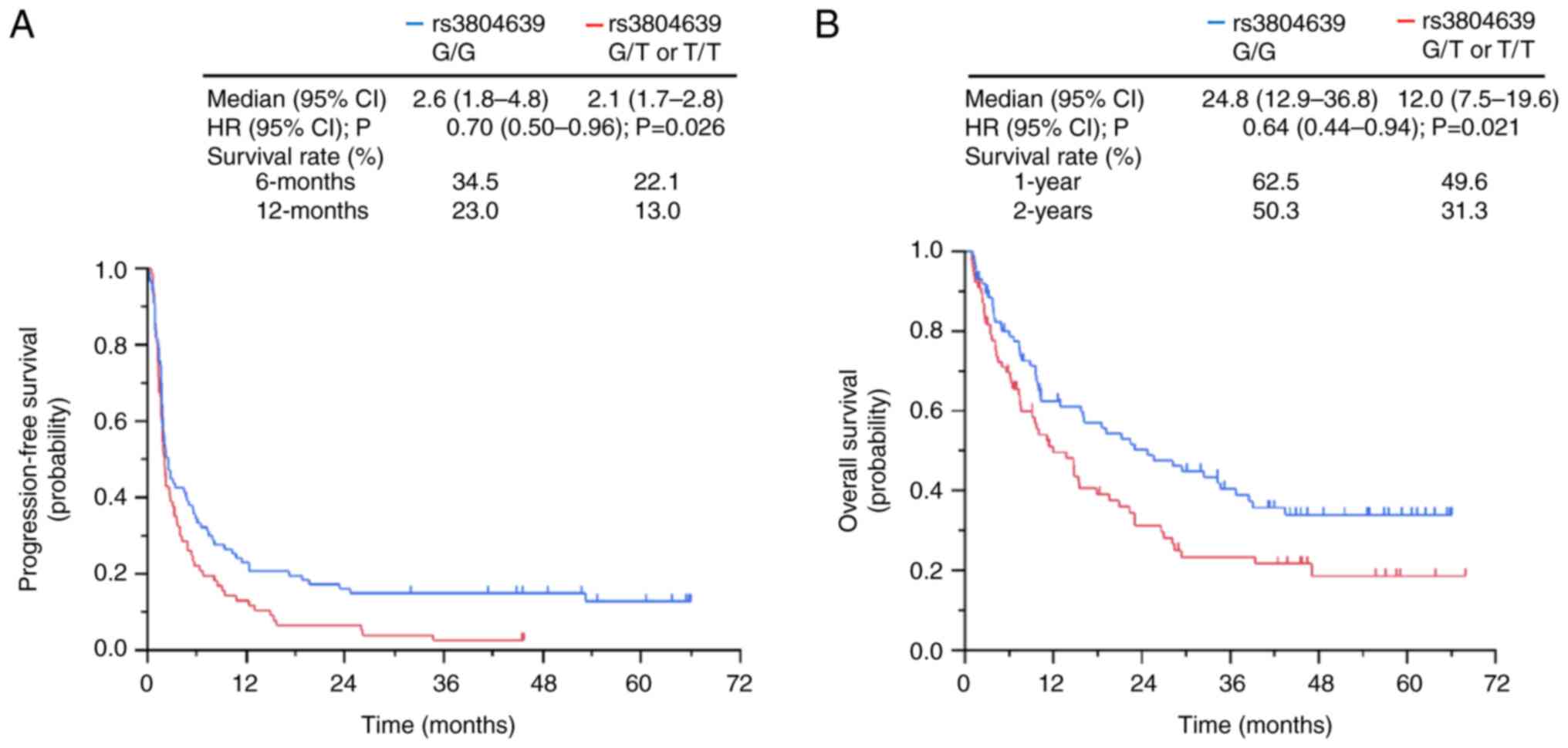
Patients with the G/G genotype of the CD47
SNP rs3804639 had significantly longer PFS than those with the G/T
or T/T genotypes of the CD47 SNP rs3804639 (2.6 months vs.
2.1 months, HR, 0.70; P=0.026; Fig.
2A). In addition, patients with the G/G genotype of CD47
SNP rs3804639 had a significantly longer OS than had those with the
G/T or T/T genotypes of CD47 SNP rs3804639 (24.8 months vs.
12.0 months, HR, 0.64; P=0.021; Fig.
2B). The 1- and 2-year survival rate of patients with the G/G
genotype of CD47 SNP rs3804639 were higher than those of
patients with the G/T or T/T genotypes of CD47 SNP rs3804639
(1-year survival rate, 62.5% vs. 49.6%; 2-year survival rate, 50.3%
vs. 31.3%; Fig. 2B).
Influencing factors of OS
Univariate analysis showed that CD47 SNP
rs3804639, ECOG PS, and the presence or absence of liver metastases
were associated with OS. Multivariate analysis showed that
CD47 SNP rs3804639 was independently associated with OS. The
results of the uni- and multivariate analyses are shown in Table II. The finding that PS and liver
metastases are associated with the prognosis of patients with NSCLC
treated with nivolumab is consistent to that of previous reports
(28,29).
 | Table II.Univariate and multivariate analyses
of influencing factors of overall survival. |
Table II.
Univariate and multivariate analyses
of influencing factors of overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥75 vs. <75
years) | 1.03 | 0.66–1.63 | 0.89 | 1.15 | 0.69–1.91 | 0.6 |
| Smoking status
(current/former vs. never) | 0.86 | 0.57–1.29 | 0.47 | 0.79 | 0.47–1.34 | 0.39 |
| ECOG PS (≥2 vs.
0–1) | 3.43 | 1.90–6.18 | <0.001 | 3.49 | 1.86–6.53 | <0.001 |
| Histology (non-Sq
vs. Sq) | 0.96 | 0.61–1.49 | 0.84 | 0.8 | 0.49–1.32 | 0.38 |
| EGFR mutation
(positive vs. negative or unknown) | 1.01 | 0.63–1.63 | 0.96 | 0.85 | 0.46–1.56 | 0.59 |
| Treatment line (2nd
vs. ≥3rd) | 0.78 | 0.53–1.14 | 0.19 | 0.76 | 0.48–1.20 | 0.24 |
| Liver metastasis
(positive vs. negative) | 1.82 | 1.17–2.84 | 0.008 | 2.19 | 1.37–3.51 | 0.001 |
| CD47
rs3804639 (G/G vs. G/T or T/T) | 0.64 | 0.44–0.94 | 0.022 | 0.66 | 0.44–0.98 | 0.04 |
Association between clinical outcomes
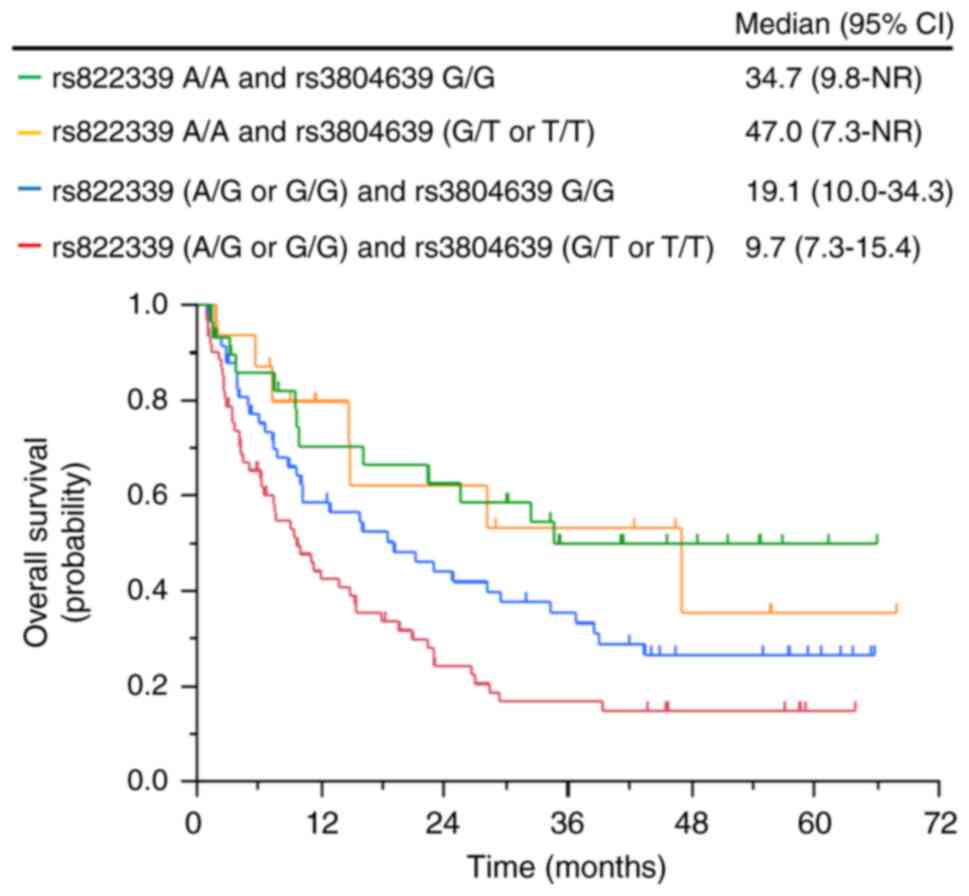
and combination of CD47 SNP rs3804639 and PD-L1 SNP rs822339
The PD-L1 SNP rs822339 has been previously
shown to predict survival outcomes in patients with NSCLC treated
with nivolumab (13). In this
study, even in patients with the A/G or G/G genotypes of the
PD-L1 SNP rs822339, which were reported to be associated
with poor prognosis, patients with the G/G genotype of the
CD47 SNP rs3804639 had longer OS than those with the G/T or
T/T genotypes of the CD47 SNP rs3804639 (19.1 months vs. 9.7
months) (Fig. 3).
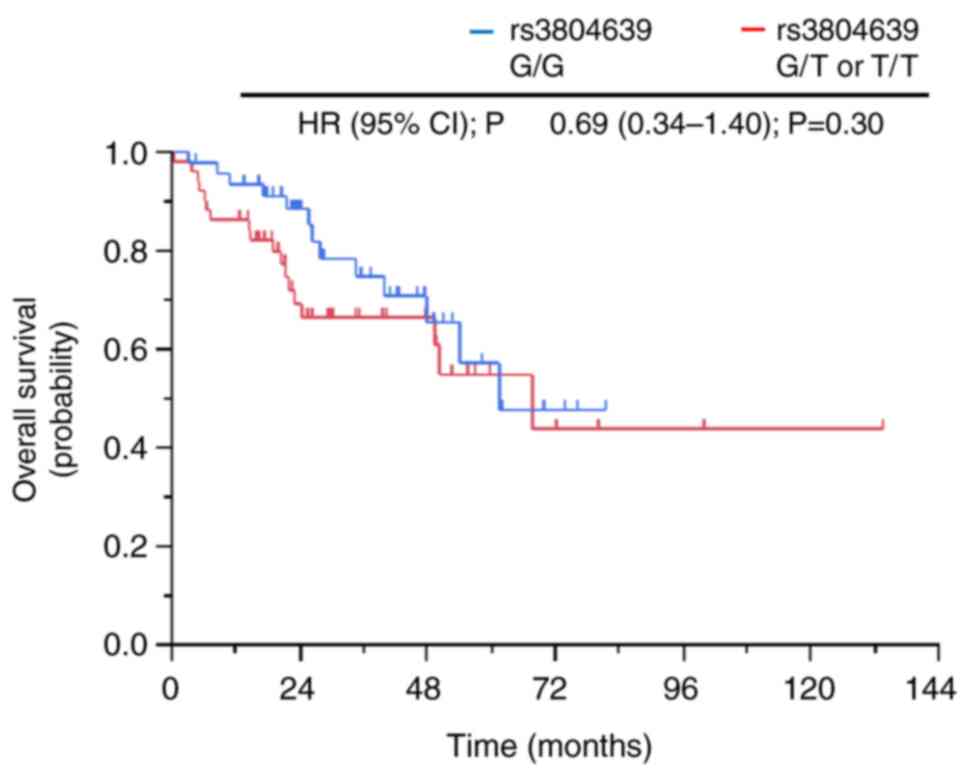
Association between clinical outcomes
and CD47 SNP rs3804639 in the non-ICI cohort
The baseline characteristics according to the
CD47 SNP rs3804639 genotype in the non-ICI cohort are
summarized in Table SI. The median
patient age was 66 years (range, 32–89 years), and 55 patients
(56.1%) were male. There were 92 (93.9%) patients with an ECOG PS
of 0 or 1, and 89 (90.8%) patients had adenocarcinoma. Further, 61
(62.2%) patients harbored EGFR mutations, and 3 (3.1%) patients had
liver metastases. The genotypes of CD47 SNP rs3804639 were
not associated with clinical factors. None of the patients in this
cohort were administered nivolumab or other ICIs during data
collection. Regarding CD47 SNP rs3804639, there was no
significant difference in OS between patients with the G/G genotype
and those with the G/T or T/T genotypes (61.5 months vs. 67.7
months, HR, 0.69; P=0.30; Fig.
4).
Discussion
Accurate predictive biomarkers of ICI efficacy in
NSCLC are lacking. This study found that the G/G genotype of the
CD47 SNP rs3804639 was associated with significantly longer
survival in patients with advanced NSCLC treated with nivolumab. In
addition, patients with the A/G or G/G genotypes of PD-L1
SNP rs822339 had longer survival than those with the G/G genotype
of CD47 SNP rs3804639. Thus, CD47 SNP rs3804639 might
be a predictive biomarker of nivolumab efficacy in patients with
advanced NSCLC. To our best knowledge, this study is the first to
show that CD47 SNP is associated with survival outcomes in
patients with advanced NSCLC treated with nivolumab.
The current study also found that the G/G genotype
of CD47 SNP rs3804639 has a survival advantage. However,
this survival advantage was not observed in the non-ICI cohort,
which involved patients who did not receive nivolumab or other ICIs
(Fig. 4). This indicates that
CD47 SNP rs3804639 can be a predictive biomarker of
nivolumab efficacy but not of prognosis in patients with advanced
NSCLC. This result is similar to that of a previous report showing
that the G/G genotype of the CD47 SNP rs3804639 is
associated with a lower frequency of distant metastases in
colorectal cancer (26); therefore,
the G/G genotype of CD47 SNP rs3804639 is suggested to be
functional in that it reduces cancer progression by suppressing
CD47 function.
The CD47 SNP rs3804639 located in the intron
region of the gene has a relatively small effect on the protein
function compared to that of SNPs in the exon region. However, SNPs
in the intron region can still regulate the protein function by
regulating alternative splicing (30). CD47 is expressed on various immune
cells other than macrophages; for example, CD47 expressed on T
cells has been associated with enhanced T cell immune responses
(31). To evaluate the role of
CD47 SNP rs3804639, we used quantitative trait locus (QTL)
data from the Genotype-Tissue Expression Program. Data on the
expression QTL, which are the quantitative effects of SNPs on gene
expression, were unavailable for CD47 SNP rs3804639.
However, data on the splicing QTL (sQTL), which are the
quantitative effects of SNPs on alternative splicing, were
available for CD47 SNP rs3804639. The sQTL for the
CD47 SNP rs3804639 in the esophagus and skin showed
different trends depending on the genotype. The sQTL data from
these organs suggest the possibility of a relationship between
CD47 SNP rs3804639 and the regulation of alternative
splicing. In the future, when surgical specimens of lung cancer are
obtained, we hope to investigate the association between
CD47 SNP rs3804639 and CD47 protein expression by staining
for the CD47 protein in tumors and surrounding immune cells.
Tumor immunity is caused not only by cytotoxic T
lymphocytes associated with PD-1/PD-L1 pathways but also by other
immune-related cells (e.g., macrophages and dendritic cells)
(32.33). This could be the reason for the insufficient predictive
ability of PD-L1 SNPs alone as biomarkers of nivolumab
efficacy in patients with NSCLC. In this study, we focused on CD47
based on previous evidence that high CD47 expression is associated
with resistance to nivolumab treatment (34). The results indicated an association
between the CD47 SNP and therapeutic effect of nivolumab,
representing the same association between CD47 expression and
nivolumab resistance in the above study. Tumor-associated
macrophages have also been reported to express PD-1 (35), which might influence differences in
macrophage function and nivolumab efficacy.
This study also found that the combination of
CD47 and PD-L1 SNP has better predictive accuracy for
nivolumab efficacy. Particularly, patients with the G/G genotype of
CD47 SNP rs3804639 treated with nivolumab had better
prognoses, even patients with the A/G or G/G genotypes of
PD-L1 SNP rs822339, which have been previously reported to
be associated with poor prognoses (13). This result suggests that in these
patients, CD47 function is suppressed and antigen-presenting
capacity is increased, but PD-L1 function is impaired. This result
might also indicate that nivolumab would be effective for these
patients, even in those with cold tumors. Although these patients
have impaired PD-L1 function, nivolumab could be effective if CD47
function is suppressed and the antigen-presenting capacity of
macrophages is restored. Thus, CD47 SNP rs3804639 could be a
predictive biomarker of nivolumab treatment in PD-L1-independent
tumors.
Given these results, other immune-related SNPs could
predict the effect of nivolumab treatment with higher accuracy when
combined with the PD-L1 SNP or CD47 SNP. We will
further investigate SNPs that could predict the effect of nivolumab
treatment, using more high-throughput methods (e.g., a genome-wide
association study). Clinical trials targeting CD47 as a potential
therapeutic target are in progress. For instance, phase I trials on
Hu5F9-G4 (5F9), a humanized IgG4 antibody targeting CD47, for
lymphoma, lung cancer, and other cancers have shown its efficacy
(36,37). A phase II study of anti-CD47
antibodies in solid tumors is also in progress. When CD47-targeted
therapies became available in clinical practice, we will
investigate the capability of CD47 SNP rs3804639 for
predicting treatment response to CD47-targeted therapies.
Our study has some limitations. First, this was a
single-center retrospective cohort study with a small sample size.
Large multicenter cohorts would provide more reliable results. We
are currently planning studies to evaluate the relevance of the
effect of nivolumab treatment and SNPs in a multicenter cohort
(UMIN000033839). Second, a CD47 SNP that has been reported
to be associated with other cancers was selected, and other SNPs
were not investigated. We plan to investigate the relationship
between various SNPs and the therapeutic effect of nivolumab
treatment in a multicenter cohort.
In conclusion, CD47 polymorphism is
associated with survival outcomes in patients with advanced NSCLC
treated with nivolumab. The CD47 SNP alone or in combination
with the PD-L1 SNP might be helpful predictive biomarkers of
nivolumab treatment in patients with advanced NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the Clinical
Bio-Resource Center at Kyoto University Hospital for their help in
the collection and quality control of human specimens.
Funding
Research support for this study was provided through a
management expense grant to Kyoto University from the Ministry of
Education, Culture, Sports, Science and Technology of Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TO and HO conceived and designed the study. HO, YS
and HY recruited and treated the patients. TO, HY, TN and TF
performed the experiments. TO, HO and SM analyzed the data. TO
drafted the manuscript. TO, HO, HY, TN, TF, HY, KHa, KHo, MY, HA,
TT, YS, KK, SM and TH contributed to the data interpretation and
discussion. TO and HO confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of Kyoto University Hospital (certification number: G0788).
Written informed consent was obtained from all study
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodríguez-Abreu D, Powell SF, Hochmair MJ,
Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, Dómine M,
Cheng SY, et al: Pemetrexed plus platinum with or without
pembrolizumab in patients with previously untreated metastatic
nonsquamous NSCLC: Protocol-specified final analysis from
KEYNOTE-189. Ann Oncol. 32:881–895. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Socinski MA, Nishio M, Jotte RM, Cappuzzo
F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D,
Moro-Sibilot D, Thomas CA, et al: IMpower150 Final overall survival
analyses for atezolizumab plus bevacizumab and chemotherapy in
first-line metastatic nonsquamous NSCLC. J Thorac Oncol.
16:1909–1924. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hellmann MD, Paz-Ares L, Bernabe Caro R,
Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A,
Lupinacci L, de la Mora Jimenez E, et al: Nivolumab plus Ipilimumab
in Advanced Non-Small-Cell lung cancer. N Engl J Med.
381:2020–2031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker
M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E,
Juan-Vidal O, et al: First-line nivolumab plus ipilimumab combined
with two cycles of chemotherapy in patients with non-small-cell
lung cancer (CheckMate 9LA): An international, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:198–211. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus Ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
Repair-Deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nomizo T, Ozasa H, Tsuji T, Funazo T,
Yasuda Y, Yoshida H, Yagi Y, Sakamori Y, Nagai H, Hirai T and Kim
YH: Clinical impact of single nucleotide polymorphism in PD-L1 on
response to nivolumab for advanced non-small-cell lung cancer
patients. Sci Rep. 7:451242017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Funazo TY, Nomizo T, Ozasa H, Tsuji T,
Yasuda Y, Yoshida H, Sakamori Y, Nagai H, Hirai T and Kim YH:
Clinical impact of low serum free T4 in patients with non-small
cell lung cancer treated with nivolumab. Sci Rep. 9:170852019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida H, Nomizo T, Ozasa H, Tsuji T,
Funazo T, Yasuda Y, Ajimizu H, Yamazoe M, Kuninaga K, Ogimoto T, et
al: PD-L1 polymorphisms predict survival outcomes in advanced
non-small-cell lung cancer patients treated with PD-1 blockade. Eur
J Cancer. 144:317–325. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonaventura P, Shekarian T, Alcazer V,
Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C and
Depil S: Cold Tumors: A therapeutic challenge for immunotherapy.
Front Immunol. 10:1682019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong N, He Z, Ma Y, Wang Z, Huang Z, Cao
H, Xu L, Zou Y, Wang W, Yi C, et al: Tumor associated macrophages,
as the dominant immune cells, are an indispensable target for
immunologically cold Tumor-Glioma therapy? Front Cell Dev Biol.
9:7062862021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strauss O, Dunbar PR, Bartlett A and
Phillips A: The immunophenotype of antigen presenting cells of the
mononuclear phagocyte system in normal human liver-a systematic
review. J Hepatol. 62:458–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehta AK, Kadel S, Townsend MG, Oliwa M
and Guerriero JL: Macrophage biology and mechanisms of immune
suppression in breast cancer. Front Immunol. 12:6437712021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller YE, Daniels GL, Jones C and Palmer
DK: Identification of a cell-surface antigen produced by a gene on
human chromosome 3 (cen-q22) and not expressed by Rhnull cells. Am
J Hum Genet. 41:1061–1070. 1987.PubMed/NCBI
|
|
19
|
Oldenborg PA, Zheleznyak A, Fang YF,
Lagenaur CF, Gresham HD and Lindberg FP: Role of CD47 as a marker
of self on red blood cells. Science. 288:2051–2054. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russ A, Hua AB, Montfort WR, Rahman B,
Riaz IB, Khalid MU, Carew JS, Nawrocki ST, Persky D and Anwer F:
Blocking ‘don't eat me’ signal of CD47-SIRPα in hematological
malignancies, an in-depth review. Blood Rev. 32:480–489. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jaiswal S, Jamieson CH, Pang WW, Park CY,
Chao MP, Majeti R, Traver D, van Rooijen N and Weissman IL: CD47 is
upregulated on circulating hematopoietic stem cells and leukemia
cells to avoid phagocytosis. Cell. 138:271–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayat SMG, Bianconi V, Pirro M, Jaafari
MR, Hatamipour M and Sahebkar A: CD47: Role in the immune system
and application to cancer therapy. Cell Oncol (Dordr). 43:19–30.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arrieta O, Aviles-Salas A, Orozco-Morales
M, Hernández-Pedro N, Cardona AF, Cabrera-Miranda L, Barrios-Bernal
P, Soca-Chafre G, Cruz-Rico G, Peña-Torres ML, et al: Association
between CD47 expression, clinical characteristics and prognosis in
patients with advanced non-small cell lung cancer. Cancer Med.
9:2390–2402. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiskopf K, Jahchan NS, Schnorr PJ,
Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer JP, Liu J, Lim
JS, et al: CD47-blocking immunotherapies stimulate
macrophage-mediated destruction of small-cell lung cancer. J Clin
Invest. 126:2610–2620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Fan J, Wang S, Li Y, Wang Y, Li
S, Luan J, Wang Z, Song P, Chen Q, et al: Targeting CD47 and
autophagy elicited enhanced antitumor effects in Non-Small cell
lung cancer. Cancer Immunol Res. 5:363–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lascorz J, Bevier M, V Schönfels W,
Kalthoff H, Aselmann H, Beckmann J, Egberts J, Buch S, Becker T,
Schreiber S, et al: Association study identifying polymorphisms in
CD47 and other extracellular matrix pathway genes as putative
prognostic markers for colorectal cancer. Int J Colorectal Dis.
28:173–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Felip E, Ardizzoni A, Ciuleanu T, Cobo M,
Laktionov K, Szilasi M, Califano R, Carcereny E, Griffiths R,
Paz-Ares L, et al: CheckMate 171: A phase 2 trial of nivolumab in
patients with previously treated advanced squamous non-small cell
lung cancer, including ECOG PS 2 and elderly populations. Eur J
Cancer. 127:160–172. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kitadai R, Okuma Y, Hakozaki T and Hosomi
Y: The efficacy of immune checkpoint inhibitors in advanced
non-small-cell lung cancer with liver metastases. J Cancer Res Clin
Oncol. 146:777–785. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng N, Zhou H, Fan H and Yuan Y: Single
nucleotide polymorphisms and cancer susceptibility. Oncotarget.
8:110635–110649. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao H, Song S, Ma J, Yan Z, Xie H, Feng Y
and Che S: CD47 as a promising therapeutic target in oncology.
Front Immunol. 13:7574802022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murata Y, Saito Y, Kotani T and Matozaki
T: CD47-signal regulatory protein α signaling system and its
application to cancer immunotherapy. Cancer Sci. 109:2349–2357.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
DeNardo DG and Ruffell B: Macrophages as
regulators of tumour immunity and immunotherapy. Nat Rev Immunol.
19:369–382. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujiwara-Tani R, Sasaki T, Ohmori H, Luo
Y, Goto K, Nishiguchi Y, Mori S, Nakashima C, Mori T, Miyagawa Y,
et al: Concurrent Expression of CD47 and CD44 in colorectal cancer
promotes malignancy. Pathobiology. 86:182–189. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gordon SR, Maute RL, Dulken BW, Hutter G,
George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al:
PD-1 expression by tumour-associated macrophages inhibits
phagocytosis and tumour immunity. Nature. 545:495–499. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Advani R, Flinn I, Popplewell L, Forero A,
Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP,
et al: CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's
Lymphoma. N Engl J Med. 379:1711–1721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sikic BI, Lakhani N, Patnaik A, Shah SA,
Chandana SR, Rasco D, Colevas AD, O'Rourke T, Narayanan S,
Papadopoulos K, et al: First-in-human, first-in-class phase I trial
of the anti-CD47 antibody Hu5F9-G4 in patients with advanced
cancers. J Clin Oncol. 37:946–953. 2019. View Article : Google Scholar : PubMed/NCBI
|