Introduction
Melanoma is a highly malignant tumor that originates
from melanocytes and can occur in different sites or tissues,
including the skin, ocular uvea, pia mater and mucosa, for example,
that of the digestive, respiratory and genitourinary tracts.
Although malignant melanoma (MM) accounts for only 1% of all
diagnosed cutaneous cancers (1), it
is responsible for the highest number of skin cancer-associated
deaths. MM of the external ear is rare. Among head and neck MMs,
the majority (70–90%) occur on the face, mainly on the cheek, while
the external ear accounts for only 7% of cases (2). The incidence of primary melanoma of
the external ear is increasing, as is the incidence of cutaneous
melanoma (3); however, case reports
of primary external ear MM are rare.
MM primarily metastasizes through lymphatic and
hematologic routes, and distant metastases are most commonly found
in the lungs, liver and brain. Gastrointestinal metastases of MM
are rare, and as the main site of gastrointestinal metastasis is
the small intestine, metastasis of MM to the stomach is even rarer
(4). Due to its rarity, to the best
of our knowledge there are no case reports or series evaluating the
gastric metastasis of MM from the external ear that provide useful
disease characterization to help clinicians choose appropriate
treatments.
The present study reports the case of a patient with
MM originating from the left auricle with initial metastasis to the
stomach and descending duodenum, followed by multiple metastases to
the brain, lungs, peritoneum, liver and left adrenal gland. The
molecular genetic alterations of the tumor were investigated to
help select the appropriate targeted therapeutic regimen, and the
presence of multiple mutated genes was identified.
Case report
Materials and methods
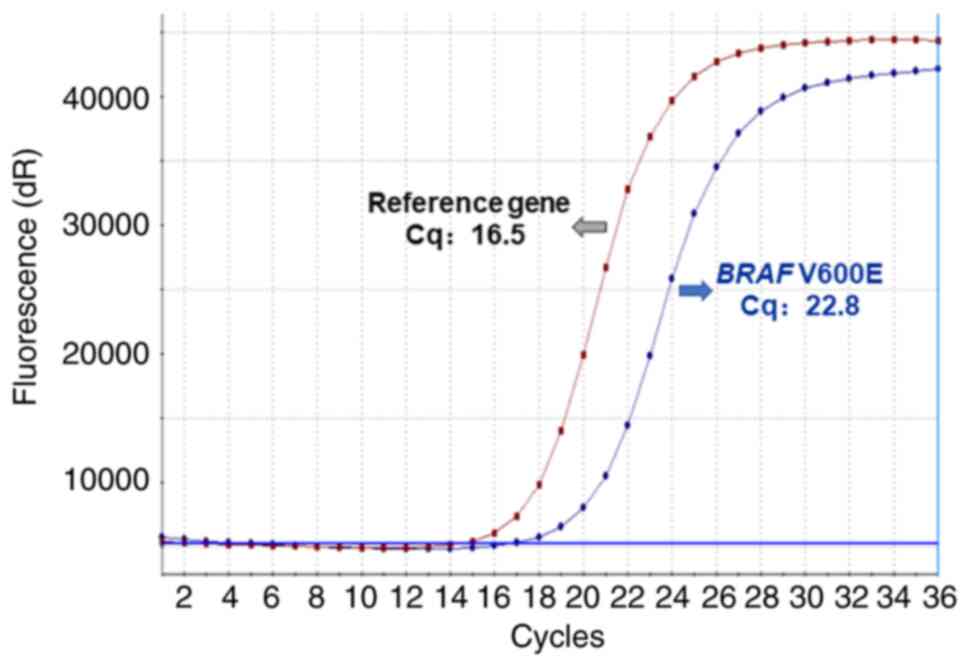
BRAF V600E mutation was detected using a Human BRAF
V600E Mutation Detection Kit (Production lot number 11221112601X;
Amoy Diagnostics Co., Ltd.) for medical diagnosis. According to the
manufacturer's instructions, amplification of the reference gene
(internal control) and BRAF V600E mutation in samples were detected
by amplification-refractory mutation system polymerase chain
reaction (ARMS-PCR) using HEX and FAM fluorescent channels,
respectively. The primers used for PCR were provided as part of the
kit. To achieve reliable results, the HEX signal of the sample to
be tested should be a positive curve, with a Cq value of between 13
and 21. The cutoff value of Cq for BRAF V600E mutation is 30.
Immunohistochemical staining was performed on 4 µm-thick paraffin
sections. The ready-to-use primary antibodies (MAB-0098, MAB-0275,
Kit-0007, MAB-0671, Kit-0024, RMA-0815, MAB-0743, MAB-0742,
MAB-0707, MAB-0672, MAB-0735, MAB-0716 and MAB-0717; MXB
Biotechnologies Co., Ltd.) and an EliVision plus Detection Kit
(KIT-9901; MXB Biotechnologies Co., Ltd.) were used for
immunohistochemical staining.
Case presentation
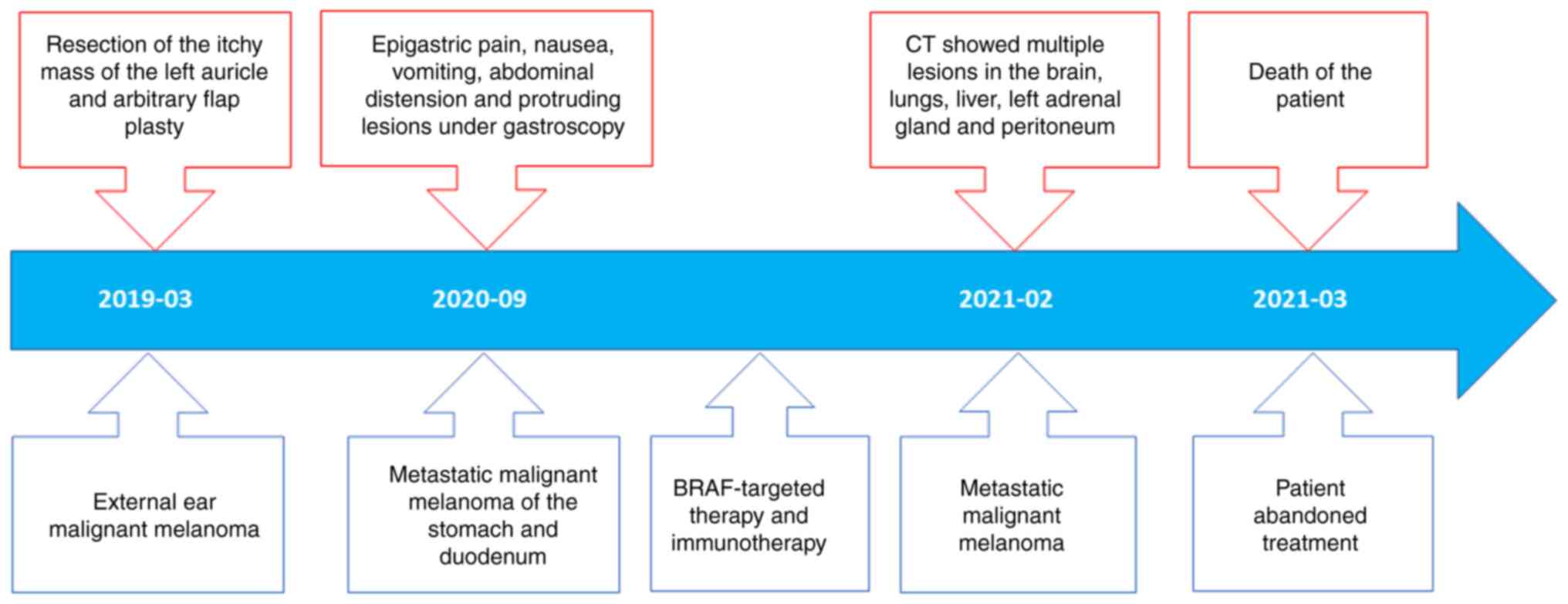
A 58-year-old female patient was hospitalized at the
People's Hospital of Xiangyun County (Xiangyun, China) in March
2019 due to a black mass the approximate size of a fava bean behind
the left ear that had been present for ~5 years with itching but no
tinnitus, stuffiness, hearing loss or pus discharge from the
external ear canal. Upon physical examination, it was observed that
the black mass was soft with clear boundaries and an absence of
pain and redness, and both auricles exhibited normal morphology.
Five days after initial hospitalization, mass resection and
arbitrary flap plasty were performed. The resected mass comprised
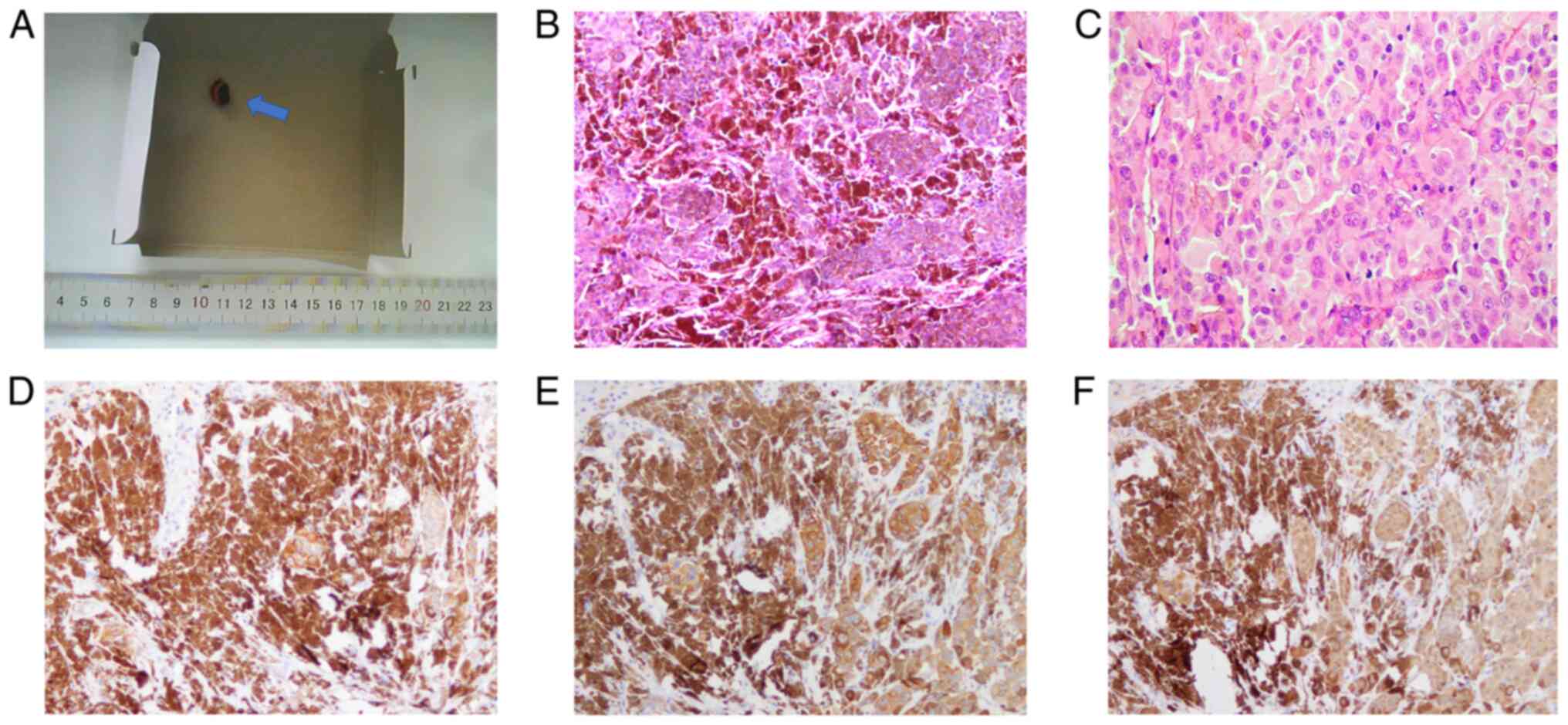
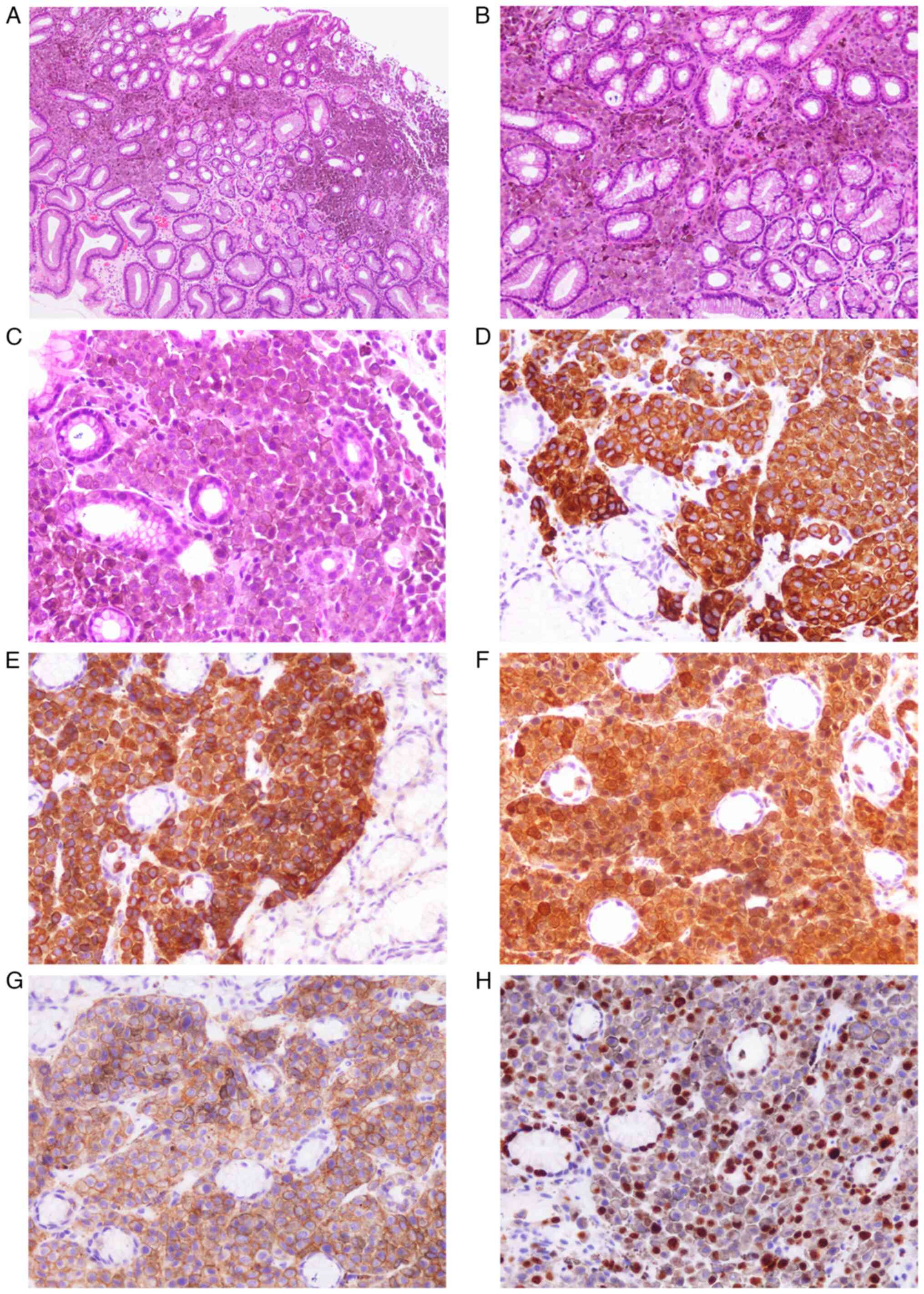
hard grayish-black tissue, 1.5×1×0.8 cm3 in size, with a
black protrusion of diameter 0.8 cm in the center (Fig. 1A). The mass was diagnosed as nodular
MM of the left auricle by intraoperative frozen section
examination. When observed under a microscope, nestlike, nodular
and diffuse melanocyte dysplasia was observed, with tumor cells
displaying marked pleomorphism and high proliferative activity
(Figs. 1B and C).
Immunohistochemical staining showed that the tumor cells were
positive for human melanoma black 45 (HMB45; Fig. 1D), melan-A (Fig. 1E) and S-100 (Fig. 1F) but negative for pan cytokeratin
(CK-pan), leukocyte common antigen and p40. Formalin-fixed
paraffin-embedded tissue sections were further analyzed to detect
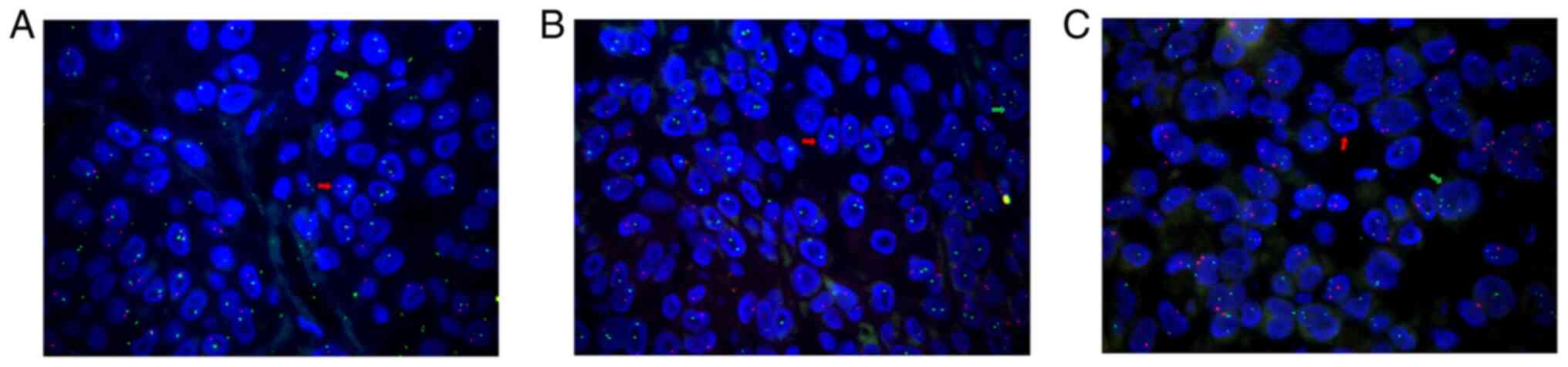
genetic variations. Fluorescence in situ hybridization
(FISH) revealed MYB deletion (Fig.
2A), p16 deletion (Fig. 2B),
MYC amplification (Fig. 2C), PTEN
deletion (Fig. 2C) and no marked
changes in the copy numbers of Ras responsive element binding
protein 1 (RREB1; Fig. 2A) and
cyclin D1 (CCND1; Fig. 2B).
ARMS-PCR revealed the BRAF V600E mutation (Fig. 3), and no common NRAS (G12D, G12S,
G13D, G13R, G12C, G12V, G12A, G13V, A59D, Q61R, Q61K, Q61L, Q61H,
K117N, and A146T) mutations were found. Furthermore, DNA sequencing
revealed no mutations in exons 9, 11, 13, or 17 of c-KIT. PCR
followed by high-resolution melting analysis showed that the tumor
was negative for seven microsatellite instability biomarkers,
namely activin A receptor type 2A; BTB domain containing 7; death
inducer-obliterator 1; MRE11 homolog, double strand break repair
nuclease; ryanodine receptor 3; SEC31 homolog A, COPII coat complex
component; and sulfatase 2. These findings indicated microsatellite
stability.
The patient was hospitalized again at the People's
Hospital of Xiangyun County in March 2020 due to epigastric pain,
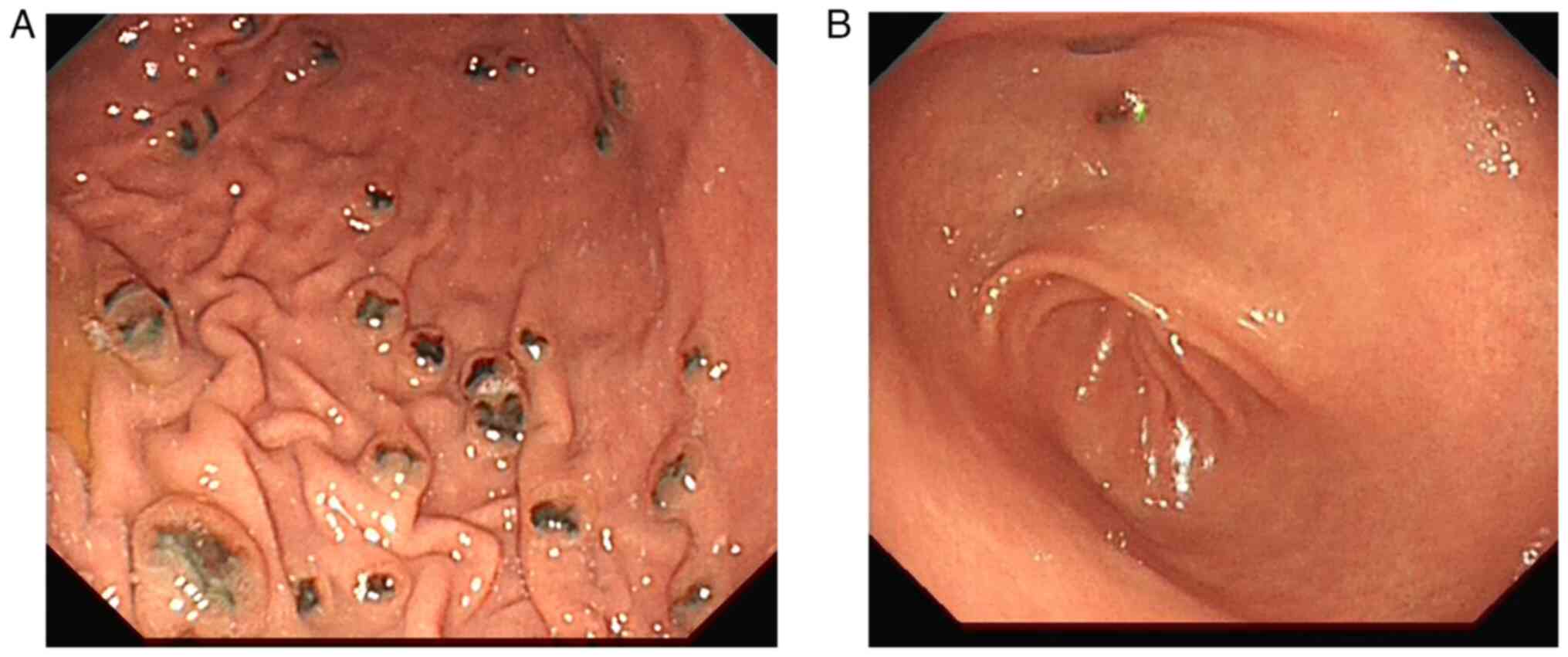
nausea, vomiting and abdominal distension. Protruding lesions with
erosion and melanin deposition were observed in the gastric body,
gastric antrum and descending part of the duodenum by gastroscopic
examination (Fig. 4). Pathological
biopsy revealed gastric mucosa hyperemia, nestlike cell clusters in
the lamina propria, and lymphocyte and plasma cell infiltration
(Fig. 5A-C). Helicobacter
pylori was not detected. Immunohistochemical staining showed
that the lesion cells were positive for HMB45 (Fig. 5D), melan-A (Fig. 5E) and S-100 (Fig. 5F), weakly positive for CD56
(Fig. 5G), and negative for CK-pan,
Syn and CgA. The Ki-67 proliferation index was ~40% (Fig. 5H). Due to the medical history,
histopathologic features and expression of protein markers in the
biopsy specimen, the patient was diagnosed with metastatic external
ear MM. Subsequently, the patient received targeted treatment with
vemurafenib, which was administered during multiple immunotherapy
sessions at a specialized oncology hospital.
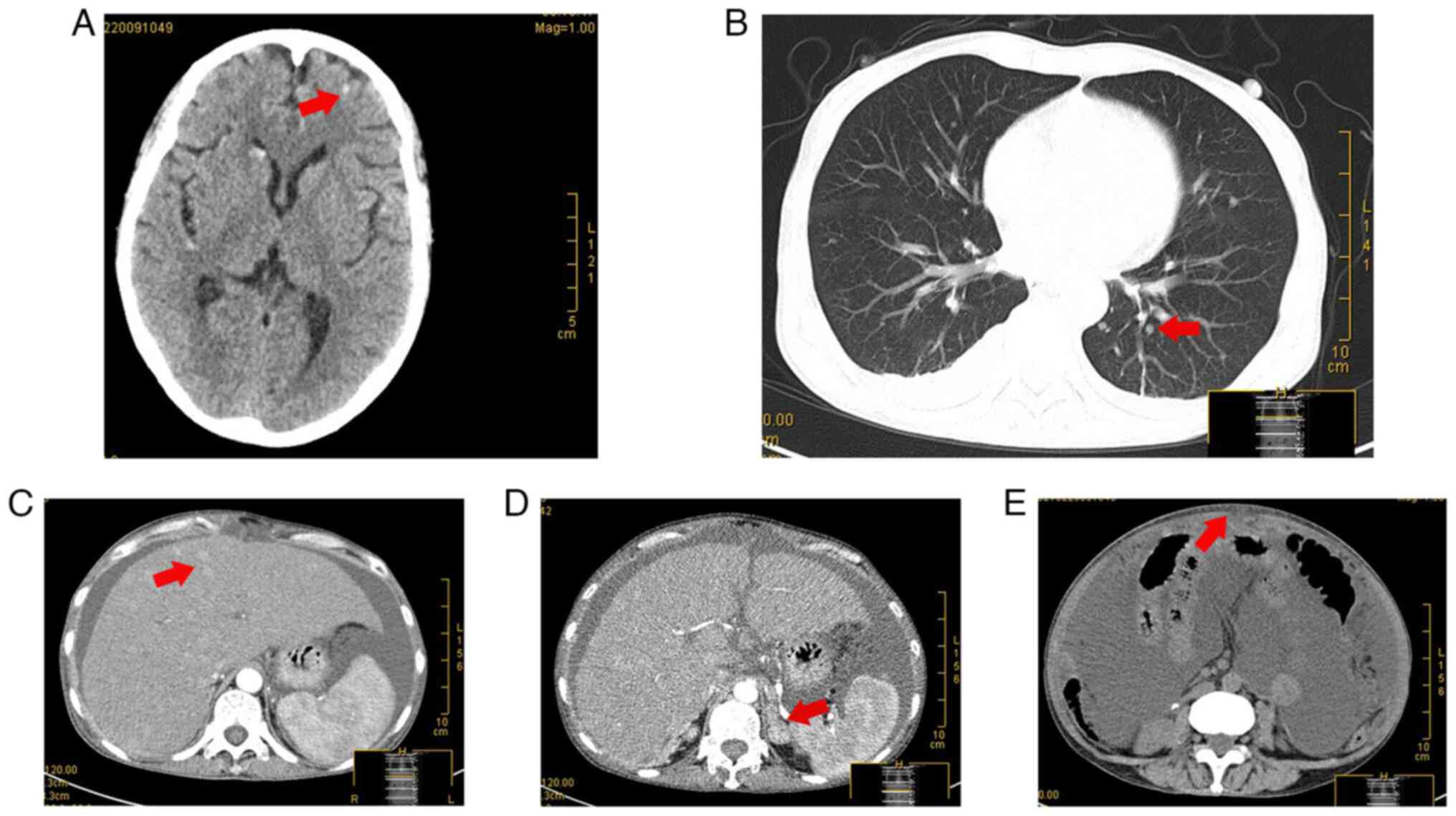
The patient experienced abdominal swelling for ~2
weeks in February 2021. Computed tomography examination revealed
multiple hyperdense shadows in the brain (Fig. 6A), bilateral small pulmonary nodules
in the lungs (Fig. 6B), diffuse
slightly hyperdense nodules of varying sizes in the liver (Fig. 6C), space-occupying lesions in the
left adrenal gland (Fig. 6D) and
peritoneal thickening (Fig. 6E),
suggesting systemic metastases of the tumor. The patient had
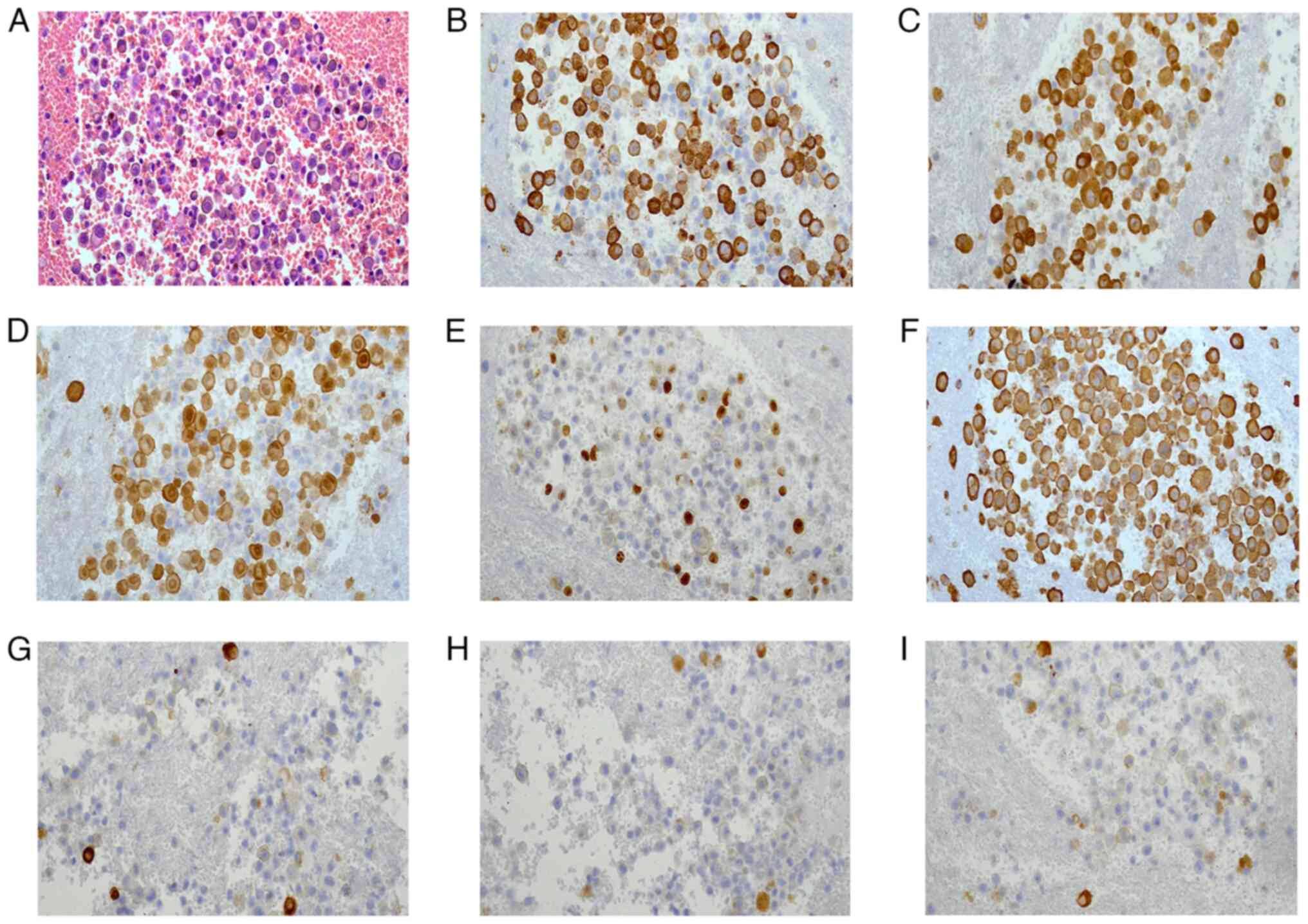
seroperitoneum in which malignant tumor cells were detected by
cytological examination (Fig. 7A).
Immunohistochemical staining showed that the tumor cells were
positive for HMB45 (Fig. 7B),
melan-A (Fig. 7C), S-100 (Fig. 7D), Ki-67 (50%) (Fig. 7E) and vimentin (Fig. 7F), and the peritoneal mesothelial
cells were positive for CK-pan (Fig.
7G), calretinin (Fig. 7H) and
mesothelial cell (Fig. 7I),
indicating metastatic MM.
In March 2021, the patient developed secondary
epilepsy, hypoproteinemia, electrolyte disturbance and cancerous
cachexia. The patient abandoned treatment and died two days after
discharge from the hospital (Fig.
8).
Literature review
A total of 6,577 cases of MM of the external ear
reported in 11 articles were reviewed in the present study
(Table I) (3,5–14). The
cases in all articles had MM of the external ear, and the specific
site was reported in some of the articles. The tumors primarily
occurred in the helix. Analysis of these cases indicated that MM of
the external ear frequently occurs in middle-aged and older adults
and occasionally in adolescents. Men (5,603 cases) were more
frequently affected than women (974 cases). Patients with MM of the
external ear were mostly asymptomatic or had some local symptoms at
diagnosis, including ear swelling, otorrhea, hearing loss and
bleeding. Regional lymph node metastasis was common, and the other
metastatic sites were primarily the liver, lung, brain, parotid
gland and retromandibular tissue. Regarding prognosis, a long
disease duration and high tumor stage were unfavorable factors for
survival.
 | Table I.Cases of malignant melanoma of the
external ear reported in the literature (n=6,577). |
Table I.
Cases of malignant melanoma of the
external ear reported in the literature (n=6,577).
| First author/s,
year | Cases, n | Age, years | Sex | Primary site | Clinical
symptoms | Metastatic
sites | Outcomes | (Refs.) |
|---|
| Amando García et
al, 2003 | 1 | 59 | Male | External ear
canal | Earache,
suppurative otorrhea, preauricular swelling | Parotid gland | Disease-free
survival for 9 months | (5) |
| Hannan and Parikh,
2006 | 1 | 40 | Male | External ear
canal | Deafness | Retromandi bular
tissue | N/A | (6) |
| Gowthami et
al, 2014 | 1 | 11 | Female | Right
post-auricular | Pain, swelling,
intermittent bleeding | Right temporal
bone | Died | (7) |
| Toia et al,
2015 | 845 | 59.4 (mean) | Male (78%), female
(22%) | Helix (57%);
earlobe (17%); post-auricular (9%); auricle (6%); antihelix and
scapha (4%); external ear canal (0.8%); other (6.2%) | N/A | Regional lymph
nodes (10%) | Five-year survival
rate: stage 0, 100%; stage I, 71%; stage II, 53%; stage III,
0% | (8) |
| Frost et al,
2017 | 45 | 63 (median) | Male (80%), female
(20%) | External ear | N/A | Sentinel lymph
nodes (7%); distant metastasis (4.4%) | Survival rate:
Overall, 80%; stage I/II,84%; stage III/ IV, 56% | (9) |
| Deep et al,
2017 | 5,481 | 66.7 (mean) | Male (86.5%) female
(13.5%) | External ear | N/A | N/A | Survival rate:
Overall, 90%; stage I, 95.3%; stage II, 81.1%; stage III, 56.6%;
stage IV, 20.5% | (3) |
| Harrison et
al, 2017 | 41 | 61 (median) | Male (73%), female
(27%) | External ear | N/A | Liver and brain
(5%) | Survival rate:
Overall, 90%; 5-year, 100%; 10-year, 71% | (10) |
| Straccia, et
al 2020 | 1 | 43 | Female | Right auricle | N/A | Pleura | N/A | (11) |
| Truong et
al, 2020 | 156 | 62.5 (median) | Male (86%), female
(14%) | Helix (61.5%);
earlobe, (13.5%); other (25%) | N/A | Sentinel lymph
nodes (14.1%) | Survival rate,
74.4% | (12) |
| Fiorio et
al, 2021 | 4 | 69 (mean), 67.5
(median) | Male (25%), female
(75%) | Right earlobe
(50%); left helix (50%) | Asymptomatic | Regional lymph
nodes (25%) | During the
follow-up, with no signs of disease progression | (13) |
| Pająk et al,
2022 | 1 | 43 | Female | Left helix | Asymptomatic | N/A | Lost to
follow-up | (14) |
A detailed literature review of 22 cases of gastric
metastatic melanoma was performed (Table II) (15–36).
The sex of one patient was not reported, and of the remaining
patients, 14 (66.7%) were male and 7 (33.3%) were female. The mean
age at diagnosis was 63.1 years (range, 36–89 years). Excluding
five patients with unclear metastatic site in the stomach, the
majority of the gastric metastatic melanomas occurred in the
gastric body (41.2%) and fundus (29.4%), and the rest occurred in
the gastric antrum, cardia and pylorus. The whole stomach was
involved in one case. On the basis of the articles containing clear
reports of 19 patients' symptoms, it was found that the main
gastrointestinal symptoms were black stools or hematemesis (52.6%),
abdominal pain (36.8%) and nausea or emesis (26.3%). Less common
symptoms included anorexia and abdominal distention. Endoscopic or
intraoperative findings were mostly masses, nodules, protruding
lumps or polyps (77.3%), ulcerations (45.5%) and melanin
pigmentation (31.8%). Multiple mucosal erosions were less common
manifestations. The primary site was not reported for one patient,
and the skin was the most common primary site in the remaining
patients (16 cases, 72.7%), including eight with acral MM. Two
cases of gastric metastatic MM originated in the eye, two in the
mucosa and one in the sphenoid sinus. Following the exclusion of
six patients with missing time-to-metastasis data, the mean and
median time intervals to metastasis were 4.4 and 2 years,
respectively, with a range from 0.5 to 15 years. Individual
differences in tumor metastasis among the patients are evident.
 | Table II.Cases of gastric metastatic malignant
melanoma reported in the literature (n=22). |
Table II.
Cases of gastric metastatic malignant
melanoma reported in the literature (n=22).
| First author/s,
year | Sex | Age, years | Metastatic
site | Clinical
symptoms | Endoscopic or
intraoperative findings | Primary site | Time interval
between primary and metastatic lesions | (Refs.) |
|---|
| Davis and
Zollinger, 1960 | Female | 76 | Greater gastric
curvature | Anorexia, nausea
and black stools | Multiple polypoid
lesions | Upper arm | 1 year | (15) |
| Morini et
al, 1980 | Female | 77 | Posterior wall of
the gastric body | Abdominal pain,
dyspepsia, lack of appetite, and black stools | Protruding
lump | Left hip | 6 years | (16) |
| Mimica and Tomić,
2002 | Male | 51 | Gastric body and
antrum | Abdominal pain | Multiple polypoid
lesions | N/A | N/A | (17) |
| Malladi et
al, 2005 | Male | 80 | Fundus, upper part
and pylorus of the stomach | Abdominal pain,
dyspepsia and weight loss | Multiple black
nodules with ulceration | Sole of the right
foot | 2 years | (18) |
| Matsubayashi et
al, 2005 | Male | 53 | Upper part of the
stomach | N/A | Dark brown lesions
with clear boundaries | Gums | Metastatic at
diagnosis | (19) |
| Wu et al,
2011 | Male | 72 | Stomach | Dysphagia | Superficial ulcers
and ulcerative nodules | Esophagus | Metastatic at
diagnosis | (20) |
| Law et al,
2011 | Female | 84 | Stomach | Hematemesis | Multiple pigmented
nodules and mucosal swelling | Right foot | 1 year | (21) |
| Eivazi-Ziaei and
Esmaili, 2014 | Male | 56 | Lesser gastric
curvature | Abdominal | Ulcers and lumps
pain | Right heel | 2 years | (22) |
| El-Sourani et
al, 2014 | Female | 43 | Lesser gastric
curvature | Black stools and
anemia | Lump | Right breast | 2 years | (23) |
| Zhao et al,
2015 | Male | 57 | Stomach | Diarrhea | Multiple erosion
and lumps | Sphenoid sinus | Half a year | (24) |
| Ruiz-Cuesta et
al, 2014 | N/A | 49 | Gastric body | Epigastric pain,
nausea, weight loss | Ulcerated lump | Right lower
limb | 13 years | (25) |
| Morita et
al, 2019 | Male | 55 | Whole stomach | N/A | Mucosa covered by
tumor cells with pitting and melanin pigmentation | Uvea | 14 years | (26) |
| Farshad et
al, 2018 | Male | 89 | Gastric cardia | Hematochezia | Bleeding mass and
ulcerations | Chest | 15 years | (27) |
| Santos-Seoane et
al, 2019 | Male | 79 | Gastric fundus | N/A | Polypoid
lesion | Left forearm | 3 years | (28) |
| Sachdeva et
al, 2020 | Male | 59 | Gastric body,
fundus and antrum | Black stools and
weight loss | Multiple black
ulcerated nodules | Multiple skin areas
throughout the body | 10 months | (29) |
| Anandabaskaran
et al, 2020 | Male | 68 | Gastric
incisura | Abdominal pain and
hematochezia | Pitted ulcerations
with melanin pigmentation around the periphery | Right foot | 7 years | (30) |
| Groudan et
al, 2020 | Female | 66 | Gastric antrum | Nausea and
hematemesis | Ulcerations and
fungoid mass | Vulva | Metastatic at
diagnosis | (31) |
| Yoshimoto et
al, 2021 | Female | 82 | Gastric body | Black stools | White semicircular
ulcerated mass | Left toe | N/A | (32) |
| Monti et al,
2021 | Male | 56 | Stomach | Severe
dyspepsia | Ulcerations and
plaques | Glans penis | Metastatic at
diagnosis | (33) |
| Zhu et al,
2022 | Male | 36 | Gastric body and
fundus | Anorexia, nausea
and emesis | Hemispherical
protrusive lesion | Sole of the right
foot | 6 months | (34) |
| Maksimaityte et
al, 2022 | Male | 46 | Gastric body and
fundus | Nausea and
abdominal enlargement | Multiple red
polyps | Right ear
auricle | 1 year | (35) |
| Bharwad et
al, 2022 | Female | 55 | Stomach | Abdominal pain,
hematochezia, dizziness and fatigue | Multiple small
pigmented lesions | Choroid of the
right eye | 2 years | (36) |
Discussion
Primary MM of the external ear is a rare type of
melanoma, accounting for ~1% of cutaneous MM and 7% of MM of the
head and neck (2). In the present
review it was identified that tumor lesions mainly occurred in the
helix and anthelix, followed by the earlobe (lobules
auriculae) and auricle (concha auriculae). The external
auditory canal was a less common site. The most common subtype of
external ear MM was superficial spreading melanoma (40.1%),
followed by malignant freckle-like nevus (33.7%) and nodular
melanoma (16.4%) (8,37). MM of the external ear is primarily
observed in the white population (99.3%), with a much lower
incidence (<0.5%) in the non-white population (37). Melanin pigmentation is well known
for protecting the skin from the harmful effects of UV radiation. A
negative association exists between the degree of skin pigmentation
and the incidence of external ear MM (37). The average incidence rates of MM are
1.015 cases per 100,000 men and 0.126 cases per 100,000 women
(37). The patient described in the
present case report is an Asian woman, and MM is extremely rare in
individuals with these demographic characteristics.
Patel et al (37) reported that the 5- and 10-year
disease-specific survival rates for MM of the external ear are 89.4
and 84.8%, respectively, and another study suggested that the
5-year overall and cancer-specific survival rates for MM of the
outer ear are 78.8 and 90.0%, respectively (3). Patients with a malignant freckle-like
nevus have been shown to have favorable survival outcomes, while
those with nodular melanoma generally have generally poor outcomes
(37). It has been reported that
the time of cancer-specific death can be independently predicted by
age, Breslow thickness, stage, ulceration and lymphatic and distant
metastasis (3). Certain studies
have implied that MM of the external ear may be more aggressive
than other head and neck MMs (7,38),
while others contend that anatomical location does not affect tumor
recurrence rates and survival outcomes (3,37,39).
MM is a highly aggressive cancer that metastasizes
relatively early, particularly to the lymph nodes, brain, liver and
lungs. The preauricular and postauricular lymph nodes, anterior and
posterior regions of the neck and parotid gland are common sites of
local metastasis for melanoma of the external ear. Consistent with
the systemic review of external ear MM published by Toia et
al (8), the literature review
in the present study observed that regional lymph nodes were the
most frequent sites of metastasis, and distant metastatic sites
were primarily liver, lung, brain and other head and neck tissues.
However, gastrointestinal metastases of MM are uncommon (4,40,41).
As reported by López et al (42), gastrointestinal metastases of MM
involve the small intestine (51–71%), stomach (27%) and colon
(22%). In the present case and literature review, it was found that
gastric metastatic MM typically occurs in the gastric body and
fundus, with various manifestations observed in endoscopy or
surgery, including masses, nodules, bulges, ulcerations, polyps,
mucosal pigmentation and erosions. A review of studies on small
intestinal melanomas suggested that 60% of deceased patients with
MM had gastrointestinal metastases, but only 1.5–4.0% were detected
before death (43). The present
retrospective literature study revealed that a total of 22 cases of
gastric MM were reported between 1960 and 2022, including only one
patient with a primary lesion in the ear, which was a 46-year-old
white male. To the best of our knowledge, this is the first case
report of gastric metastasis from auricular MM in a non-white
female.
Prophylactic removal of nevus of the external ear is
advocated in the literature to prevent the development of MM
(44). Approximately 8% of cases of
MM in the ear are non-pigmented, which can easily lead to
misdiagnosis. As these tumors are insensitive to radiotherapy, wide
local excision should be performed as soon as possible if
malignancy is suspected. Surgery, with or without lymph node
dissection, remains the preferred treatment strategy for MM
(8). Numerous adjuvant therapies
have been investigated for the treatment of cutaneous MM following
complete surgical excision. The US Food and Drug Administration
(FDA) has approved ipilimumab, nivolumab and pembrolizumab for the
adjuvant treatment of MM at stage III and above (45). Immune checkpoint inhibitors
represent a promising new type of systemic therapy for the adjuvant
treatment of MM. Regarding the surgical treatment of MM from the
external ear, the safe margin of resection remains unclear. A
minimum resection margin of 1 cm is commonly reported; however,
some authors advocate a resection margin of 2 cm if the tumor
thickness is >2.0 mm. There is a trend towards surgical
approaches with reduced resection margins, with the advised minimum
extent being >0.5 cm (3,46). The resection margin in the present
case was much smaller than the minimum resection standard advocated
in the aforementioned studies, which may a primary reason for the
postoperative metastasis of the tumor.
Positive results for specific immunohistochemical
markers, including HMB45, melan-A, S-100, SRY-box transcription
factor 10, melanoma-associated antigen (PNL-2), tyrosinase and
melanocyte inducing transcription factor, are critical for the
diagnosis and differential diagnosis of MM (47). HMB45 is a glycoprotein expressed in
melanosomes with extremely high specificity; melan-A, PNL-2 and
tyrosinase also have high specificity; while S-100 is more
sensitive but less specific for the detection of MM. The
appropriate marker for detection and diagnosis can be selected on
the basis of the marker characteristics. In the present study,
HMB45, melan-A, and S-100 were found to be expressed in the tumor
cells in the primary lesion, gastric metastatic lesion and
seroperitoneum of the patient.
The initiation and development of MM involve
multiple genes. BRAF gene mutation is the most prevalent genetic
alteration in 40–60% of cutaneous MM cases, and this mutation
results in constitutive activation of the MAPK pathway (48–50).
Vemurafenib, dabrafenib and encorafenib are US FDA-approved
inhibitors targeting the BRAF V600 mutation, and a combination of
BRAF inhibitors and MEK inhibitors, including trametinib and
cobimetinib, has become the standard treatment for advanced MM with
BRAF V600 mutation (51,52). Furthermore, atezolizumab plus
vemurafenib plus cobimetinib was approved by the FDA for patients
with BRAF V600 mutation-positive unresectable or metastatic MM in
July 2020 (53). Therefore,
molecular testing for BRAF mutations in MM is critical for
selecting the most appropriate treatment. Genetic information is
rarely mentioned in reported cases of MM of the external ear, and
the present case was positive for the BRAF V600E gene mutation.
BRAF mutations are associated with a higher tumor stage, lymph node
metastasis and an increased depth of tumor infiltration (54). Brain metastases are more likely to
occur in patients in which the MM has BRAF mutations than in those
with wild-type BRAF (55).
Consistent with this, 11 months following the gastric metastasis of
external ear MM, the involvement of multiple organs, including the
brain, had occurred in the present case.
Numerous molecular genetic studies have demonstrated
that although BRAF mutations have been detected in both MM and
melanocytic nevi, the genetic alterations of the two presentations
differ substantially. MM frequently shows a large number and
variety of chromosomal abnormalities, including 6q23 (MYB), 6q25
(RREB1), 9p21 (p16), 11q23 (CCND1), 8q24 (MYC) and 10q23 (PTEN),
which can be amplified, deleted or translocated (56). By contrast, these genetic loci are
rarely changed in melanocytic nevi. Multiple molecular testing by
FISH confirmed the amplification of the MYC gene and deletion of
the MYB, p16 and PTEN genes in the present case. Additionally,
RREB1 and CCND1 copy numbers were not markedly changed.
The proto-oncogene c-MYC is strongly associated with
various types of cancer. It has been demonstrated that the copy
number of c-MYC is increased in the tumor region of nodular MM
(57). In a study of 43 cases of
uveal melanoma, extra copies of c-MYC were detected in 70% of cases
(58). Of the 30 patients with
extra copies of c-MYC, 13 (43%) had amplification, 14 (47%) had an
intermediate relative increase in copy number, and 3 (10%) had a
simple gain of chromosome 8 (58).
In a study of 44 cases of primary acral melanoma, c-MYC was found
to be amplified in 54.5% of cases (59). In addition, c-MYC gene expression
may be positively associated with the metastatic potential of MM
(57). An association has also been
identified between larger tumor size and c-MYC amplification
(58).
The MYB gene is localized in chromosome 6q22-q23.
Deletions and translocations involving this region of the long arm
of chromosome 6 occur frequently in MM, and rearrangements of the
MYB gene have been reported in MM (60). Romano et al (61) observed deletion of the MYB gene in
five of seven cases (71%) of melanoma of the nail apparatus, with
the percentage of abnormal cells ranging from 52 to 94%. Two of the
patients in the study had lymph node metastasis and died from
widespread metastatic disease, and these two patients had
amplification of RREB1, CCND1 and MYC as well as MYB deletion
(61).
As a tumor suppressor, p16 has been shown to play a
crucial role in tumor progression rather than initiation. In
healthy cells, p16 blocks the phosphorylation of retinoblastoma
protein by binding to CDK4/6, leading to cell cycle arrest.
Dysfunction of the p16 protein diminishes its ability to regulate
cell proliferation, resulting in uncontrolled cell division and the
growth and development of tumors (62). p16 alterations frequently occur in
sporadic melanomas (62). In a
study of vertical growth phase melanoma, absent or minimal nuclear
p16 protein expression was found in 45% of primary melanomas, and
further loss of p16 expression was observed in 77% of metastatic
lesions; the absence of p16 expression independently predicted poor
survival in the patients (62).
PTEN is a well-known tumor suppressor that is
frequently mutated, inactivated or deleted in various malignancies.
The MEK/ERK pathway contributes to the inhibition of PTEN by
activating c-Jun, which activates the PI3K/AKT pathway, causing
apoptosis resistance, tumor growth and metastasis in melanoma cells
(63,64). Loss of PTEN function has been
reported in 35% of melanomas and is often detected in advanced
melanomas along with BRAF mutations (65). Dankort et al (66) induced the expression of BRAF V600E
specifically in the melanocytes of mice; the mice developed benign
melanocytic hyperplasias that did not progress to melanoma within
15–20 months. By contrast, when the expression of BRAF V600E was
combined with PTEN gene silencing, melanoma with 100% penetrance, a
short latency period and metastases to the lymph nodes and lungs
developed, suggesting that the BRAF V600E mutation combined with
PTEN deletion induces MM metastasis (66). It has been reported that PTEN
deletion is a possible predicting factor of intrinsic resistance to
BRAF inhibitor therapy (67).
Notably, BRAF inhibitor treatment was ineffective in the present
patient. In addition, PTEN deletion has been described as a driver
of immune evasion, with PTEN deletion in MM being associated with
the increased expression of programmed death-ligand 1 and
immunosuppressive cytokines, decreased T-cell infiltration, and
resistance to T-cell-mediated tumor cell lysis (64). PTEN deletion has also been suggested
to mediate immune evasion by the attenuation of tumor antigen
cross-presentation, resulting in T-cell exclusion and indirectly
influencing B-cell function (67).
In conclusion, the present report describes a rare
case of MM in an Asian female with postoperative metastasis to the
stomach and descending duodenum from the auricle and secondary
metastases to multiple organs, including the brain, lungs, liver,
left adrenal gland and peritoneum. Molecular testing revealed
genetic aberrations in BRAF, MYB, p16, MYC and PTEN. The small
resection margin, nodular histological type and number of genetic
aberrations may be strongly associated with high-metastatic
potential, resistance to targeted therapy and poor prognosis in
this case.
Acknowledgements
The authors would like to thank Guangzhou LBP
Medical Technology Co., Ltd. (Guangzhou, Guangdong, China), for
providing FISH technical services.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82160582), the Yunnan Province
Fundamental Research Project (grant no. 202201AT070003) and the
Scientific Research Foundation of Dali (grant no. 2021KBG036).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YCY detected the gene mutation, collected the
pathological images, drafted the manuscript and analyzed the data
for the literature review. YL and YP contributed to the
pathological diagnosis and clinical data collection. BG contributed
to the analysis and interpretation of the case report and revised
the manuscript. YCY, YL, YP and BG confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Dali University
(approval no. 20221202001). Informed consent was obtained from the
patient's family.
Patient consent for publication
Consent for publication was obtained from the
patient's family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shashanka R and Smitha BR: Head and neck
melanoma. ISRN Surg. 2012:9483022012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deep NL, Glasgow AE, Habermann EB,
Kasperbauer JL and Carlson ML: Melanoma of the external ear: A
population-based study. Am J Otolaryngol. 38:309–315. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hao M, Zhao G, Du X, Yang Y and Yang J:
Clinical characteristics and prognostic indicators for metastatic
melanoma: Data from 446 patients in north China. Tumour Biol.
37:10339–10348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amando García L, Suárez Nieto C, Madrigal
Rubiales B and García García J: External ear melanoma. Acta
Otorrinolaringol Esp. 54:89–93. 2003.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hannan SA and Parikh A: Malignant melanoma
of the external ear canal. Lancet. 368:16802006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gowthami C, Kumar P, Ravikumar A, Joseph
LD and Rajendiran S: Malignant melanoma of the external auditory
canal. J Clin Diagn Res. 8:FD04–FD06. 2014.PubMed/NCBI
|
|
8
|
Toia F, Garbo G, Tripoli M, Rinaldi G,
Moschella F and Cordova A: A systematic review on external ear
melanoma. J Plast Reconstr Aesthet Surg. 68:883–894. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frost J, Dunne JA and Powell BW: External
ear melanoma: A 10 year assessment of management and outcomes. J
Plast Reconstr Aesthet Surg. 70:551–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harrison C, Mikhail M, Potter M, Gore S
and Cassell O: Sentinel lymph node biopsy for external ear
melanoma: A 17 year experience with long term survival data. J
Plast Reconstr Aesthet Surg. 70:1301–1303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Straccia P, Martini M and Pierconti F:
Pleural metastasis from auricular melanoma: A brief report. Diagn
Cytopathol. 48:376–379. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Truong A, Hyngstrom JR, Andtbacka RHI,
Noyes RD, Wright M, Snyder J, Winters A, Sause WT, Grossmann KF,
Khong HT, et al: Recurrence risk of early-stage melanoma of the
external ear: An investigation of surgical approach and sentinel
lymph node status. Melanoma Res. 30:173–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiorio LM, Diniz LM, Spelta K and Badaró
BA: Ear melanoma: A four-case series. An Bras Dermatol. 96:64–67.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pająk J, Lelonek E, Chlebicka I and
Szepietowski JC: Nodular melanoma of the external ear: A rare
tumour successfully treated with simple excision. Postepy Dermatol
Alergol. 39:635–636. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis GH and Zollinger RW: Metastatic
melanoma of the stomach. Am J Surg. 99:94–96. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morini S, Bassi O and Colavolpe V:
Malignant melanoma metastatic to the stomach: Endoscopic diagnosis
and findings. Endoscopy. 12:86–89. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mimica M and Tomić I: Endoscopic diagnosis
of malignant melanoma metastatic to the stomach. Am J
Gastroenterol. 97:1572–1573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malladi V, Palanivelu C, Mathew S, Rajan
PS, Jani K, Senthilkumar R, Senthilkumaran S and Kavalkat AJ:
Malignant melanoma metastatic to the stomach and duodenum. Indian J
Gastroenterol. 24:1332005.PubMed/NCBI
|
|
19
|
Matsubayashi H, Takizawa K, Nishide N and
Ono H: Metastatic malignant melanoma of the gastric mucosa. Intern
Med. 49:1243–1244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu PR, Yen HH and Chen CJ:
Gastrointestinal: Primary esophageal melanoma with gastric
metastasis. J Gastroenterol Hepatol. 26:13382011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Law ST, Li MK and Lo WH: Gastric melanoma.
Hong Kong Med J. 17:503–504. 2011.PubMed/NCBI
|
|
22
|
Eivazi-Ziaei J and Esmaili H: Metastatic
malignant melanoma affecting stomach. J Cancer Res Ther.
10:733–736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Sourani N, Troja A, Raab HR and
Antolovic D: Gastric metastasis of malignant melanoma: Report of a
case and review of available literature. Viszeralmedizin.
30:273–275. 2014.PubMed/NCBI
|
|
24
|
Zhao L, Yan J, Li L, Wei J, Li L, Qian X,
Liu B and Zou Z: Gastric metastasis from sphenoid sinus melanoma: A
case report. Oncol Lett. 9:609–613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruiz-Cuesta P, Hervás-Molina AJ,
Villar-Pastor CM, Jurado-García J and Barrera-Baena P: Late gastric
metastasis from cutaneous melanoma. Gastroenterol Hepatol.
37:564–565. 2014.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morita S, Suda T and Terai S: Gastric
metastasis from uveal melanoma. Clin Gastroenterol Hepatol.
7:A202019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Farshad S, Keeney S, Halalau A and Ghaith
G: A case of gastric metastatic melanoma 15 years after the initial
diagnosis of cutaneous melanoma. Case Rep Gastrointest Med.
2018:76849642018.PubMed/NCBI
|
|
28
|
Santos-Seoane SM, Pérez-Casado L,
Helguera-Amezua C and Diez-Fernández M: Metastatic melanoma of the
stomach. Cir Esp (Engl Ed). 97:512019.(In English, Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sachdeva S, Dalal A, Kumar A and George R:
Multifocal gastric metastasis of malignant melanoma: An ominous
endoscopic appearance: Gastric metastasis of malignant melanoma.
Dig Liver Dis. 52:15122020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anandabaskaran S, Fewtrell M, Ismail K and
Prakoso E: A case of delayed metastatic melanoma to the stomach.
Intern Med J. 50:1590–1591. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Groudan K, Ma W and Joshi K: Metastatic
melanoma presenting as a gastric mass. Cureus.
12:e118742020.PubMed/NCBI
|
|
32
|
Yoshimoto T, Okamoto T and Fukuda K: Giant
gastric metastasis of malignant melanoma. Oxf Med Case Reports.
2021:omab0502021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monti M, Guidoboni M, Oboldi D, Bartolini
G, Pieri F, Ruscelli S, Passardi A, Ridolfi L, De Rosa F, Sullo FG
and Frassineti GL: Melanoma metastasis mimicking gastric cancer: A
challenge that starts from diagnosis. Therap Adv Gastroenterol.
14:17562848219895592021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu M, Zhang DY, Zhang GJ, Wang ZB and Lid
MY: Amelanotic metastatic gastric malignant melanoma: A case
report. Anticancer Drugs. 33:e808–e812. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maksimaityte V, Reivytyte R, Milaknyte G,
Mickys U, Razanskiene G, Stundys D, Kazenaite E, Valantinas J and
Stundiene I: Metastatic multifocal melanoma of multiple organ
systems: A case report. World J Clin Cases. 10:10136–10145. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bharwad A, Shah H and Salyers WJ Jr:
Malignant melanoma of the stomach. Eur J Case Rep Intern Med.
9:0036402022.PubMed/NCBI
|
|
37
|
Patel TD, Chin OY, Baredes S, Eloy JA and
Ying YM: A population based analysis of melanoma of the external
ear. Otol Neurotol. 39:e137–e142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oliver JD, Boczar D, Sisti A, Huayllani
MT, Restrepo DJ, Spaulding AC, Gabriel E, Bagaria S, Rinker BD and
Forte AJ: National Analysis of Patients With External Ear Melanoma
in the United States. J Craniofac Surg. 30:e787–e790. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Augenstein AC, Capello ZJ, Little JA,
McMasters KM and Bumpous JM: The importance of ulceration of
cutaneous melanoma of the head and neck: A comparison of ear
(pinna) and nonear sites. Laryngoscope. 122:2468–2472. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang S, Barnhill RL, Graeme-Cook F,
Randolph G, Nadol JB and Sober AJ: Primary malignant melanoma of
the external auditory canal: A case report with presentation as an
aural polyp. Am J Otol. 13:194–196. 1992.PubMed/NCBI
|
|
41
|
Hoshikawa H, Miyashita T and Mori N:
Surgical procedures for external auditory canal carcinoma and the
preservation of postoperative hearing. Case Rep Surg.
2012:8413722012.PubMed/NCBI
|
|
42
|
López RG, Santomé PM, Porto EI, Moreiras
MI, Gómez CG, Villanueva JM, Canosa MA, Veiga OR, Gutiérrez AE,
Ares IM and Val FA: Intestinal perforation due to cutaneous
malignant melanoma mestastatic implants. Rev Esp Enferm Dig.
103:386–388. 2011.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lens M, Bataille V and Krivokapic Z:
Melanoma of the small intestine. Lancet Oncol. 10:516–521. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Benmeir P, Baruchin A, Weinberg A,
Nahlieli O, Neuman A and Wexler MR: Rare sites of melanoma:
Melanoma of the external ear. J Craniomaxillofac Surg. 23:50–53.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Appelbaum EN, Gross ND, Diab A, Bishop AJ,
Nader ME and Gidley PW: Melanoma of the external auditory canal: A
review of seven cases at a tertiary care referral center.
Laryngoscope. 131:165–172. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jahn V, Breuninger H, Garbe C and Moehrle
M: Melanoma of the ear: Prognostic factors and surgical strategies.
Br J Dermatol. 154:310–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohsie SJ, Sarantopoulos GP, Cochran AJ and
Binder SW: Immunohistochemical characteristics of melanoma. J Cutan
Pathol. 35:433–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Melis C, Rogiers A, Bechter O and van den
Oord JJ: Molecular genetic and immunotherapeutic targets in
metastatic melanoma. Virchows Arch. 471:281–293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Long GV, Menzies AM, Nagrial AM, Haydu LE,
Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scolyer RA and
Kefford RF: Prognostic and clinicopathologic associations of
oncogenic BRAF in metastatic melanoma. J Clin Oncol. 29:1239–1246.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hauschild A, Grob JJ, Demidov LV, Jouary
T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr,
Kaempgen E, et al: Dabrafenib in BRAF-mutated metastatic melanoma:
A multicentre, open-label, phase 3 randomised controlled trial.
Lancet. 380:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sarkisian S and Davar D: MEK inhibitors
for the treatment of NRAS mutant melanoma. Drug Des Devel Ther.
12:2553–2565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Robert C, Lewis KD, Gutzmer R,
Stroyakovskiy D, Gogas H, Protsenko S, Pereira RP, Eigentler T,
Rutkowski P, Demidov L, et al: Biomarkers of treatment benefit with
atezolizumab plus vemurafenib plus cobimetinib in
BRAFV600 mutation-positive melanoma. Ann Oncol.
33:544–555. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abd Elmageed ZY, Moore RF, Tsumagari K,
Lee MM, Sholl AB, Friedlander P, AI-Qurayshi Z, Hassan M, Wang AR,
Boulares HA and Kandil E: Prognostic role of BRAFV600E
cellular localization in melanoma. J Am Coll Surg. 226:526–537.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cheng L, Lopez-Beltran A, Massari F,
MacLennan GT and Montironi R: Molecular testing for BRAF mutations
to inform melanoma treatment decisions: A move toward precision
medicine. Mod Pathol. 31:24–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bandarchi B, Jabbari CA, Vedadi A and
Navab R: Molecular biology of normal melanocytes and melanoma
cells. J Clin Pathol. 66:644–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Greulich KM, Utikal J, Peter RU and Krähn
G: c-MYC and nodular malignant melanoma. A case report. Cancer.
89:97–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Parrella P, Caballero OL, Sidransky D and
Merbs SL: Detection of c-myc amplification in uveal melanoma by
fluorescent in situ hybridization. Invest Ophthalmol Vis Sci.
42:1679–1684. 2001.PubMed/NCBI
|
|
59
|
Su J, Yu W, Liu J, Zheng J, Huang S, Wang
Y, Qi S, Ma X, Chen J and Zhang Y: Fluorescence in situ
hybridisation as an ancillary tool in the diagnosis of acral
melanoma: A review of 44 cases. Pathology. 49:740–749. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Meese E, Meltzer PS, Witkowski CM and
Trent JM: Molecular mapping of the oncogene MYB and rearrangements
in malignant melanoma. Genes Chromosomes Cancer. 1:88–94. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Romano RC, Shon W and Sukov WR: Malignant
melanoma of the nail apparatus: A fluorescence in situ
hybridization analysis of 7 cases. Int J Surg Pathol. 24:512–518.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Straume O, Sviland L and Akslen LA: Loss
of nuclear p16 protein expression correlates with increased tumor
cell proliferation (Ki-67) and poor prognosis in patients with
vertical growth phase melanoma. Clin Cancer Res. 6:1845–1853.
2000.PubMed/NCBI
|
|
63
|
Ince FA, Shariev A and Dixon K: PTEN as a
target in melanoma. J Clin Pathol jclinpath-2021-208008.
9–May;2022.(Epub ahead of print).
|
|
64
|
Giles KM, Rosenbaum BE, Berger M, Izsak A,
Li Y, Illa Bochaca I, Vega-Saenz de Miera E, Wang J, Darvishian F,
Zhong H and Osman I: Revisiting the clinical and biologic relevance
of partial PTEN loss in melanoma. J Invest Dermatol. 139:430–438.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Aguissa-Touré AH and Li G: Genetic
alterations of PTEN in human melanoma. Cell Mol Life Sci.
69:1475–1491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dankort D, Curley DP, Cartlidge RA, Nelson
B, Karnezis AN, Damsky WE Jr, You MJ, DePinho RA, McMahon M and
Bosenberg M: Braf(V600E) cooperates with Pten loss to induce
metastatic melanoma. Nat Genet. 41:544–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cabrita R, Mitra S, Sanna A, Ekedahl H,
Lövgren K, Olsson H, Ingvar C, Isaksson K, Lauss M, Carneiro A and
Jönsson G: The role of PTEN loss in immune escape, melanoma
prognosis and therapy response. Cancers (Basel). 12:7422020.
View Article : Google Scholar : PubMed/NCBI
|