Introduction
Immunotherapy is a new promising anticancer
strategy. Immune checkpoint inhibitors (ICPIs) block certain immune
inhibitory ‘checkpoints’, such as programmed cell death receptor 1
(PD-1), programmed cell death ligand 1 (PD-L1) or cytotoxic
T-lymphocyte antigen 4, preventing these inhibitors from blocking
T-cell proliferation and activation against tumor cells (1). In patients with early-stage,
triple-negative breast cancer, neoadjuvant treatment with
chemotherapy in combination with ICPIs improves pathological
complete response (pCR) rates (2,3). Among
patients with metastatic triple-negative breast cancer with
positive PD-L1 status, chemotherapy in combination with ICPIs has
demonstrated a notable and clinically meaningful improvement in
progression-free survival (4,5).
Despite the clinical benefit of ICPI treatment, ICPI use is
associated with a spectrum of adverse effects involving any organ,
most commonly those of the gastrointestinal tract, endocrine
glands, skin and liver (6,7). Thyroid dysfunction is one of the most
frequent endocrine adverse events associated with ICPIs and is
present in 3–15% of patients treated with either anti-PD-1 or
anti-PD-L1 (2–5).
In the present study, the case of a 36-year-old
female patient pathologically diagnosed with left, triple-negative
breast cancer and left axillary lymph node metastasis is reported.
Prior to surgery, this patient received neoadjuvant
anthracycline-free chemotherapy and anti-PD-1 treatment for six
cycles. The postoperative pathology showed a pCR for both the
breast and the axillary lymph node. Despite the meaningful clinical
improvement, the patient suffered from transient hyperthyroidism
for 4 weeks and subsequent hypothyroidism requiring sustained
hormone replacement.
Case report
In June 2020, a 36-year-old female patient attended
The Outpatient Department of The Union Hospital, Tongji Medical
College, Huazhong University of Science and Technology (Wuhan,
China) due to a lump present in the left breast for half a year.
Physical examination revealed a hard and poorly mobile mass in the
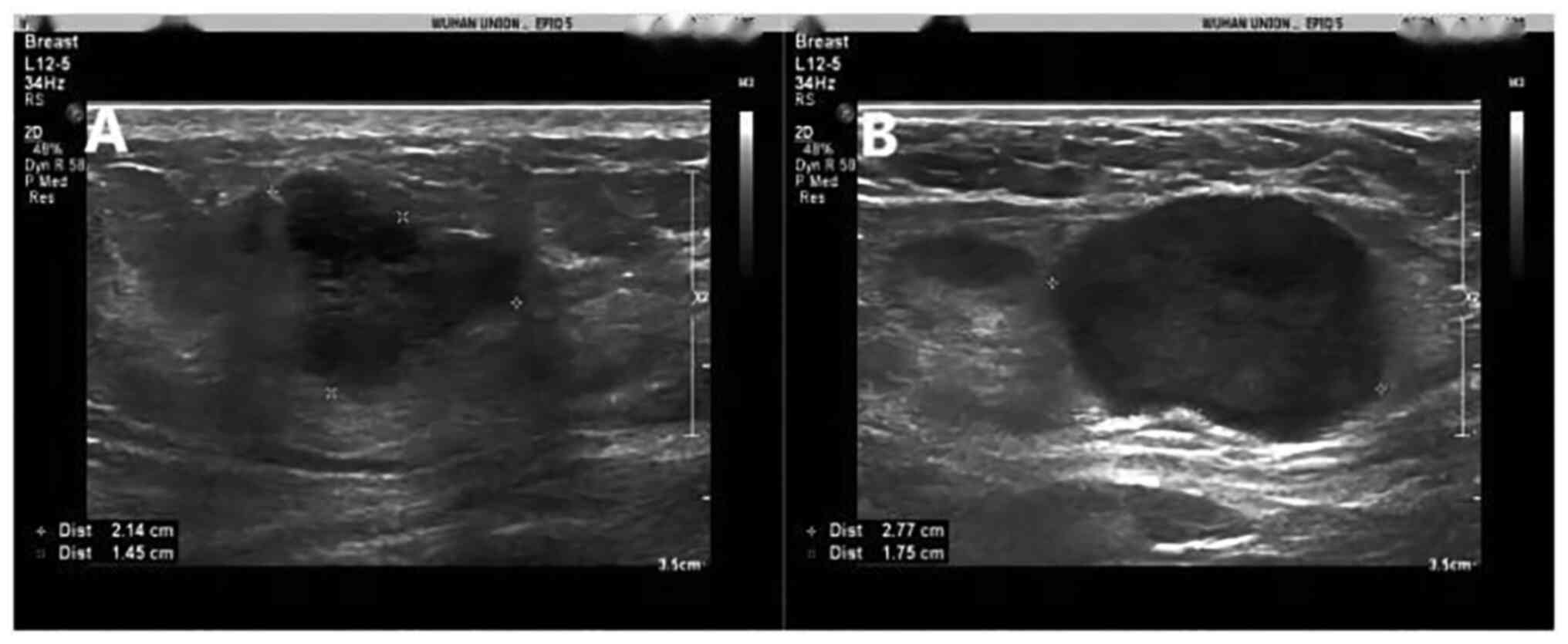
outer upper quadrant of the left breast. An ultrasound displayed an
irregular hypoechoic mass of 21.4×14.5 mm in the 2 o'clock
orientation of the left breast with several abnormal axillary lymph
nodes (Fig. 1). Magnetic resonance
imaging (MRI) of the breast revealed an irregularly-shaped and
lobulated mass in the outer upper quadrant of the left breast with
enlarged axillary lymph nodes. Any distant metastases were not
detected by radiological examinations, including brain, chest and
upper abdominal computed tomography and bone scintigraphy.
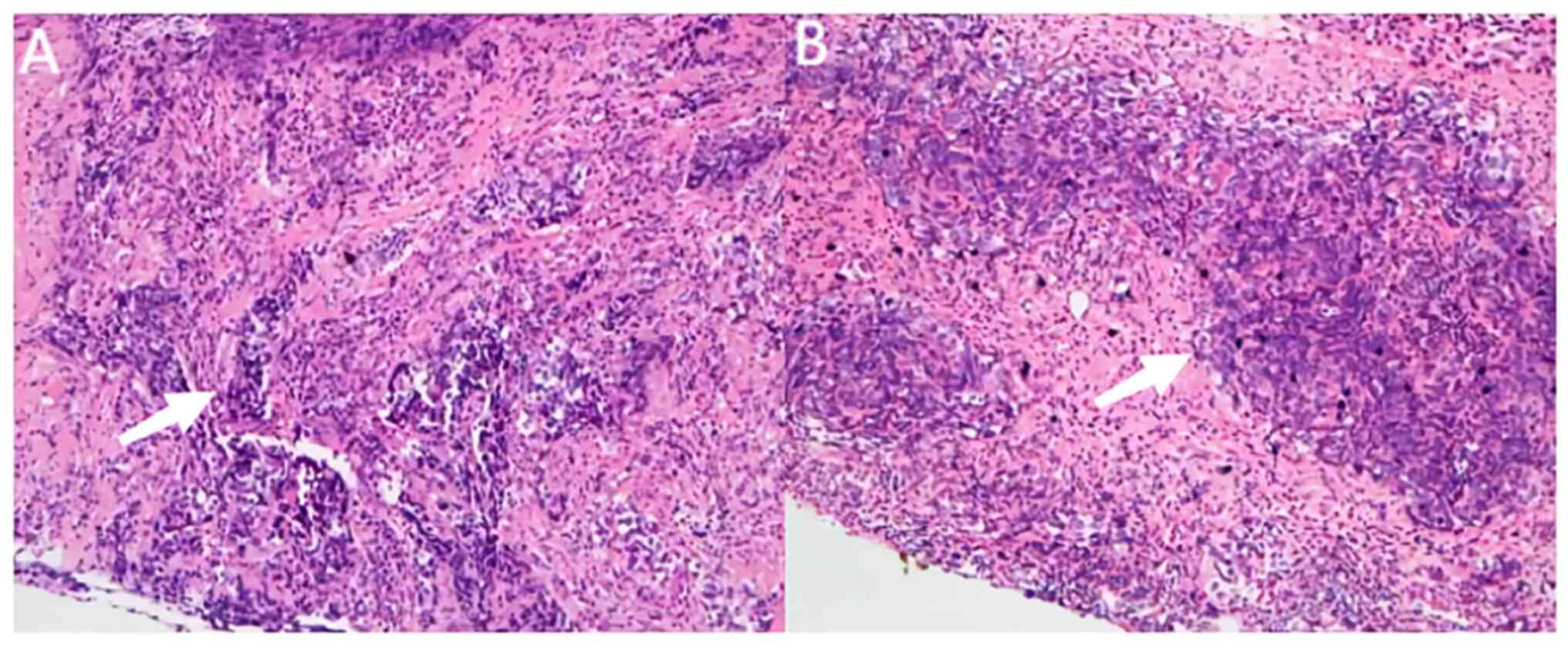
In addition, a core needle biopsy confirmed left
breast-invasive ductal carcinoma and left axillary lymph node
metastasis (Fig. 2).
Immunohistochemistry of paraffin-embedded breast tissues was
negative for both hormone receptor (HR) and human epidermal growth
factor receptor 2 (HER2). For immunohistochemistry, the breast
samples of core needle biopsy were fixed in 10% formalin at room
temperature for 24 h. The sections (3-µm thick) of
paraffin-embedded breast cancer samples were heated at 58°C for 2 h
and then deparaffinized in xylene for 10 min and hydrated in a
series of graded alcohols (100% ethanol for 2 min, 95% ethanol for
2 min, 80% ethanol for 2 min, 70% ethanol for 2 min). After
blocking with working fluid (cat. no. DM841; Dako; Agilent
Technologies, Inc.) at room temperature for 3 min, the samples were
immersed in citrate buffer (0.01M, pH 9.0) and heated at 121°C in a
microwave oven for 10 min. To block endogenous peroxidase activity,
samples were incubated with 3% hydrogen peroxide at 20°C for 30
min. Subsequently, the samples were probed with primary antibodies
against estrogen receptor (working fluid; cat. no. SP1; Roche
Diagnostics Co., Ltd.), progesterone receptor (working fluid; cat.
no. 1E2; Roche Diagnostics Co., Ltd.) or HER2 (working fluid; cat.
no. 4B5; Roche Diagnostics Co., Ltd.) at 37°C for 32 min. The
samples were then incubated with a biotinylated anti-mouse/rabbit
secondary antibody (1:500 dilution; cat. no. D0486; Dako; Agilent
Technologies, Inc.) at 37°C for 15 min. The ultraview DAB IHC
detection kit (version 1.7; Roche Diagnostics Co., Ltd.) was used
visualize the bands. The sections were counterstained with
haematoxylin and mounted. The sections were visualized using a
light microscope (Olympus BX53; Olympus Corp.) at ×100
magnification.
Following the National Comprehensive Cancer Network
breast cancer guidelines (8),
neoadjuvant therapy was suggested for treatment after discussions
with the patient. The patient received 260 mg/m2 of
nab-paclitaxel every 2 weeks with anti-PD-1 antibody, 200 mg of
camrelizumab, every 2 weeks for the first two cycles. Clinical CR,
partial response (PR) and stable disease (SD) were evaluated with
reference to the definitions of the Response Evaluation Criteria in
Advanced Solid Tumors version 1.1 (9). Before the third cycle of therapy, the
breast ultrasound showed clinical SD; thus, the patient received
additional platinum therapy consisting of 50 mg lobaplatin every 2
weeks for the next two cycles. Before the fifth cycle, the breast
ultrasound indicated clinical CR, but the MRI showed clinical PR.
Therefore, the decision was made to continue with an additional two
cycles of the prior therapy regimen after communicating with the
patient. The last preoperative evaluation occurred after completing
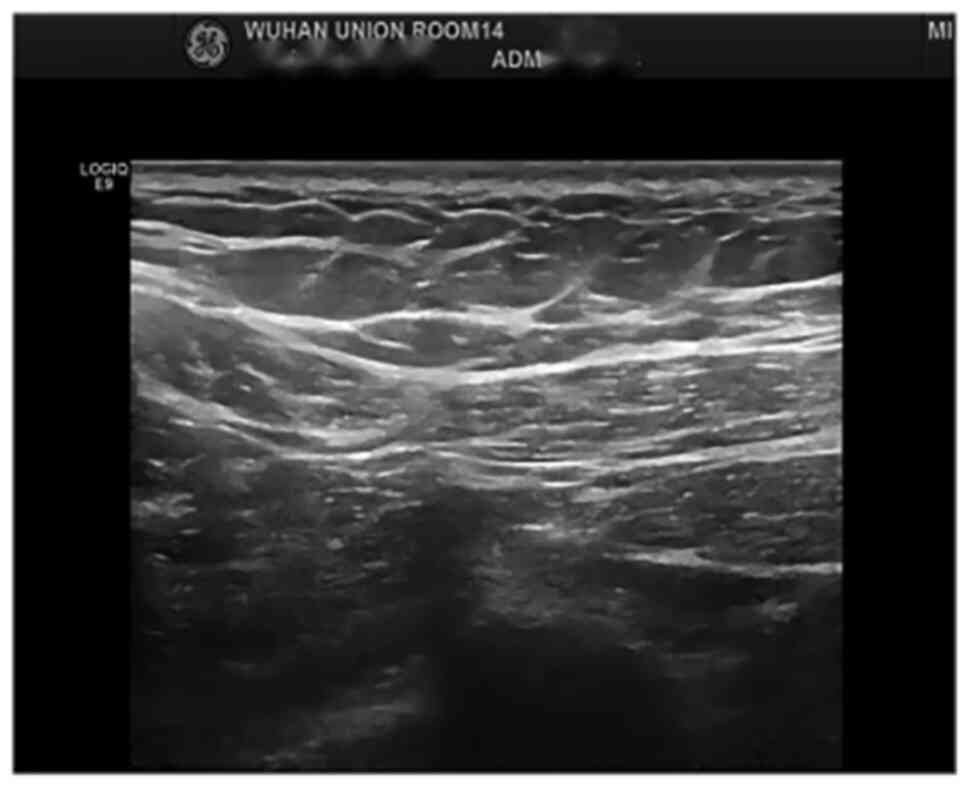
six cycles of neoadjuvant therapy in total, and the breast
ultrasound (Fig. 3) and the MRI
showed clinical CR and PR, respectively. Subsequently, both the
clinicians and the patient decided to pursue surgical treatment.
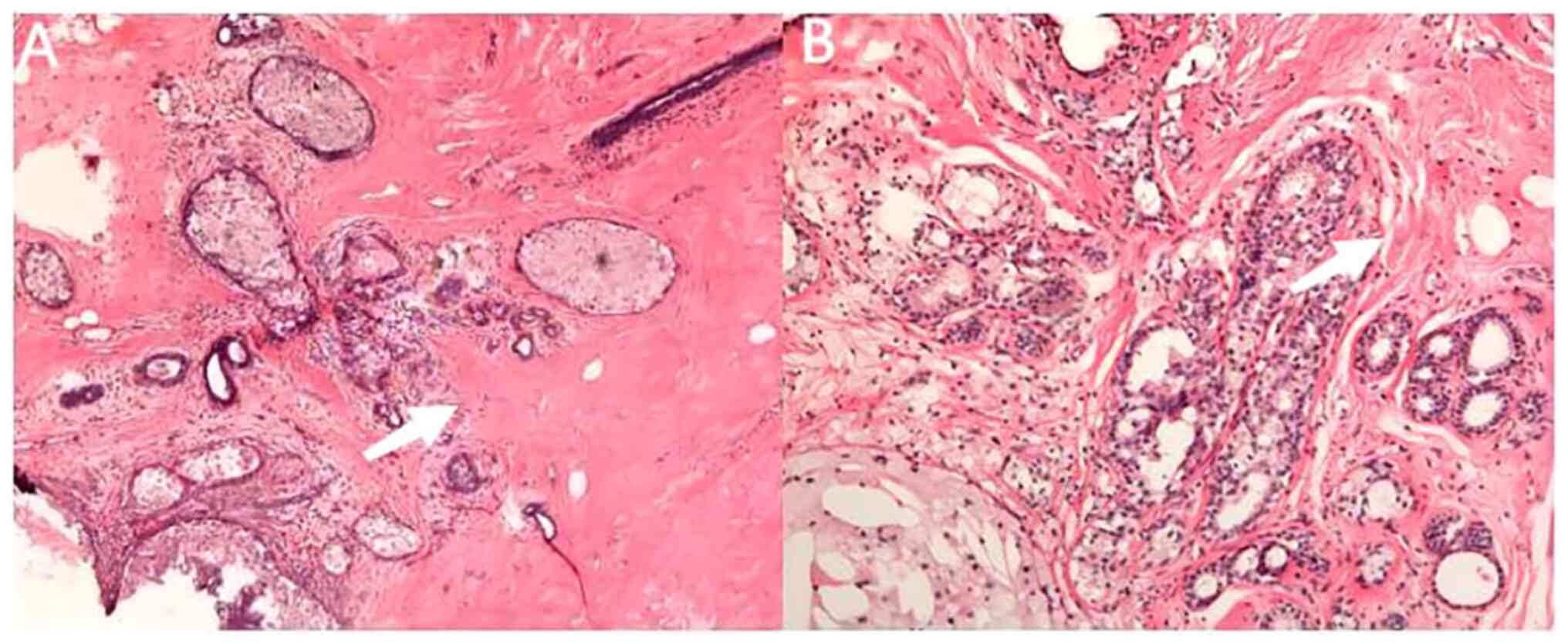
After surgery, both the left breast and axillary lymph nodes
achieved pCR based on analyzing the specimens of the modified
radical mastectomy (Fig. 4).
Although the patient exhibited a good therapeutic
response, the patient did suffer from asymptomatic thyroid
dysfunction by empirical testing for thyroid stimulating hormone
(TSH). Hypothyroidism was preceded by transient hyperthyroidism.
The first TSH levels were 0.2732 mIU/l (normal reference range,
0.35–4.94 mIU/l) after two cycles of neoadjuvant therapy. The
second TSH levels were 0.0171 mIU/l after four cycles of
neoadjuvant therapy without any additional interventions. The third
TSH levels were 96.2476 mIU/l after six cycles of neoadjuvant
therapy; thus, the hypothyroidism was classified as G2 according to
the Common Terminology Criteria for Adverse Events grading system
(6). Hormone replacement therapy
with levothyroxine and the monitoring of TSH and free thyroxine
(FT4) levels are currently ongoing. The first visit was 3 months
after radical surgery, which was performed in October 2020 and the
latest visit was 2 years after surgery. The CR status has been
maintained for >2 years.
Discussion
Monotherapy with the anti-PD-1 antibody
pembrolizumab has demonstrated durable antitumor activity for
previously-treated metastatic, triple-negative breast cancer
(10). Treatment with pembrolizumab
plus chemotherapy has also achieved a notable and clinically
meaningful improvement in progression-free survival among patients
with metastatic, triple-negative breast cancer with positive PD-L1
status (5). Treatment with the
anti-PD-L1 antibody atezolizumab plus nab-paclitaxel also extended
progression-free survival among patients with metastatic,
triple-negative breast cancer (4).
Pembrolizumab plus neoadjuvant chemotherapy doubled the estimated
pCR rate in female patients with early-stage
HR+/HER2− or triple-negative breast cancer
compared with the standard neoadjuvant chemotherapy (11). In another study, the number of
patients with early-stage, triple-negative breast cancer who
demonstrated a pCR was markedly higher among those patients who
received pembrolizumab plus neoadjuvant chemotherapy (64.8%) than
among those who received placebo plus neoadjuvant chemotherapy
(51.2%) (3). Neoadjuvant treatment
with atezolizumab in combination with nab-paclitaxel and
anthracycline-based chemotherapy has also been indicated to
markedly improve the pCR rate with an acceptable safety profile,
i.e., safety was consistent with the known profiles of the
individual drugs, compared with placebo plus nab-paclitaxel and
anthracycline-based chemotherapy (2). In the present case report, six cycles
of neoadjuvant anthracycline-free chemotherapy with camrelizumab
treatment achieved a pCR. To the best of our knowledge, this is the
first report of immunotherapy in combination with neoadjuvant
anthracycline-free chemotherapy for triple-negative early breast
cancer. It is considered a challenge to evaluate clinical CR after
neoadjuvant therapy, particularly when ultrasonography and MRI show
different results. Usually, ultrasonography is combined with MRI to
evaluate the response of neoadjuvant therapy, as in the present
case report.
Immune-related adverse events refer to inflammatory
side effects induced by immune checkpoint inhibitors.
Immune-related adverse events most commonly involve the
gastrointestinal tract, endocrine glands, skin and liver.
Immune-related events also involve the central nervous,
cardiovascular, pulmonary, musculoskeletal and hematologic systems,
but less frequently (7,12). Most adverse events are spontaneously
relieved; however, hypothyroidism may be a permanent side effect.
Hyperthyroidism is almost always resolving, followed or not by a
hypothyroidism phase (1). In the
present case report, persistent hypothyroidism occurred after
transient hyperthyroidism lasting for four weeks.
Immune-related thyroid dysfunction is relatively
common. In total, 7–15% of the population suffered from
hypothyroidism and 3–6% of the population suffered from
hyperthyroidism, as demonstrated by the data of several phase II
and III clinical trials (2–5,10,11).
The pathophysiological mechanism of thyroid disorder is similar to
that of destructive thyroiditis. Thyroid destruction appears to be
independent of thyroid autoantibodies and may involve T-cell-,
natural killer cell- and/or monocyte-mediated pathways. Activated
monocytes infiltrate the thyroid tissue after recognition of
antigens similar to tumor antigens and exert their cytotoxic
action, indicating the reason behind the temporary nature of the
first phase of thyrotoxicosis before returning to normal, or before
a possible evolution towards hypothyroidism (1,13). As
with the thyroid function reversal of the patient in the present
case report, persistent hypothyroidism occurred after four-week
hyperthyroidism. The American Society of Clinical Oncology clinical
practice guidelines recommend supportive treatment with
levothyroxine after ICPI therapy in symptomatic patients with any
degree of TSH elevation or in patients with asymptomatic TSH levels
that persist at >10 mIU/l, measured 4 weeks apart (6). For persistent hyperthyroidism of >6
weeks, clinical suspicion or work-up for Grave's disease,
guidelines recommend treatment with thionamide. In the patient of
the present case report, FT4 and TSH levels were monitored every 2
weeks during the transient four-week hyperthyroid phase. Hormone
supplementation and monitoring of thyroid function are ongoing
since the diagnosis of hypothyroidism. It remains to be determined
whether hypothyroidism is permanent and whether the patient will
require lifelong support with levothyroxine.
In conclusion, as a promising treatment strategy,
immunochemotherapy has demonstrated a meaningful improvement in pCR
and survival of patients with triple-negative breast cancer. The
present case report provides a new combination regimen of
immunotherapy and anthracycline-free chemotherapy for the treatment
of early-stage, triple-negative breast cancer, which is worth
examining further in a clinical trial. Most immune-related adverse
events are reversible and manageable, but hypothyroidism typically
requires sustained levothyroxine replacement therapy. How
immune-induced thyroid destruction occurs and whether it may be
prevented and reversed still needs further exploration.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
XZ and FD provided substantial contributions to the
design and drafting of the current report. WC interpreted the
pathology of the breast and axillary node before and after
treatment. CL managed the treatment of the patient in the present
report throughout. XZ and FD confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Wuhan Union Hospital (Wuhan, China; approval no.
2023-IEC-302).
Patient consent for publication
The patient provided written informed consent for
the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jannin A, Penel N, Ladsous M, Vantyghem MC
and Do Cao C: Tyrosine kinase inhibitors and immune checkpoint
inhibitors-induced thyroid disorders. Crit Rev Oncol Hematol.
141:23–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mittendorf EA, Zhang H, Barrios CH, Saji
S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al:
Neoadjuvant atezolizumab in combination with sequential
nab-paclitaxel and anthracycline-based chemotherapy versus placebo
and chemotherapy in patients with early-stage triple-negative
breast cancer (IMpassion031): A randomised, double-blind, phase 3
trial. Lancet. 396:1090–1100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: Pembrolizumab plus chemotherapy versus placebo plus
chemotherapy for previously untreated locally recurrent inoperable
or metastatic triple-negative breast cancer (KEYNOTE-355): A
randomised, placebo-controlled, double-blind, phase 3 clinical
trial. Lancet. 396:1817–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
Society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Postow MA, Sidlow R and Hellmann MD:
Immune-Related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
NCCN Clinical Practice Guidelines in
Oncology, . (NCCN Guidelines®). Breast Cancer. (version
4.2020). NCCN; May 8–2020
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams S, Schmid P, Rugo HS, Winer EP,
Loirat D, Awada A, Cescon DW, Iwata H, Campone M, Nanda R, et al:
Pembrolizumab monotherapy for previously treated metastatic
triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086
study. Ann Oncol. 30:397–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai
L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H, et al:
Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic
complete response in women with early-stage breast cancer: An
analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial.
JAMA Oncol. 6:676–684. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weber JS, Hodi FS, Wolchok JD, Topalian
SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, et
al: Safety profile of nivolumab monotherapy: A pooled analysis of
patients with advanced melanoma. J Clin Oncol. 35:785–792. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delivanis DA, Gustafson MP, Bornschlegl S,
Merten MM, Kottschade L, Withers S, Dietz AB and Ryder M:
Pembrolizumab-Induced Thyroiditis: Comprehensive clinical review
and insights into underlying involved mechanisms. J Clin Endocrinol
Metab. 102:2770–2780. 2017. View Article : Google Scholar : PubMed/NCBI
|