Introduction
Malignant melanoma continues to be a major challenge
for clinicians with 324,635 newly diagnosed cases and 57,043 deaths
worldwide in 2020 (1). According to
the GLOBOCAN 2020 database, Austria has a moderate incidence of
13.5 cases per 100,000 person years (1). The data might however be
underestimated as private pathology laboratories are not required
to report the data to the national registry (2). Superficial spreading melanomas (SSM)
represent the most common histopathological subtype in Central
Europe, including Austria, followed by not otherwise specified
(NOS) malignant melanomas and nodular melanomas (NM) (3). The majority of cutaneous melanomas
confirmed using histopathology in Austria are classified as Tis or
T1 (2). Melanomas in a more
advanced stage, such as regional (III), distant (IV) or with a
Breslow thickness >4 mm (pT4), were reported to present
gender-related differences and to be more common in men than in
women in Austria (2,4).
It previously been reported that ~50% of metastatic
melanomas harbor a BRAF V600 activating mutation (5). Therefore, current therapeutic
strategies include the combination of specific BRAF and MEK
inhibitors (6–8). The use of the immune-checkpoint
inhibitor ipilimumab, an anti-cytotoxic T-lymphocyte-associated
protein 4 antibody, and the programmed cell death protein 1
inhibitors nivolumab and pembrolizumab demonstrate progress in the
treatment of malignant melanoma (9–13).
Despite the remarkable advances in targeted therapies and
immunotherapy during the last decade, ~50% of patients with
metastatic melanoma do not survive >5 years after diagnosis
(13,14). Therefore, there is an urgent need
for additional therapeutic targets to enable better management of
melanoma patients.
In the search for new treatment approaches in
melanoma therapy the chondroitin sulfate proteoglycan 4 (CSPG4) has
been reported to be an important molecule involved in the oncogenic
potential of melanoma (15). CSPG4,
also termed human high molecular weight-melanoma associated antigen
or melanoma-associated chondroitin sulfate proteoglycan, was
reported as a glycoprotein-proteoglycan complex on human melanoma
cells nearly 40 years ago (16). It
is composed of a large extracellular domain, a transmembrane region
and a short cytoplasmic tail (17).
CSPG4 does not possess any catalytic function on its own but it
associates with receptors that contain an intrinsic receptor
tyrosine kinase activity (15). The
mitogen-activated protein kinase/extracellular signal-regulated
kinase (ERK) and the integrin-regulated focal adhesion kinase
signaling pathway were proposed as the two major signaling pathways
associated with CSPG4 activity (18). The enhanced downstream signaling of
these pathways can promote tumor progression via cellular functions
such as adhesion, migration, proliferation and survival (18). It has been reported that the
cytoplasmic domain of CSPG4 contains a phospho-acceptor site at
Thr-2314, which is phosphorylated by ERK1/2, which results in the
stimulation of cell proliferation (19). Moreover, the expression of full
length CSPG4 has been reported to be necessary for the maximal
ERK1/2 activation in melanoma cells possessing a BRAF V600E
mutation (20). Inhibition of CSPG4
with short interfering RNA has been reported to lead to a reduction
in constitutive ERK1/2 activation (20). Consequently, inhibition of ERK1/2
with specific MEK inhibitors reduces CSPG4-dependent growth and the
motility of BRAF-mutant cells (20).
Originally, expression of CSPG4 was only associated
with malignant melanomas (21). In
recent years the proteoglycan has also been reported to be present
in numerous other cancer entities, including triple-negative breast
cancer, glioma, squamous carcinoma of the head and neck (22), certain types of leukemia (23), pancreatic tumors (24), soft-tissue sarcomas (25), anaplastic thyroid cancer (26), osteosarcomas (27,28)
and ovarian cancer (29).
CSPG4 expression in malignant melanoma has been
reported to vary among the different histopathological subtypes
(30–32). An early study by Kageshita et
al (30) reported that CSPG4
expression in acral lentiginous melanoma (ALM) was significantly
higher in metastatic lesions compared with primary lesions. This
was observed both in terms of the number of tissue samples
positively stained for CSPG4 and the percentage of stained melanoma
cells within each lesion (30).
Furthermore, Kageshita et al (30) reported that CSPG4 expression was
more prevalent in primary NM lesions compared with primary ALM
lesions and that the percentage of positively stained cells within
each lesion was significantly higher in this subtype. Notably,
CSPG4 expression in primary ALM lesions has been reported to be
negatively associated with survival (30,33).
In NM, the expression of CSPG4 was reported to be consistently high
in both primary and metastatic lesions, with >90% of tissue
samples demonstrating positive staining in each group (30). Similarly, in SSM, CSPG4 was
expressed in the majority of stained cases (31). However, in mucosal melanoma, the
frequency of CSPG4 expression was reported to be lower in primary
lesions compared with metastatic lesions (32). A high expression of CSPG4 was
demonstrated in ≤95% of uveal melanoma samples (34).
Certain approaches which make use of CSPG4 as a
potential therapeutic target in the treatment of melanoma have
already been reported, including monoclonal antibodies (35–40),
mimotope vaccines (41,42), anti-idiotypic monoclonal antibodies
(43–45), fusion proteins (46–48),
CAR-T cells (27,49–54),
bispecific T-cell engagers (55),
antibody-drug conjugate (56) or
targeted radioimmunotherapy (57–59).
Those strategies rely on different mechanisms of action, as
reviewed previously (60). Among
these approaches, CAR-T cells targeting CSPG4 hold particular
promise due to their ability to specifically recognize and
eliminate CSPG4-expressing tumor cells, which makes them a highly
attractive therapeutic option for further investigation in a
clinical setting.
A more detailed analysis of CSPG4 expression in
melanoma samples, including primary tumors as well as metastases,
could support new approaches for melanoma therapy. Therefore, the
present study assessed CSPG4 protein expression in melanoma tissue
samples, both primary tumors and metastases, to evaluate the
histopathological data as well as detailed patient
characteristics.
Materials and methods
Patients and tissue samples
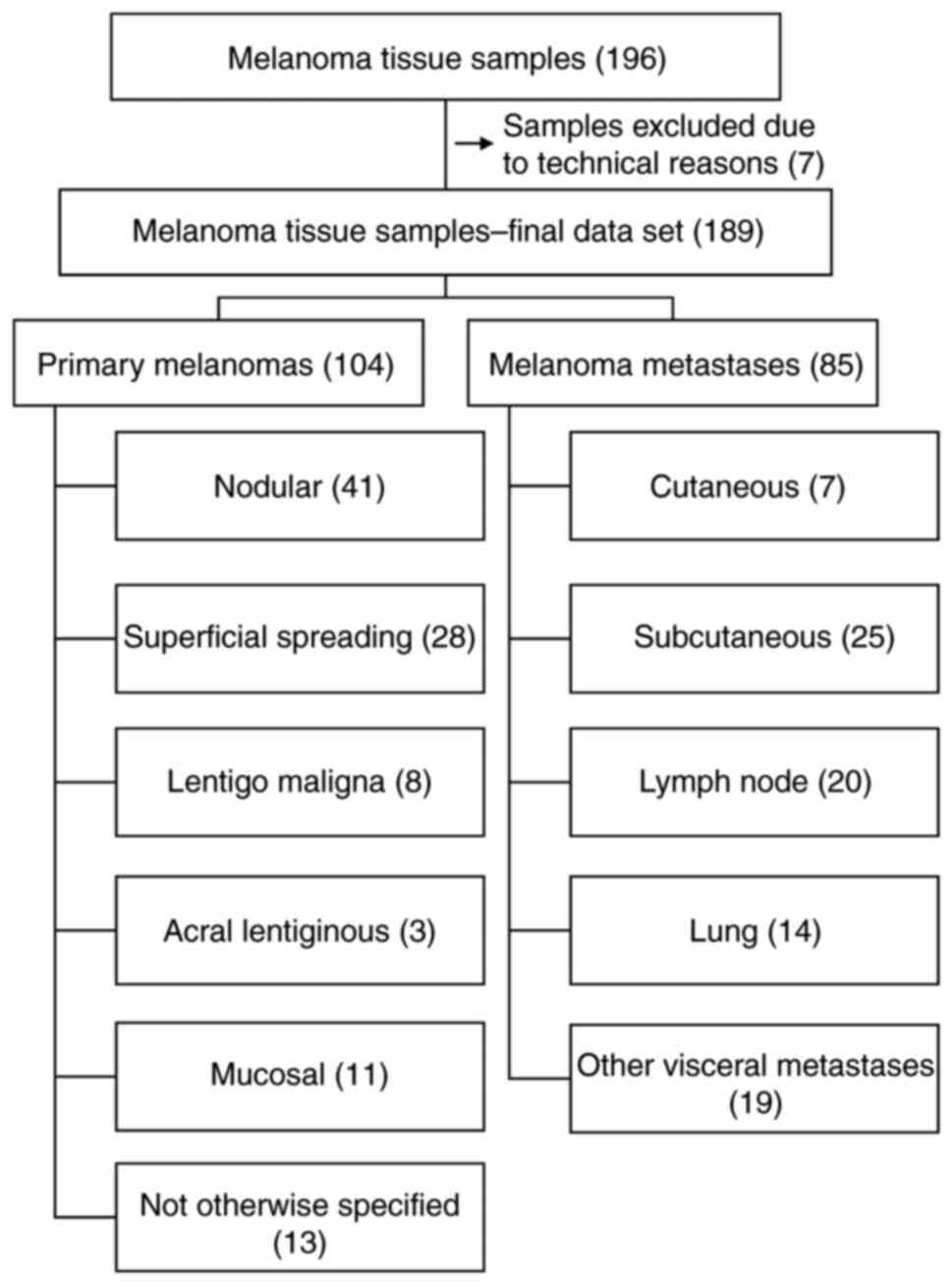
A total of 189 melanoma tissue samples were obtained
from 159 Caucasian patients, who had been histologically diagnosed
with primary melanoma (n=104) or a melanoma metastasis (n=85) at
the Department of Pathology at the University Hospital Krems (Krems
an der Donau, Austria) or at the Department of Pathology at the
University Hospital St. Poelten (St. Poelten, Austria) between
January 2010 and August 2018. Residual tissue samples were used for
the present study. Clinicopathological data recorded with the
samples included sex, age at diagnosis, histopathological subtype
of primary melanomas (nodular, superficial spreading, lentigo
maligna, acral lentiginous, mucosal or NOS), site of melanoma
metastases (cutaneous, subcutaneous, lymph node, lung or other
visceral metastases) and BRAF mutation status (wild type, V600E or
V600K).
For primary melanoma tissue samples, the tumor
thickness in mm (according to Breslow), the ulceration status and T
classification were also evaluated (61). For routine histopathology, the
formalin-fixed samples (10% buffered formalin) were placed in a
Tissue-Tek VIP machine (Sakura Finetek Europe) overnight for
dehydration and clearing, following the manufacturer's
instructions. Samples were then embedded in paraffin, cut into 2–3
µm thick sections and stained using hematoxylin and eosin
(Tissue-Tek Prisma H&E Stain Kit#1, cat. no. 6190; Sakura
Finetek Europe), following the manufacturer's instruction. Routine
immunohistochemistry (IHC) for HMB45, cytokeratin AE1/AE3, Melan A,
S100, Vimentin, Ki-67 was performed using the fully-automated
Benchmark ULTRA staining platform (Roche Tissue Diagnostics; Roche
Diagnostics, Ltd) to support the histopathological diagnosis of
primary malignant melanoma or melanoma metastasis. The following
ready-to-use primary antibodies (Roche Diagnostics Ltd.) were used
and the recommended staining procedure (temperature and duration of
incubation) was applied: Anti-HMB45 mouse mAb (cat. no.
05479282001; incubation, 8 min at 36°C), anti-Pan Keratin mouse
mAbs (cat. no. 05267145001; incubation, 8 min at 36°C), anti-Melan
A mouse mAb (cat. no. 05278350001; incubation, 32 min at 36°C),
anti-S100 mouse mAb (cat. no. 05278104001; incubation, 8 min at
36°C), anti-Vimentin mouse mAb (cat. no. 05278139001; incubation,
16 min at 36°C) and anti-Ki-67 rabbit mAb (cat. no. 05278384001;
incubation, 16 min at 36°C). The antibodies were visualized using
an UltraView Universal Detection Kit (cat. no. 760-501; Roche
Diagnostics Ltd.) according to the manufacturer's instructions.
Stained sections were examined using a light microscope (Olympus
BX53; Olympus Europa SE & Co. KG). NOS melanomas were mainly
punch biopsies that did not allow a more detailed diagnosis of the
primary melanoma. The group ‘other visceral metastases’ comprised
different parts of the gastrointestinal tract and other organ
systems that occurred in a scattered manner in the sample group and
were therefore pooled. The tumor (T) classification for primary
melanomas was determined according to the eighth edition of the
American Joint Committee on Cancer melanoma staging system
(61). Information regarding the
BRAF mutation status was obtained from data files for each sample.
The routine testing procedure involved utilizing either the cobas
4800 BRAF V600 mutation test (Roche Diagnostics, Ltd) or the
BRAF-strip Assay (ViennaLab Diagnostics GmbH). The study was
performed in accordance with the Declaration of Helsinki and was
approved by the Ethics Committee of the Karl Landsteiner University
of Health Sciences (Krems an der Donau, Austria; approval no.
1031/2018).
CSPG4 staining and scoring
All tissue samples were stained for CSPG4 expression
at the Department of Pathology at the University Hospital St.
Poelten. Formalin-fixed paraffin-embedded tissue samples were
deparaffinized and stained using the BenchMark XT automated
immune-staining platform (Roche Tissue Diagnostics; Roche
Diagnostics, Ltd) and the ultraView Universal DAB detection system
(Roche Tissue Diagnostics; Roche Diagnostics, Ltd) according to the
manufacturer's protocol. Briefly, heat-induced antigen retrieval
was performed for 10 min at 95°C. Then the tissue sections were
incubated in 3% H2O2 for 14 min at room
temperature. Following this, Inhibitor CM (Roche Diagnostics Ltd.)
was applied, and slides were incubated for 4 min at 37°C. Next, the
sections were incubated with a mouse monoclonal antibody specific
to CSPG4 (1:200; cat. no. ab50009; Abcam) overnight at 4°C. The
slides were then incubated with OmniMap anti-Ms HRP for 16 min in
conjunction with the ultraView Universal DAB detection system (cat.
no. 760-700; Roche Diagnostics Ltd.), following the manufacturer's
instructions. Between the steps, slides were washed with the
Reaction buffer (Tris-based buffer, pH 7.6–7.8; cat. no. 950-300;
Roche Diagnostics Ltd.). Finally, the samples were counterstained
with Hematoxylin II (cat. no. 790-2208; Roche Diagnostics Ltd.) and
Bluing Reagent (cat. no. 760-2037; Roche Diagnostics Ltd.) for 3
min at room temperature, and dehydrated in graded ethanol and
xylene.
Scoring of tissue slides was performed independently
by two investigators. Stained sections were examined using an
Olympus BX53 microscope (Olympus Europa SE & Co. KG).
Expression of CSPG4 was categorized visually according to a
four-tiered scale as follows: i) 0, negative (a complete loss of
CSPG4 expression); ii) +, weakly positive staining (<25% cells
stained positive for CSPG4); iii) ++, moderately positive staining
(25–50% cells stained positive for CSPG4); and iv) +++, strongly
positive staining (>50% cells stained positive for CSPG4).
Statistical analysis
For statistical analysis, CSPG4 expression was
dichotomized into positive (including +, ++ and +++) and negative
groups. The T classification of primary melanomas was simplified to
pT1-pT4 (pT1, tumor thickness according to Breslow ≤1 mm; pT2,
>1-2 mm; pT3, >2-4 mm; and pT4, >4 mm) (61). The supplementary staging criterion
non-ulcerated or ulcerated was considered as the separate variable
‘ulceration status of primary melanomas’. The numeric parameters,
age at diagnosis and tumor thickness were tested for an association
with CSPG4 expression using the Mann-Whitney-U test. Pearson's
χ2 test was used to analyze associations between
qualitative clinicopathological data and CSPG4 expression. When
cells had an expected count of <5 in the crosstabulation,
Fisher's Exact test was used instead of Pearson's χ2.
For a more detailed analysis of significant results in the
χ2 test or Fisher's Exact test the column proportion
test (Z-test) and the Bonferroni method to adjust P-values for
multiple comparisons were used. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS Statistics, version 24.0 (IBM
Corp.).
Results
Histopathological and clinical
characteristics of the sample group
A total of 196 melanoma tissue samples were stained
for CSPG4. CSPG4 expression could not be evaluated in seven samples
due to technical reasons or missing tumor tissues. Therefore, the
final data set included 189 melanoma tissue samples. Tables I and II presented the absolute frequencies for
all characteristics of the sample group. A flowchart indicated the
division and classification of the melanoma tumor samples (Fig. 1).
 | Table I.Association of CSPG4 expression and
clinicopathological parameters. |
Table I.
Association of CSPG4 expression and
clinicopathological parameters.
|
|
| Expression of
CSPG4 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Samples, n | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Sex |
|
|
| 0.182a |
|
Female | 92 | 36 (39.1) | 56 (60.9) |
|
|
Male | 97 | 29 (29.9) | 68 (70.1) |
|
| Diagnosis |
|
|
| 0.813a |
| Primary
melanoma | 104 | 35 (33.7) | 69 (66.3) |
|
|
Melanoma metastasis | 85 | 30 (35.3) | 55 (64.7) |
|
| Ulceration status
of primary melanomas |
|
|
| 0.742a |
|
Ulcerated | 36 | 12 (33.3) | 24 (66.7) |
|
|
Non-ulcerated | 50 | 15 (30.0) | 35 (70.0) |
|
| T classification of
primary melanomas |
|
|
| 1.000b |
|
pT1 | 33 | 11 (33.3) | 22 (66.7) |
|
|
pT2 | 13 | 4 (30.8) | 9 (69.2) |
|
|
pT3 | 14 | 4 (28.6) | 10 (71.4) |
|
|
pT4 | 26 | 8 (30.8) | 18 (69.2) |
|
| BRAF mutation
status |
|
|
| 0.214b |
| Wild
type | 40 | 14 (35.0) | 26 (65.0) |
|
|
V600E | 32 | 6 (18.8) | 26 (81.3) |
|
|
V600K | 2 | 1 (50.0) | 1 (50.0) |
|
 | Table II.Association of CSPG4 expression and
histopathological diagnosis. |
Table II.
Association of CSPG4 expression and
histopathological diagnosis.
|
|
| Expression of
CSPG4 |
|
|---|
|
|
|
|
|
|---|
| Histopathological
diagnosis | Samples, n | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Primary melanoma
subtypes |
|
|
| 0.009a |
|
Nodular | 41 | 10 (24.4) | 31 (75.6) |
|
|
Superficial spreading | 28 | 7 (25.0) | 21 (75.0) |
|
| Lentigo
maligna | 8 | 7 (87.5) | 1 (12.5) |
|
| Acral
lentiginous | 3 | 2 (66.7) | 1 (33.3) |
|
|
Mucosal | 11 | 5 (45.5) | 6 (54.5) |
|
| Not
otherwise specified | 13 | 4 (30.8) | 9 (69.2) |
|
| Site of melanoma
metastases |
|
|
| 0.882a |
|
Cutaneous | 7 | 3 (42.9) | 4 (57.1) |
|
|
Subcutaneous | 25 | 7 (28.0) | 18 (72.0) |
|
| Lymph
node | 20 | 7 (35.0) | 13 (65.0) |
|
|
Lung | 14 | 5 (35.7) | 9 (64.3) |
|
| Other
visceral metastases | 19 | 8 (42.1) | 11 (57.9) |
|
A total of 104 of the tissue samples (55.0%) were
histologically diagnosed as primary melanomas and 85 (45.0%) as
melanoma metastases (Table I and
Fig. 1). Ninety-two of the tissue
samples (48.7%) were obtained from female patients and 97 (51.3%)
from male patients (Table I). The
median age at diagnosis of the sample group was 71 years (range,
25–93 years). Data for tumor thickness, ulceration status and T
classification were available for 86 primary melanomas. These data
were not available for mucosal melanomas and for most of the NOS
primary melanomas. The median tumor thickness (according to
Breslow) of primary melanoma tissue samples was 2 mm (range, 0.2–25
mm), and 36 (41.9%) presented with an ulceration and 50 (58.1%) did
not (Table I). A total of 33 of the
primary melanoma tissue samples (38.4%) were classified as pT1, 13
(15.1%) as pT2, 14 (16.3%) as pT3 and 26 (30.2%) as pT4 (Table I). BRAF mutation status analyses
were available for 74 tissue samples, including primary melanomas
and melanoma metastases, and 32 (43.2%) samples demonstrated a BRAF
V600E mutation (Table I). In terms
of the histopathological subtype of the primary melanomas, NM were
represented with the highest number (n=41; 39.4%), followed by SSM
(n=28; 26.9%) (Table II and
Fig. 1). Among the melanoma
metastases group, the most common were subcutaneous metastases
(n=25; 29.4%), followed by lymph node metastases (n=20; 23.5%)
(Table II and Fig 1).
Immunohistochemical analysis of the
expression of CSPG4
After staining the samples using CSPG4-specific
antibodies, 124 samples (65.6%) were demonstrated to be positive
for the expression of CSPG4. Among these, 47 (24.9%) were
classified as +, 62 (32.8%) as ++, and 15 (7.9%) as +++. Sixty-five
(34.4%) samples demonstrated no expression of CSPG4. No
immunohistochemical staining for CSPG4 was demonstrated in the
adjacent normal skin of the tumor tissues within the tissue
samples, including both primary melanomas and cutaneous metastases
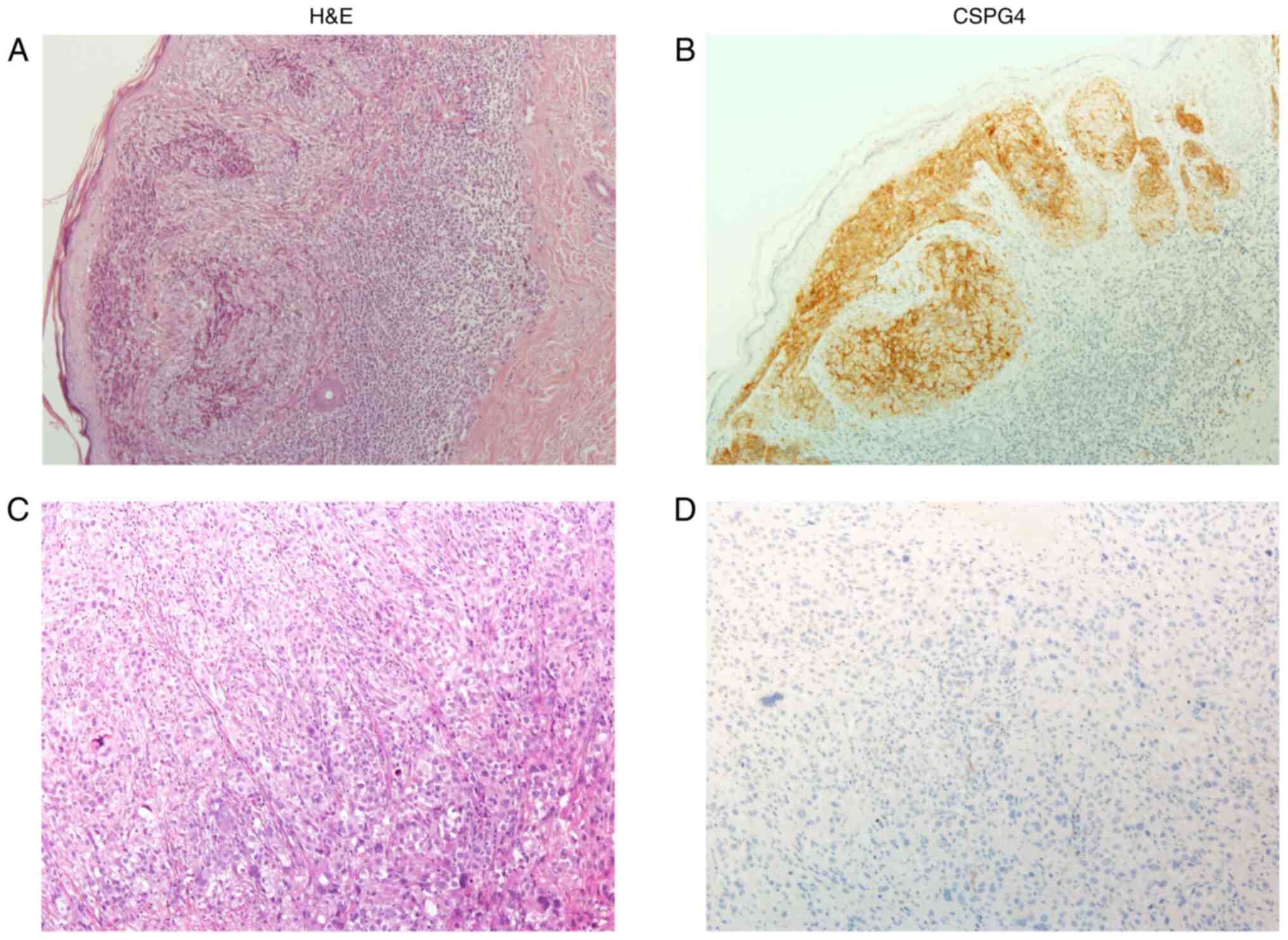
(data not shown). Immunohistochemical analysis of CSPG4 expression
in the sample group, which included a total of 189 melanoma tissue
samples, was performed (Table
III). Examples of H&E staining and CSPG4 staining for
primary and metastatic melanomas were presented in Fig. 2.
 | Table III.Immunohistochemical expression of
CSPG4 in the sample group. |
Table III.
Immunohistochemical expression of
CSPG4 in the sample group.
| Expression of
CSPG4 | Samples, n (%) |
|---|
| Negative | 65 (34.4) |
| Positive |
|
| + | 47 (24.9) |
| ++ | 62 (32.8) |
|
+++ | 15 (7.9) |
|
Combined total | 124 (65.6) |
Association between CSPG4 expression
and clinicopathological characteristics
Details of the frequencies of positive and negative
CSPG4 staining among the different clinicopathological parameters
were presented (Table I). There was
no significant association between the expression of CSPG4 in
melanoma tissue samples and the demographic parameters sex
(P=0.182) and age at diagnosis (P=0.121). Furthermore, no
significant association with CSPG4 expression was demonstrated for
tumor thickness (P=0.713), ulceration status (P=0.742) and T
classification (P=1.000). The vast majority of BRAF V600E mutated
tissue samples was stained positive for CSPG4 expression (n=26;
81.3%) and only few samples with a BRAF V600E mutation demonstrated
no CSPG4 expression (n=6; 18.8%).
CSPG4 expression in primary melanomas
and melanoma metastases
Frequencies of CSPG4 expression among the different
primary melanoma subtypes and the different sites of melanoma
metastases were summarized (Table
II). There was a significant association between primary
melanoma subtypes and CSPG4 protein expression (P=0.009).
Therefore, a further evaluation was performed using the column
proportion test (Z-test) in SPSS to evaluate which subtypes
demonstrated significant differences. This analysis demonstrated
that the number of primary nodular (P=0.009) and SSM lesions
(P=0.021) stained positive for CSPG4 was significantly higher than
the number of primary lentigo maligna melanomas (LMM). Primary
nodular (n=31; 75.6%), primary superficial spreading (n=21; 75.0%)
and mucosal (n=6; 54.5%) melanomas demonstrated positive staining
for CSPG4 expression. However, only 1/8 (12.5%) primary LMM and 1/3
(33.3%) ALM demonstrated CSPG4 expression (Table II). No significant association was
demonstrated (P=0.882) regarding the site of melanoma metastases.
CSPG4 expression demonstrated a comparable distribution between
negative and positive CSPG4 expression across different metastatic
sites, including cutaneous, subcutaneous, lymph node, lung and
other visceral metastases (Table
II).
In total 69 (66.3%) of the primary melanoma tissue
samples stained positive for the expression of CSPG4 and 35 (33.7%)
samples were negative (Table I).
Fifty-five (64.7%) samples in the melanoma metastases group were
positive for CSPG4 expression and 30 (35.3%) were negative
(Table I). There was no significant
difference in the frequency of CSPG4 expression between the primary
melanoma and melanoma metastases groups (P=0.813) (Table I).
Discussion
CSPG4 is a transmembrane proteoglycan involved in
the oncogenic potential of malignant melanoma through numerous
cellular mechanisms (15).
Determining the expression of CSPG4 among a large number of primary
and metastatic melanoma lesion samples may contribute to the
demonstration of the clinical significance of CSPG4 and to defining
its role in new treatment approaches. Previous studies have
reported differences in the expression of the proteoglycan among
the different histopathological subtypes of primary and metastatic
malignant melanoma lesions (30–32).
The present study demonstrated significant differences in CSPG4
protein expression in an analysis of a large number of archived
melanoma tissue samples, including primary and metastatic lesions,
from different histopathological subtypes.
As it has been reported that melanoma represents an
underestimated disease burden in Austria, research on this topic is
of importance (2). To the best of
our knowledge, the present study represents the largest collection
of melanoma samples systematically analyzed for the expression of a
melanoma antigen in the region of Lower Austria to date. It should
be noted that patients who underwent primary surgical intervention
in a hospital setup tended to have thicker primary melanomas.
Consequently, there was a higher frequency of nodular melanomas
observed in these cases (Table
II). It is important to acknowledge, that the data presented in
the present study represents the collection of tumor materials from
a specific group of institutions within a defined period of
time.
The majority of tissue samples included in the
present study demonstrated positive staining for the expression of
CSPG4, both in the primary melanoma and melanoma metastases groups
(Table I). An early study reported
by Natali et al (21) in
1983 reported higher (>75%) percentages of melanoma tissue
samples which stained positive for the expression of CSPG4. The
reported differences in the number of tissue samples staining
positive for the expression of CSPG4 may be attributed to the use
of different antibodies that target distinct determinants of the
proteoglycan. The monoclonal antibody used in the present study was
validated for its suitability to stain formalin-fixed
paraffin-embedded tissue samples specifically for CSPG4.
There was no significant difference in the frequency
of CSPG4 expression between primary melanoma and melanoma
metastasis lesions in the study population (Table I), which supported the previous
hypothesis that CSPG4 already has an impact in the formation
process of metastases (15). A
higher frequency of cells which expressed CSPG4 in metastatic
lesions than in primary ones has, to the best of our knowledge,
only previously been described for ALM and mucosal melanomas
(30,32). Given that the sample cohort in the
present study primarily included NM and SSM, no statistically
significant differences were demonstrated between primary and
metastatic tumor samples. Further studies examining the detailed
mechanisms of up- and down-regulation of CSPG4 expression in
primary and metastatic lesions would therefore be of interest.
No correlation was demonstrated between CSPG4
expression and clinicopathological parameters, such as sex, age,
BRAF mutation status, tumor thickness, ulceration status and
T-classification of primary melanomas in the present study
(Table I). A previous study by
Kageshita et al (33) which
also tested for an association between the factors such as age and
stage of disease, and CSPG4 expression among primary ALM tissue
samples, reported a significance between these clinical parameters
and CSPG4 expression in this subtype of primary melanomas. The same
study also reported an inverse correlation between CSPG4 expression
and survival in primary ALM lesions, which emphasized the
prognostic significance of CSPG4 (33). A significant finding in survival
analysis has only been observed thus far in primary ALM lesions for
CSPG4-expressing melanomas. Unfortunately, due to limitations of
the study protocol, no information on the treatment status of the
patients from whom samples were collected was available; as a
result, it was not possible to calculate the correlation between
the expression of CSPG4 and treatment modalities or overall
survival within the study cohort.
The BRAF mutational status was available for 74
(39.1%) tissue samples in the present study (Table I). As analysis of the BRAF
mutational status has been available only in the recent years, it
has not been performed for all tissue samples in the present study,
some of which date back to the year 2010.
The present study demonstrated that the vast
majority of tissue samples with a confirmed BRAF V600E mutation
also expressed CSPG4 (Table I).
This finding supported the observations made in an earlier study
which reported that CSPG4 seemed to function with an important role
in a strong and sustained activation of ERK1,2 in BRAF-mutant
melanoma cell lines (20). A
detailed analysis of CSPG4 expression in BRAF-mutant melanomas is
of importance when defining the role of this proteoglycan in tumor
advancement and regarding it as a potential therapeutic target.
Indeed, Yu et al (36)
reported that a combination therapy of the BRAF-selective inhibitor
vemurafenib with the CSPG4-specific monoclonal antibody (mAb)
225.28 resulted in a more effective inhibition of CSPG4-positive
melanoma cells with a BRAF V600E mutation. The addition of mAb
225.28 to vemurafenib was also reported to delay the development of
resistance to this therapy (36),
which further supported the oncogenic potential of CSPG4 in BRAF
mutant melanomas. It has also been reported that CSPG4-specific
anti-225D9+-TT polyclonal antibodies enhanced the
anti-proliferative effects of the BRAF inhibitor, PLX4032, in
melanoma cells (62). In addition,
a recent study reported that when combined with PLX4032, the
anti-CSPG4 mAb 9.2.27 contributed to a significant, additional
inhibition of melanoma cell viability, compared with cells treated
with BRAF inhibitor alone (37).
The present study demonstrate a high prevalence of
CSPG4 expression in primary NM and primary SSM (Table II), and in line with previous
reports (30,31), CSPG4 was expressed in a distinct
majority of stained tissue samples in these subtypes of primary
melanomas. In primary LMM the frequency of CSPG4 expression was
significantly lower than that in NM and SSM (Table II) and, to the best of our
knowledge, the present study is the first to describe this
significant difference. Previous studies had only reported a
significantly lower frequency of cells which expressed CSPG4 in
primary ALM tissue samples (30,31).
In this retrospective analysis of CSPG4 expression in primary
melanomas a low frequency of primary ALM tissue samples that
stained positive for CSPG4 were observed. However, this result was
not significant, which might be due to the small sample number of
primary ALMs included in the present study (Table II). As ALMs are not that common in
the Caucasian population (63),
only a limited number of samples were available for inclusion in
the present study.
It would be valuable to perform a prospective
analysis of CSPG4 expression in a substantial number of melanoma
tissue samples both before and after treatment with kinase
inhibitors, as well as other treatment approaches such as
immunotherapy. Our group has previously reported that CSPG4
expression is downregulated after treatment with a BRAF/MEK
inhibitor by retrospectively analyzing a small number of
patient-derived tumor samples (64). It has been reported that shedding of
tumor antigens, such as carcinoembryonic antigen into the blood
circulation could potentially suppress antitumor CD8+ T-cell
function (65). It could be
hypothesized that a similar phenomenon might occur for CSPG4.
In summary, the present study utilized a cohort of
melanoma tissue samples from Lower Austria, which provided valuable
insights into the expression profile of CSPG4 in this specific
Caucasian population. By correlating CSPG4 expression with both
histopathological characteristics and patient characteristics, w
the clinical relevance and potential implications of CSPG4
expression in melanoma subtypes was demonstrated, which laid the
foundation for personalized treatment strategies. Notably, the
present study demonstrated a previously unreported finding of low
CSPG4 expression in LMM within this particular population, which
adds to our understanding of the heterogeneity of CSPG4 expression
in melanoma.
Acknowledgements
The authors wish to acknowledge Mag. Konrad Kogler
and Dipl. Ing. Alfred Zens (NÖ Landesgesundheitsagentur, the legal
entity of University Hospitals in Lower Austria), for their
contribution in providing the organizational framework for
conducting this research.
Funding
The present study was funded by the NÖ Forschungs-und
Bildungsges.m.b.H. (grant no. LSC15-007). The authors also
acknowledge the support of the Open Access Publishing Fund of the
Karl Landsteiner University of Health Sciences.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
AG and CH conceived and designed the study, and
analyzed and interpreted the data. KU and HB participated in
designing the study and in analyzing the data. MK and MM analyzed
and interpreted the data. CH and HB confirm the authenticity of all
the raw data. AG, CH, KU and HB wrote the manuscript. All authors
have read and revised the manuscript, and approved the final
version.
Ethics approval and consent to
participate
All histopathological samples and clinical data were
obtained from the collection of patient samples at the Department
of Pathology at the University Hospital St. Poelten, Karl
Landsteiner University of Health Sciences and the Department of
Pathology at the University Hospital Krems, Karl Landsteiner
University of Health Sciences. The collection and storage of
archival tissue samples and data were performed according to local
ethical guidelines. The study was conducted in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
the Karl Landsteiner University (Krems an der Donau, Austria;
approval no. 1031/2018). Informed patient consent was not required
due to the retrospective nature of this study, in accordance with
local ethical guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALM
|
acral lentiginous melanoma
|
|
CAR
|
chimeric antigen receptor
|
|
CSPG4
|
chondroitin sulfate proteoglycan 4
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
H&E
|
hematoxylin and eosin
|
|
LMM
|
lentigo maligna melanoma
|
|
mAb
|
monoclonal antibody
|
|
MEK
|
mitogen-activated protein kinase
kinase
|
|
NM
|
nodular melanoma
|
|
NOS
|
not otherwise specified
|
|
SSM
|
superficial spreading melanoma
|
References
|
1
|
World Health Organization, International
Agency for Research on Cancer (IARC), . GLOBOCAN 2020: Estimated
incidence, mortality and prevalence rates in 2020, melanoma of
skin. Available from:. http://gco.iarc.fr/today/homeFeb 15–2022
|
|
2
|
Monshi B, Vujic M, Kivaranovic D, Sesti A,
Oberaigner W, Vujic I, Ortiz-Urda S, Posch C, Feichtinger H, Hackl
M and Rappersberger K: The burden of malignant melanoma-lessons to
be learned from Austria. Eur J Cancer. 56:45–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crocetti E, Mallone S, Robsahm TE, Gavin
A, Agius D, Ardanaz E, Lopez MC, Innos K, Minicozzi P, Borgognoni
L, et al: Survival of patients with skin melanoma in Europe
increases further: Results of the EUROCARE-5 study. Eur J Cancer.
51:2179–2190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duschek N, Skvara H, Kittler H, Delir G,
Fink A, Pinkowicz A and Waldhor T: Melanoma epidemiology of Austria
reveals gender-related differences. Eur J Dermatol. 23:872–878.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Platz A, Egyhazi S, Ringborg U and Hansson
J: Human cutaneous melanoma; a review of NRAS and BRAF mutation
frequencies in relation to histogenetic subclass and body site. Mol
Oncol. 1:395–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Larkin J, Ascierto PA, Dreno B, Atkinson
V, Liszkay G, Maio M, Mandala M, Demidov L, Stroyakovskiy D, Thomas
L, et al: Combined vemurafenib and cobimetinib in BRAF-mutated
melanoma. N Engl J Med. 371:1867–1876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robert C, Karaszewska B, Schachter J,
Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R,
Grange F, Mortier L, et al: Improved overall survival in melanoma
with combined dabrafenib and trametinib. N Engl J Med. 372:30–39.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long GV, Stroyakovskiy D, Gogas H,
Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A,
Grob JJ, et al: Dabrafenib and trametinib versus dabrafenib and
placebo for Val600 BRAF-mutant melanoma: A multicentre,
double-blind, phase 3 randomised controlled trial. Lancet.
386:444–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Adjuvant ipilimumab versus placebo after
complete resection of high-risk stage III melanoma (EORTC 18071): A
randomised, double-blind, phase 3 trial. Lancet Oncol. 16:522–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber J, Mandala M, Del Vecchio M, Gogas
HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al: Adjuvant Nivolumab versus ipilimumab in
resected stage III or IV melanoma. N Engl J Med. 377:1824–1835.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schachter J, Ribas A, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab for advanced melanoma: Final
overall survival results of a multicentre, randomised, open-label
phase 3 study (KEYNOTE-006). Lancet. 390:1853–1862. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ribas A, Lawrence D, Atkinson V, Agarwal
S, Miller WH Jr, Carlino MS, Fisher R, Long GV, Hodi FS, Tsoi J, et
al: Combined BRAF and MEK inhibition with PD-1 blockade
immunotherapy in BRAF-mutant melanoma. Nat Med. 25:936–940. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Price MA, Wanshura LE, Yang J, Carlson J,
Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA and McCarthy JB:
CSPG4, a potential therapeutic target, facilitates malignant
progression of melanoma. Pigment Cell Melanoma Res. 24:1148–1157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bumol TF and Reisfeld RA: Unique
glycoprotein-proteoglycan complex defined by monoclonal antibody on
human melanoma cells. Proc Natl Acad Sci USA. 79:1245–1249. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pluschke G, Vanek M, Evans A, Dittmar T,
Schmid P, Itin P, Filardo EJ and Reisfeld RA: Molecular cloning of
a human melanoma-associated chondroitin sulfate proteoglycan. Proc
Natl Acad Sci USA. 93:9710–9715. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Price MA, Neudauer CL, Wilson C,
Ferrone S, Xia H, Iida J, Simpson MA and McCarthy JB: Melanoma
chondroitin sulfate proteoglycan enhances FAK and ERK activation by
distinct mechanisms. J Cell Biol. 165:881–891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Makagiansar IT, Williams S, Mustelin T and
Stallcup WB: Differential phosphorylation of NG2 proteoglycan by
ERK and PKCalpha helps balance cell proliferation and migration. J
Cell Biol. 178:155–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang J, Price MA, Li GY, Bar-Eli M, Salgia
R, Jagedeeswaran R, Carlson JH, Ferrone S, Turley EA and McCarthy
JB: Melanoma proteoglycan modifies gene expression to stimulate
tumor cell motility, growth, and epithelial-to-mesenchymal
transition. Cancer Res. 69:7538–7547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Natali PG, Giacomini P, Russo C, Steinbach
G, Fenoglio C and Ferrone S: Antigenic profile of human melanoma
cells. Analysis with monoclonal antibodies to histocompatibility
antigens and to melanoma-associated antigens. J Cutan Pathol.
10:225–237. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Wang Y, Yu L, Sakakura K, Visus C,
Schwab JH, Ferrone CR, Favoino E, Koya Y, Campoli MR, et al: CSPG4
in cancer: Multiple roles. Curr Mol Med. 10:419–429. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fenton M, Whiteside TL, Ferrone S and
Boyiadzis M: Chondroitin sulfate proteoglycan-4 (CSPG4)-specific
monoclonal antibody 225.28 in detection of acute myeloid leukemia
blasts. Oncol Res. 22:117–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keleg S, Titov A, Heller A, Giese T,
Tjaden C, Ahmad SS, Gaida MM, Bauer AS, Werner J and Giese NA:
Chondroitin sulfate proteoglycan CSPG4 as a novel hypoxia-sensitive
marker in pancreatic tumors. PLoS One. 9:e1001782014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu SC, Nadesan P, Puviindran V, Stallcup
WB, Kirsch DG and Alman BA: Effects of chondroitin sulfate
proteoglycan 4 (NG2/CSPG4) on soft-tissue sarcoma growth depend on
tumor developmental stage. J Biol Chem. 293:2466–2475. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Egan CE, Stefanova D, Ahmed A, Raja VJ,
Thiesmeyer JW, Chen KJ, Greenberg JA, Zhang T, He B, Finnerty BM,
et al: CSPG4 is a potential therapeutic target in anaplastic
thyroid cancer. Thyroid. 31:1481–1493. 2021.PubMed/NCBI
|
|
27
|
Beard RE, Zheng Z, Lagisetty KH, Burns WR,
Tran E, Hewitt SM, Abate-Daga D, Rosati SF, Fine HA, Ferrone S, et
al: Multiple chimeric antigen receptors successfully target
chondroitin sulfate proteoglycan 4 in several different cancer
histologies and cancer stem cells. J Immunother Cancer. 2:252014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riccardo F, Tarone L, Iussich S, Giacobino
D, Arigoni M, Sammartano F, Morello E, Martano M, Gattino F, De
Maria R, et al: Identification of CSPG4 as a promising target for
translational combinatorial approaches in osteosarcoma. Ther Adv
Med Oncol. 11:17588359198554912019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J, Liao Q, Price M, Moriarity B, Wolf
N, Felices M, Miller JS, Geller MA, Bendzick L, Hopps R, et al:
Chondroitin sulfate proteoglycan 4, a targetable oncoantigen that
promotes ovarian cancer growth, invasion, cisplatin resistance and
spheroid formation. Transl Oncol. 16:1013182022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kageshita T, Nakamura T, Yamada M, Kuriya
N, Arao T and Ferrone S: Differential expression of melanoma
associated antigens in acral lentiginous melanoma and in nodular
melanoma lesions. Cancer Res. 51:1726–1732. 1991.PubMed/NCBI
|
|
31
|
Nishi H, Inoue Y, Kageshita T, Takata M
and Ihn H: The expression of human high molecular weight
melanoma-associated antigen in acral lentiginous melanoma. Biosci
Trends. 4:86–89. 2010.PubMed/NCBI
|
|
32
|
Kageshita T, Kimura T, Yoshi A, Hirai S,
Ono T and Ferrone S: Antigenic profile of mucosal melanoma lesions.
Int J Cancer. 56:370–374. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kageshita T, Kuriya N, Ono T, Horikoshi T,
Takahashi M, Wong GY and Ferrone S: Association of high molecular
weight melanoma-associated antigen expression in primary acral
lentiginous melanoma lesions with poor prognosis. Cancer Res.
53:2830–2833. 1993.PubMed/NCBI
|
|
34
|
Li Y, Madigan MC, Lai K, Conway RM,
Billson FA, Crouch R and Allen BJ: Human uveal melanoma expresses
NG2 immunoreactivity. Br J Ophthalmol. 87:629–632. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hafner C, Breiteneder H, Ferrone S,
Thallinger C, Wagner S, Schmidt WM, Jasinska J, Kundi M, Wolff K,
Zielinski CC, et al: Suppression of human melanoma tumor growth in
SCID mice by a human high molecular weight-melanoma associated
antigen (HMW-MAA) specific monoclonal antibody. Int J Cancer.
114:426–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu L, Favoino E, Wang Y, Ma Y, Deng X and
Wang X: The CSPG4-specific monoclonal antibody enhances and
prolongs the effects of the BRAF inhibitor in melanoma cells.
Immunol Res. 50:294–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uranowska K, Samadaei M, Kalic T, Pinter
M, Breiteneder H and Hafner C: A chondroitin sulfate proteoglycan
4-specific monoclonal antibody inhibits melanoma cell invasion in a
spheroid model. Int J Oncol. 59:702021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schroff RW, Woodhouse CS, Foon KA, Oldham
RK, Farrell MM, Klein RA and Morgan AC Jr: Intratumor localization
of monoclonal antibody in patients with melanoma treated with
antibody to a 250,000-dalton melanoma-associated antigen. J Natl
Cancer Inst. 74:299–306. 1985.PubMed/NCBI
|
|
39
|
Schroff RW, Morgan AC Jr, Woodhouse CS,
Abrams PG, Farrell MM, Carpenter BE, Oldham RK and Foon KA:
Monoclonal antibody therapy in malignant melanoma: Factors
effecting in vivo localization. J Biol Response Mod. 6:457–472.
1987.PubMed/NCBI
|
|
40
|
Oldham RK, Foon KA, Morgan AC, Woodhouse
CS, Schroff RW, Abrams PG, Fer M, Schoenberger CS, Farrell M and
Kimball E: Monoclonal antibody therapy of malignant melanoma: In
vivo localization in cutaneous metastasis after intravenous
administration. J Clin Oncol. 2:1235–1244. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wagner S, Hafner C, Allwardt D, Jasinska
J, Ferrone S, Zielinski CC, Scheiner O, Wiedermann U, Pehamberger H
and Breiteneder H: Vaccination with a human high molecular weight
melanoma-associated antigen mimotope induces a humoral response
inhibiting melanoma cell growth in vitro. J Immunol. 174:976–982.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wagner S, Krepler C, Allwardt D, Latzka J,
Strommer S, Scheiner O, Pehamberger H, Wiedermann U, Hafner C and
Breiteneder H: Reduction of human melanoma tumor growth in severe
combined immunodeficient mice by passive transfer of antibodies
induced by a high molecular weight melanoma-associated antigen
mimotope vaccine. Clin Cancer Res. 14:8178–8183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mittelman A, Chen ZJ, Yang H, Wong GY and
Ferrone S: Human high molecular weight melanoma-associated antigen
(HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody
MK2-23: Induction of humoral anti-HMW-MAA immunity and prolongation
of survival in patients with stage IV melanoma. Proc Natl Acad Sci
USA. 89:466–470. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Ko EC, Peng L, Gillies SD and
Ferrone S: Human high molecular weight melanoma-associated antigen
mimicry by mouse anti-idiotypic monoclonal antibody MK2-23:
Enhancement of immunogenicity of anti-idiotypic monoclonal antibody
MK2-23 by fusion with interleukin 2. Cancer Res. 65:6976–6983.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mittelman A, Chen ZJ, Kageshita T, Yang H,
Yamada M, Baskind P, Goldberg N, Puccio C, Ahmed T and Arlin Z:
Active specific immunotherapy in patients with melanoma. A clinical
trial with mouse antiidiotypic monoclonal antibodies elicited with
syngeneic anti-high-molecular-weight-melanoma-associated antigen
monoclonal antibodies. J Clin Invest. 86:2136–2144. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
de Bruyn M, Rybczynska AA, Wei Y,
Schwenkert M, Fey GH, Dierckx RA, van Waarde A, Helfrich W and
Bremer E: Melanoma-associated chondroitin sulfate proteoglycan
(MCSP)-targeted delivery of soluble TRAIL potently inhibits
melanoma outgrowth in vitro and in vivo. Mol Cancer. 9:3012010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jordaan S, Chetty S, Mungra N, Koopmans I,
van Bommel PE, Helfrich W and Barth S: CSPG4: A target for
selective delivery of human cytolytic fusion proteins and TRAIL.
Biomedicines. 5:372017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schwenkert M, Birkholz K, Schwemmlein M,
Kellner C, Kugler M, Peipp M, Nettelbeck DM, Schuler-Thurner B,
Schaft N, Dörrie J, et al: A single chain immunotoxin, targeting
the melanoma-associated chondroitin sulfate proteoglycan, is a
potent inducer of apoptosis in cultured human melanoma cells.
Melanoma Res. 18:73–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Geldres C, Savoldo B, Hoyos V, Caruana I,
Zhang M, Yvon E, Del Vecchio M, Creighton CJ, Ittmann M, Ferron S
and Dotti G: T lymphocytes redirected against the chondroitin
sulfate proteoglycan-4 control the growth of multiple solid tumors
both in vitro and in vivo. Clin Cancer Res. 20:962–971. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abken H, Hombach A, Heuser C and Reinhold
U: A novel strategy in the elimination of disseminated melanoma
cells: Chimeric receptors endow T cells with tumor specificity.
Recent Results Cancer Res. 158:249–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Burns WR, Zhao Y, Frankel TL, Hinrichs CS,
Zheng Z, Xu H, Feldman SA, Ferrone S, Rosenberg SA and Morgan RA: A
high molecular weight melanoma-associated antigen-specific chimeric
antigen receptor redirects lymphocytes to target human melanomas.
Cancer Res. 70:3027–3033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Y, Geldres C, Ferrone S and Dotti G:
Chondroitin sulfate proteoglycan 4 as a target for chimeric antigen
receptor-based T-cell immunotherapy of solid tumors. Expert Opin
Ther Targets. 19:1339–1350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Krug C, Birkholz K, Paulus A, Schwenkert
M, Schmidt P, Hoffmann N, Hombach A, Fey G, Abken H, Schuler G, et
al: Stability and activity of MCSP-specific chimeric antigen
receptors (CARs) depend on the scFv antigen-binding domain and the
protein backbone. Cancer Immunol Immunother. 64:1623–1635. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wiesinger M, Marz J, Kummer M, Schuler G,
Dorrie J, Schuler-Thurner B and Schaft N: Clinical-scale production
of CAR-T cells for the treatment of melanoma patients by mRNA
transfection of a CSPG4-specific CAR under full GMP compliance.
Cancers (Basel). 11:11982019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Torisu-Itakura H, Schoellhammer HF, Sim
MS, Irie RF, Hausmann S, Raum T, Baeuerle PA and Morton DL:
Redirected lysis of human melanoma cells by a MCSP/CD3-bispecific
BiTE antibody that engages patient-derived T cells. J Immunother.
34:597–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hoffmann RM, Crescioli S, Mele S, Sachouli
E, Cheung A, Chui CK, Andriollo P, Jackson PJM, Lacy KE, Spicer JF,
et al: A novel antibody-drug conjugate (ADC) delivering a DNA
mono-alkylating payload to chondroitin sulfate proteoglycan
(CSPG4)-expressing melanoma. Cancers (Basel). 12:10292020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Allen BJ, Singla AA, Rizvi SM, Graham P,
Bruchertseifer F, Apostolidis C and Morgenstern A: Analysis of
patient survival in a phase I trial of systemic targeted
alpha-therapy for metastatic melanoma. Immunotherapy. 3:1041–1050.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Allen BJ, Raja C, Rizvi S, Li Y, Tsui W,
Graham P, Thompson JF, Reisfeld RA and Kearsley J: Intralesional
targeted alpha therapy for metastatic melanoma. Cancer Biol Ther.
4:1318–1324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Raja C, Graham P, Rizvi SM, Song E,
Goldsmith H, Thompson J, Bosserhoff A, Morgenstern A, Apostolidis
C, Kearsley J, et al: Interim analysis of toxicity and response in
phase 1 trial of systemic targeted alpha therapy for metastatic
melanoma. Cancer Biol Ther. 6:846–852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ilieva KM, Cheung A, Mele S, Chiaruttini
G, Crescioli S, Griffin M, Nakamura M, Spicer JF, Tsoka S, Lacy KE,
et al: Chondroitin sulfate proteoglycan 4 and its potential as an
antibody immunotherapy target across different tumor types. Front
Immunol. 8:19112017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gershenwald JE, Scolyer RA, Hess KR,
Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM,
McArthur GA, et al: Melanoma staging: evidence-based changes in the
American Joint Committee on Cancer eighth edition cancer staging
manual. CA Cancer J Clin. 67:472–492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pucciarelli D, Lengger N, Takacova M,
Csaderova L, Bartosova M, Breiteneder H, Pastorekova S and Hafner
C: Anti-chondroitin sulfate proteoglycan 4-specific antibodies
modify the effects of vemurafenib on melanoma cells differentially
in normoxia and hypoxia. Int J Oncol. 47:81–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang Y, Zhao Y and Ma S: Racial
differences in six major subtypes of melanoma: Descriptive
epidemiology. BMC Cancer. 16:69112016. View Article : Google Scholar
|
|
64
|
Uranowska K, Kalic T, Valtsanidis V,
Kitzwögerer M, Breiteneder H and Hafner C: Expression of
chondroitin sulfate proteoglycan 4 (CSPG4) in melanoma cells is
downregulated upon inhibition of BRAF. Oncol Rep. 45:142021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hochst B and Diehl L: Antigen shedding
into the circulation contributes to tumor immune escape.
Oncoimmunology. 1:1620–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|