Introduction
Prostate cancer is the second most common type of
malignancy in men and also has the fifth highest mortality rate in
men with cancer (1). In the past 20
years, due to an aging society and changes in Chinese diet and
lifestyle, the incidence of prostate cancer has gradually increased
in China, and the standardized incidence and mortality of prostate
cancer in China are 6.15/100,000 and 2.48/100,000 individuals
(2). Prostate cancer is the most
common male genitourinary malignancy in China, higher than bladder
cancer (3). However, the
pathogenesis of prostate cancer is still poorly understood and may
be related to genetic background, age, ethnicity, lifestyle, diet,
chronic infection and inflammatory response (4). Previous studies have reported that
chronic inflammation is strongly associated with the development of
prostate tumors (4,5). Inflammation also plays a key role in
tumorigenesis and development, interfering with the ability of the
immune system to target tumor cells and affecting the response of
the tumor to therapy (6). A
previous study reported that untreated chronic lower urinary and
reproductive tract infections can lead to prostatic inflammatory
hyperplasia, which can lead to the development of prostate tumors
(4). A previous study on chronic
inflammation and prostate tumors by Gurel et al (5) reported that chronic prostate infection
was associated with prostate cancer and high-grade malignancies,
even in men with levels of prostate-specific antigen (PSA) in the
expected range. Therefore, prostate cancer can be considered to be
closely related to the chronic inflammatory response.
The innate immune system is the body's first line of
defense against invading pathogens or danger signals. The innate
immune system can induce an inflammatory response, activating
phagocytic cells to rapidly recognize and eliminate invading
microorganisms and can also induce and activate the acquired immune
response (7). The innate immune
system, using pathogen-associated and damage-associated molecular
pattern recognition, activate inflammatory signaling pathways and
cause inflammation (8).
Nucleotide-binding oligomerization domain-like receptors (NLRs) in
the cytoplasm are activated in response to intracellular danger
signals and form inflammasomes (8).
Inflammatory bodies are comprised of NLRs, the apoptosis-associated
speck-like protein (ASC) containing a caspase recruitment domain
(CARD) and the effector protein cysteinyl aspartate specific
proteinase-1 (caspase-1) (9).
Effects of the inflammasome are closely related to a number of
human diseases associated with dysfunctional immunoregulation,
including autoimmune diseases (8),
asthma (10), psoriasis (11), lupus nephritis (12), intestinal inflammation (13) and Alzheimer's disease (14), and have also been reported to serve
crucial roles in tumor progression, prognosis and treatment
response (15,16). In previous years, the pathogenic
mechanism of inflammasome action has been reported to occur through
activation of caspase-1, which promotes the expression of
inflammatory factors and thus serves a role in the inflammatory
response (9). The classic
inflammatory response pathway is mainly activated through
caspase-1-mediated induction of active subunits p10 and p20, which
promote maturation of inflammatory cytokines, such as IL-1β and
IL-18, and initiate inflammation-associated programmed cell death,
namely pyroptosis. Activated caspase-1 provides host cells with a
dual defense mechanism by releasing mature cytokines and removing
infected or damaged cells (17).
Inflammasomes initiate appropriate immune responses after infection
or aseptic injury to resist pathogenic injury and avoid damage to
the host. NLRP1, NLRP3, NLRC4 and other inflammasomes participate
in the maintenance and resolution of chronic inflammatory response
(18).
In a previous study, numerous inflammasomes were
reported to be found in prostate tissue, including nucleotide
binding oligomerization-like receptor family pyrin domain
containing (NLRP)1, NLRP3 and nucleotide-binding oligomerization
domain leucine-rich repeat and caspase recruitment
domain-containing 4 (NLRC4) inflammasomes (19). Although the mechanisms of action of
these inflammasomes are not currently fully understood, the process
of inflammation may be closely related to tumor development
(1). Presently, the pathogenesis of
prostate cancer is unclear. Therefore, studying the process of
inflammation in the context of prostate cancer and exploring its
role in the occurrence and development of tumors can provide
insight into the mechanism of prostate cancer development and a
future direction for new diagnostic and treatment options.
Materials and methods
Ethical approval
In the present study, data were collected from 112
patients who underwent prostate puncture biopsy at the Urology
Department of The First People's Hospital of Pinghu (Pinghu, China)
from January to May 2022. All patients signed the informed consent
forms. Ethical approval was obtained from The First People's Ethics
Committee of Pinghu (Pinghu, China; approval no. 002).
Patient inclusion and exclusion
criteria
The patient inclusion criteria used in the present
study were as follows: i) All patients were >18 years of age;
ii) prostate specific antigen (PSA) 4–10 ng/ml and/or free/total
PSA (F/TPSA) <0.16; iii) PSA >10 ng/ml; iv) rectal ultrasound
or prostate MRI reported suspicious lesions; and v) the digital
rectal examination reached the prostate nodules. The patient
exclusion criteria used in the present study were as follows: i)
Patients in the acute stage of urinary tract or suffering from
systemic infection; ii) patients with serious heart complications
or major heart disease; iii) poor control of hypertension and
diabetes; iv) patients with severe perianal rectal lesions; v)
patients with severe hemorrhagic disease; vi) preoperative
long-term oral administration of a 5A-reductase specific inhibitor;
and vii) prior history of prostate surgery.
Sample collection
Between January and May 2022, a total of 112
patients who met the aforementioned inclusion criteria underwent
transrectal prostatic needle biopsies. An ultrasound system was
used for intraoperative positioning and an automatic biopsy gun was
used to perform 12 needle punctures (six needles on each side of
the prostate). Tissue samples obtained from the punctures were
preserved in a 10% formaldehyde solution (at room temperature for
12–24 h) for pathological examination. According to the results,
patients were divided into two groups: The prostate hyperplasia and
prostate cancer groups.
Immunohistochemistry
A paraffin-embedded continuous slice (4–8°C; section
thickness, 3 mm) and hematoxylin and eosin (H&E) staining was
performed to analyze tissue structure and extent of the lesions in
patients with prostatic hyperplasia and prostate cancer.
Immunohistochemical staining was performed as previously described
(20). Briefly, conventional
dewaxing was performed, then samples were placed in citrate buffer
solution (pH 6.0), heated in a pressure cooker (>95°C) for
antigen repair then washed thrice with PBS (pH 7.4; Xiamen Tongling
Biomedical Technology Co., Ltd.) for 3 min after natural cooling
occurred. For rehydration, the slices were soaked in: i) Anhydrous
alcohol for 3 min twice; ii) 95% alcohol 3 min twice; and iii) 85%
alcohol 3 min. The slices were then washed thoroughly with double
steamed water. Endogenous peroxidase activity was inactivated by
incubating samples with H2O2 (3%; 18–28°C)
for 10 min, then washed thrice with PBS for 3 min. IL-1β (cat. no.
AP8531C; 1:100; Abcepta Biotech Ltd. Co.), NLRC4 (cat. no.
bs20016R; 1:200; BIOSS), IL-18 (cat. no. AP20583c; 1:100; Abcepta
Biotech Ltd. Co.,) and NLRP1 (cat. no. 862764; 1:100; Zenbio;
Chengdu Zhengneng Biotechnology Co., Ltd.) primary antibody working
solutions were incubated with samples at 37°C for 1 h, then washed
thrice with PBS for 3 min. HRP goat anti-mouse/anti-rabbit
secondary antibody (cat. no. DD13; 1:200; Xiamen Tongling
Biomedicine Technology Co., Ltd.) was added to samples and
incubated at 37°C for 30 min, then washed thrice in PBS for 3 min.
Samples were stained with 3,3′-diaminobenzidine (Tongling
Biomedicine Technology) and incubated at room temperature. Slides
were subsequently stained with H&E (18–28°C for 5–10 min),
washed with water then dehydrated (85% alcohol twice for 3 min each
time, 98% alcohol twice for 3 min each time, and anhydrous alcohol
twice for 3 min each time), before samples were sealed using clear,
neutral gum. Samples were imaged using a light microscope (Olympus
Corporation).
Immunohistochemical grade
determination
Two senior pathologists reviewed and scored the
radiographs (20). Positive protein
expression was indicated by a brownish-yellow color in the cell
membrane or cytoplasm. The proportion of positive cells and the
intensity of the stain were subsequently scored. The proportion of
positive cells was scored as follows: Five fields of view
(magnification, ×200) were observed on each sample section and the
percentage of positive cells was calculated. Positive proportion of
cells <5%=0 points, 5–25%=1 point, 26–50%=2 points, 51–75%=3
points and 76–100%=4 points. The stain intensity of positive cells
was scored as follows: 0 for colorless, 1 for light yellow, 2 for
brown and 3 for dark brown. The two scores were multiplied to give
the final score. The expression levels of NLRP1 and NLRC4 in
patients with prostate hyperplasia and prostate cancer were
compared, and their expression characteristics were analyzed.
Protein expression levels of proinflammatory cytokines IL-1β, IL-18
and the NLRP1 and NLRC4 inflammasomes were determined and
correlation analysis performed. Microscope images were produced
using cellSens (Olympus Corporation).
Differences in inflammatory cell
expression between different prostate cancer groups
Based on data from The Cancer Genome Atlas (TCGA)
dataset (https://portal.gdc.com), differences in
the expression of NLRP1 and NLRC4 inflammasomes in patients with
different stages of prostate cancer were compared to further
explore the clinical value of the diagnosis and treatment of
prostate cancer (The data of 495 patients were collected, including
177 patients in the T1 stage, 205 patients in the T2 stage and 113
patients in the T3/T4 stage).
Statistical analysis
SPSS (version 25.0; IBM Corp.) statistical software
was used for data analysis and the mean ± standard deviation was
calculated. Data with a non-normal distribution were presented as
the median ± interquartile range. An unpaired Student's t-test was
used to compare data from normally distributed groups and a
Wilcoxon rank sum test was used when data were not normally
distributed. According to the normal distribution of measurement
data in both groups, Pearson correlation was used to conduct a
correlation analysis. The difference in expression of NLRP1 or
NLRC4 in different stages of prostate cancer was also analyzed. The
Kruskal-Wallis test was used to compare the significant differences
between the three groups, and the Dunn post hoc test was used for
pairwise comparisons between the groups. P<0.05 was considered
to indicate a statistically significant difference. The R (version
4.0.3) GTEx package (https://www.gtexportal.org/home/datasets) was used for
statistical analysis.
Results
Comparison of basic patient
characteristics
Characteristics of patients with prostatic
hyperplasia (n=54) and prostate cancer (n=58) were assessed. The
mean age of patients with prostatic hyperplasia was 72.98±6.57
years and those with prostate cancer was 72.52±6.89 years. There
was no significant difference in the age, BMI, prostate volume (PV)
and International Prostate System Score (21) between the two groups of patients
(P>0.05), which demonstrated that the clinical data were
comparable (Table I).
 | Table I.Comparison of basic data from
patients with prostate cancer or prostate hyperplasia. |
Table I.
Comparison of basic data from
patients with prostate cancer or prostate hyperplasia.
| Patient
characteristic | Prostate
hyperplasia (n=54) | Prostate cancer
(n=58) | T-value | P-value |
|---|
| Age, years | 72.98±6.57 | 72.52±6.89 | 1.151 | 0.252 |
| BMI | 22.84±2.93 | 23.19±2.90 | −0.652 | 0.516 |
| Prostate volume,
mm | 38.26±18.23 | 39.35±18.56 | −0.235 | 0.815 |
| International
Prostate Symptom Score | 14.81±5.15 | 15.09±5.16 | −0.289 | 0.773 |
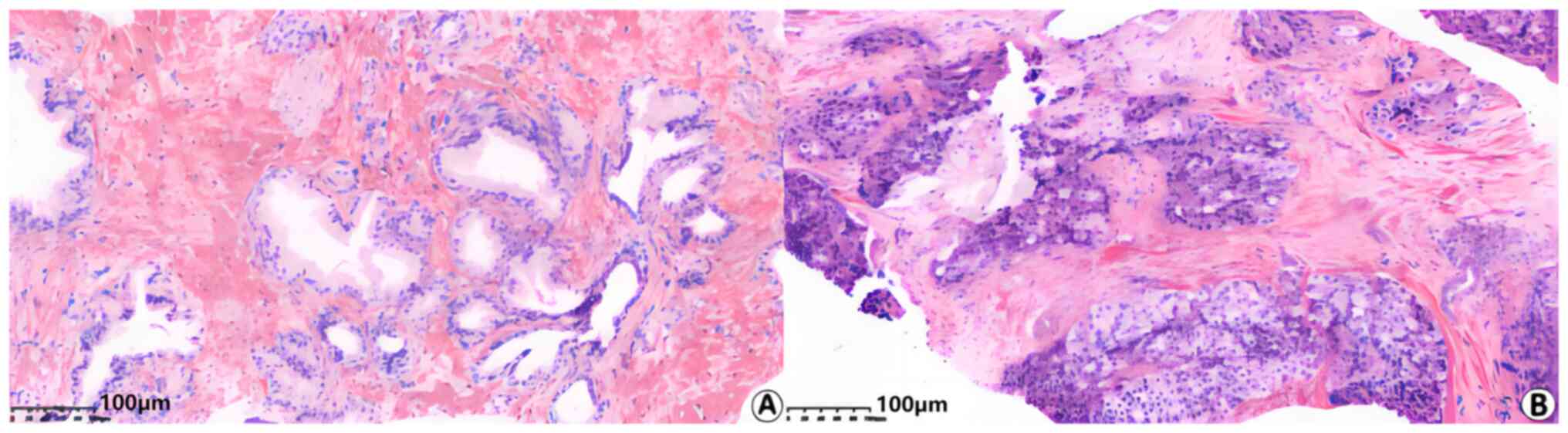
H&E staining
Arrangement of the glands in the patients with
prostatic hyperplasia were regular and the outlines of individual
glands were well defined (Fig. 1).
There was no infiltration of lymphocytes, plasma cells or other
inflammatory cells in samples of patients with prostatic
hyperplasia. In the group of patients with prostate cancer, minor
acinar structures were observed, some of the glands were damaged
and not precisely defined, solid flakes, nests or single-cell
structures were observed and some nuclei were distinct. There was
no infiltration of lymphocytes or plasma cells observed in samples
of patients with prostate cancer.
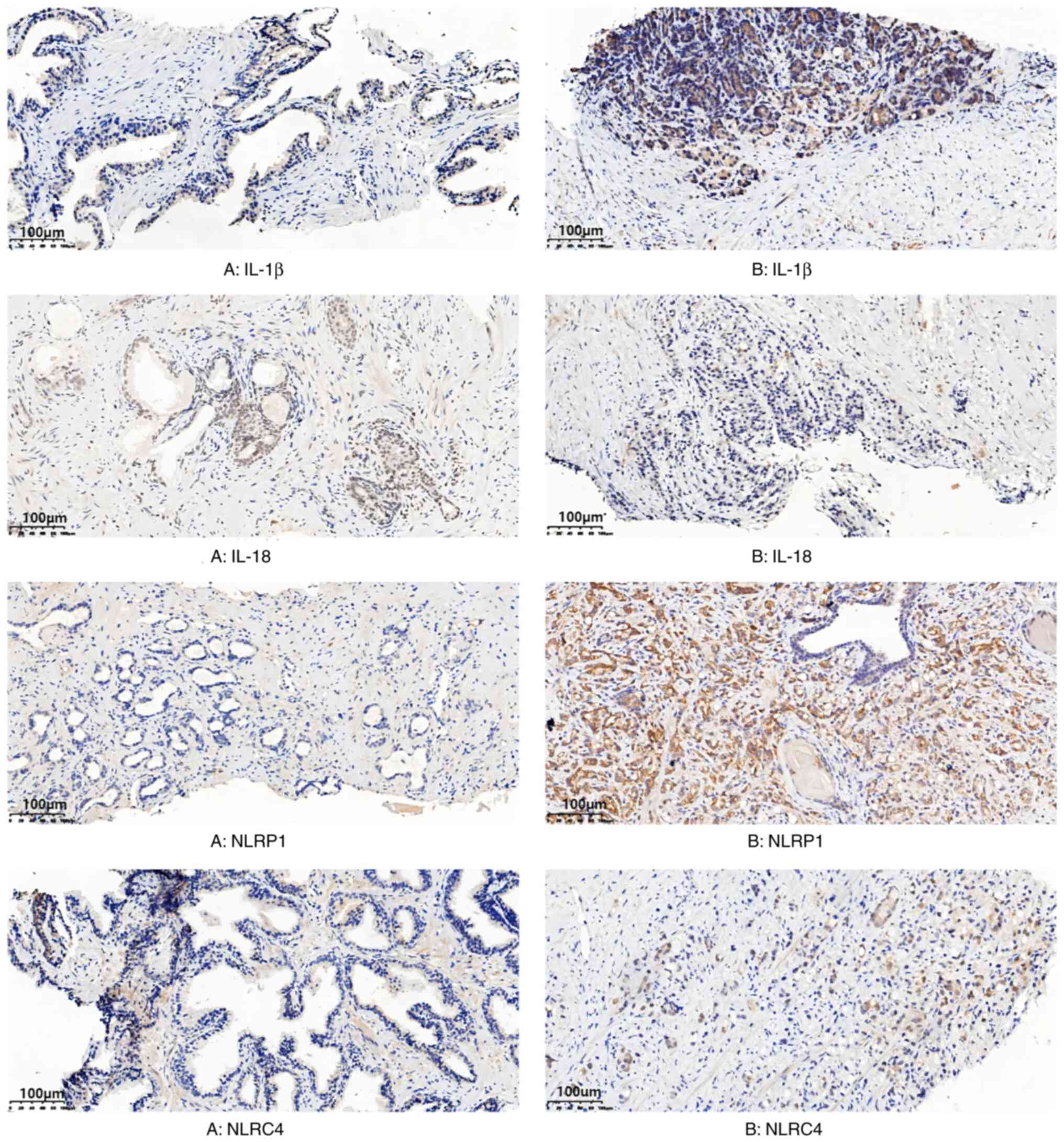
Expression of inflammasomes
NLRP1 and NLRC4 inflammasome expression was observed
in prostate hyperplasia and prostate cancer tissue, primarily in
the cytoplasm and membrane of cells, and were diffusely distributed
(Fig. 2). Compared with patients
with prostate hyperplasia, the expression of NLRP1 and NLRC4
inflammasomes in patients with prostate cancer was significantly
increased (P<0.001; Table II).
The protein expression level of IL-18 in patients with prostatic
hyperplasia was not significantly different compared with patients
with prostate cancer (P>0.05; Table
II). However, the protein expression level of IL-1β in patients
with prostate cancer was significantly increased compared with
patients with prostatic hyperplasia (P<0.001; Table II).
 | Table II.Expression of inflammasome in
patients with prostate cancer or prostate hyperplasia. |
Table II.
Expression of inflammasome in
patients with prostate cancer or prostate hyperplasia.
| Inflammasome | Prostate
hyperplasia (n=54) | Prostate cancer
(n=58) | T-value | P-value |
|---|
| NLRP1, points | 3.28±1.43 | 6.60±3.25 | 6.919 | >0.001 |
| NLRC4, points | 0.09±0.56 | 1.26±2.30 | 3.629 | >0.001 |
| IL-18, points | 1.43±1.92 | 1.72±2.34 | 0.735 | 0.464 |
| IL-1β, points | 3.22±1.11 | 6.59±1.97 | 11.005 | >0.001 |
Correlation analysis of the expression
of NLRP1 and NLRC4 with IL-18 and IL-1β
The protein expression levels of NLRP1, NLRC4 and
the proinflammatory cytokines IL-18 and IL-1β in tissue samples
were analyzed and a correlation analysis was performed. Protein
expression levels of IL-18 and IL-1β demonstrated a significant
positive correlation with the protein expression levels of NLRP1,
with a Pearson correlation coefficient of 0.208 and 0.568,
respectively (P<0.05; Table
III). The protein expression level of NLRC4 demonstrated a
significant positive correlation with IL-1β protein expression
level, with a Pearson correlation coefficient of 0.379
(P<0.01).
 | Table III.Correlation analysis of the
expression NLRP1 and NLRC4 with IL-18 and IL-1β expression. |
Table III.
Correlation analysis of the
expression NLRP1 and NLRC4 with IL-18 and IL-1β expression.
|
| IL-18 | IL-1β |
|---|
|
|
|
|
|---|
| Inflammasome | r | P-value | r | P-value |
|---|
| NLRP1 | 0.208 | 0.020 | 0.568 | <0.001 |
| NLRC4 | 0.145 | 0.108 | 0.379 | <0.001 |
Correlation analysis of NLRP1 and
NLRC4 expression with the prostate cancer index
In the group of patients with prostate cancer, the
protein expression levels of NLRP1 and NLRC4 demonstrated a
significant positive correlation with the Gleason score, with a
Pearson correlation coefficient of 0.578 and 0.279, respectively
(P<0.05; Table IV). The protein
expression levels of IL-18 and IL-1β were not significantly
correlated with the Gleason score (P>0.05) and the protein
expression levels of NLRP1, NLRC4, IL-18 and IL-1β were not
significantly correlated with TPSA, FPSA and F/TPSA
(P>0.05).
 | Table IV.Correlation analysis of NLRP1 and
NLRC4 expression with the prostate cancer index. |
Table IV.
Correlation analysis of NLRP1 and
NLRC4 expression with the prostate cancer index.
|
| Gleason score | TPSA | FPSA | F/TPSA |
|---|
|
|
|
|
|
|
|---|
| Inflammasome | r | P-value | r | P-value | r | P-value | r | P-value |
|---|
| NLRP1 | 0.578 | 0.000 | 0.183 | 0.169 | −0.004 | 0.977 | −0.078 | 0.560 |
| NLRC4 | 0.279 | 0.034 | 0.028 | 0.832 | −0.060 | 0.654 | −0.106 | 0.429 |
| IL-18 | 0.132 | 0.323 | 0.171 | 0.200 | −0.060 | 0.655 | −0.134 | 0.316 |
| IL-1β | 0.117 | 0.382 | −0.360 | 0.786 | 0.157 | 0.245 | −0.201 | 0.130 |
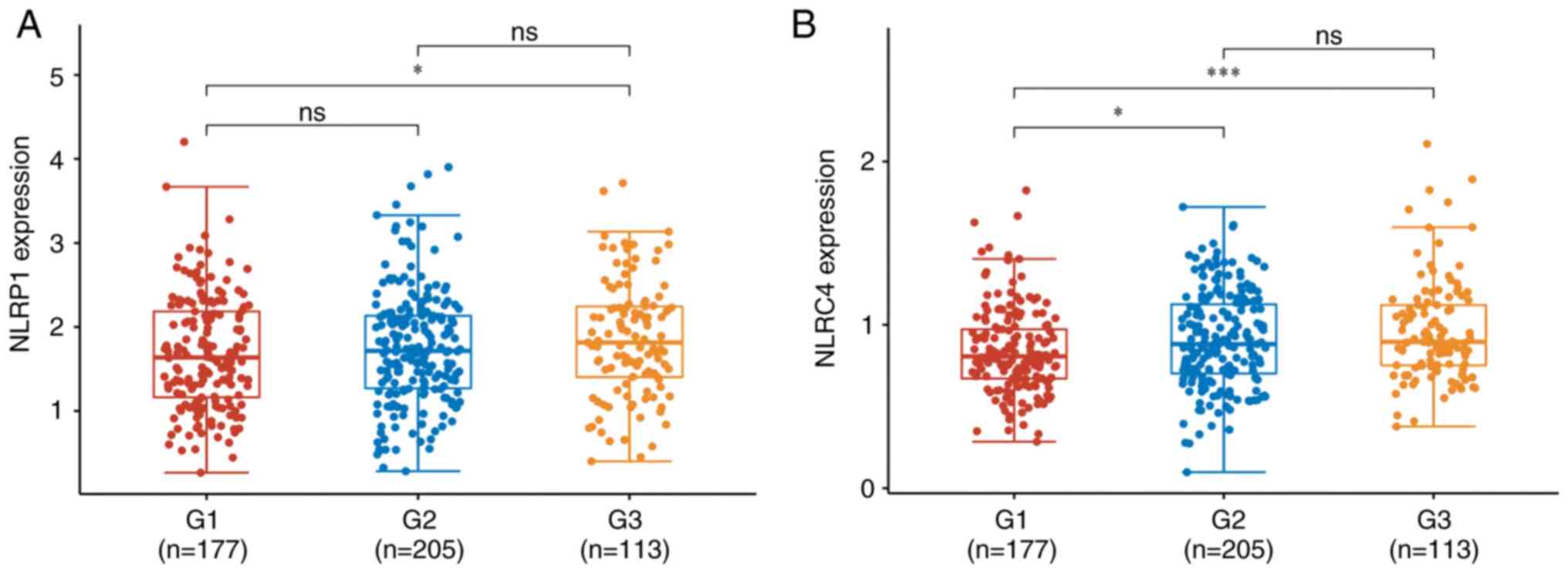
Comparison of NLRP1 and NLRC4
expression in different stages of prostate cancer
The expression levels of NLRP1 and NLRC4 in
different stages of prostate cancer were obtained from the TCGA
database (22). The expression
level of NLRP1 in G3 was significantly higher compared with G1
(P<0.05; Fig. 3). The expression
level of NLRC4 was significantly increased at higher stages of
prostate cancer, with the expression levels in cases with G2>G1
(P<0.05) and G3>T1 (P<0.001). However, there is currently
a lack of reliable evidence to support this finding which needs to
be confirmed by statistical analysis on a larger sample size of
patients with prostate cancer.
Discussion
Prostate cancer is a common malignancy of the
genitourinary system in older men (>60 years old), with the
highest incidence and the third highest mortality rate of all types
of cancers among men worldwide (23). For nearly 20 years, with the
improvement of living standards, including the formation of a
sedentary lifestyle and high-fat diet, as well as the aging of the
population, the incidence of prostate cancer has increased yearly,
which has brought an increasing social burden. Prostate cancer has
therefore become a major public health problem in the male
population (24).
The pathogenesis of prostate cancer remains unclear
and may be related to genetic background, age, ethnicity,
lifestyle, diet, chronic infection and inflammatory responses.
Previous studies have reported that chronic inflammation is
associated with the occurrence of prostate tumors and serves a key
role in certain stages of tumor development (4,5). The
inflammatory response can be generated by the activation of
inflammasome expression, thus inflammasomes may serve a role in the
occurrence and development of prostate cancer (4). Inflammasomes are involved in the
construction of the tumor microenvironment (TME) as they can induce
the release of proinflammatory cytokines IL-18, IL-1β, TNF-α and
human Toll-like receptor 4 and promote the proliferation of
peripheral cells, thus constructing the TME, which is conducive to
the occurrence of cancer (25).
Inflammasomes can also promote the growth and survival of cancer
cells. The IL-6/androgen receptor (AR) pathway serves an essential
role in the occurrence and development of prostate cancer (26). Activation of the inflammasomes
promotes the maturation and release of proinflammatory mediators
IL-6 and IL-18 (27). Under the
regulation of NF-kB, AR can be activated to induce PSA expression
and promote the growth and proliferation of tumor cells (6). Inflammasomes can also promote
angiogenesis, as NLRC4 mediates production of proinflammatory
factor IL-1β, which acts on adipocytes and can mediate vascular
endothelial growth factor A expression, which promotes the
formation of blood vessels associated with trophoblast tumor cells
(27).
As the first identified inflammasome, NLRP1 belongs
to the NLR protein family and is comprised of pyrin domain (PYD),
leucine-rich repeat (LRR), function to α-find domain (FIIND) and
C-terminal CARD, in addition to other domains and can independently
activate caspase-1 (16). Mutation
of the PYD domain of NLRP1 can lead to cancer and familial
bryophyte-like keratosis, whilst a gain-of-function mutation in the
FIIND domain can lead to systemic inflammation, arthritis and
dyskeratosis (28). In the absence
of external stimuli, NLRP1 suppresses autoregulation (29). Once stimulated, the N-terminal PYD
domain of NLRP1 binds to ASC, initiating a cascade reaction and
participating in numerous inflammatory diseases by regulating the
innate and adaptive immune responses (30). In a previous study of prostatitis
and prostatic hyperplasia in rats, Kashyap et al (31) reported that assembly and activation
of NLRP1 in the prostate promoted the production of proinflammatory
cytokines IL-1β and IL-18 after the autoproteolysis of caspase-1
and maturation. Glinskii et al (32) reported that the expression of the
NLRP1 inflammasome is involved in the occurrence of prostate
tumors, and NLRP1 expression is enhanced in highly metastatic
prostate cancer cells in prostate cancer. In the present study, the
NLRP1 inflammasome demonstrated a significant increase in
expression in prostate cancer compared with prostatic hyperplasia.
This result suggested that NLRP1 may be involved in the development
of prostate cancer. The expression of NLRP1 demonstrated a
significant positive correlation with the expression of IL-18 and
IL-1β, which was consistent with the results of previous studies
(6). Thus, further indicating that
activation of the NLRP1 inflammasome may promote the maturation and
release of inflammatory cytokines IL-1β and IL-18, serving an
essential role in the occurrence and development of prostate
cancer.
The NLRC4 inflammasome, also known as
ICE-protease-activating factor, belongs to the NLR protein family
(31). NLRC4 is comprised of CARD,
neuronal apoptosis inhibitory protein (NAIP), MHC class II
transcription activator, incompatibility locus protein from
Podospora anserina and telomerase-associated protein and LRR
domains (31). NLRC4 can directly
bind to pro-caspase-1 through CARD-CARD interactions, which
triggers caspase-1 processing and activation (33). In addition, the adaptor molecule
ASC, which contains apoptosis-associated blotch-like proteins
encoded by the PYCARD gene, can also facilitate such
interactions (31). ASC contains
both PYD and CARD domains, and CARD can activate caspase-1, promote
the protein expression of pro-IL-1β and pro-IL-18 and eliminate
infected cells through apoptosis (34). In animal experiments, NLRC4 has been
reported to serve a defensive role in maintaining intestinal
stability, and NAIP5-NLRC4 influence can be activated to promote
apoptosis of infected cells when infected with Escherichia
coli and Salmonella typhimurium (35). Expression of IL-18 was increased in
patients with active idiopathic thrombocytopenia compared with
normal and reactive-idiopathic thrombocytopenia, which may be
related to activation of the NLRC4 inflammasome (17). However, previous studies of NLRC4
have focused on the maintenance of intestinal balance and immune
disorders, therefore information on the expression of NLRC4 in
prostate disease is currently lacking. In the present study, the
NLRC4 inflammasome demonstrated a significant increase in
expression in patients with prostate cancer compared with prostatic
hyperplasia, which indicated that NLRC4 inflammasomes may be
involved in the occurrence and development of prostate cancer.
Expression of NLRC4 demonstrated a significant positive correlation
with IL-1β expression. These results demonstrated that the
expression of IL-1β was closely related to NLRC4 expression.
Therefore, NLRC4 inflammasome expression may be involved in the
development and progression of prostate cancer by promoting the
production of the pro-inflammatory cytokine IL-18 following
auto-proteolysis of caspase-1.
Previous studies have reported that IL-18 expression
promotes tumorigenesis (26). The
present study demonstrated that IL-18 was not expressed in patients
with prostate cancer. However, Dupaul-Chicoine et al
(36) reported that NLRP3
inflammatory vesicle-mediated IL-18 production could inhibit the
proliferation of hepatic colorectal cancer. NLRP3/IL-18-mediated
downregulation may also provide protection against intestinal
tissue damage during peaks of the inflammatory process. However,
sample limitations in the present study may affect these
conclusions, which need to be confirmed by a large sample
study.
Tumorigenesis is strongly correlated with
interactions between cancer cells and their TME (37). The major components of the TME are
the extracellular matrix, fibroblasts, myofibroblasts, mesenchymal
stem cells, neuroendocrine cells, fatty cells, immune and
inflammatory cells, and blood and lymphatic networks (38). Galectin-1 overexpression in
cancer-associated fibroblasts is associated with poor prognosis in
certain types of cancer, including prostate cancer (39). Neuroendocrine cells serve a key role
in the development of prostate cancer cells by influencing their
proliferation and invasiveness (39). Abnormal AR reactions in the
epithelium and stroma may lead to tumor occurrence (40). Immune cells such as regulatory T
cells, T helper 17 cells and macrophages are also involved in
prostate cancer progression. However, cytokines secreted by cells
in the TME, such as IL-1β, IL-6, and RANKL have been reported to
have pleiotropic effects on prostate cancer cells (37). These findings suggest that the TME
serves an essential role in the occurrence and progression of
prostate cancer.
The present study demonstrated that the NLRP1 and
NLRC4 inflammasome-mediated inflammatory response may be involved
in the occurrence of prostate cancer, as a significant increase in
the expression of inflammasomes in tumor tissues compared with
non-tumor tissues was demonstrated. In addition, expression of the
NLRP1 and NLRC4 inflammasomes positively correlated with the
Gleason score of prostate cancer, which indicated that NLRP1 and
NLRC4 expression was closely related to the risk assessment score
of prostate cancer and could predict the prognosis of patients with
prostate cancer. Expression of NLRP1 and NLRC4 was significantly
increased in intermediate and advanced prostate cancer tissues
compared with early-stage prostate cancer tissues, which suggested
that these inflammasomes also serve a role in prostate cancer
progression and metastasis. Therefore, expression of the NLRP1 and
NLRC4 inflammasomes and their downstream products IL-1β and IL-18
were involved in the construction of TME, thus promoting the
occurrence and development of prostate cancer. This result
demonstrated potential clinical value for predicting the
progression and prognosis evaluation of prostate cancer.
Inflammasomes promote the occurrence and development
of inflammation, which can lead to the development of prostate
cancer. Future research into inflammasome inhibitors could give
rise to potential candidates for the treatment of prostate cancer.
The main types of inflammasome inhibitors currently in use function
by blocking IL-1β activation (40).
MCC950, CY-09, trails and OLT1177 can reduce the activity of
caspase-1 and the production of IL-1β in mouse experiments, thus
inhibiting inflammation (41,42).
Although research into the efficacy of these compounds in humans is
currently lacking. However, the present study provides a valuable
direction for the diagnosis and treatment of prostate diseases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ conceptualized the study, gave final approval of
the version to be published, and agreed to be accountable for the
work in ensuring that questions related to the integrity of any
part of the work are appropriately investigated and resolved
(according to the ICMJE). KL wrote and edited the manuscript, made
substantial contributions to the design of the study, and the
acquisition and analysis of data. JH performed the data analysis.
ZK contributed to the interpretation of data for the manuscript,
drafting the manuscript or revising it critically for important
intellectual content. All authors read and approved the final
version of the manuscript. XZ and KL confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The project was approved by The Ethics Committee of
The First People's Hospital of Pinghu (Pinghu, China; approval no.
002) and all patients provided written, informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NLRP
|
nucleotide binding
oligomerization-like receptor family pyrin domain containing
|
|
NLRC4
|
nucleotide-binding oligomerization
domain leucine-rich repeat and caspase recruitment
domain-containing 4
|
|
PSA
|
prostate specific antigen
|
|
TPSA
|
total prostate specific antigen
|
|
FPSA
|
free prostate specific antigen
|
|
ASC
|
apoptosis-associated speck-like
protein containing CARD
|
|
CARD
|
caspase recruitment domain
|
|
NLR
|
nucleotide-binding oligomerization
domain-like receptor
|
|
H&E
|
hematoxylin and eosin
|
|
PYD
|
pyrin domain
|
|
LRR
|
leucine-rich repeat
|
|
TME
|
tumor microenvironment
|
|
IL
|
interleukin
|
|
TCGA
|
The Cancer Genome Atlas
|
|
PV
|
prostate volume
|
|
BMI
|
body mass index
|
|
TNF-α
|
tumor necrosis factor alpha
|
|
AR
|
androgen receptor
|
|
NF-kB
|
nuclear factor-kappa B
|
|
caspase-1
|
cysteinyl aspartate specific
proteinase-1
|
|
T
|
tumor
|
|
FIIND
|
function to α-find domain
|
|
G
|
grade
|
References
|
1
|
Boehm BJ, Colopy SA, Jerde TJ, Loftus CJ
and Bushman W: Acute bacterial inflammation of the mouse prostate.
Prostate. 72:307–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo YL, Na YQ, Ye ZQ and Huang J:
Guidelines for Diagnosis and Treatment of Urology and Andrology
Diseases in China. Science Press; Beijing, China: pp. pp4392019
|
|
3
|
Xie Jinbo and Peng Bo: Research progress
on the epidemiological characteristics and risk factors of benign
prostatic hyperplasia. J Tongji Univ Med Edition. 42:62021.(In
Chinese).
|
|
4
|
Leela SCA, Paes Batista da Silva A, Verma
S, Fu P, Shen DL, MacLennan G, Gupta S and Bissada NF: Presence of
specific periodontal pathogens in prostate gland diagnosed with
chronic inflammation and adenocarcinoma. Cureus.
13:e177422021.PubMed/NCBI
|
|
5
|
Gurel B, Lucia MS, Thompson IM Jr, Goodman
PJ, Tangen CM, Kristal AR, Parnes HL, Hoque A, Lippman SM,
Sutcliffe S, et al: Chronic inflammation in benign prostate tissue
is associated with high-grade prostate cancer in the placebo arm of
the prostate cancer prevention trial. Cancer Epidemiol Biomarkers
Prev. 23:847–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishra V and Pathak C: Human Toll-Like
Receptor 4 (hTLR4): Structural and functional dynamics in cancer.
Int J Biol Macromol. 122:425–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krysko DV, Agostinis P, Krysko O, Garg AD,
Bachert C, Lambrecht BN and Vandenabeele P: Emerging role of
damage-associated molecular patterns derived from mitochondyria in
inflammation. Trends Immunol. 32:157–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Costa FRC, Leite JA, Rassi DM, da Silva
JF, Elias-Oliveira J, Guimarães JB, Foss-Freitas MC, Câmara NOS,
Pontillo A, Tostes RC, et al: NLRP1 acts as a negative regulator of
Th17 cell programming in mice and humans with autoimmune diabetes.
Cell Rep. 35:1091762021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alehashemi S and Goldbach-Mansky R: Human
autoinflammatory diseases mediated by NLRP3-, Pyrin-, NLRP1-, and
NLRC4-Inflammasome dysregulation updates on diagnosis, treatment,
and the respective roles of IL-1 and IL-18. Front Immunol.
11:18402020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moecking J, Laohamonthonkul P, Chalker K,
White MJ, Harapas CR, Yu CH, Davidson S, Hrovat-Schaale K, Hu D,
Eng C, et al: NLRP1 variant M1184V decreases inflammasome
activation in the context of DPP9 inhibition and asthma severity. J
Allergy Clin Immunol. 147:2134–2145.e20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ciążyńska M, Olejniczak-Staruch I,
Sobolewska-Sztychny D, Narbutt J, Skibińska M and Lesiak A: The
role of NLRP1, NLRP3, and AIM2 inflammasomes in psoriasis: Review.
Int J Mol Sci. 22:58982021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang T, Yin H, Ning W, Wang X, Chen C,
Lin W, Li J, Zhou Y, Peng Y, Wang M, et al: Expression of
inflammasomes NLRP1, NLRP3 and AIM2 in different pathologic
classification of lupus nephritis. Clin Exp Rheumatol. 38:680–690.
2020.PubMed/NCBI
|
|
13
|
Gong Q, Robinson K, Xu C, Huynh PT, Chong
KHC, Tan EYJ, Zhang J, Boo ZZ, Teo DET, Lay K, et al: Structural
basis for distinct inflammasome complex assembly by human NLRP1 and
CARD8. Nat Commun. 12:1882021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Pei L, Zang D, Xue Y, Wang X,
Chen Y, Li J, Yu J, Gao Q, Di W, et al: Gender differences of NLRP1
inflammasome in mouse model of Alzheimer's disease. Front Aging
Neurosci. 12:5120972020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karki R and Kanneganti TD: Diverging
inflammasome signals in tumorigenesis and potential targeting. Nat
Rev Cancer. 19:197–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gouravani M, Khalili N, Razi S,
Keshavarz-Fathi M, Khalili N and Rezaei N: The NLRP3 inflammasome:
A therapeutic target for inflammation-associated cancers. Expert
Rev Clin Immunol. 16:175–187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sundaram B and Kanneganti TD: Advances in
understanding activation and function of the NLRC4 inflammasome.
Int J Mol Sci. 22:10482021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Broz P: Recognition of intracellular
bacteria by inflammasomes. Microbiol Spectr. 7:2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chear CT, Nallusamy R, Canna SW, Chan KC,
Baharin MF, Hishamshah M, Ghani H, Ripen AM and Mohamad SB: A novel
de novo NLRC4 mutation reinforces the likely pathogenicity of
specific LRR domain mutation. Clin Immunol. 211:1083282020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang J, Gu J, Hu Y, Wang N, Gao J and Wang
P: Molecular cloning and characterization of HSP60 gene in domestic
pigeons (Columba livia) and differential expression patterns under
temperature stress. Cell Stress Chaperones. 26:115–127. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barry MJ, Fowler FJ jr, O'Leary MP,
Bruskewitz RC, Holtgrewe HL, Mebust WK and Cockett AT: The American
Urological Association symptom index for benign prostatic
hyperplasia The Measurement Committee of the American Urological
Association. J Urol. 148:1549–1557; discussion 1564. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YJ, Luo SN, Dong L, Liu TT, Shen XZ,
Zhang NP and Liang L: Interferon regulatory factor family
influences tumor immunity and prognosis of patients with colorectal
cancer. J Transl Med. 19:3792021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou T, Cai Z, Ma N, Xie W, Gao C, Huang
M, Bai Y, Ni Y and Tang Y: A novel ten-gene signature predicting
prognosis in hepatocellular carcinoma. Front Cell Dev Biol.
8:6292020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I and Jemal Aand Bray F: Global cancer statistics
2020: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Puhr M, De Marzo A, Isaacs W, Lucia MS,
Sfanos K, Yegnasubramanian S and Culig Z: Inflammation, microbiota,
and prostate cancer. Eur Urol Focus. 2:374–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohashi K, Wang Z, Yang YM, Billet S, Tu W,
Pimienta M, Cassel SL, Pandol SJ, Lu SC, Sutterwala FS, et al:
NOD-like receptor C4 inflammasome regulates the growth of colon
cancer liver metastasis in NAFLD. Hepatology. 70:1582–1599. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schillaci O, Scimeca M, Trivigno D,
Chiaravalloti A, Facchetti S, Anemona L, Bonfiglio R, Santeusanio
G, Tancredi V, Bonanno E, et al: Prostate cancer and inflammation:
A new molecular imaging challenge in the era of personalized
medicine. Nucl Med Biol. 68–69. 66–69. 2019.
|
|
29
|
Yu CH, Moecking J, Geyer M and Masters SL:
Mechanisms of NLRP1-mediated autoinflammatory disease in humans and
Mice. J Mol Biol. 430:142–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan Y, Yang L, Wei Q, Ding Y, Tang Z, Tan
P, Lin T, Guo D and Qiu S: Toll-like receptor 10 (TLR10) exhibits
suppressive effects on inflammation of prostate epithelial cells.
Asian J Androl. 21:393–399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kashyap M, Pore S, Wang Z, Gingrich J,
Yoshimura N and Tyagi P: Inflammasomes are important mediators of
prostatic inflammation associated with BPH. J Inflamm (Lond).
12:372015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Glinskii AB, Ma S, Ma J, Grant D, Lim CU,
Guest I, Sell S, Buttyan R and Glinsky GV: Networks of intergenic
long-range enhancers and snpRNAs drive castration-resistant
phenotype of prostate cancer and contribute to pathogenesis of
multiple common human disorders. Cell Cycle. 10:3571–3597. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen J, Xuan B, Liu Y, Wang L, He L, Meng
X, Zhou T and Wang Y: Updating the NLRC4 inflammasome: From
bacterial infections to autoimmunity and cancer. Front Immunol.
12:7025272021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Fu TM, Lu A, Witt K, Ruan J, Shen C
and Wu H: Inaugural Article: Cryo-EM structures of ASC and NLRC4
CARD filaments reveal a unified mechanism of nucleation and
activation of caspase-1. Proc Natl Acad Sci USA. 115:10845–10852.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Man SM, Tourlomousis P, Hopkins L, Monie
TP, Fitzgerald KA and Bryant CE: Salmonella infection induces
recruitment of caspase-8 to the inflammasome to modulate IL-1β
production. J Immunol. 191:5239–5246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dupaul-Chicoine J, Arabzadeh A, Dagenais
M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton
V, Colpitts SL, Beauchemin N and Saleh M: The Nlrp3 inflammasome
suppresses colorectal cancer metastatic growth in the liver by
promoting natural killer cell tumoricidal activity. Immunity.
43:751–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv Y, Ruan G, Liu Y, Cui D, Zhao Y, Yan C,
Lv M, Xu D, Mao Y, Cao J, et al: Aberrant expression of NLRP3,NLRC4
and NLRP6 inflammasomes in patients with primary im-mune
thrombocytopenia. Thromb Res. 176:101–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Di Donato M, Giovannelli P, Cernera G, Di
anti A, Marino I, Bilancio A, Galasso G, Auricchio F, Migliaccio A
and Castoria G: Non-genomic androgen action regulates
proliferative/migratory signaling in stromal cells. Front
Endocrinol (Lausanne). 5:2252014.PubMed/NCBI
|
|
39
|
Van den Brule FA, Waltregny D and
Castronovo V: Increased expression of galectin-1 in
carcinoma-associated stroma predicts poor outcome in prostate
carcinoma patients. J Pathol. 193:80–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dai J, Lu Y, Roca H, Keller JM, Zhang J,
McCauley LK and Keller ET: Immune mediators in the tumor
microenvironment of prostate cancer. Chin J Cancer. 36:292017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang H, He H, Chen Y, Huang W, Cheng J,
Ye J, Wang A, Tao J, Wang C, Liu Q, et al: Identification of a
selective and direct NLRP3 inhibitor to treat inflammatory
disorders. J Exp Med. 214:3219–3238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang Y, Jiang H, Chen Y, Wang X, Yang Y,
Tao J, Deng X, Liang G, Zhang H, Jiang W and Zhou R: Tranilast
directly targets NLRP3 to treat inflammasome-driven diseases. EMBO
Mol Med. 10:e86892018. View Article : Google Scholar : PubMed/NCBI
|