Introduction
Globally, lung cancer ranks as the second most
prevalent type of cancer and is the leading cause of cancer-related
mortality. Non-small cell lung carcinoma (NSCLC) is responsible for
~85% of these cases, thus indicating its substantial contribution
to the global cancer burden (1,2). In
accordance with the Comprehensive Cancer Network guidelines,
surgery is upheld as the primary and most effective therapeutic
modality for resectable stage IA-IIIA NSCLC (3). However, enhancing overall survival
duration and minimizing postoperative recurrence pose complex
challenges in clinical practice. While perioperative management can
marginally extend survival, the cumulative benefit remains
restricted (4–6).
A number of studies have indicated that
immunotherapy can markedly enhance the therapeutic outcome for
patients with advanced NSCLC (7,8). The
application of immunotherapy in advanced NSCLC provides a new
therapeutic strategy for neoadjuvant treatment of resectable NSCLC.
Additionally, a study on nivolumab (9) demonstrated that preoperative nivolumab
induction therapy can lead to the expansion of T-cell clones, which
may be an advantage of neoadjuvant immunotherapy in combating
tumors. Currently, numerous clinical trials (10–12)
are investigating neoadjuvant immune checkpoint inhibitor (ICI)
therapy for stage IB-IIIA NSCLC. For patients with unresectable
locally advanced lung cancer, the prevailing standard of care is
concurrent chemoradiotherapy (13);
however, a subset of patients still succumb due to disease
progression or tumor metastasis (14). The demonstrated efficacy of
immunotherapy in advanced NSCLC has generated renewed optimism for
patients with unresectable NSCLC. Furthermore, no definitive
biomarkers predictive of the effectiveness of neoadjuvant
immunotherapy have been identified. Research has proposed
programmed death-ligand 1 (PD-L1) expression levels and tumor
mutation burden as two concurrent and potentially predictive
indicators of efficacy in neoadjuvant immunotherapy. However, the
predictive value of these markers remains contentious, with
different studies yielding disparate conclusions (9,15,16).
Tislelizumab is a novel humanized IgG4 monoclonal
antibody that acts as an ICI targeting programmed death-1 (PD-1).
It exhibits distinct binding epitopes from other PD-1 monoclonal
antibodies to enhance its antitumor activity (17). Tislelizumab was approved by the
National Medical Products Administration (NMPA) on December 27,
2019, for the treatment of relapsed or refractory classical Hodgkin
lymphoma following at least second-line systemic therapy (18). Subsequently, in January and June
2021, it was approved for first-line treatment of metastatic NSCLC
of both squamous and non-squamous histology (19,20).
The present case report suggested tislelizumab as
neoadjuvant immunotherapy, which may provide significant benefits
to patients with PD-L1-negative, potentially resectable stage IIIB
NSCLC (21). Furthermore,
neoadjuvant immunotherapy has the potential to modulate the immune
microenvironment of the tumor without compromising surgical
outcomes, thus providing a favorable immune microenvironment for
subsequent immunotherapy.
Case report
In August 2022, a 64-year-old Chinese male patient
presented for a medical consultation at The Second Affiliated
Hospital of Harbin Medical University (Harbin, China). The visit
was prompted by the discovery of a pulmonary mass during a physical
examination conducted 10 days prior. The patient was asymptomatic,
demonstrating no symptoms such as sputum production, hemoptysis,
chest pain, hot flushes or night sweats. Additionally, they had
neither a history of smoking nor any prior medical issues, and
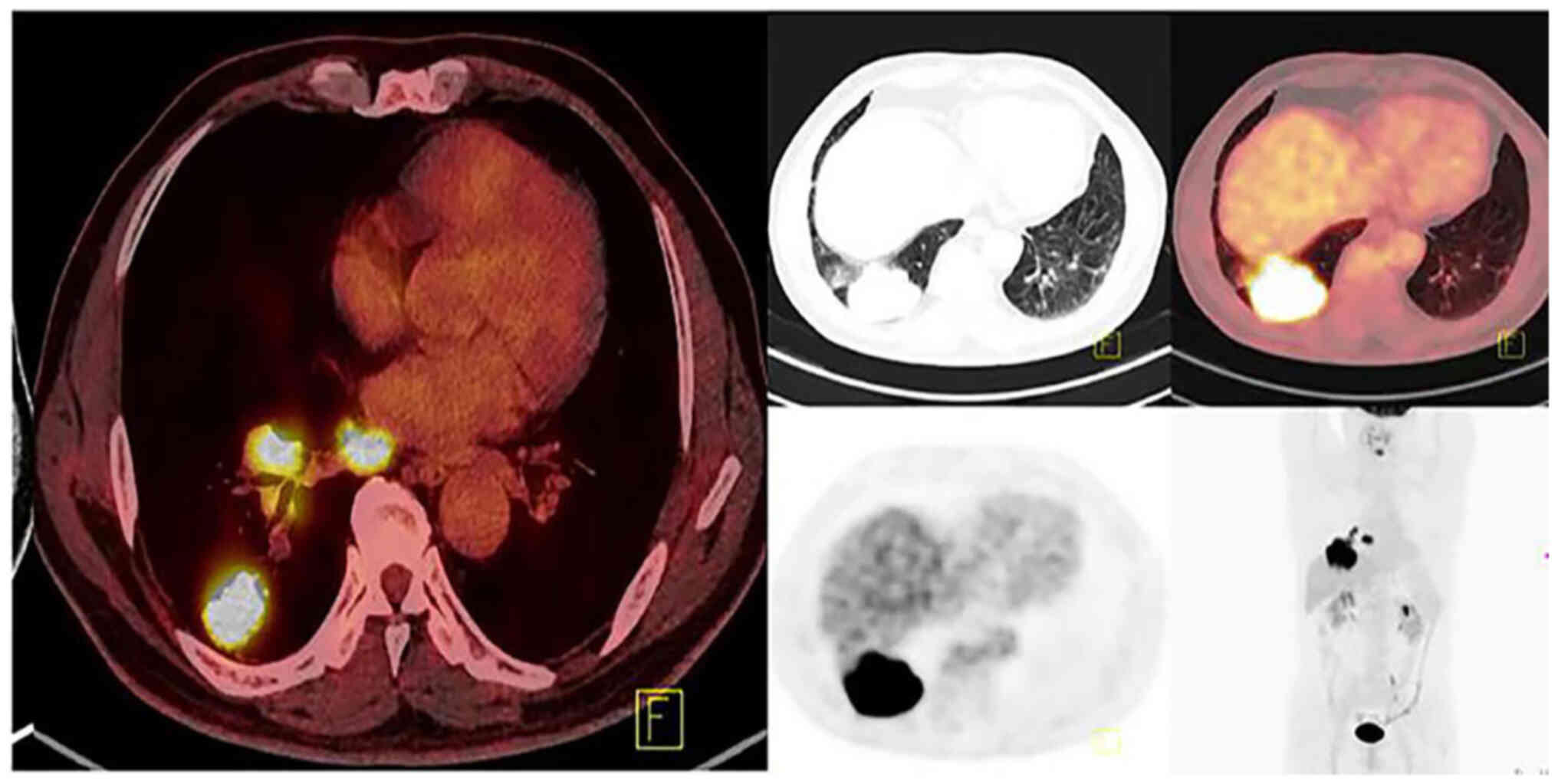
there was no known familial history of cancer. An enhanced computed
tomography (CT) scan of the chest and a positron emission
tomography-CT (PET-CT) scan of the chest revealed a subpleural
mass, measuring 70×59×56 mm, situated in the posterior lateral
basal segment of the lower lobe of the right lung. Mediastinal
lymph nodes (~20 mm in diameter) and right hilar lymph nodes (~23
mm in diameter) showed enlargement and increased radiological
uptake, suggestive of metastases (Fig.
1). However, no notable metastases were detected elsewhere
based on the brain magnetic resonance imaging and whole-body PET-CT
scans.
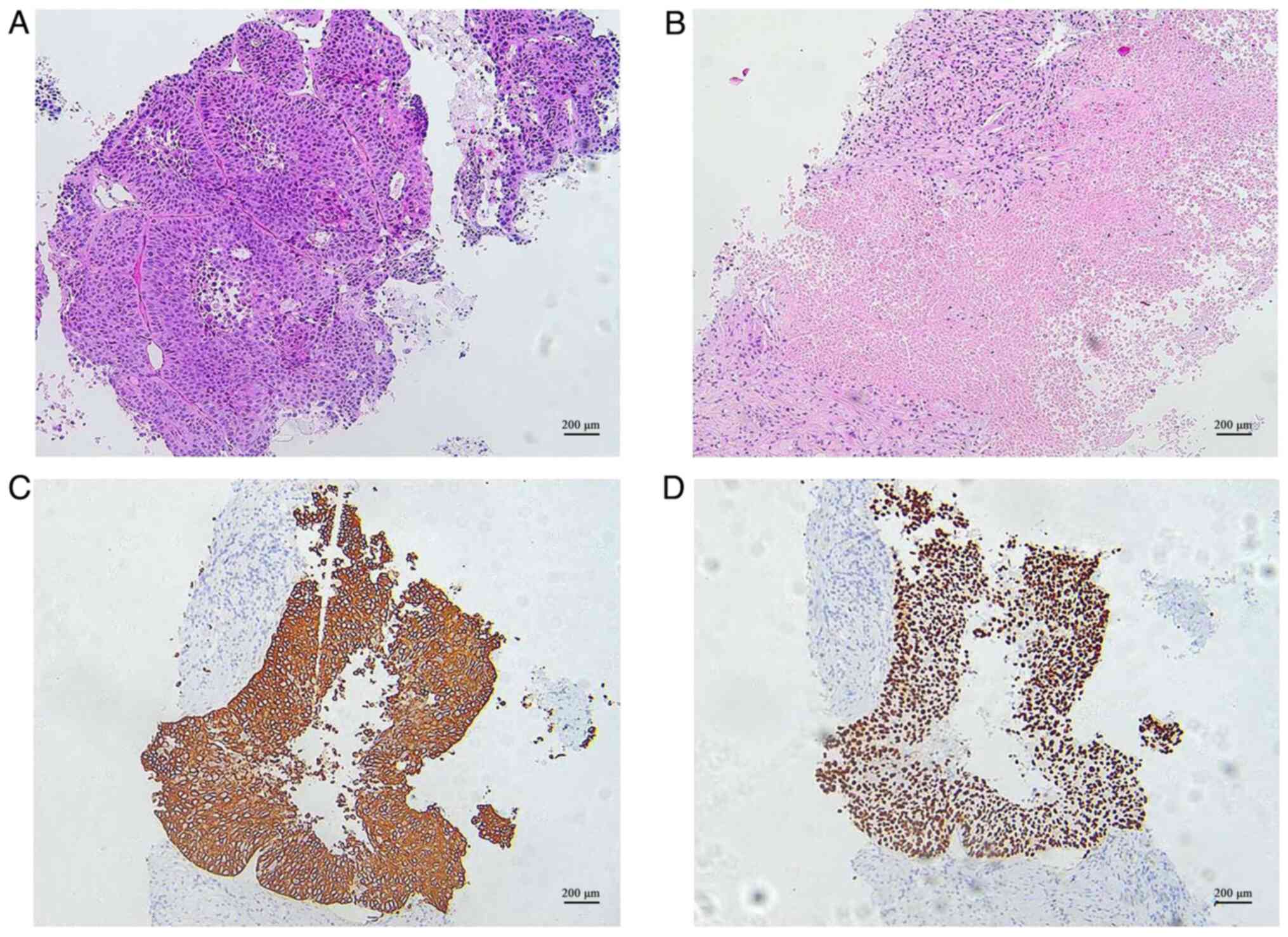
A pathological biopsy from the right lung confirmed
the presence of squamous cell carcinoma with necrosis in the lower
lobe of the right lung (Fig. 2A and
B). The patient's blood was analyzed for tumor markers using
chemiluminescent immunoassay methods, and the results revealed
elevated levels of squamous cell carcinoma antigen at 2.5 ng/ml,
surpassing the reference value of ≤2.0 ng/ml. Notably, tests for
cytokeratin (CK)19 fragment, neuron-specific enolase, serum
carbohydrate antigen 125 and carcinoembryonic antigen returned
negative results.
Immunohistochemical staining was also performed.
Briefly, tissue was fixed in 4% paraformaldehyde solution at room
temperature for 24 h. Subsequently, the tissue was dehydrated in a
gradient alcohol series, embedded in paraffin and sectioned (4 µm).
Paraffin-embedded sections were then dewaxed, and hydrated in
xylene, anhydrous ethanol, gradient alcohol and distilled water.
The tissue sections were then placed in citric acid antigen repair
buffer (pH 6.0) for antigen repair in a water bath at 100°C for 15
min; after cooling, the sections were washed three times with PBS
(5 min/wash). Sections were blocked for endogenous peroxidase by
placing them in 3% hydrogen peroxide solution for at 37°C for 25
min, and then washed three times with PBS (5 min/wash). The
sections were then blocked using 3% BSA (Beijing Coolaber
Technology Co., Ltd.) for 30 min at 37°C and incubated with the
following primary antibodies (all antibodies were ready-to-use and
supplied by Fuzhou Maixin Biotech Co., Ltd.) for 1 h at 37°C: CK5/6
(cat. no. MAB-0744; clone no. MX040), P40 [cat. no. RMA-1006; clone
no. MXR010], TTF-1 (cat. no. MAB-0599; clone no. SPT24), CD38 (cat.
no. MAB-0755; clone no. MX044) and CD68 (cat. no. KIT-0026; clone
no. KPI). The sections were washed three times with PBS (5
min/wash). Subsequently, the appropriate secondary antibody
(Elivision plus mouse/rabbit; ready-to-use; cat. no. KIT-9902;
Fuzhou Maixin Biotech Co., Ltd.) was added dropwise to the section
and incubated for 20 min at room temperature. The sections were
washed three times with PBS (5 min/wash) and were slowly rinsed
with tap water to terminate the color development. Finally, the
nuclei were stained with hematoxylin for 3 min, the sections were
rinsed with running tap water, dehydrated and sealed. Images were
captured under a light microscope.
PD-L1 expression was assessed by
immunohistochemistry, as aforementioned. After antibody incubation,
the sections were placed on a specific detection platform for 3 h
to get the final results and the sections were then detected under
a light microscope. Ready-to-use rabbit monoclonal anti-PD-L1
antibodies (cat. no. 8.17.0002; clone no. E1L3N; Amoy Diagnostics
Co., Ltd.) were used. The testing platform was Leica Bond-MAx
(supplied by Leica Microsystems GmbH). PD-LI protein expression was
assessed according to the tumor proportion score (TPS), where TPS
is the percentage of live tumor cells that are partially or
completely membrane-stained at any intensity. TPS <1% indicates
no PD-LI expression, TPS ≥1% indicates PD-L1 expression, and TPS
≥50% indicates high PD-L1 expression. Immunohistochemical staining
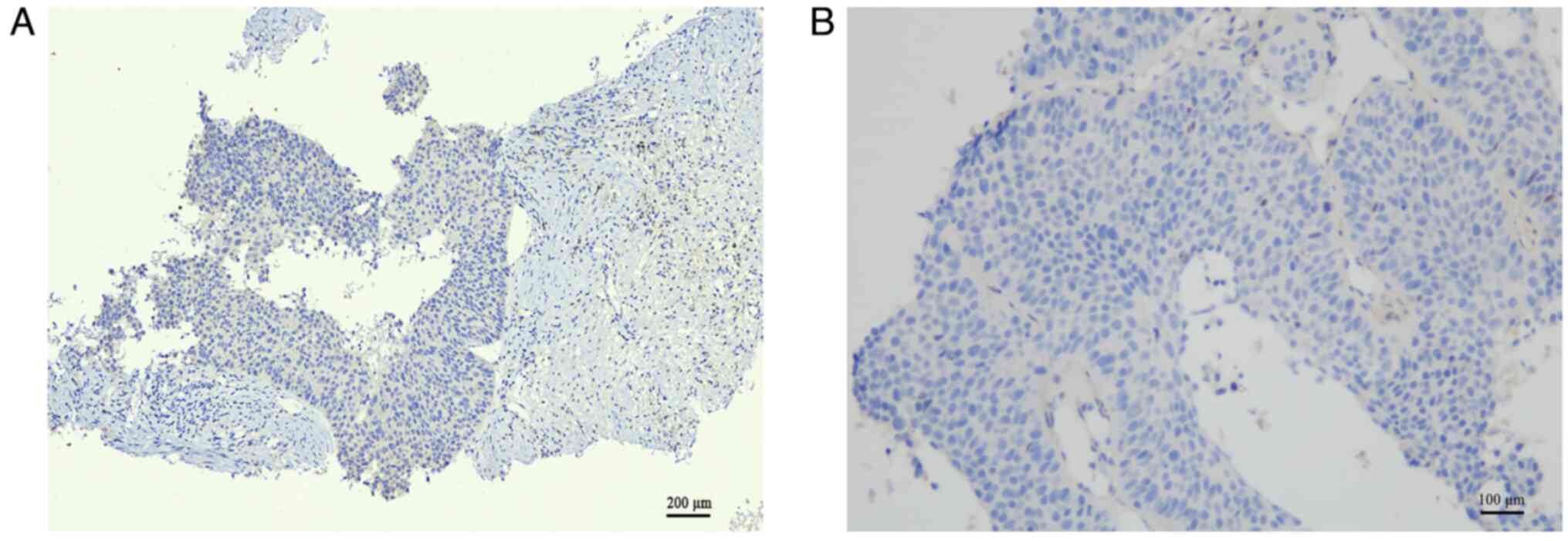
demonstrated positive staining for CK5/6 and P40 (Fig. 2C and D), whereas TTF-1and PD-LI
staining were negative (Fig. 3A and
B). The patient's biopsy tissue was subjected to whole-genome
sequencing, and the results revealed the absence of mutations in
target genes, such as EGFR, ALK or ROS1. In light of the
chest-enhanced CT, PET-CT and pathological findings, the patient
was diagnosed with locally advanced NSCLC at TNM stage cT3N2M0,
stage IIIB. Despite the patient's preference for surgical
intervention, a thoracic surgery consultation determined that R0
resection was unfeasible using current surgical techniques.
Following a multidisciplinary diagnosis and treatment (MDT)
approach, the patient was advised to undertake two cycles of
neoadjuvant therapy. Should the tumor decrease in size, the patient
could be considered suitable for NSCLC resection. Conversely, if
the tumor and lymph nodes remained unchanged, radical
chemoradiotherapy would be the recommended course.
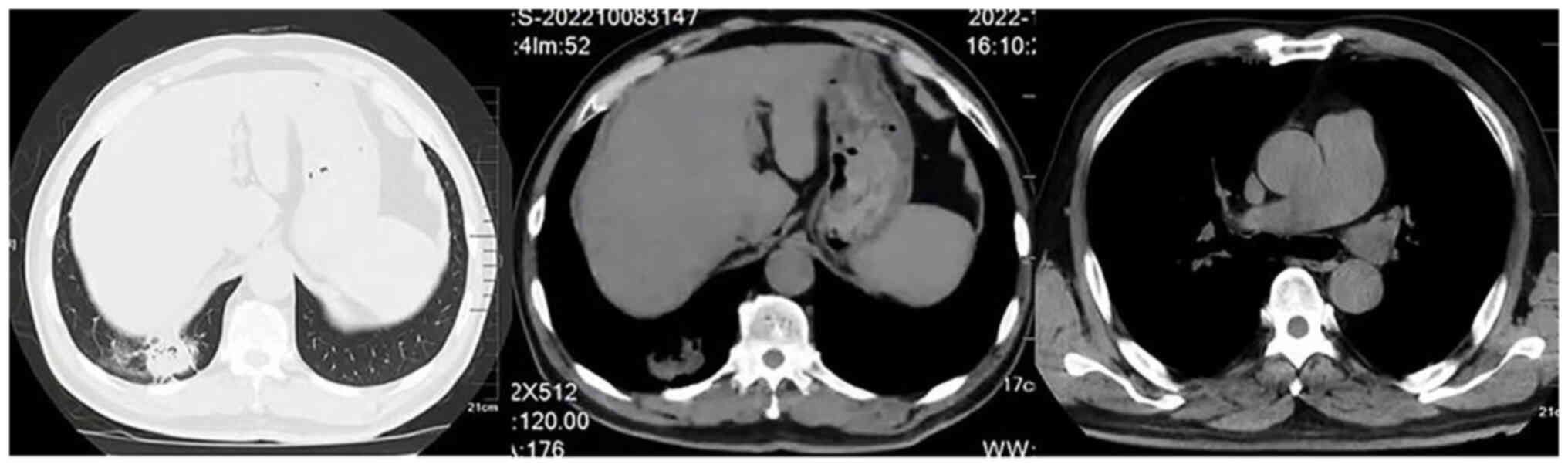
From August to October 2022, the patient underwent
two cycles of neoadjuvant chemotherapy [carboplatin 500 mg (area
under the curve, 5) and albumin-bound paclitaxel 300 mg] combined
with immunotherapy (tislelizumab 200 mg). After two cycles of
neoadjuvant chemotherapy combined with immunotherapy, the patient
underwent a follow-up chest contrast-enhanced CT scan in October
2022. The results showed a significant therapeutic effect. In
comparison to before the treatment, the tumor in the lower lobe of
the right lung was reduced to ~31×27 mm, and there was also a
noticeable decrease in the size of the mediastinal lymph nodes
(Fig. 4). Following an MDT
discussion, surgical intervention was recommended. A total of 6
days after treatment was completed, after ruling out
contraindications, the patient underwent radical thoracoscopic lung
cancer surgery. Intraoperative cryopathology revealed no metastasis
in the lymph nodes of the 7th group (the lymph nodes situated below
the tracheal prominence). Postoperative pathology revealed the
following: Resected specimen of squamous cell carcinoma of the
right lower lobe (below the tracheal prominence) of the lung after
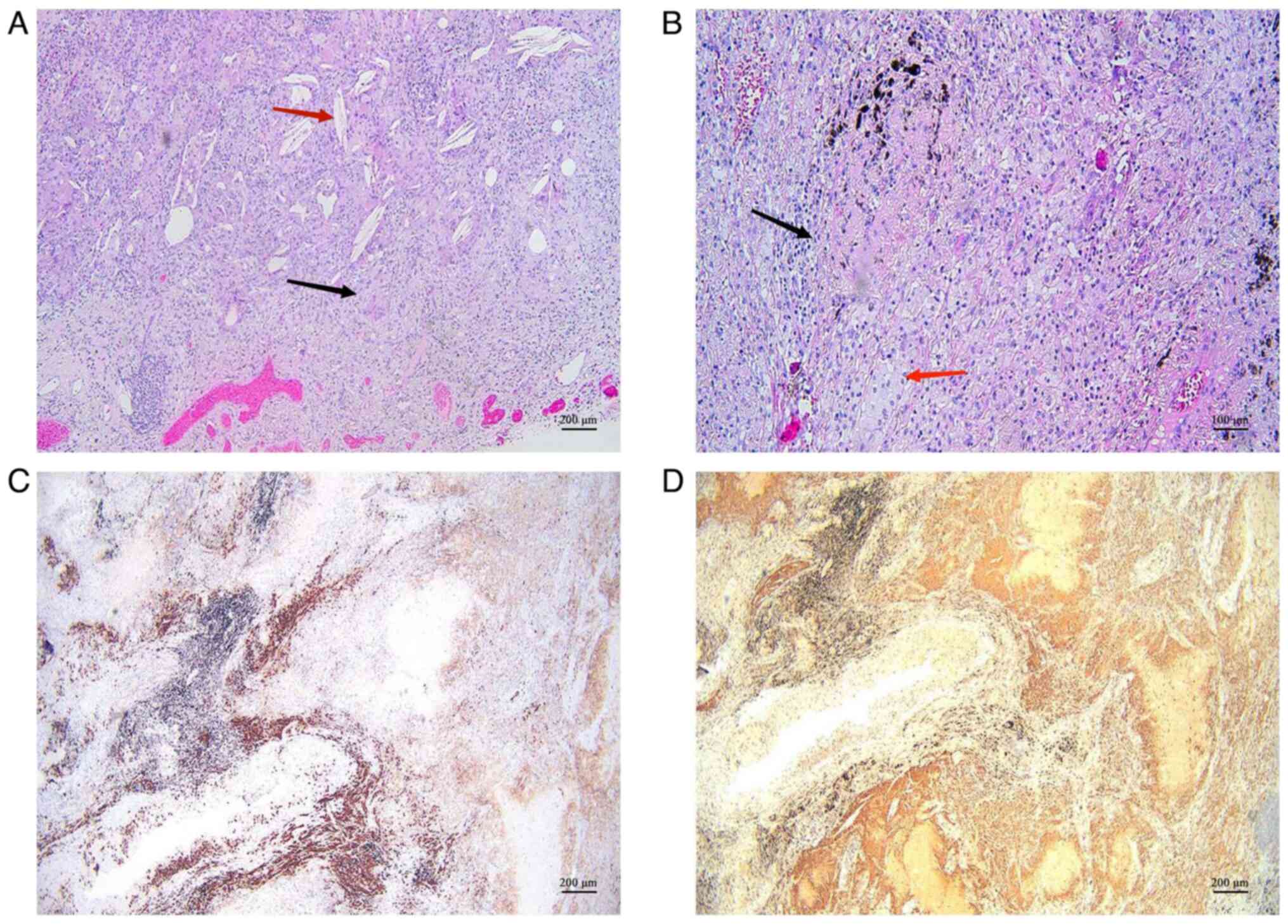
chemotherapy; no residual cancer observed in the tumor bed area.
Patchy necrosis, fibrosis, and extensive lymphoplasmacytic and
foamy histiocytic infiltration were noted. The localized presence
of cholesterol crystals and a multinucleated giant cell reaction
(~4×2.5 cm) were observed. Bronchial stumps (−) and visceral pleura
(−) were unaffected. Lymph nodes in groups 2, 4, 7, 9, 10 and 11
were uninvolved (0/1, 0/2, 0/12, 0/1, 0/3 and 0/6, respectively).
Immunohistochemistry results of the postoperative tissue showed
CD38 (+) and CD68 (+) expression, predominantly in plasma cells and
histiocytes, respectively. CD38 is a surface molecule expressed on
plasma cells, whereas CD68 is a reliable marker for macrophages.
These findings indicated the infiltration of plasma cells and
macrophages in the surgical tissue of the patient (Fig. 5). The patient had an uneventful
recovery and was discharged on postoperative day 7. In November
2022, 1 month post-surgery, the patient underwent two further
cycles of the pre-surgery chemotherapy and immunotherapy regimen.
Subsequently, the patient received maintenance therapy of
tislelizumab 200 mg every 21 days. The patient had grade II
myelosuppression during tislelizumab treatment, which was
controlled by subcutaneous administration of recombinant human
granulocyte growth factor and did not prevent the use of
tislelizumab. Initial follow-up results were encouraging; however,
long-term outcomes are yet to be assessed.
Discussion
Lung cancer is the second most prevalent type of
cancer and the leading cause of cancer-related mortality worldwide.
In 2020, ~1.8 million fatalities were attributed to lung cancer,
with NSCLC responsible for ~85% of these deaths (1,22).
Squamous lung cancer represents the most prevalent subtype of
NSCLC. The occurrence rate of common driver gene mutations, such as
EGFR mutations and ALK gene rearrangements, in lung squamous cell
carcinoma is generally low, ~2.7 and 1.5–2.5%, respectively
(23–25). Therefore, only a small proportion of
patients with squamous cell carcinoma have the opportunity to
receive treatment with EGFR-tyrosine kinase inhibitors or ALK
inhibitors. Chemotherapy continues to be the main treatment of
advanced lung squamous cell carcinoma. Furthermore, several trials
on the development of new targets for lung squamous cancer have
been terminated due to a lack of efficacy of the drugs or drug
toxicity (26,27), thus hampering the advancement of
targeted therapies for this disease (28). Immunotherapy has successfully
altered the clinical guidelines for advanced lung cancer, including
squamous lung cancer. Neoadjuvant immunotherapy, albeit still in
the early stages of development, may allow for enhanced activation
of antigenic immune activity, control of distant metastases and
reduction of disease recurrence, dependent on the preoperative
antigens of the patient, an intact lymphatic system and a robust
physical condition (29). Previous
research has demonstrated that during the initial phase of ICI
treatment, patients with NSCLC exhibit a substantial increase in
tumor-specific cytotoxic T cells in their blood; this is
accompanied by a significant increase in regulatory T cells, and a
decrease in natural killer cells and dendritic cells (30,31).
Initial findings from prior phase I/II studies have suggested that
the combination of immunotherapy and neoadjuvant chemotherapy can
yield major pathological response rates ranging from 50–80% in
resectable NSCLC (32). Table I provides a summary of several
neoadjuvant immunotherapy trials, for which data are currently
available.
 | Table I.Trials and results of several
neoadjuvant immunotherapies for which data are available. |
Table I.
Trials and results of several
neoadjuvant immunotherapies for which data are available.
| A,
Immunotherapy |
|---|
|
|---|
|
|
|
|
|
|
| R0 resection |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Trial | Phase | Patient
population | N | Treatment
regimen | Initial
findings | N | Rate, % | (Refs.) |
|---|
| CheckMate159 | II | Stage I–IIIA | 22 | Nivolumab | MPR 45% (9/20) | 20 | 100 | (9) |
|
|
| NSCLC |
|
|
|
|
|
|
| LCMC3 | II | Stage IB-IIIB | 181 | Atezolizumab | MPR 21%
(30/144); | 145 | 80.1 | (45) |
|
|
| (T3N2) |
|
| pCR 7%
(10/144) |
|
|
|
|
|
| NSCLC |
|
|
|
|
|
|
| ChiCTR-OIC- | I | Stage IA-IIIB | 49 | Sintilimab | MPR 40.5%
(15/37); | 37 | 92.5 | (46) |
| 17013726 |
| NSCLC |
|
| pCR 16.2%
(6/37) |
|
|
|
|
| B, Immunotherapy
+ ChT |
|
|
|
|
|
|
|
| R0
resection |
|
|
|
|
|
|
|
|
|
|
| Trial | Phase | Patient
population | N | Treatment
regimen | Initial
findings | N | Rate, % | (Refs.) |
|
| NCT02987998 | I | Stage IIIA | 9 | ChT + | pCR 67% (4/6) | 6 | 66.7 | (31) |
|
|
| NSCLC |
| pembrolizumab |
|
|
|
|
| NCT04326153 | II | Stage
IIIA/IIIB | 20 | Sintilimab +
ChT | MPR 62.5%
(10/16); | 16 | 80 | (47) |
|
|
| NSCLC |
|
| pCR 31.25%
(5/16) |
|
|
|
| NADIM | II | Stage IIIA
(N2) | 46 | ChT +
nivolumab | MPR 83%; pCR
63% | 41 | 89.1 | (48) |
|
|
| NSCL |
|
|
|
|
|
|
| NCT02716038 | II | Stage IB-IIIA | 14 | ChT + | MPR 50%
(7/14); | 11 | 78.6 | (49) |
|
|
| NSCLC |
| atezolizumab | pCR 21% (3/14) |
|
|
|
| CheckMate 816 | III | Stage IB-IIIA | 358 | Arm A: ChT + | Arm A: MPR
36.9%; | Arm A: | Arm A: | (32) |
|
|
| NSCLC |
| nivolumab | pCR 24% Arm B: | 124 | Arm B: |
|
|
|
|
|
| Arm B: ChT | MPR 8.9%; | Arm B: | 83 |
|
|
|
|
|
|
| pCR 2.2% | 105 | 75 |
|
| SAKK16/14 | II | Stage IIIIA | 67 | Docetaxel | MPR 62%; | 55 | 82 | (50) |
|
|
| NSCLC |
| combined with | pCR 18% |
|
|
|
|
|
|
|
| cisplatin + |
|
|
|
|
|
|
|
|
| durvalumab |
|
|
|
|
|
| C, Double
Immunotherapy |
|
|
|
|
|
|
|
| R0
resection |
|
|
|
|
|
|
|
|
|
|
| Trial | Phase | Patient
population | N | Treatment
regimen | Initial
findings | N | Rate, % | (Refs.) |
|
| NEOSTAR | II | Stage I–IIIA | 44 | Arm A:
Nivolumab | Arm A: MPR
22%; | Arm A: | Arm A: | (15) |
|
|
| NSCLC |
| arm Arm B: | pCR 9% | 21 | 24 |
|
|
|
|
|
| Nivolumab+ | Arm B: MPR
38%; | Arm B: | Arm B: |
|
|
|
|
|
| ipilimumab | pCR 29% | 16 | 50 |
|
In recent years, the adverse impacts of coronavirus
disease 2019 have limited patient access to care, and subsequently,
the diagnosis and treatment of lung cancer. This has led to an
increase in patients presenting with unresectable NSCLC and
advanced lung cancer (33). NSCLC
is a complex disease with diverse clinical treatment modalities,
including surgery, chemotherapy, radiation therapy, targeted
therapy and immunotherapy. The selection of treatment strategies is
closely related to tumor staging. For patients with stage IIIA or
IIIB NSCLC without N2 lymph node metastasis, surgical intervention
is the preferred approach; however, for patients with positive N2
lymph node metastasis, surgical resection can still be considered
following chemotherapy or radiation therapy (34); however, complete microsurgical
resection (R0) combined with adjuvant chemotherapy only enhances
survival by ~5% (35). Given the
demonstrated benefits of immunotherapy in lung cancer, the
preferred treatment for temporarily unresectable but potentially
resectable patients now lies in surgical intervention following
tumor load reduction via neoadjuvant immunotherapy. However,
current trials of neoadjuvant immunotherapy combined with
chemotherapy for NSCLC have included only a limited number of
patients with stage IIIB cancer.
The present case details a patient with stage IIIB
squamous cell carcinoma of the lung, characterized by considerable
hilar lymph node enlargement. In this scenario, complete
microsurgical resection (R0) may not be feasible and a direct
surgical approach might not yield optimal long-term survival
benefits. After MDT discussions, and considering the successful
application and good accessibility of tislelizumab, the present
case employed a neoadjuvant regimen of tislelizumab in combination
with liposomal paclitaxel and carboplatin in a patient with stage
IIIB squamous cell NSCLC. Despite preoperative genetic testing
yielding negative results for PD-L1 expression, the patient
achieved a favorable outcome. The procedure was successful and
pathological complete response was attained postoperatively.
Tislelizumab is an innovative anti-programmed death-1 (PD-1)
antibody independently developed by BeiGene (17). The Fc segment of tislelizumab has
been engineered to diminish antibody-dependent cellular
phagocytosis, T-cell depletion and the potential risk associated
with resistance to anti-PD-1 therapy. The RATIONALE307 study
(36), which was announced at the
2020 American Society of Clinical Oncology conference, confirmed
the effectiveness and safety of tislelizumab in combination with
either paclitaxel + carboplatin or albumin-bound paclitaxel +
carboplatin, compared with paclitaxel + carboplatin alone, as
first-line treatment for advanced squamous NSCLC. Based on the
excellent data from the RATIONALE307 study, tislelizumab officially
received approval from the NMPA on January 12, 2021, for use as
first-line treatment for advanced squamous NSCLC (20); this treatment was revealed to
significantly prolong progression-free survival in patients.
Relevant investigations regarding the perioperative use of
tislelizumab in NSCLC are currently in progress. The RATIONALE 315
(NCT04379635) study (37), which
holds the distinction of being the largest perioperative phase III
clinical study involving a predominantly Chinese NSCLC patient
population, is actively comparing tislelizumab (or placebo) in
tandem with platinum-based doublet chemotherapy. This is employed
as a neoadjuvant therapy in patients exhibiting resectable stage II
or IIIA NSCLC. In May 2023, it was officially announced that the
study yielded positive results, with specific data yet to be
released. However, it is noteworthy that patients with stage IIIB
lung squamous carcinoma were not included in the study.
It is widely acknowledged that the expression level
of PD-L1 serves as a critical biomarker for predicting the
effectiveness of PD-1 inhibitors. Generally, enhanced PD-L1
expression is considered to correspond to greater efficacy of PD-1
inhibitors; however, emerging evidence in recent years has
suggested that the association between the effectiveness of
immunotherapy and PD-L1 expression in patients with lung squamous
carcinoma is not as robust as in those with non-squamous carcinoma
(38). The following potential
reasons have been suggested: i) The inherent bias of the assay may
be a factor, owing to the absence of a uniform standard for the
detection of PD-L1 expression; ii) biological attributes of PD-L1
itself could contribute, such as the non-uniform distribution of
PD-L1 within the tumor; iii) the instability of PD-L1 expression in
tumor tissues may serve a role. PD-L1 expression can be influenced
by various molecular signals and may change dynamically.
Consequently, the PD-L1 expression level determined during a
particular sampling may not accurately represent the overall PD-L1
expression level within tumor tissues. Additionally, the efficacy
of immunotherapy is deeply intertwined with the molecular
pathological characteristics of tumors. Lung squamous cell
carcinoma, being a highly mutated and immunogenic type of cancer,
tends to exhibit a reduced dependency on PD-L1 expression (39). In the present case report, a
pathological assessment of the patient's postoperative specimen
revealed that the tissue, after neoadjuvant immunotherapy combined
with chemotherapy treatment, exhibited patchy necrosis, fibrosis,
and significant lymphoplasmacytic and foamy histiocytic
infiltration. Furthermore, local observations of cholesterol
crystals and multinucleated giant cell reactions were noted. These
findings are consistent with the immune-related pathological
response characteristics reported in a previous study (40). The emergence of this response is
primarily related to the mechanism of action of immunotherapeutic
drugs (41). Neoadjuvant
immunotherapy kills tumor cells indirectly by activating
tumor-specific T cells, and the stroma that provides nutrients to
tumor cells is destroyed, thus leading to the sudden death of the
entire tumor cell nest; subsequently, tumor cell debris is rapidly
phagocytosed by macrophages to form granulomas, and consequently, a
lower proportion of necrosis is pathologically evaluated after
neoadjuvant chemotherapy alone (29).
With the continuous emergence of immunotherapy
resistance, determining the interaction between immunotherapy and
the tumor microenvironment may represent a critical breakthrough to
address tumor resistance to immunotherapy. Recently, Hu et
al (42) performed single-cell
sequencing of primary tumor samples from 15 patients with stage
IIIA NSCLC, taken before and after neoadjuvant immunotherapy, in
order to characterize alterations in the tumor microenvironment
during the course of immunotherapy. The study suggested that the
presence of FCRL4+ FCRL5+ B cells,
CD16+ CX3CR1+ monocytes and alterations in
plasma estrogen signatures could serve as novel biomarkers. These
findings were further corroborated through validation with
independent and publicly available transcriptome data, providing
valuable insights for subsequent studies focusing on the
interaction between immunotherapy and the tumor microenvironment.
According to the imaging assessment, the patient still had lesions
after two cycles of neoadjuvant therapy. However, postoperative
pathological findings showed complete remission of the tumor and
lymph nodes. These results suggested that the conventional criteria
(The Response Evaluation Criteria In Solid Tumors) used to evaluate
the efficacy of cytotoxic chemotherapy might not be applicable to
ICIs (43). Previous research has
suggested that pronounced fibrosis, which is typically induced by
an effective immunotherapy response, may potentially complicate
surgical procedures (44). This
can, in some instances, necessitate a transition from thoracoscopy
to a more invasive thoracotomy (44); however, such a scenario did not
transpire in the current case report. It is important to note that
the follow-up period for this case has been relatively brief,
necessitating an extended follow-up duration to accurately evaluate
long-term efficacy. This finding presents a contrast to previous
studies that have suggested PD-L1 positivity as a potential
predictor of a favorable response to immunotherapy. Therefore, it
is necessary to continue to identify specific predictors of
response to neoadjuvant immunotherapy.
In conclusion, the present case report indicated
that using tislelizumab in tandem with chemotherapy as a
neoadjuvant treatment may serve as an effective therapeutic
strategy for patients with stage IIIB lung squamous cell carcinoma
who are PD-L1-negative. However, this conclusion warrants further
validation through an expanded study with a larger sample size to
provide more robust evidence.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GHC and DQ are the primary physicians who performed
the diagnosis and treatment of the patient. GHC, YB, XKS and YJL
collected and analyzed clinical data. YJL collected and processed
the images. GHC and YY wrote the manuscript. DQ and YY conceived
and designed the study. GHC, DQ and YY confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of their
clinical details and clinical images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 2.2021. J Natl Compr Canc Netw. 19:254–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NSCLC Meta-analyses Collaborative Group, .
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le
Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, et al: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NSCLC Meta-analysis Collaborative Group, :
Preoperative chemotherapy for non-small-cell lung cancer: A
systematic review and meta-analysis of individual participant data.
Lancet. 383:1561–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft
A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, et al:
Durvalumab with or without tremelimumab vs standard chemotherapy in
first-line treatment of metastatic non-small cell lung cancer: The
MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 6:661–674.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forde PM, Chaft JE, Smith KN, Anagnostou
V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR,
Broderick S, et al: Neoadjuvant PD-1 blockade in resectable lung
cancer. N Engl J Med. 378:1976–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al:
Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell
lung cancer (NADIM): An open-label, multicentre, single-arm, phase
2 trial. Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eichhorn F, Klotz LV, Kriegsmann M,
Bischoff H, Schneider MA, Muley T, Kriegsmann K, Haberkorn U,
Heussel CP, Savai R, et al: Neoadjuvant anti-programmed death-1
immunotherapy by pembrolizumab in resectable non-small cell lung
cancer: First clinical experience. Lung Cancer. 153:150–157. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong BC, Gu L, Wang X, Wigle DA, Phillips
JD, Harpole DH Jr, Klapper JA, Sporn T, Ready NE and D'Amico TA:
Perioperative outcomes of pulmonary resection after neoadjuvant
pembrolizumab in patients with non-small cell lung cancer. J Thorac
Cardiovasc Surg. 163:427–436. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schuchert MJ, Normolle DP, Awais O,
Pennathur A, Wilson DO, Luketich JD and Landreneau RJ: Factors
influencing recurrence following anatomic lung resection for
clinical stage I non-small cell lung cancer. Lung Cancer.
128:145–151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
William WN Jr, Pataer A, Kalhor N, Correa
AM, Rice DC, Wistuba II, Heymach J, Lee JJ, Kim ES, Munden R, et
al: Computed tomography RECIST assessment of histopathologic
response and prediction of survival in patients with resectable
non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac
Oncol. 8:222–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong
W, Zhang Y, Zhou X, Wang Z, Wang Y, et al: The binding of an
anti-PD-1 antibody to FcγRI has a profound impact on its biological
functions. Cancer Immunol Immunother. 67:1079–1090. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee A and Keam SJ: Tislelizumab: First
approval. Drugs. 80:617–624. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma
Z, Li X, Zhuang W, Liu Y, et al: Tislelizumab plus chemotherapy as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol.
16:1512–1522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z,
Zhao J, Yu Y, Hu C, Yang K, et al: Tislelizumab plus chemotherapy
vs chemotherapy alone as first-line treatment for advanced squamous
non-small-cell lung cancer: A phase 3 randomized clinical trial.
JAMA Oncol. 7:709–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kutob L and Schneider F: Lung cancer
staging. Surg Pathol Clin. 13:57–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Shen Q, Shi Q, Yu B, Wang X, Cheng
K, Lu G and Zhou X: Detection of ALK protein expression in lung
squamous cell carcinomas by immunohistochemistry. J Exp Clin Cancer
Res. 33:1092014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forbes SA, Bhamra G, Bamford S, Dawson E,
Kok C, Clements J, Menzies A, Teague JW, Futreal PA and Stratton
MR: The catalogue of somatic mutations in cancer (COSMIC). Curr
Protoc Hum Genet Chapter 10. Unit 10.11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei Y, Lei Y, Shi X and Wang J: EML4-ALK
fusion gene in non-small cell lung cancer. Oncol Lett. 24:2772022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chae YK, Hong F, Vaklavas C, Cheng HH,
Hammerman P, Mitchell EP, Zwiebel JA, Ivy SP, Gray RJ, Li S, et al:
Phase II study of AZD4547 in patients with tumors harboring
aberrations in the FGFR pathway: results from the NCI-MATCH trial
(EAY131) subprotocol W. J Clin Oncol. 38:2407–2417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brunner AM, Costa DB, Heist RS, Garcia E,
Lindeman NI, Sholl LM, Oxnard GR, Johnson BE and Hammerman PS:
Treatment-related toxicities in a phase II trial of dasatinib in
patients with squamous cell carcinoma of the lung. J Thorac Oncol.
8:1434–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thatcher N, Hirsch FR, Luft AV, Szczesna
A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy
G, et al: Necitumumab plus gemcitabine and cisplatin versus
gemcitabine and cisplatin alone as first-line therapy in patients
with stage IV squamous non-small-cell lung cancer (SQUIRE): An
open-label, randomised, controlled phase 3 trial. Lancet Oncol.
16:763–774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Topalian SL, Taube JM and Pardoll DM:
Neoadjuvant checkpoint blockade for cancer immunotherapy. Science.
367:eaax01822020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Broderick SR and Bott MJ: Neoadjuvant
immunotherapy in patients with resectable non-small cell lung
cancer. J Thorac Cardiovasc Surg. 158:1471–1474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lavin Y, Kobayashi S, Leader A, Amir ED,
Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH,
et al: Innate immune landscape in early lung adenocarcinoma by
paired single-cell analyses. Cell. 169:750–765.e17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Williams PA, Zaidi SK and Sengupta R: AACR
report on the impact of COVID-19 on cancer research and patient
care. Clin Cancer Res. 28:609–610. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN guidelines® insights: Non-small cell lung
cancer, version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arriagada R, Dunant A, Pignon JP, Bergman
B, Chabowski M, Grunenwald D, Kozlowski M, Le Péchoux C, Pirker R,
Pinel MI, et al: Long-term results of the international adjuvant
lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy
in resected lung cancer. J Clin Oncol. 28:35–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Yu X, Lu S, Hu Y, Sun Y, Wang Z,
Zhao J, Yu Y, Hu C, Yang K, et al: Phase III study of tislelizumab
plus chemotherapy vs chemotherapy alone as first-line (1L)
treatment for advanced squamous non-small cell lung cancer (sq
NSCLC). J Clin Oncol. 38 (Suppl):S95542020. View Article : Google Scholar
|
|
37
|
BeiGene, . Comparing the efficacy and
safety of a new additional treatment with tislelizumab in non-small
cell lung cancer (NSCLC). U.S. National Library of Medicine; 2023,
Available from:. https://clinicaltrials.gov/ct2/show/NCT04379635
|
|
38
|
Hu X, Hu C, Liu X, Ma F, Xie J, Zhong P,
Tang C, Fan D, Gao Y, Feng X, et al: Tumor regression rate, PD-L1
expression, pembrolizumab/nab-paclitaxel-based regimens, squamous
cell carcinoma, and comorbidities were independently associated
with efficacy of neoadjuvant chemoimmunotherapy in non-small cell
lung cancer. Front Oncol. 12:10576462023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
La Fleur L, Falk-Sörqvist E, Smeds P,
Berglund A, Sundström M, Mattsson JS, Brandén E, Koyi H, Isaksson
J, Brunnström H, et al: Mutation patterns in a population-based
non-small cell lung cancer cohort and prognostic impact of
concomitant mutations in KRAS and TP53 or STK11. Lung Cancer.
130:50–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Travis WD, Dacic S, Sholl LM and Wistuba
II: Pathologic assessment of lung squamous cell carcinoma after
neoadjuvant immunotherapy. J Thorac Oncol. 16:e9–e10. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Szeto GL and Finley SD: Integrative
approaches to cancer immunotherapy. Trends Cancer. 5:400–410. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu J, Zhang L, Xia H, Yan Y, Zhu X, Sun F,
Sun L, Li S, Li D, Wang J, et al: Tumor microenvironment remodeling
after neoadjuvant immunotherapy in non-small cell lung cancer
revealed by single-cell RNA sequencing. Genome Med. 15:142023.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bott MJ, Yang SC, Park BJ, Adusumilli PS,
Rusch VW, Isbell JM, Downey RJ, Brahmer JR, Battafarano R, Bush E,
et al: Initial results of pulmonary resection after neoadjuvant
nivolumab in patients with resectable non-small cell lung cancer. J
Thorac Cardiovasc Surg. 158:269–276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee J, Chaft J, Nicholas A, Patterson G,
Waqar S, Toloza E, Haura E, Raz D, Reckamp K, Merritt R, et al:
P2.04–88 surgical outcomes of a multicenter phase II trial of
neoadjuvant atezolizumab in resectable stages Ib-IIIb NSCLC: Update
on LCMC3 clinical trial. J Thorac Oncol. 14 (Suppl):S7442019.
View Article : Google Scholar
|
|
46
|
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S,
Tao X, Zhao J, Mao Y, Wang B, et al: Neoadjuvant PD-1 inhibitor
(Sintilimab) in NSCLC. J Thorac Oncol. 15:816–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun C, Liu Y, Zhang P, Wang X, Xu Y, Lin
X, Ma X, Guo Y, Qiu S, Shao G, et al: Interim analysis of the
efficiency and safety of neoadjuvant PD-1 inhibitor (sintilimab)
combined with chemotherapy (nab-paclitaxel and carboplatin) in
potentially resectable stage IIIA/IIIB non-small cell lung cancer:
A single-arm, phase 2 trial. J Cancer Res Clin Oncol. 149:819–831.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Provencio-Pulla M, Nadal-Alforja E, Cobo
M, Insa A, Costa Rivas M, Majem M, Rodriguez-Abreu D, Lopez-Vivanco
G, Domine M, Del Barco Morillo E, et al: Neoadjuvant
chemo/immunotherapy for the treatment of stages IIIA resectable
non-small cell lung cancer (NSCLC): A phase II multicenter
exploratory study-NADIM study-SLCG. J Clin Oncol. 36
(Suppl):S85212018. View Article : Google Scholar
|
|
49
|
Shu CA, Gainor JF, Awad MM, Chiuzan C,
Grigg CM, Pabani A, Garofano RF, Stoopler MB, Cheng SK, White A, et
al: Neoadjuvant atezolizumab and chemotherapy in patients with
resectable non-small-cell lung cancer: An open-label, multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:786–795. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Goldstraw P, Ball D, Jett JR, Le Chevalier
T, Lim E, Nicholson AG and Shepherd FA: Non-small-cell lung cancer.
Lancet. 378:1727–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|