Introduction
Renal cell carcinoma (RCC) is a prominent tumor
within the urinary system; it accounts for ~2% of global cancer
diagnoses and deaths, and is projected to increase in burden
worldwide (1). Clear cell RCC
(ccRCC) is the predominant subtype of RCC. Despite recent advances
in treating advanced and metastatic ccRCC, the 5-year survival rate
of metastatic ccRCC is <10% (2).
Surgical resection is currently the main treatment option for
ccRCC; however, it has been reported that 30–40% of patients with
local lesions experience post-surgery recurrence (3). Despite gradual improvements in immune
and targeted therapies, these approaches have failed to achieve
desirable progression-free survival in patients with ccRCC.
Moreover, subsequent treatments for recurrent ccRCC have yielded
suboptimal outcomes (4). Therefore,
exploring the mechanisms underlying ccRCC development, and
identifying highly sensitive and specific tumor biomarkers have
emerged as current research trends.
Recent studies have highlighted the role of specific
transcription factor families in the malignant progression of ccRCC
(5,6). Within these families, the E-twenty-six
(ETS) transcription factor family serves major roles in
tumorigenesis, including that of ccRCC, with some members
functioning as oncogenes and others as tumor suppressors (7). Among the ETS family, various E47-like
factors (ELFs) influence the biological activity of ccRCC cells
through transcriptional regulation. For example, ELF1 exhibits
bidirectional suppression of the tumor suppressor TSC2 and the
repair-related gene NTH1 (8).
Additionally, ELF2 has been reported to promote ccRCC cell
proliferation by mediating the transcription of c-Myc-induced ELF2
regulator (9). However, the
molecular mechanisms underlying the carcinogenic or
tumor-suppressive effects of these ELFs in ccRCC remain poorly
understood.
Previous reports have highlighted the association of
various ELFs with malignant progression, prognosis and infiltration
in numerous types of cancer. For example, ELF1, which has been
identified as a carcinogen, has been observed to regulate the cell
proliferation of multiple types of cancer, including prostate and
lung cancer (7,10). ELF4 has been implicated in the
malignant progression of gastric cancer by regulating CDX2
(11). In tumor prognosis research,
ELF4 expression has emerged as an independent predictor of poor
prognosis in colorectal cancer (12). Furthermore, ELF5 expression levels
have also been linked to the survival and prognosis of patients
with epithelial ovarian cancer (13). As immunotherapy gains wider
application, research on the regulatory mechanisms of ELFs in the
tumor immune microenvironment have gained attention. For example, a
decrease in T-cell receptor (TCR) ζ chain transcription factor ELF1
and its binding to DNA may contribute to reduced or absent TCR ζ
chain transcripts in tumor-infiltrating lymphocytes (14). In breast cancer, ELF5 has been
identified as a key transcriptional determinant of tumor subtype
and increased levels of ELF5 have been associated with enhanced
leukocyte infiltration (15).
Despite the significant roles played by ELFs in other cancer types,
their specific functions and related mechanisms in ccRCC remain
unclear.
In the present study, a comprehensive analysis of
ELF1-5 in ccRCC was conducted using multiple databases and the
clinical significance of ELF3-5 was confirmed in patients with
ccRCC. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) enrichment analyses were also performed. Notably,
the effects of ELF4 on the proliferation, migration and invasion of
ccRCC cells were assessed, as were its effects on macrophage
polarization and chemotaxis. The present study is expected to
reveal the clinical significance, biological activity and immune
infiltration of ELF4, in order to identify a potential new target
for patients with ccRCC.
Materials and methods
Analysis of differentially expressed
genes in multiple databases
The Gene Expression Profiling Interactive Analysis
(GEPIA) database (http://gepia.cancer-pku.cn/) was used to analyze
ELF1-5 expression data (16). GEPIA
is a newly developed interactive web server for analyzing the RNA
sequencing expression data from 9,736 tumor tissues and 8,587
normal tissues of patients obtained from The Cancer Genome Atlas
(TCGA) and The Genotype-Tissue Expression projects (16). For Gene Expression Omnibus (GEO)
analysis, raw sequencing data were obtained from the GEO database
(GEO accession: GSE53757) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE53757)
(17). Differential expression
analysis and gene expression data normalization were performed
using the R package edgeR (18).
The differential expression levels of ELF1-5 in ccRCC and normal
tissues were also illustrated using UALCAN (http://ualcan.path.uab.edu) (19). UALCAN is a comprehensive,
user-friendly web resource for analyzing cancer omics data in TCGA
project. The differential expression levels of ELF1-5 in various
cancer types and normal tissues were illustrated using the Oncomine
database (https://www.oncomine.org) (20). Oncomine, the largest cancer gene
chip database and integrated data mining platform is designed to
extract valuable cancer gene information. The threshold parameters
of P-value and fold-change were demarcated as 0.05 and 2,
respectively.
Clinical significance analysis
The expression levels and promoter methylation
levels of ELF3-5 were assessed in relation to cancer stage, subtype
and tumor grade using UALCAN (19).
The threshold parameters of P-value and fold-change were demarcated
as 0.05 and 2, respectively. The clinical significance of ELF3-5 on
the overall survival (OS) and disease-free survival (DFS) of
patients with ccRCC was evaluated using GEPIA. Kaplan-Meier
survival analysis and log-rank test were performed using GEPIA
database, and log-rank P-values and hazard ratio (HR) values were
obtained (16). A log-rank test
with P<0.05 was considered to indicate a statistically
significant difference.
cBioPortal analysis
Genetic alterations of ELF3-5 were obtained and
analyzed from the cBioPortal based on TCGA project (11). As a comprehensive web resource, the
cBioPortal database (http://www.cbioportal.org) is used for visualizing and
analyzing multidimensional cancer genomics data.
GO and KEGG enrichment analysis
The LinkedOmics (https://www.linkedomics.org/login.php) database was
used to search for ELF3-5-related co-expressed genes in ccRCC
(21). LinkedOmics is a publicly
accessible portal integrating multi-omics data from all 32 TCGA
cancer types and 10 Clinical Proteomics Tumor Analysis Consortium
cancer cohorts. It is a valuable platform for biologists and
clinicians to access, analyze and compare multi-omics data across
various tumor types. Co-expression analysis was performed using the
Pearson correlation coefficient as a statistical measure. GO
analysis and KEGG pathway enrichment analysis were conducted on the
ELF3-5-related co-expressed genes using the LinkInterpreter module
of LinkedOmics to obtain descriptive information. The Gene Set
Enrichment Analysis tool (https://www.linkedomics.org/lo_batchfile/qindex_gsea.php?fn=122773)
was employed to explore the functional network of co-expressed
genes, including GO (biological process, cellular component,
molecular function) and KEGG pathway analyses. The rank criterion
for significance was set at a false-discovery rate <0.05 and
1,000 simulations were performed.
Tumor immune estimation resource
(TIMER) analysis
The TIMER web server (https://cistrome.shinyapps.io/timer/) is a
comprehensive resource for analyzing immune infiltrates in various
cancer types (22). The gene module
of TIMER allows users to select any gene of interest and visualize
the correlation of its expression with immune infiltration level in
diverse cancer types. The partial Spearman's correlation analysis
was performed to determine the relationship between the
RNA-sequencing expression profiles of ELF3-5 in ccRCC and immune
cells.
Cell culture and transfection
The 786-O and 769-P ccRCC cell lines, and the HK-2
normal human renal tubular epithelial cell line were purchased from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. 769-P and 786-O cells were cultured in RPMI-1640 medium
(cat. no. C11875500BT; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% heat-inactivated fetal bovine serum (FBS; cat. no.
16140089; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (cat. no. P4333; MilliporeSigma). HK-2
cells were cultured in minimum Eagle's medium (cat. no. SH30244.01;
Hyclone; Cytiva) containing 10% FBS. The THP-1 human monocytic
leukemia cell line was also purchased from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences and were
cultured in RPMI-1640 containing 10% FBS and 1%
penicillin-streptomycin. THP-1 cells were differentiated into
macrophages by treating them with 10 ng/ml
phorbol-12-myristate-13-acetate (PMA; cat. no. P8139;
MilliporeSigma) for 24 h at 37°C. All cells were cultured at 37°C
in a humidified atmosphere with 5% CO2.
The small interfering RNA (siRNA) constructs
targeting ELF4 (si-ELF4) and the corresponding negative control
(si-NC) were purchased from Guangzhou RiboBio Co., Ltd. 769-P and
786-O cells were seeded into 6-well plates at 1×106
cells/well. According to the manufacturer's instructions, the
transfection of the aforementioned siRNAs into 769-P and 786-O
cells was performed using Lipofectamine® 2000
transfection reagent (cat. no. 11668030; Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h at 37°C. Approximately 48 h
post-transfection, cells were collected for further studies. The
siRNAs were used at a concentration of 100 nM and the sequences
were as follows: si-ELF4 sense, 5′-GCUGGACGACGUUCACAAUTT-3′ and
antisense, 5′-AUUGUGAACGUCGUCCAGCTT-3′; si-NC sense,
5′-AUCAACGAUAUCCGGUUGG-3′ TT and antisense,
5′-CCAACCGGAUAUCGUUGAUTT-3′.
Macrophage polarization assay
The macrophage polarization experiment was performed
as previously described (23).
Si-NC and si-ELF4 groups of 769-P and 786-O cells were seeded at
1×106 cells/ml in 6-well plates (3 ml/well). The
supernatant of ccRCC cells was collected. PMA-induced THP-1 cells
(macrophages) were seeded at 1×106 cells/ml in 6-well
plates (3 ml/well) in RPMI-1640 medium containing ccRCC cell
supernatant and were incubated for 48 h. The mRNA expression levels
of the M1 macrophage markers (IL-6, CXCL10 and CD80) and M2
macrophage markers (CD206, fibronectin and CCL22) were determined
to study the effects of ccRCC cell supernatant on polarization of
macrophages.
Macrophage chemotaxis assay
A chemotaxis assay was performed as previously
described (24,25). Briefly, PMA-induced THP-1 cells
(macrophages) were incubated with IL-4 and IL-13 (20 ng/ml IL-4 and
IL-13; cat. nos. 6507IL and 213ILB; R&D Systems, Inc.) for 48 h
at 37°C to obtain M2 macrophages. Similarly, PMA-induced THP-1
cells (macrophages) were incubated with lipopolysaccharide (100
ng/ml; cat. no. L2880; MilliporeSigma) and IFN-γ (20 ng/ml; cat.
no. 285-IF; R&D Systems, Inc.) for 48 h at 37°C to obtain M1
macrophages. The supernatant of 769-P and 786-O cells (400 µl) was
added to the lower compartment of 6 Transwell inserts (pore size, 3
µm; cat. no. 3414; Corning, Inc.). M1 or M2 macrophages
(4×104 cells/well) were then overlaid onto the upper
chamber. After 16 h at 37°C, the migrated cells were counted using
a hemocytometer (cat. no. Z359629; MilliporeSigma).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® (cat. no. 15596026; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. RNA
purity (OD260/OD280 nm, 1.8–2.2) was assessed
using NanoDrop 2000 (NanoDrop; Thermo Fisher Scientific, Inc.). RT
was performed with 1 µg total RNA as the template using the
PrimeScript™ RT reagent Kit (cat. no. RR037Q; Takara Bio, Inc.)
according to the manufacturer's instructions. The relative mRNA
expression levels were determined using RT2 SYBR® Green
qPCR Mastermixes (cat. no. 330509; Qiagen GmbH) on the LightCycler
480 system (Roche Diagnostics). The reaction conditions included an
initial single cycle at 95°C for 10 min, followed by 45 cycles at
95°C for 15 sec and 95°C for 1 min. The following primer sets were
used for qPCR: ELF4 forward (F), 5′-CATCATAACAGACGGGACCTTG-3′,
reverse (R), 5′-GCTGGGAGACTCCATATTGAGTA-3′; GAPDH F,
5′-GAATGGGCAGCCGTTAGGAA-3′, R, 5′-AAAAGCATCACCCGGAGGAG-3′; IL-6 F,
5′-CCTGAACCTTCCAAAGATGGC-3′, R, 5′-CACCAGGCAAGTCTCCTCATT-3′; CXCL10
F, 5′-TGAATCCAGAATCGAAGGCCA-3′, R, 5′-TGCATCGATTTTGCTCCCCT-3′; CD80
F, 5′-ACGCCCTGTATAACAGTGTCC-3′, R, 5′-GAGGAAGTTCCCAGAAGAGGTC-3′;
CD206 F, 5′-GCTAAACCTACTCATGAATT-3′, R, 5′-GGCAAGGCCAGCACCCGTTA-3′;
fibronectin F, 5′-CCATCGCAAACCGCTGCCAT-3′, R,
5′-AACACTTCTCAGCTATGGGCTT-3′; CCL22 F, 5′-GAGATCTGTGCCGATCCCAG-3′,
R, 5′-AGGGAATGCAGAGAGTTGGC-3′; RPS9 F, 5′-CTGGATGAGGGCAAGATGAAG-3′,
R, 5′-GTCTGCAGGCGTCTCTCTAAGAA-3′. The relative mRNA expression
levels were normalized to the average Cq values of GAPDH plus RPS9,
and were quantified using the 2−ΔΔCq cycle threshold
method (26).
Cell counting Kit-8 (CCK-8) assay
Cell proliferation was assessed using the CCK-8
assay. Cancer cells were seeded in 96-well plates at
5×103 cells/well density. According to the
manufacturer's instructions, the cells were assessed at 0, 24, 48,
72 and 96 h using the CCK-8 Kit (10 µl/well; cat. no. ab228554;
Abcam). The plates were incubated in the dark for 1 h at 37°C. Cell
proliferation was measured using a microplate reader (cat. no.
168-1130; Bio-Rad Laboratories, Inc.) at 450 nm.
Colony formation assay
Approximately 48 h post-transfection, ccRCC cells
were cultured in 6-well plates at 2×103 cells/well and
the medium was changed every 3 days. The medium was aspirated once
cell colonies became visible to the naked eye. The cells were then
washed twice with 1×PBS and fixed with 4% paraformaldehyde (cat.
no. 158127; MilliporeSigma) for 15 min at room temperature.
Following the removal of paraformaldehyde, cells were stained with
0.25% crystal violet (cat. no. C6158; MilliporeSigma) at room
temperature for 25 min. Finally, the cells were washed with sterile
water, dried and images were captured under a light microscope. The
numbers of colonies with >50 cells were counted manually under a
light microscope.
Transwell assay
The Transwell assay was performed as previously
described (27). Briefly, cells
were suspended in FBS-free medium and 200 µl cell suspension
(1×105 cells/well) was inoculated into the upper layer
of 24 Transwell inserts (pore size, 8 µm; cat. no. 3422; Corning,
Inc.). The lower layer was filled with 600 µl complete medium
containing 10% FBS. For the invasion assay, Matrigel (cat. no.
356234; Corning, Inc.) was diluted to a concentration of 1 mg/ml
using FBS-free medium and was then added to the upper chamber of
the Transwell inserts and incubated at 37°C for 1 h. After 36 h of
incubation at 37°C, non-penetrating cells on the membrane were
removed using cotton swabs. The cells that passed through the
membrane were fixed with 4% paraformaldehyde for 30 min and stained
with 0.1% crystal violet for 20 min at room temperature.
Subsequently, cell counting was performed under a light microscope
(Olympus Corporation) at a magnification of ×100.
Statistical analysis
The experimental data are presented as the mean ±
standard deviation. Unpaired Student's t-test was used for
two-group comparisons. Statistical analyses involving multiple
group comparisons were performed using one-way ANOVA followed by
Tukey's post hoc test. Data analyses were conducted using GraphPad
Prism 8 (Dotmatics). The normality of data distribution was
assessed using the Shapiro-Wilk or Kolmogorov-Smirnov normality
test. Macrophage polarization, macrophage chemotaxis, RT-qPCR,
CCK-8, colony formation and Transwell assays were repeated three
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of ELF3-5 between
tumor and normal tissues
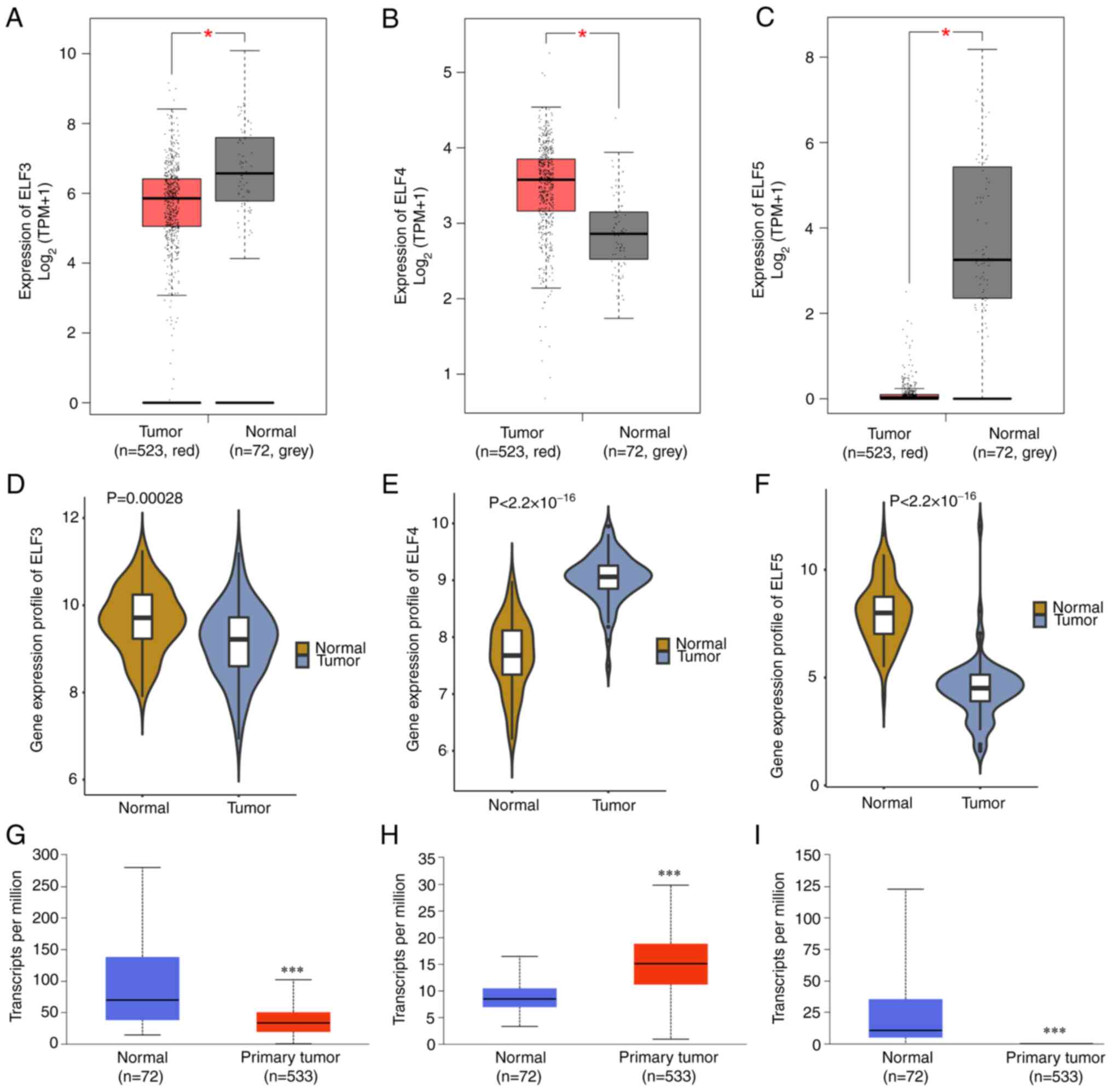
The present study investigated the function of five
key ELFs from the ETS family in ccRCC development. Differential
analysis was performed using GEPIA, GEO and UALCAN databases. All
three databases showed significantly higher expression levels of
ELF4 in ccRCC tissues compared with those in normal tissues
(Fig. 1B, E and H). Conversely, the
expression levels of ELF3 and ELF5 showed an opposite pattern
(Fig. 1A, C, D, F, G and I).
Furthermore, GEPIA and UALCAN databases indicated no significant
difference in the expression levels of ELF1 and 2 between ccRCC
cancer tissues and normal tissues (Fig. S1A, B, E and F). Additionally, the
expression profiles of ELF1-5 were analyzed in various cancer types
using Oncomine, revealing differential expression across multiple
types of cancer, including breast cancer, cholangiocarcinoma,
chromophobe RCC, thyroid cancer and endometrial cancer (Fig. S2). Specifically, ELF3 and ELF5
exhibited lower expression levels in ccRCC compared with those in
normal tissues (Fig. S2C and E).
ELF4 exhibited higher expression levels in ccRCC compared with in
normal tissues (Fig. S2D).
Consequently, ELF3-5 were identified as key genes in the present
study and further investigated for their functional relevance in
subsequent investigations.
Clinical significance of ELF3-5 in
ccRCC
DNA promoter methylation levels of ELF3 and ELF5
were significantly higher in ccRCC tissues compared with those in
normal tissues (Fig. 2A and C). DNA
promoter methylation level of ELF4 did not show a significant
difference (Fig. 2B). There was no
significant difference in the expression of ELF3-5 in patient
tissues at different cancer stages (Fig. 2D-F). By contrast, ELF3 and ELF4
expression exhibited differences depending on tumor grade (Fig. 2G and H). There was no significant
difference in the expression of ELF5 in patient tissues at
different tumor grades (Fig. 2I).
Additionally, ELF3-5 exhibited different expression levels in clear
cell type A (ccA) and B (ccB) subtypes (Fig. 2J-L). Moreover, genetic variations of
ELF3-5 were analyzed using the cBioPortal database, revealing
mutations, amplifications and deep deletions in these three genes
in some types of cancer (Fig.
S3A-C). Amplification variation existed in all three genes;
however, only ELF4 showed a high amplification variation in ccRCC,
exhibiting 1.27 and 0.02% incidence rates of amplification and
mutation, respectively (Fig. S3B).
Furthermore, the prognostic significance of ELF3-5 was investigated
in patients with ccRCC by assessing OS and DFS. The results
indicated no significant association between ELF3-5 expression and
patient survival (Fig. S4).
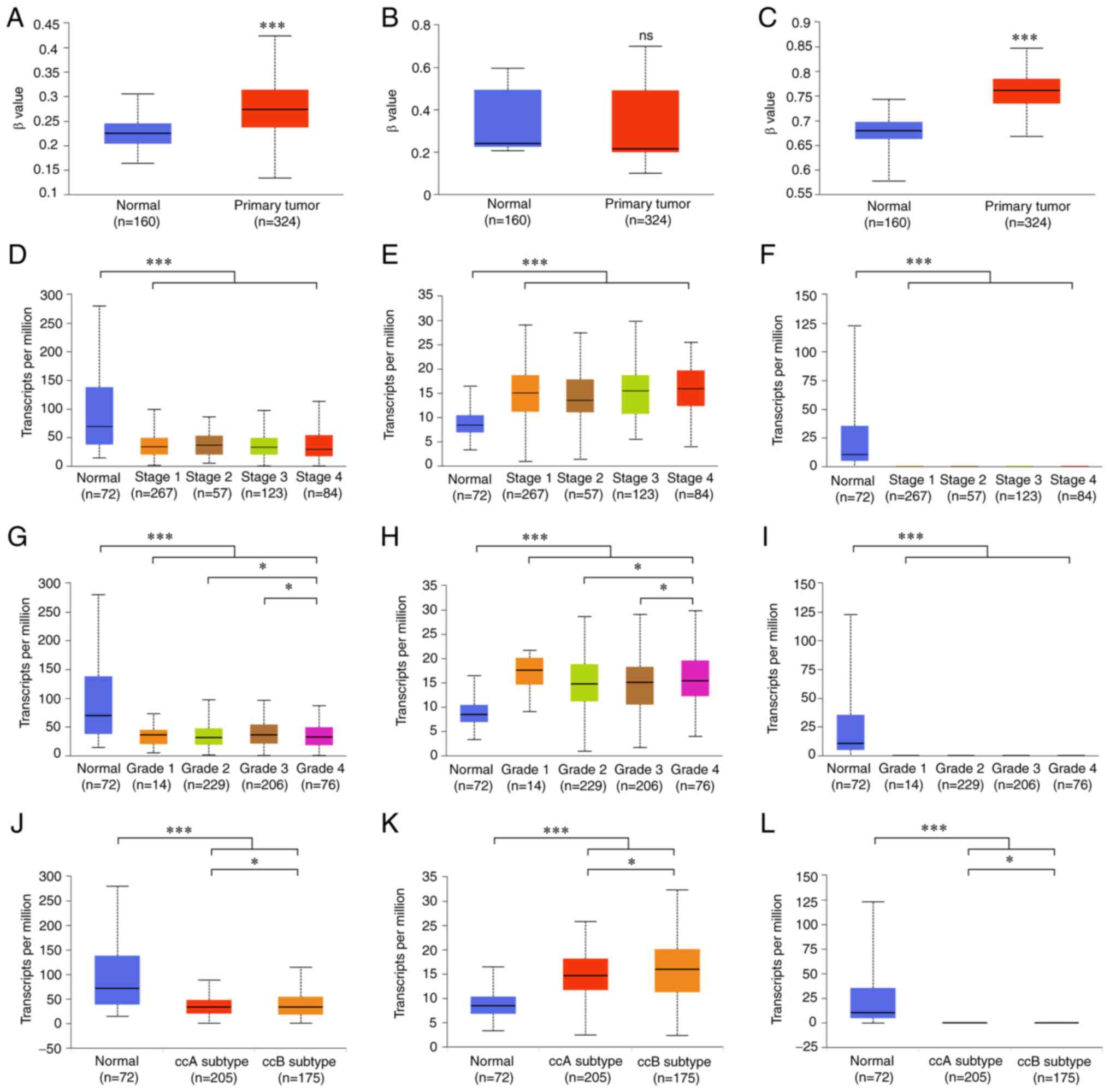 | Figure 2.Clinical significance of ELF3-5 in
ccRCC. Promoter methylation levels of (A) ELF3, (B) ELF4 and (C)
ELF5 in normal tissues and primary ccRCC tissues in the UALCAN
database. Expression levels of (D) ELF3, (E) ELF4 and (F) ELF5 in
ccRCC cancer tissues of various tumor stages. Expression levels of
(G) ELF3, (H) ELF4 and (I) ELF5 in ccRCC cancer tissues of various
tumor grades. Expression levels of (J) ELF3, (K) ELF4 and (L) ELF5
in ccRCC cancer tissues of ccA and ccB subtypes. ns, no
significance; *P<0.05, ***P<0.001. ELF, E47-like factor;
ccRCC, clear cell renal cell carcinoma; ccA, clear cell type A;
ccB, clear cell type B. |
Enrichment analysis of ELF3-5 in
ccRCC
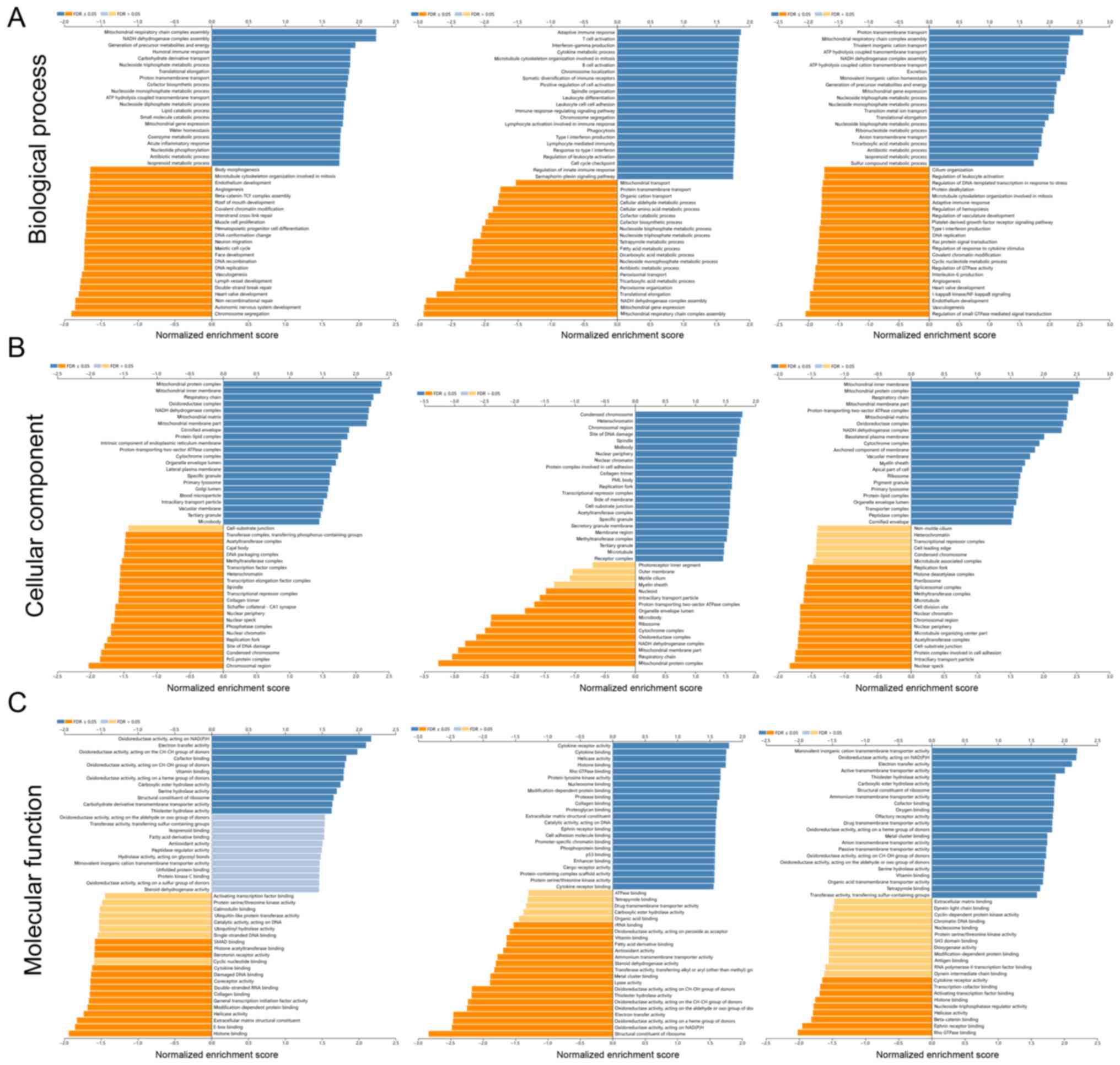
GO and KEGG enrichment analyses were performed to
investigate the potential functions and pathways associated with
the differential expression of ELF3-5. Among the enrichment
functions showing the strongest association with genes co-expressed
with ELF3, ‘mitochondrial respiratory chain complex’, ‘chromosome
segregation’, ‘oxidoreductase activity, acting on NAD(P)H’ and
‘histone binding’ were associated with tumorigenesis and tumor
progression (Fig. 3A-C). The
ELF4-related functions included ‘adaptive immune response’, ‘T cell
activation’, ‘mitochondrial respiratory chain complex assembly’,
‘mitochondrial protein complex’, and ‘cytokine receptor activity’,
which are associated with immune response and malignant progression
(Fig. 3A-C). The ELF5-related
functions included ‘proton transmembrane transport’, ‘regulation of
small GTPase-mediated signal transduction’, ‘mitochondrial inner
membrane’, and ‘nuclear speck’ (Fig.
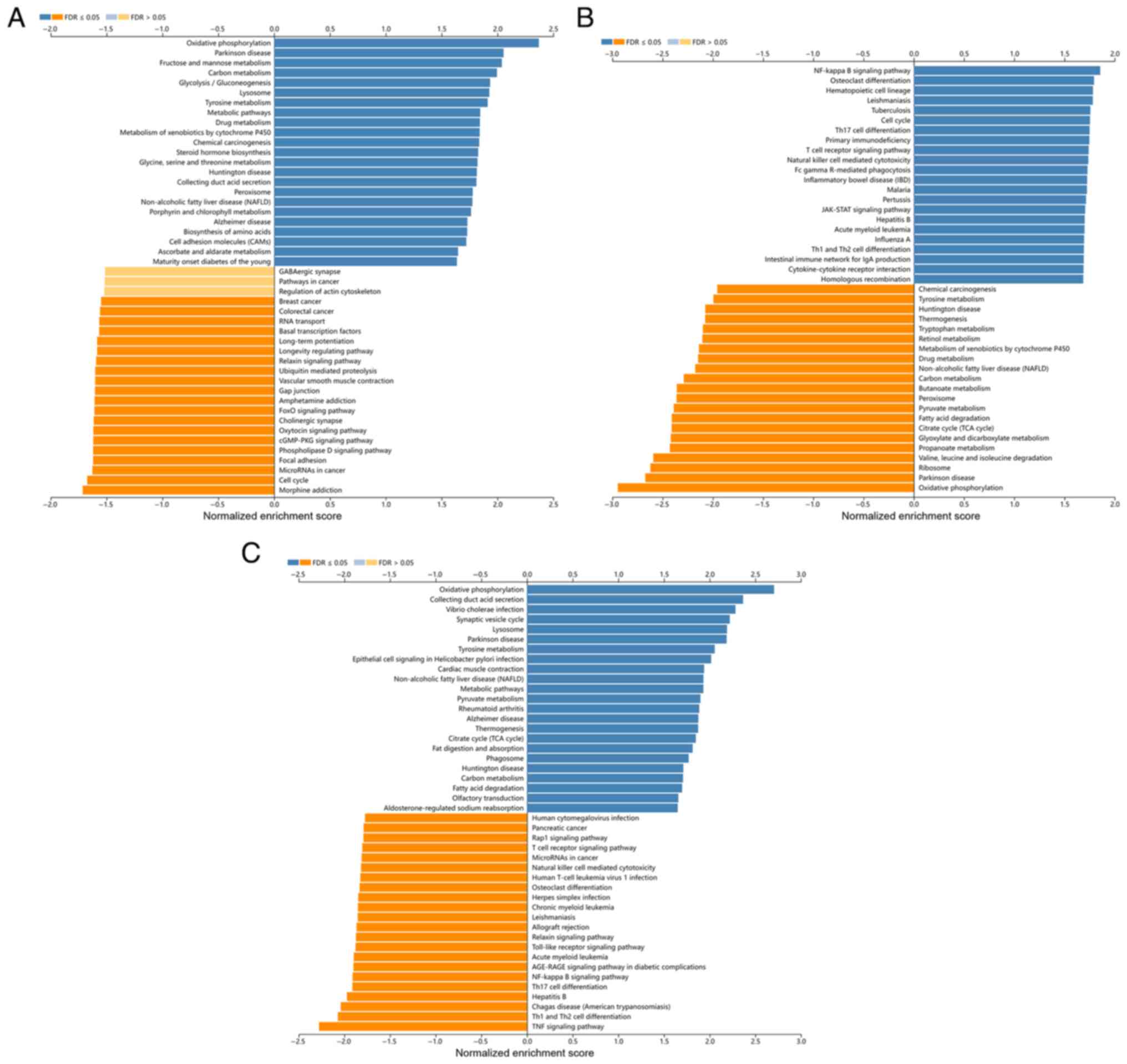
3A-C). KEGG pathway analysis revealed that ELF3 was mainly
enriched in ‘Oxidative phosphorylation’, ‘Cell cycle’,
‘Phospholipase D signaling pathway’ and ‘cGMP-PKG signaling
pathway’ (Fig. 4A). KEGG pathway
analysis revealed that ELF5 was mainly enriched in ‘Oxidative
phosphorylation’, ‘Collecting duct acid secretion’ and ‘TNF
signaling pathway’ (Fig. 4C). These
pathways were closely related to ccRCC development. By contrast,
ELF4 was associated with more immune-related signaling pathways,
including ‘Th17 cell differentiation’, ‘Primary immunodeficiency’,
‘T cell receptor signaling pathway’, ‘Natural killer cell mediated
cytotoxicity’, and ‘Th1 and Th2 cell differentiation’ (Fig. 4B). These results indicated that the
ELF4 expression network was closely related to immune response and
the immune microenvironment in ccRCC.
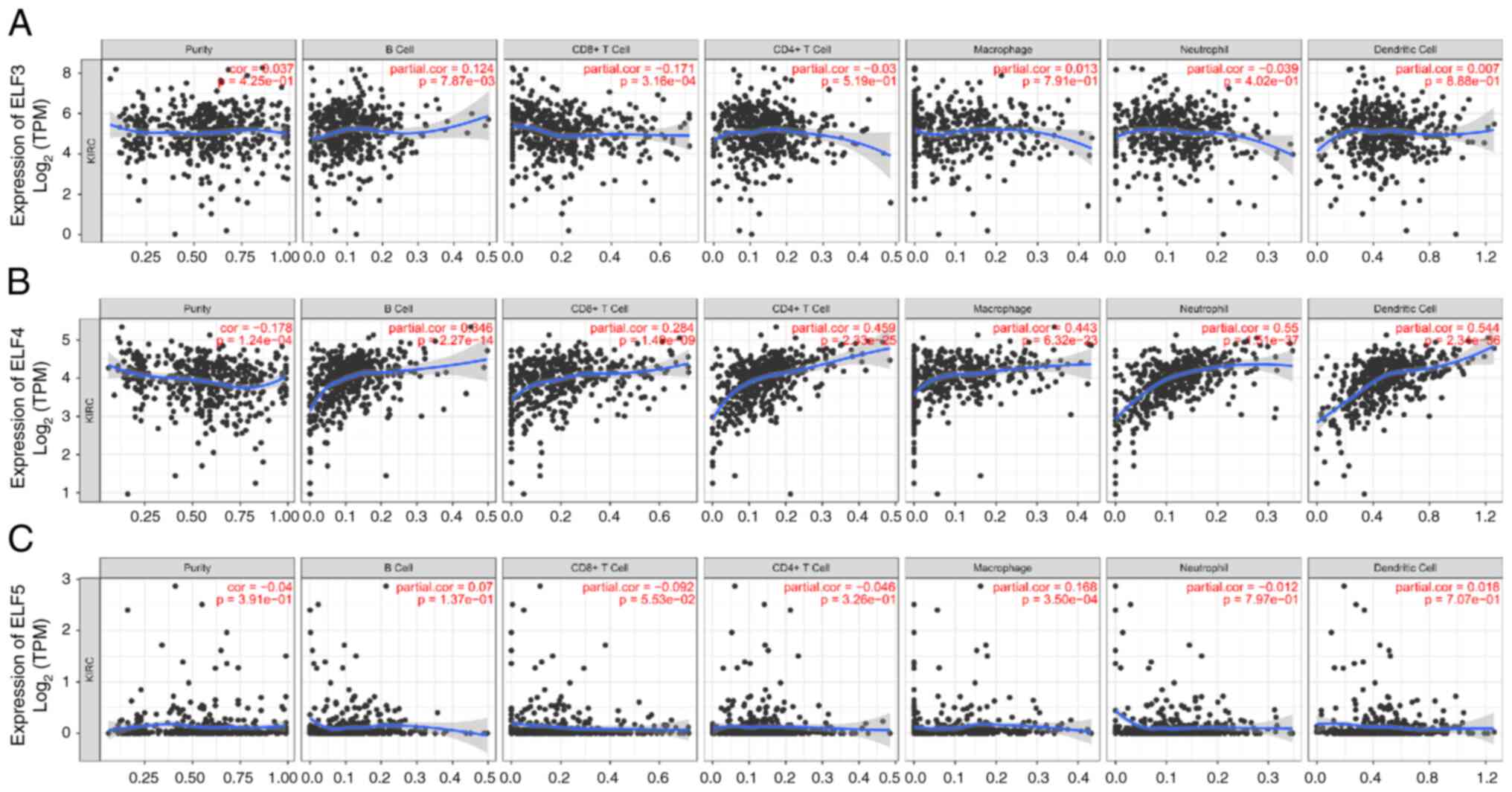
Correlation analysis between ELF3-5
expression and immune infiltrate
The TIMER database was used to investigate the
relationship between ELF3-5 expression and immune infiltrate. The
expression levels of ELF3 and ELF5 showed no significant
association with most immune cell infiltration levels (Fig. 5A and C). However, ELF4 exhibited a
notable correlation with various immune cells, including B cells,
CD4+ T cells, macrophages, neutrophils and dendritic
cells (Fig. 5B). These findings
suggested a specific role for ELF4 in immune infiltration in ccRCC.
Therefore, the present study further investigated the effects of
ELF4 on the proliferation, migration, invasion and immune escape of
ccRCC cells.
ELF4 promotes ccRCC cell
proliferation, migration and invasion
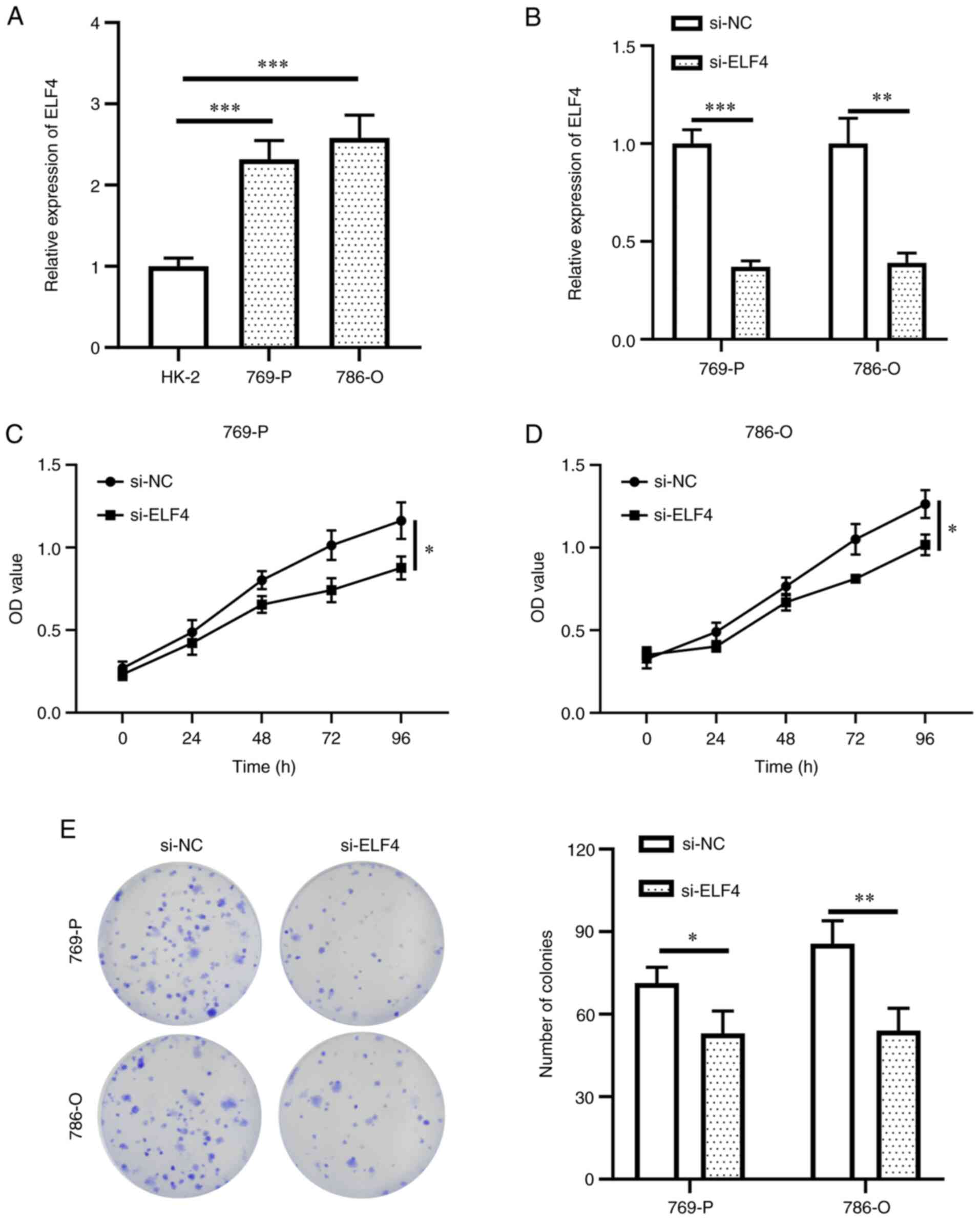
ELF4 expression was detected in HK-2 and ccRCC cells
to validate its potential function. ELF4 exhibited high expression
levels in 769-P and 786-O cells compared with those in HK-2 cells
(Fig. 6A). Subsequently, ELF4
expression was knocked down in the two ccRCC cell lines (Fig. 6B). A decrease in 769-P and 786-O
cell proliferation was detected upon ELF4 knockdown compared with
that in the si-NC group (Fig. 6C and
D). The colony formation assay results also revealed that
knockdown of ELF4 expression could reduce the colony formation of
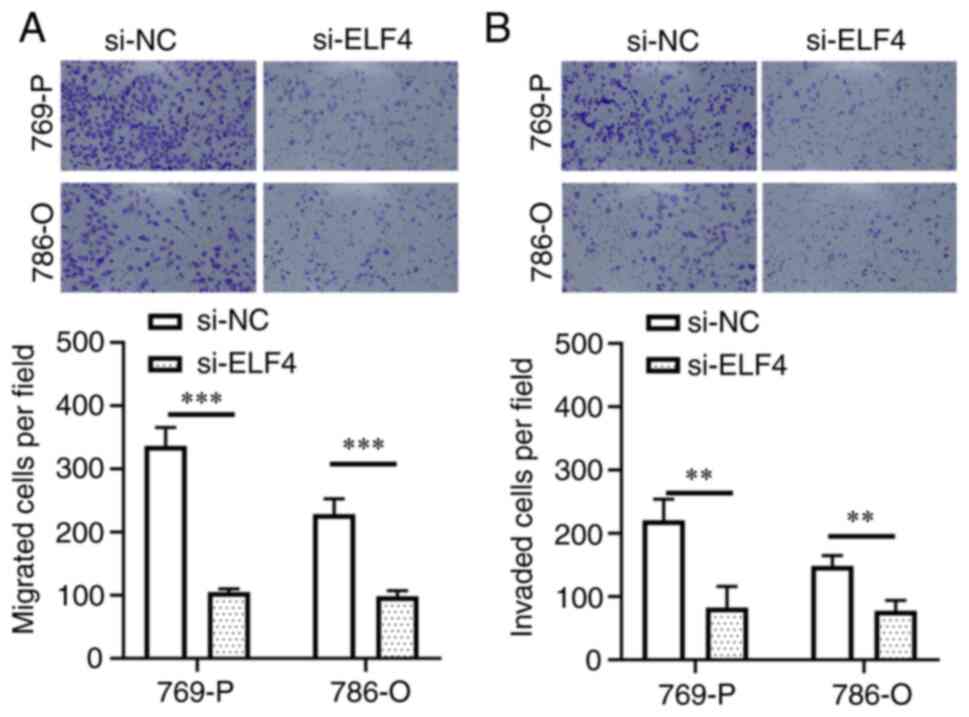
ccRCC cells compared with that in the si-NC group (Fig. 6E). Furthermore, Transwell assay
results indicated a reduction in cell migration and invasion in the
si-ELF4 group compared with those in the si-NC group (Fig. 7A and B). These findings suggested
that activating ELF4 may promote ccRCC cell proliferation,
migration and invasion.
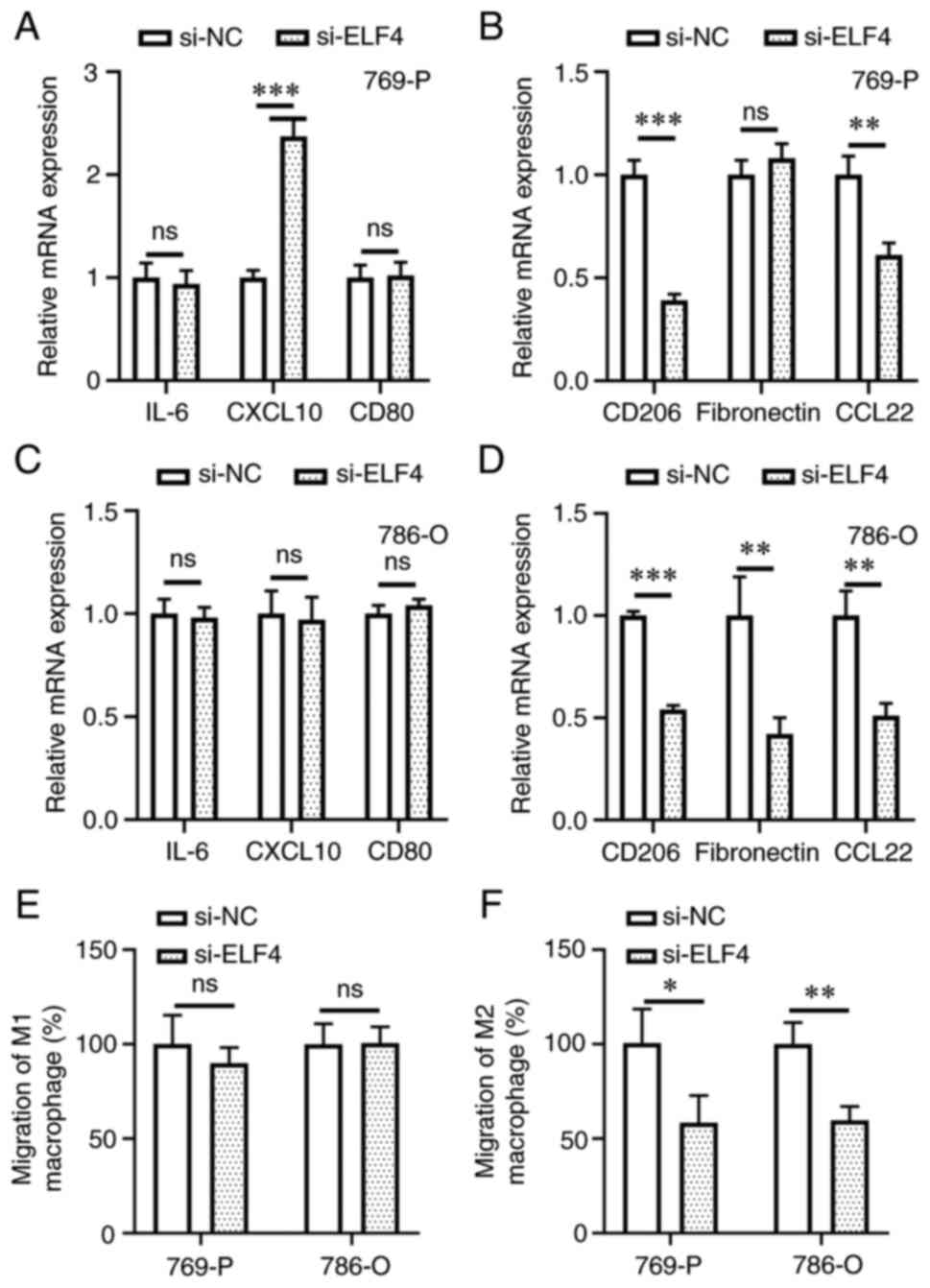
ELIF4 regulates M2 macrophage
polarization and chemotaxis of M2 macrophages to ccRCC cells
M1 and M2 macrophage marker expression levels were
detected in ccRCC and macrophage co-culture experiments. In 769-P
cells, higher transcription levels of the M1 macrophage marker
CXCL10 were detected in the si-ELF4 group compared with that in the
si-NC group (Fig. 8A). Conversely,
the expression levels of the M2 macrophage markers CD206 and CCL22
were lower in the si-ELF4 group compared with those in the si-NC
group (Fig. 8B). Knockdown of ELF4
expression had no impact on M1 marker expression in the 786-O cell
and macrophage co-culture system (Fig.
8C); however, it did decrease the expression levels of M2
markers (Fig. 8D). Regarding
macrophage chemotaxis, the present findings revealed that knockdown
of ELF4 in ccRCC cells did not regulate the migration rate of M1
macrophages towards cancer cells (Fig.
8E); however, it did inhibit the migration rate of M2
macrophages towards cancer cells (Fig.
8F). These results suggested that ELF4 could promote M2
macrophage polarization and chemotaxis of M2 macrophages to ccRCC
cells.
Discussion
Abnormal expression of ELFs has been identified in
various malignant tumors, influencing their biological processes
(28); however, the regulatory
mechanisms and clinical significance of certain ELFs in ccRCC
remain unclear. The present study comprehensively analyzed the
clinical significance and key pathways associated with ELF3-5 in
ccRCC using multiple databases. Moreover, the
proliferation-promoting effects of the core gene ELF4 and its
regulation of macrophages were assessed in vitro.
The present study demonstrated that ELF3 and ELF5
exhibited lower expression levels in ccRCC tissues compared with
those in normal tissues, whereas ELF4 expression was higher.
Furthermore, the clinical significance of these three key genes
were explored in ccRCC. Previous research has highlighted ELF3 as a
methylation-driven gene in lung adenocarcinoma (29). In addition, DNA methylation levels
at the ELF5 promoter region have been identified as potential
breast-specific biological clocks for identifying the risk of
breast cancer (30). Demethylation
of ELF5 has also been explored as a potential therapeutic strategy
in urothelial cancer (31). The
present study detected higher methylation levels of ELF3 and ELF5
in primary tumor tissues compared with those in normal tissues.
Notably, ELF4 methylation has previously been reported to be
significantly upregulated during liver cell carcinogenesis
(32), and hypermethylation of the
ELF4 promoter region in colitis preparations has been associated
with disease progression to colorectal cancer (33). However, no significant difference
was observed in the methylation level of ELF4 between ccRCC tissues
and normal tissues in the present study. Clinical significance
serves a crucial role in exploring the diagnostic value of
biomarkers. ELF3 has been shown to have clinical significance in
non-small cell lung cancer, where the inhibition of ELF3 mediated
the synthetic lethality of PARP inhibitor (34). In epithelial ovarian cancer, the
expression levels of ELF5 were related to pathological surgical
stage, pathological grade and lymph node metastasis (13). Clinical analysis has also revealed
associations between ELF4 expression and tumor size, pathological
grade and clinical stage in squamous cell carcinoma of the cervix
(35). In the present study, ELF3
and 4 exhibited different expression patterns across different
grades, and ELF3-5 showed differential expression levels in ccA and
ccB subtypes. Regarding genetic variations, all three genes
exhibited amplification variations, but it was only ELF4 that
showed a high amplification variation in ccRCC. Kafita et al
(36) reported that high
amplification variation of ELF4 in cancer was associated with worse
disease outcomes and increased resistance to anticancer drugs.
These reports and findings highlighted the high clinical
significance of ELF3-5, particularly ELF4, in ccRCC.
ELF3-5, as members of a transcription factor family,
have been implicated in regulating tumor progression through
various signaling pathways. ELF3 can promote resistance in
gallbladder cancer cells via the PKMYT1/CDK1 signaling pathway
(37), whereas ELF5 can inhibit the
p53/p21 pathway, leading to the induction of acute myeloid leukemia
(38). In glioblastoma, ELF4
controls genes associated with receptor tyrosine kinase and
receptor tyrosine kinase pathways (39). Therefore, identifying the key
regulatory pathways of these three ELF genes was crucial for
understanding the molecular mechanisms underlying the impact of ETS
family genes on cancer cell development. ELF3-5 were revealed to be
associated with various functions in the present study, including
biological regulation, metabolic processes, membrane functions,
protein binding and nucleic acid binding. Notably, ELF4 was
particularly linked to immune-related signaling pathways. A
previous study highlighted the critical involvement of ELF4 in the
cancer immune response (40). It
has also been reported to be associated with immune cell
infiltration and immune-related feature genes (CD14, CD163, CD33)
in cholangiocarcinoma (41).
Similarly, the present study revealed that ELF4 expression in ccRCC
was closely related to the infiltration levels of multiple immune
cell types compared with ELF3 and ELF5. These results suggested an
important role for ELF4 in regulating cancer cell activity and
tumor-related immune cell infiltration.
Previous cancer studies have indicated that ELF4
functions as an oncogene. It has been shown to promote
neuroblastoma proliferation and maintain an undifferentiated state
(42). ELF4 has also been
implicated in endometrial cancer, where it acts as an oncogene by
binding to the CTNNB1 promoter in cancer cells (43). The present findings in ccRCC cell
lines further support the role of ELF4 in promoting cell
proliferation, migration and invasion in 769-P and 786-O cell
lines. Moreover, abnormal ELF4 expression was shown to influence
the regulation of M2 polarization and the chemotaxis of macrophages
to cancer cells. In lung cancer, ELF4 in macrophages has been shown
to rescue immunotherapy efficacy (44). ELF4 also exhibits transcriptional
activation of macrophage colony-stimulating factors in ovarian
cancer (45). These findings
underscore the significance of ELF4 in regulating cancer cell
abilities, inducing M2 polarization of macrophages, and their
chemotaxis towards ccRCC cells. This highlights the crucial role of
ELF4 in the tumor microenvironment of ccRCC.
The present study has certain limitations that
should be acknowledged. Firstly, the clinical significance of ELFs
was primarily assessed through bioinformatics analysis using public
databases; therefore, it is crucial to gather larger clinical
samples of ccRCC to further validate the clinical significance of
ELFs. Secondly, although the study uncovered the involvement of
ELF4 in macrophage polarization and chemotaxis, the immune escape
mechanism of ELF4 in ccRCC remains to be elucidated. Future
investigations should explore the regulatory effects of ELF4 on
other immune cell types in ccRCC. Moreover, inconsistencies in the
expression results of certain ELFs across different databases
necessitate additional sequencing data for further verification.
Finally, the specific molecular mechanism by which ELF4 regulates
ccRCC cells and M2 macrophages warrants in-depth exploration. A
number of the findings from in vitro experiments also
require future in vivo validation.
In conclusion, ELF members display varying degrees
of abnormal expression and serve important roles in ccRCC
tumorigenesis and progression. The present study comprehensively
analyzed the clinical significance and tumor-immune interaction of
ELF4. The results revealed that ELF4 was significantly upregulated
in ccRCC tumor tissues, indicating its high clinical significance
in ccRCC. The present study further elucidated the promoting
effects of ELF4 on ccRCC cell proliferation, migration and
invasion. Additionally, the results suggested that ELF4 could
regulate macrophage polarization and chemotaxis to ccRCC cells.
These findings provide novel insights into our understanding of the
involvement of ELFs in ccRCC development.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Province Health Science
and Technology Plan Project (grant no. 2023KY1343).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the GEPIA (http://gepia.cancer-pku.cn/), GEO database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE53757),
UALCAN (http://ualcan.path.uab.edu), Oncomine
(https://www.oncomine.org), cBioPortal (http://www.cbioportal.org), LinkedOmics (https://www.linkedomics.org/login.php)
and TIMER web server (https://cistrome.shinyapps.io/timer/) databases. All
other datasets used and/or analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
JL and WC conceived and designed the study. WC
acquired funding. LMo, LMa and QZ conducted the experiments and
bioinformatics analysis. JL drafted the paper and WC revised the
manuscript. JL and WC confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ljungberg B, Albiges L, Abu-Ghanem Y,
Bedke J, Capitanio U, Dabestani S, Fernández-Pello S, Giles RH,
Hofmann F, Hora M, et al: European association of urology
guidelines on renal cell carcinoma: The 2022 update. Eur Urol.
82:399–410. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W, Liu R, Zhang L, Wang C, Dong Z,
Feng J, Luo M, Zhang Y, Xu Z, Lv S and Wei Q: Downregulation of
miR-335 exhibited an oncogenic effect via promoting KDM3A/YAP1
networks in clear cell renal cell carcinoma. Cancer Gene Ther.
29:573–584. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choueiri TK and Motzer RJ: Systemic
therapy for metastatic renal-cell carcinoma. N Engl J Med.
376:354–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin E, Liu X, Liu Y, Zhang Z, Xie L, Tian
K, Liu J and Yu Y: Roles of the dynamic tumor immune
microenvironment in the individualized treatment of advanced clear
cell renal cell carcinoma. Front Immunol. 12:6533582021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li F, Feng Y, Jiang Q, Zhang J, Wu F, Li
Q, Jing X, Wang X and Huang C: Pan-cancer analysis, cell and animal
experiments revealing TEAD4 as a tumor promoter in ccRCC. Life Sci.
293:1203272022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren X, Diao X, Zhuang J and Wu D:
Structural basis for the allosteric inhibition of hypoxia-inducible
factor (HIF)-2 by belzutifan. Mol Pharmacol. Sep 27–2022.doi:
10.1124/molpharm.122.000525 (Epub ahead of print). View Article : Google Scholar
|
|
7
|
Budka JA, Ferris MW, Capone MJ and
Hollenhorst PC: Common ELF1 deletion in prostate cancer bolsters
oncogenic ETS function, inhibits senescence and promotes docetaxel
resistance. Genes Cancer. 9:198–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Honda S, Kobayashi T, Kajino K, Urakami S,
Igawa M and Hino O: Ets protein Elf-1 bidirectionally suppresses
transcriptional activities of the tumor suppressor Tsc2 gene and
the repair-related Nth1 gene. Mol Carcinogenesis. 37:122–129. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Yao B, Guo X, Wang Z, Xie W, Wu X,
Wang F and Mei Y: c-Myc-induced long noncoding RNA MIRE cooperates
with hnRNPK to stabilize ELF2 mRNA and promotes clear cell renal
cell carcinogenesis. Cancer Gene Ther. May 29–2023.doi:
10.1038/s41417-023-00631-0 (Epub ahead of print). View Article : Google Scholar
|
|
10
|
Xiao XH and He SY: ELF1 activated long
non-coding RNA CASC2 inhibits cisplatin resistance of non-small
cell lung cancer via the miR-18a/IRF-2 signaling pathway. Eur Rev
Med Pharmacol Sci. 24:3130–3142. 2020.PubMed/NCBI
|
|
11
|
Brunner M, Mullen L, Jauk F, Oliver J,
Cayol F, Minata J, Herrera V, Pavicic W, Luna D, Risk M, et al:
Automatic integration of clinical and genetic data using
cBioPortal. Stud Health Technol Inform. 290:799–803.
2022.PubMed/NCBI
|
|
12
|
Chen X, Chen J, Feng W, Huang W, Wang G,
Sun M, Luo X, Wang Y, Nie Y, Fan D, et al: FGF19-mediated ELF4
overexpression promotes colorectal cancer metastasis through
transactivating FGFR4 and SRC. Theranostics. 13:1401–1418. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Y, Yan Y, Xu Y, Yang H, Fang L, Liu Y,
Li X, Li Q and Yan H: Expression and clinical significance of WWOX,
Elf5, Snail1 and EMT related factors in epithelial ovarian cancer.
Oncol Lett. 19:1281–1290. 2020.PubMed/NCBI
|
|
14
|
Kulkarni DP, Wadia PP, Pradhan TN, Pathak
AK and Chiplunkar SV: Mechanisms involved in the down-regulation of
TCR zeta chain in tumor versus peripheral blood of oral cancer
patients. Int J Cancer. 124:1605–1613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gallego-Ortega D, Ledger A, Roden DL, Law
AM, Magenau A, Kikhtyak Z, Cho C, Allerdice SL, Lee HJ, Valdes-Mora
F, et al: ELF5 drives lung metastasis in luminal breast cancer
through recruitment of Gr1+ CD11b+ myeloid-derived suppressor
cells. PLoS Biol. 13:e10023302015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47:W556–W560. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
von Roemeling CA, Radisky DC, Marlow LA,
Cooper SJ, Grebe SK, Anastasiadis PZ, Tun HW and Copland JA:
Neuronal pentraxin 2 supports clear cell renal cell carcinoma by
activating the AMPA-selective glutamate receptor-4. Cancer Res.
74:4796–4810. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vasaikar SV, Straub P, Wang J and Zhang B:
LinkedOmics: Analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46:D956–D963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malekghasemi S, Majidi J, Baradaran B and
Aghebati-Maleki L: Prostate cancer cells modulate the
differentiation of THP-1 cells in response to etoposide and TLR
agonists treatments. Cell Biol Int. 44:2031–2041. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Cui J, Zhao Y, Liu X, Chen L, Xia
Y, Wang Y, Chen S, Sun S, Shi B and Zou Y: KDM6A-ARHGDIB axis
blocks metastasis of bladder cancer by inhibiting Rac1. Mol Cancer.
20:772021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye J, Chen X and Lu W: Identification and
experimental validation of immune-associate lncRNAs for predicting
prognosis in cervical cancer. Onco Targets Ther. 14:4721–4734.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo X, Li H, Zhang M and Li R: LncRNA GAS6
antisense RNA 1 facilitates the tumorigenesis of clear cell renal
cell carcinoma by regulating the AMP-activated protein kinase/mTOR
signaling pathway. Oncol Lett. 22:7272021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galang CK, Muller WJ, Foos G, Oshima RG
and Hauser CA: Changes in the expression of many Ets family
transcription factors and of potential target genes in normal
mammary tissue and tumors. J Biol Chem. 279:11281–11292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Enfield KSS, Marshall EA, Anderson C, Ng
KW, Rahmati S, Xu Z, Fuller M, Milne K, Lu D, Shi R, et al:
Epithelial tumor suppressor ELF3 is a lineage-specific amplified
oncogene in lung adenocarcinoma. Nat Commun. 10:54382019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyano M, Sayaman RW, Shalabi SF, Senapati
P, Lopez JC, Angarola BL, Hinz S, Zirbes A, Anczukow O, Yee LD, et
al: Breast-specific molecular clocks comprised of ELF5 expression
and promoter methylation identify individuals susceptible to cancer
initiation. Cancer Prev Res (Phila). 14:779–794. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu B, Cao X, Liang X, Zhang X, Zhang W,
Sun G and Wang D: Epigenetic regulation of Elf5 is associated with
epithelial-mesenchymal transition in urothelial cancer. PLoS One.
10:e01175102015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goncharova IA, Zarubin AA, Babushkina NP,
Koroleva IA and Nazarenko MS: Changes in DNA methylation profile in
liver tissue during progression of HCV-induced fibrosis to
hepatocellular carcinoma. Vavilovskii Zhurnal Genet Selektsii.
27:72–82. 2023.PubMed/NCBI
|
|
33
|
Du H, Xia H, Liu T, Li Y, Liu J, Xie B,
Chen J, Liu T, Cao L, Liu S, et al: Suppression of ELF4 in
ulcerative colitis predisposes host to colorectal cancer. iScience.
24:1021692021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Zuo M, Jin H, Lai M, Luo J and
Cheng Z: Inhibition of ELF3 confers synthetic lethality of PARP
inhibitor in non-small cell lung cancer. J Recept Signal Transduct
Res. 41:304–311. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo Y, Ma D, Jia SF, Liu J, Fan SB, Zhang
M, Shi LR, Jiang LL, Shi JX, Wang HQ, et al: Proliferation of
MicroRNA-365 and E74-like factor 4 in cervical cancer cells and its
clinical significance. Zhongguo Yi Xue Ke Xue Yuan Xue Bao.
41:220–227. 2019.(In Chinese). PubMed/NCBI
|
|
36
|
Kafita D, Daka V, Nkhoma P, Zulu M, Zulu
E, Tembo R, Ngwira Z, Mwaba F, Sinkala M and Munsaka S: High ELF4
expression in human cancers is associated with worse disease
outcomes and increased resistance to anticancer drugs. PloS one.
16:e02489842021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang L, Wang H, Guo M, He M, Zhang W, Zhan
M and Liu Y: ELF3 promotes gemcitabine resistance through
PKMYT1/CDK1 signaling pathway in gallbladder cancer. Cell Oncol
(Dordr). Mar 29–2023.doi: 10.1007/s13402-023-00799-5 (Epub ahead of
print). View Article : Google Scholar
|
|
38
|
Endo A, Tomizawa D, Aoki Y, Morio T,
Mizutani S and Takagi M: EWSR1/ELF5 induces acute myeloid leukemia
by inhibiting p53/p21 pathway. Cancer Sci. 107:1745–1754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kosti A, Chiou J, Guardia GDA, Lei X,
Balinda H, Landry T, Lu X, Qiao M, Gilbert A, Brenner A, et al:
ELF4 is a critical component of a miRNA-transcription factor
network and is a bridge regulator of glioblastoma receptor
signaling and lipid dynamics. Neuro Oncol. 25:459–470. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suico MA, Shuto T and Kai H: Roles and
regulations of the ETS transcription factor ELF4/MEF. J Mol Cell
Biol. 9:168–177. 2017.PubMed/NCBI
|
|
41
|
Jin H, Liu W, Xu W, Zhou L, Luo H, Xu C,
Chen X and Chen W: Identification of prognostic factors in
cholangiocarcinoma based on integrated ceRNA network analysis.
Comput Math Methods Med. 2022:71027362022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kosti A, Du L, Shivram H, Qiao M, Burns S,
Garcia JG, Pertsemlidis A, Iyer VR, Kokovay E and Penalva LOF: ELF4
is a target of miR-124 and promotes neuroblastoma proliferation and
undifferentiated state. Mol Cancer Res. 18:68–78. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang WL, Hong GC, Chien PJ, Huang YH, Lee
HT, Wang PH, Lee YC and Chang WW: Tribbles pseudokinase 3
contributes to cancer stemness of endometrial cancer cells by
regulating β-catenin expression. Cancers. 12:37852020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao J, Ao YQ, Zhang LX, Deng J, Wang S,
Wang HK, Jiang JH and Ding JY: Exosomal circZNF451 restrains
anti-PD1 treatment in lung adenocarcinoma via polarizing
macrophages by complexing with TRIM56 and FXR1. J Exp Clin Cancer
Res. 41:2952022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yao JJ, Liu Y, Lacorazza HD, Soslow RA,
Scandura JM, Nimer SD and Hedvat CV: Tumor promoting properties of
the ETS protein MEF in ovarian cancer. Oncogene. 26:4032–4037.
2007. View Article : Google Scholar : PubMed/NCBI
|