Introduction
Renal cell carcinoma (RCC), a large group of cancers
originating from renal epithelial cells, includes ~10 subtypes
based on molecular and histopathological characteristics (1). Mucinous tubular and spindle cell
carcinoma (MTSCC) is a low-grade carcinoma composed of tightly
packed tubules separated by pale mucinous stroma and a spindle cell
component. MTSCC has low-grade malignant potential. Among RCCs,
MTSCC is rare, accounting for <1% of all renal tumors (2). Additionally, MTSCC is slow-growing
(3). The age distribution of
patients with MTSCC is wide and these patients are predominantly
female (2–5). Most patients have asymptomatic,
incidentally discovered tumors (2).
Some patients with uncommon histologic features, including
mucin-poor stroma and high nuclear grade, may have painless gross
hematuria or lower back pain (6).
The overall imaging characteristics of MTSCC have not yet been
clearly described.
Case report
Materials and methods
Image acquisition
Computed tomography (CT) examinations were performed
with 64-slice spiral dual-energy CT (SOMATOM Definition Flash;
Siemens Healthcare GmbH). The CT protocols were as follows:
Non-contrast CT, corticomedullary phase (35 sec), nephrographic
phase (60 sec) and dual-energy phase (300 sec). All CT examinations
was performed using similar scanning parameters with tube voltage,
100 kV (non-contrast CT: 120KV); tube current, 300 mA; slice
thickness, 5.0 mm; display field of view: 36.8×43.7 cm;
reconstruction thickness, 1.5 mm. The contrast agent iohexol was
intravenously injected at a dose of 1.5–2.0 mmol/kg via a power
injector at an injection rate of 3.0 ml/sec.
Magnetic resonance imaging (MRI) examinations were
performed with a 3.0-Tesla units (MAGNETOM Skyra; Siemens
Healthcare GmbH). The MRI protocols were as follows: T1-weighted
imaging (T1WI), T2-weighted imaging (T2WI), diffusion weighted
imaging (DWI; slice thickness, 5 mm; gap, 1 mm; Display field of
view, 38.0×44.9 cm), DWI (b value of 0 and 800 sec/mm2,
respectively), apparent diffusion coefficient (ADC) maps were
reconstructed by subtracting the DWI with the high b value (800
sec/mm2) from the DWI with the low b value (0
sec/mm2). The present study was approved by the Ethics
Committee of the First People's Hospital of Zunyi (Zunyi, China;
approval no. 2023201). The patient provided written informed
consent.
Histological and immunohistochemical
methods
A piece of tissue (0.4 cm) was removed from the
tumor. The biopsy material was fixed in 10% formalin (1 h; 25°C).
The tissue is gradually dehydrated in 75, 85 and 95% alcohol, and
then it was made transparent in dimethylbenzene. The tissue were
embedded in paraffin wax and cut into 3-µm sections. The tissue was
stained with hematoxylin and eosin (10 min; 25°C). Finally, the
mount was sealed with neutral gum. The process of
immunohistochemistry from sampling to embedding was the same as for
histology, with the difference that the tissue was sectioned at 2
µm. Then, the sections were placed in a 65°C oven for 30 min and
dewaxed with dimethylbenzene (5 min; 2 times), dehydration with
anhydrous ethanol (1 min; 2 times), 95% ethanol (1 min) and 85%
ethanol (1 min) before being placed into a pressure cooker filled
with sodium citrate buffer repair solution for 3 min. After
rinsing, the sections were washed with phosphate buffer saline
(PBS; 3 min; 3 times) and inactivated with 3% hydrogen peroxide (10
min; 25°C), washed again in PBS (3 min; 3 times) and primary and
secondary antibodies added for incubation (50 min; 37°C), each
incubation being followed by a rinse in PBS (3 min; 3 times). The
color was developed with 3,3′-diaminobenzidine (5 min) and the
reaction blocked. The sections were stained with hematoxylin (1
min; 25°C) and differentiation with 1% hydrochloric alcohol (10
sec; 25°C), rinsed with tap water (5 min; 25°C) and dehydrated with
85 and 95% ethanol (2 min each; 25°C) and, finally, with anhydrous
ethanol (2 min; 2 times; 25°C), cleared with dimethylbenzene (1
min; 25°C) and sealed with neutral gum.
The catalog numbers of all primary and secondary
antibodies were: RCC cat. no. GT210902, PAX8 cat. no. GT210202,
vimentin (Vim) cat. no. GM072502, epithelial membrane antigen (EMA)
cat. no. GM061302, CD10 cat. no. GT200402, CK8/18 cat. no.
GT207802, HMB45 cat. no. GM063402, S100 cat. no. GT242002, Ki67
cat. no. GM724002, CD68 cat. no. GM081402 with secondary antibodies
cat. no. Gk600711A (conjugated with horseradish peroxidase).
Supplier of all primary and secondary antibodies was GeneTech
(Shanghai) Co., Ltd. They were all ready-to-use antibodies and no
dilution was required.
Literature review
In order to present the literature review, case
reports of MTSCC in the English language were searched from the
PubMed (pubmed.ncbi.nlm.nih.gov), Medline
(lib.cpu.edu.cn/3b/12/c1172a80658/page.htm) databases. Key words
were used for the search, which included ‘mucinous tubular and
spindle cell carcinoma of the kidney’, ‘mucinous tubular and
spindle cell carcinoma’, ‘bilateral atrialmyxomas’, ‘mucinous
tubular and spindle cell carcinoma of the renal’, ‘clinical’,
‘treatment’ and ‘image’. Inclusion criteria for screening studies
were: Full text articles assessed for eligibility. Exclusion
criteria for screening studies: duplicates removed, irrelevant
publication, review only or full text not found.
Case
A 65-year-old woman with no painless gross hematuria
or lower back pain was admitted to The First People's Hospital of
Zunyi (Guizhou, China) on April 6, 2021 due to space-occupying
lesions of the kidney found on conventional ultrasound images. The
physical examinations performed on the patient at the time of
admission included sight, touch, tapping and listening to check
abdominal double renal areas. The double ureteral running areas
were checked by touch and the bladder area with tapping and
touch.
The laboratory tests were completed as follows:
Serum myocardial zymogram, plasma D-dimer, troponin, blood routine,
liver function, hepatitis B, hepatitis C, AIDS and syphilis
antibody tests. Physical and laboratory examinations revealed no
abnormalities. Then, the patient underwent abdominal enhanced CT
and MRI. Enhanced CT has four stages: Noncontrast (NC),
corticomedullary (CM), nephrographic (Ne) and excretory (Ex). The
CT image showed an isodense mass with clear boundaries in the
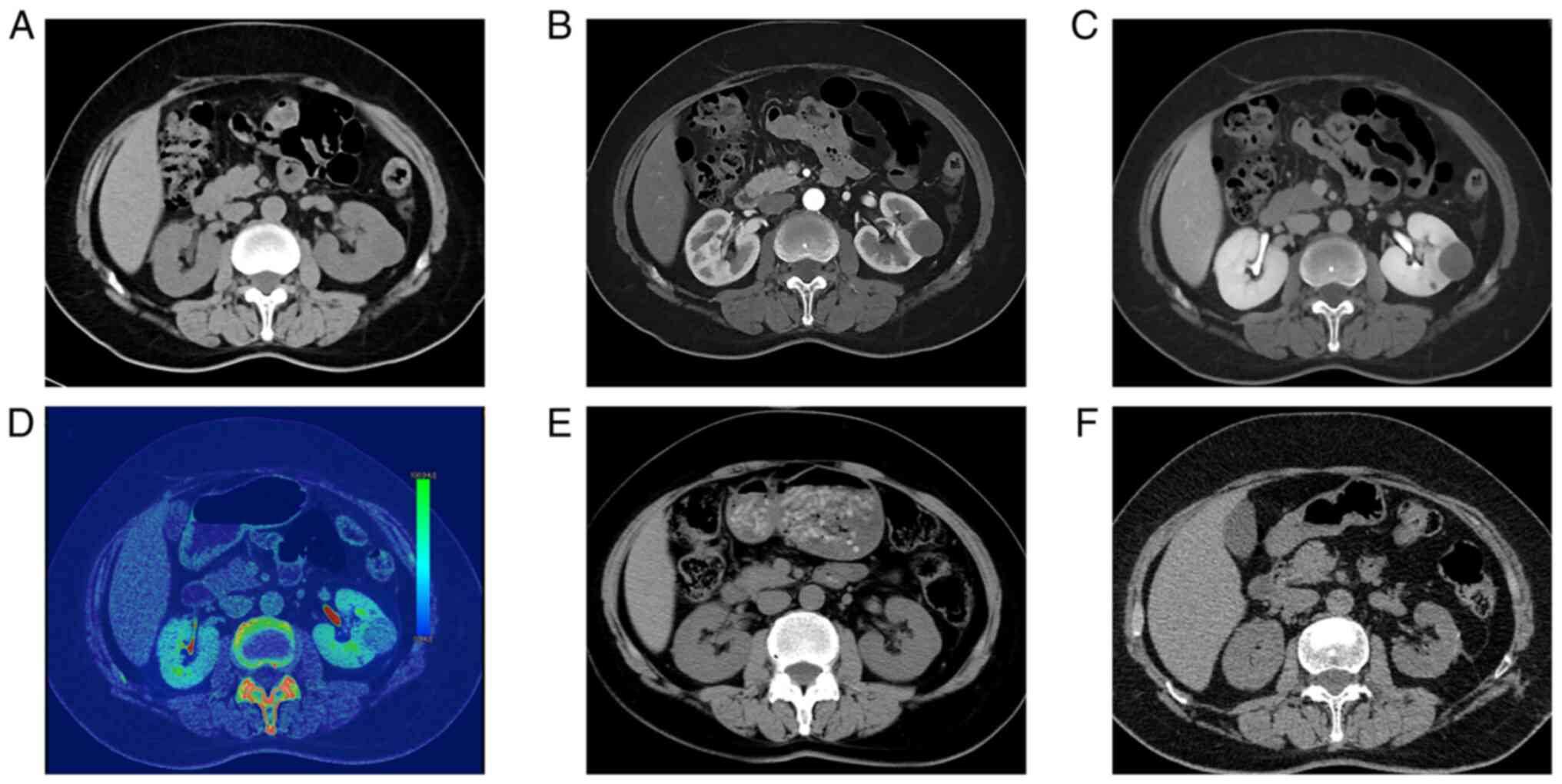
submiddle pole of the left kidney. A homogeneous solid soft mass
2.8×2.6×2.5 cm in size was identified on unenhanced CT (Fig. 1A). The Hounsfield units (HU) in the
region of the mass were measured. In the NC, CM, Ne and Ex phases
of the CT scan, the average attenuation values of the tumor were
21, 42, 61 and 69 HU, respectively. Dual-energy CT revealed iodine
uptake within the lesion (Fig. 1D).
Enhanced CT showed that the mass had mild to moderate, uniform,
progressive enhancement (Fig. 1B and
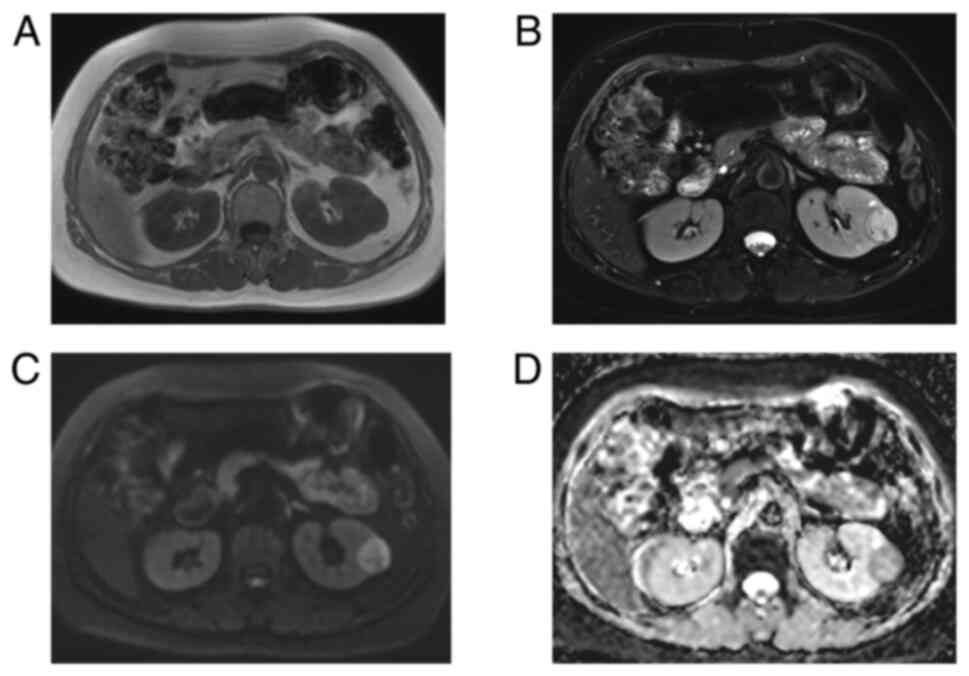
C). MRI revealed a round isointense lesion in the left kidney
on T1-weighted imaging (T1WI; Fig.
2A). The mass was slightly hyperintense with a small
hypointense area on T2-weighted imaging (T2WI; Fig. 2B). DWI (b=800 sec/mm2)
presented a high signal (Fig. 2C).
The ADC (ADC=1.47×10−3 mm2/sec) presented a
low signal (Fig. 2D). DWI and the
ADC indicated obviously restricted diffusion at the margin of the
tumor. No obvious lipid content was observed in any of the MRI
images. These imaging features suggested a diagnosis of renal
cancer.
The patient then underwent laparoscopic left renal
tumor removal. The mass was grayish-white and pale yellow with
intact capsules. No invasion of the renal pelvis, perinephric fat
or hilar vessels was observed. Neither the adrenal glands nor the
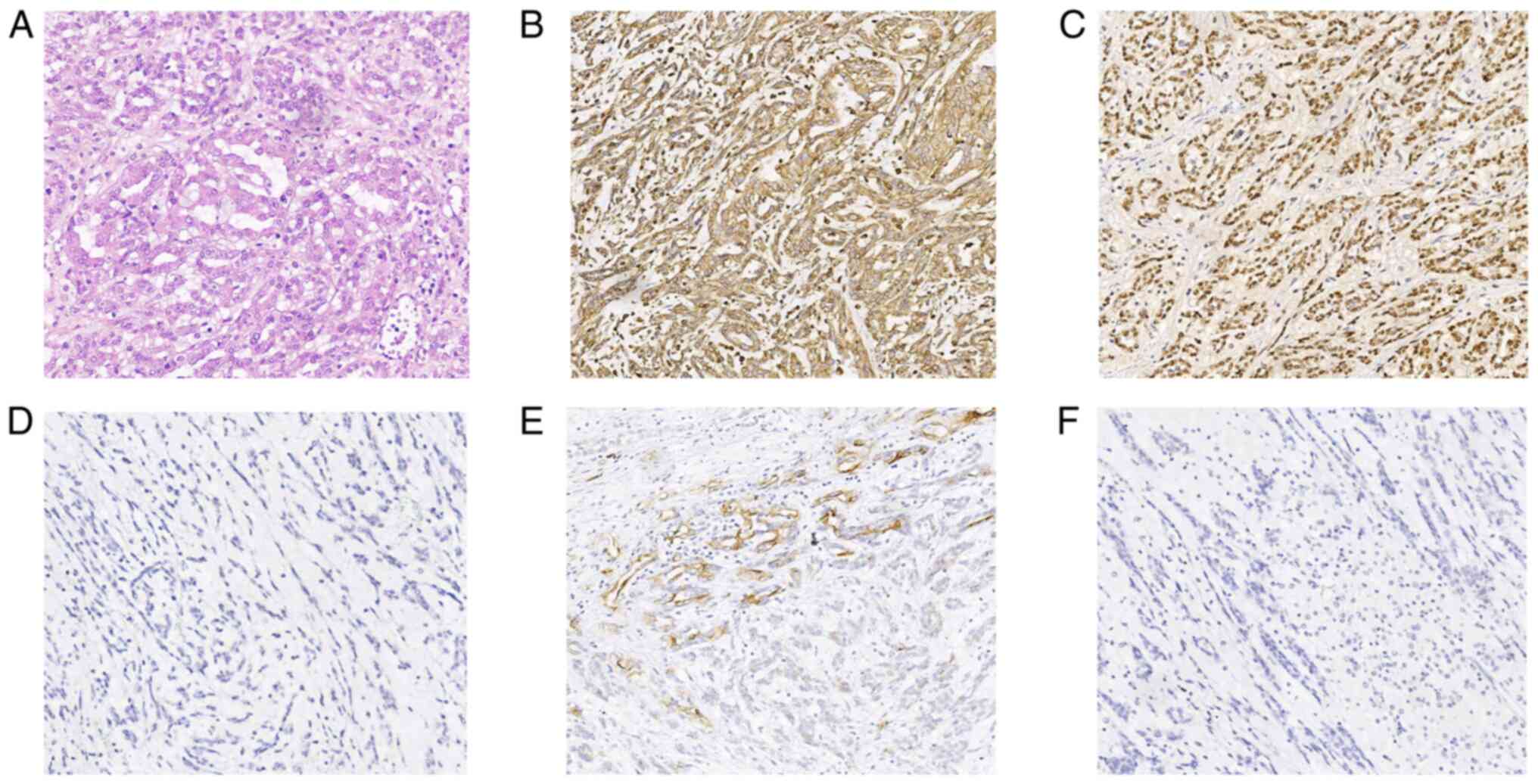
lymph nodes showed signs of metastasis. In the histopathological
examination of the lesion, mucinous tubular and spindle cells were
found (Fig. 3A), and
immunohistochemistry showed Vim (positive; Fig. 3B), PAX8 (positive; Fig. 3C), RCC (positive; data not shown),
EMA (focally positive; Fig. 3E),
Ki-67 (positive; ~5%), S-100 (negative), CD68 (negative), HMB45
(negative) (data not shown), CD10 (negative; Fig. 3F) and CK8/18 (negative; Fig. 3D). The patient was given
anti-infection treatment by intravenous drip of cefoxitin,
hemostasis treatment by intravenous drip of carbazochrome sodium
sulfonate for injection, analgesia treatment by intravenous drip of
propacetamo 1 hydrochloride for injection and nutritional support
following the operation. The patient was discharged 4 days after
the operation. At two and a half years following the operation, the
chest and abdomen of the patient was reexamined by CT and no signs
of tumor recurrence and metastasis were found (Fig. 1F).
The medical history of the patient was reviewed.
This renal mass may have existed at 5 years from first
presentation. The patient underwent chest CT, which revealed that
the local renal parenchyma has protruded 1–2 mm on the lateral side
of the left kidney due to thoracic trauma in 2016 (Fig. 1E).
Discussion
Previous reports have indicated the indolent
behavior of MTSCC, with growth of 0.33 cm/year (3). In general, MTSCC has a better
prognosis than other RCCs, including slower growth and
significantly lower rates of progression, metastases and mortality
(7). In the patient described in
the present study, the left kidney local morphology was herniated
five years ago, and the postoperative tumor size was 3.0×2.6×1.7
cm. According to the change in this mass size, it was concluded
that this MTSCC was a slow-growing carcinoma. However, some MTSCCs
of the kidney are highly malignant with locally advanced
metastasis. Nephron-sparing surgery has been recommended for MTSCCs
by a number of authors (8) and a
number of studies have confirmed good long-term results and
excellent patient survival (2,9).
Histologically, MTSCCs are composed of a white
mucinous matrix, long narrow tubular epithelial cells and spindle
cells. Clear cell renal cell carcinoma is characterized by cells
with clear cytoplasm and a delicate capillary network. Papillary
renal cell carcinoma shows a focal papillary architecture. Renal
collecting duct carcinoma presents irregular adenoid and small
tubular structure, with high nuclear grade and evident nucleoli
(10). In 2006, according to the
percentage of extracellular mucin in the tumor after adequate
sampling, Fine et al (8)
expanded the histological spectrum of MTSCC into two variants:
Classic, with ample mucin stroma, and mucin-poor, with little to no
mucin. Classic tumors have an indolent behavior. Lack of mucin may
be related to sarcomatoid transformation and metastasis. A few
MTSCCs have been reported to exhibit sarcomatoid changes and
high-grade epithelial elements (10). Some studies (10,11)
have shown that the variability in the imaging features of MTSCCs
is based on their histological diversity. The signal intensity on
T2WI images is determined by the amount of mucin; the greater the
amount of mucin is, the higher the signal intensity (11,12).
In the current patient, the tumor had an obviously high signal on
the T2WI image and contained a large amount of mucin.
Genetically, some studies have revealed that MTSCCs
with aggressive clinical behavior have progressed through clonal
evolution; CDKN2A/B deletion and additional complex genomic
abnormalities may contribute to this process (13,14).
Locally advanced/metastatic MTSCCs share typical MTSCC genomic
profiles with loss of chromosomes 1, 4, 6, 8, 9, 13, 14, 15 and 22,
while some exhibit additional complex genomic alterations, most
frequently a relative gain of 1q (7/8) (14,15).
Wang et al (16) identified
VSTM2A and IRX5 as novel cancer-specific and lineage-specific
biomarkers in MTSCC. Immunohistochemically, the neoplastic cells of
both the tubules and spindle cells stain consistently positive for
PAX2/8, low-molecular-weight cytokeratins (CK8/18, CK19 and CK7),
EMA, alpha-methylacyl-CoA racemase and E-cadherin (17). In the present case report the
results were Vim (positive), PAX8 (positive), RCC (positive), EMA
(focally positive), Ki-67 (positive) and CK8/18 (negative). The
present case was negative for CK8/18 but previous cases of MTSCC
have been positive (17). The
phenomenon may be the same as that of CD10 and CD15 described in
Zhao et al (17), which are
usually negative and occasionally positive. CD10 marker is
sensitive to renal cell neoplasms derived from proximal tubules,
including clear cell and papillary RCCs (18). Only 15% of the mucinous tubular and
spindle cell carcinoma displayed immunoreactivity with CD10
(19). CD15 interacts with E-, L-
and P-selectins, which allows for adhesion with endothelial cells.
CK8/18 are the sole keratins present in the proximal tubular
epithelial cells of the kidney (20). The negative expression of CD10, CD15
and CK8/18 in MTSCC is related to the histological structure and
differentiation degree of MTSCC (21). The positive expression of CD10, CD15
and CK8/18 in other tumors can represent that the tumor is
malignant (20,22).
MTSCCs are usually a solitary, well-circumscribed,
isodense mass originating from the renal medulla on CT imaging.
They can be exophytic, partially exophytic, or endophytic tumors.
Enhancement is less than that of the cortex and medulla in all CT
phases and lesions show mild to moderate, uniform and progressive
enhancement but may be homogenous when tumors are <5 cm
(23). Dual-energy imaging can be
used to distinguish mild enhancement from nonenhancement. Cystic
components and calcification are rarely detected (24). Renal vein invasion, perinephric
extension, and metastatic disease are extremely rare (4,5).
However, in a few studies, metastasis has been reported in 10–27%
of patients (9,25). Common metastatic sites include the
lymph nodes, bone and retroperitoneum (13). The studies by Lu et al
(24) and Zhu et al
(26) show that MTSCCs are isodense
or hypodense masses on unenhanced CT scans. In the present report,
the mass was slightly hypodense. It was isointense on T1WI and
usually had a high signal on T2WI (classical). DWI indicated a high
signal; the ADC presented a low signal. Benign tumors of the kidney
typically show low enhancement on contrast-enhanced CT imaging,
high signal on DWI and low signal on ADC (27).
MTSCC, together with collecting duct carcinoma (CDC)
and papillary renal cell carcinoma (PRCC), are thought to be
hypovascular tumors due to their enhancement pattern. However,
their treatment modalities and prognoses are different. Therefore,
it is necessary for radiologists to differentially diagnose MTSCC,
papillary RCC and CDC. PRCC is a slight hyperattenuating tumor;
however, MTSCC is an isodense or hypodense mass. Some researchers
consider that the pathological basis of hyperdensity on unenhanced
CT is mainly hemosiderin deposition (24). PRCCs usually have calcification,
necrosis and cystic changes on CT. Enhancement is greater with PRCC
than with MTSCC tumors during all phases of CT. MTSCC is
homogeneously slightly hyperintense, whereas PRCC is
heterogeneously hypointense on T2WI. The T2 signal intensity ratio
of MTSCC is 0.96, and that of PRCC is 0.67 (28). Most patients with MTSCC show
homogeneous enhancement and no retroperitoneal lymph node
metastasis, whereas those with CDC show heterogeneous enhancement
and retroperitoneal lymph node metastasis. Normal renal cortex and
medulla enhancement are more than MTSCC and CDC tumor enhancement
in all phases of CT (5). However,
the degree of enhancement of MTSCC is less than that of CDC in all
phases.
As this type of tumor is rare, the best treatment
scheme has yet to be properly determined. Nephrectomy and tumor
cryoablation are performed in patients without metastasis and the
effect of treatment is good. According to Li et al (3), follow-up observation and delayed
treatment of patients with MTSCC does not reduce the 3-year
survival rate or increase the recurrence rate. No therapeutic
strategy has yet been established for metastatic MTSCC. One case
report described the surgical removal of MTSCC with lymph node
metastasis. One year after the operation, repeated imaging showed
no signs of recurrence (7). It has
been reported that sunitinib and the combination of nivolumab plus
ipilimumab are effective in the treatment of metastatic patients
(9,29). Ivey et al (7) demonstrated a large left renal mass and
associated retroperitoneal lymphadenopathy on CT. The pathological
features of the mass is a tubulocystic pattern with mucin
interspersed with a spindle cell pattern. Ged et al
(9) presented specific pathological
features that high-grade histological features or sarcomatoid
dedifferentiation were diagnosed in 5 of 6 patients with metastatic
disease compared with 0 of 19 non-metastatic patients. There was
revealed a large tumor at the upper pole of the left kidney with
bone metastases on CT in Furubayashi et al (29). From these cases, it is known that
MTSCC with high degree of malignancy is either a larger tumor or a
higher histological grade. As far as the treatment results are
concerned, compared with the treatment of patients in Ivey et
al (7) and Li et al
(3), there is no difference in the
rate of recurrence one year after operation. Since the present
patient's tumor was small, there were no high-grade histological
features and metastases. The patient in the present case report
lived longer and treatment effect is superior compared with the
treatment of patients in Ged et al (9) and Furubayashi et al (29).
Most patients are asymptomatic and tumors are found
by accident. Some patients with uncommon histologic features may
have painless gross hematuria or lower back pain. In the present
case, the tumor was discovered by accident without any clinical
symptoms. Previous case reports showed that MTSCC is an indolent
tumor with a long course of disease (2,3).
However, in previous case reports, it can be explained that there
are no comprehensive image of the case with a long course of the
tumor and a case report without an image cannot provide a longer
course of the disease. The novelty of the present case lies in
providing a morphological change of the patient's tumor for 5
years, dual-energy CT image, a comprehensive image consistent with
the pathological diagnosis and postoperative reexamination image.
The present case report can supplement the image performance of
MTSCC. The limitation of the present study is that it does not
include an image of the tumor after surgical removal.
MTSCCs grow slowly. Early in the cancer, the mass
may not be visible, but the local morphology of the kidney may have
changed based on CT images. However, MTSCCs are not always indolent
tumors. It is necessary to raise radiologists' awareness of the
risks of MTSCC. Any lump in the kidney or any localized change in
the morphology of the kidney should be of concern to the
radiologist. MTSCCs generally have low malignancy. Therefore, with
improved preoperative diagnosis, nephron-sparing surgery should be
considered as the principal treatment choice. This will be helpful
in preserving the postoperative renal function of patients.
Acknowledgments
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GRW performed data collection and wrote the
manuscript. JRZ and LJ were responsible for the analysis of case
data and literature and edited the manuscript. JJL performed data
collection. LYZ performed the staining of this tumor and provided
the pathological procedures. WY performed data analysis and
supervised the present study. WY and JJL confirm the authenticity
of all the raw data. All authors agreed to the journal to which the
article was submitted and agreed to take responsibility for all
aspects of the work. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First People's Hospital of Zunyi (Zunyi, China;
approval no. 2023201). The patient provided written informed
consent. The patient agreed to cooperate with us to have a
chest/abdomen CT examination every year to observe the
postoperative recurrence/metastasis. Thus, an ethical review was
conducted.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu X, Zhong J, Zhou X, Wei Z, Xia Q, Huang
P, Shi C, Da J, Tang C, Cheng W and Ge J: Mucinous tubular and
spindle cell carcinoma of the kidney: A study of clinical, imaging
features and treatment outcomes. Front Oncol. 12:8652632022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li XS, Yao L, Gong K, Yu W, He Q, Zhou LQ
and He ZS: Growth pattern of renal cell carcinoma (RCC) in patients
with delayed surgical intervention. J Cancer Res Clin Oncol.
138:269–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cornelis F, Ambrosetti D, Rocher L, Derchi
LE, Renard B, Puech P, Claudon M, Rouvière O, Ferlicot S, Roy C, et
al: CT and MR imaging features of mucinous tubular and spindle cell
carcinoma of the kidneys. A multi-institutional review. Eur Radiol.
27:1087–1095. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu J, Zhu Q, Zhu W, Chen W and Wang S:
Comparative study of CT appearances in mucinous tubular and spindle
cell carcinoma and collecting duct carcinoma of the kidney. Br J
Radiol. 88:201404342015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nathany S and Monappa V: Mucinous tubular
and spindle cell carcinoma: A review of histopathology and clinical
and prognostic implications. Arch Pathol Lab Med. 144:115–118.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ivey JA III, Cortese C, Baird BA, Thiel DD
and Lyon TD: Mucinous tubular and spindle cell carcinoma of the
kidney with nodal metastasis managed with surgical resection. Eur
Urol Open Sci. 29:10–14. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fine SW, Argani P, DeMarzo AM, Delahunt B,
Sebo TJ, Reuter VE and Epstein JI: Expanding the histologic
spectrum of mucinous tubular and spindle cell carcinoma of the
kidney. Am J Surg Pathol. 30:1554–1560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ged Y, Chen YB, Knezevic A, Donoghue MTA,
Carlo MI, Lee CH, Feldman DR, Patil S, Hakimi AA, Russo P, et al:
Mucinous tubular and spindle-cell carcinoma of the kidney: Clinical
features, genomic profiles, and treatment outcomes. Clin Genitourin
Cancer. 17:268–274.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trpkov K, Hes O, Williamson SR, Adeniran
AJ, Agaimy A, Alaghehbandan R, Amin MB, Argani P, Chen YB, Cheng L,
et al: New developments in existing WHO entities and evolving
molecular concepts: The genitourinary pathology society (GUPS)
update on renal neoplasia. Mod Pathol. 34:1392–1424. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farghaly H: Mucin poor mucinous tubular
and spindle cell carcinoma of the kidney, with nonclassic
morphologic variant of spindle cell predominance and psammomatous
calcification. Ann Diagn Pathol. 16:59–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thway K, du Parcq J, Larkin JMG, Fisher C
and Livni N: Metastatic renal mucinous tubular and spindle cell
carcinoma. Atypical behavior of a rare, morphologically bland
tumor. Ann Diagn Pathol. 16:407–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang C, Cimera RS, Aryeequaye R,
Jayakumaran G, Sarungbam J, Al-Ahmadie HA, Gopalan A, Sirintrapun
SJ, Fine SW, Tickoo SK, et al: Adverse histology, homozygous loss
of CDKN2A/B, and complex genomic alterations in locally
advanced/metastatic renal mucinous tubular and spindle cell
carcinoma. Mod Pathol. 34:445–456. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brandal P, Lie AK, Bassarova A, Svindland
A, Risberg B, Danielsen H and Heim S: Genomic aberrations in
mucinous tubular and spindle cell renal cell carcinomas. Mod
Pathol. 19:186–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cossu-Rocca P, Eble JN, Delahunt B, Zhang
S, Martignoni G, Brunelli M and Cheng L: Renal mucinous tubular and
spindle carcinoma lacks the gains of chromosomes 7 and 17 and
losses of chromosome Y that are prevalent in papillary renal cell
carcinoma. Mod Pathol. 19:488–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Zhang Y, Chen YB, Skala SL,
Al-Ahmadie HA, Wang X, Cao X, Veeneman BA, Chen J, Cieślik M, et
al: VSTM2A overexpression is a sensitive and specific biomarker for
mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney.
Am J Surg Pathol. 42:1571–1584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao M, He XL and Teng XD: Mucinous
tubular and spindle cell renal cell carcinoma: A review of
clinicopathologic aspects. Diagn Pathol. 10:1682015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Truong LD and Shen SS: Immunohistochemical
diagnosis of renal neoplasms. Arch Pathol Lab Med. 135:92–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paner GP, Srigley JR, Radhakrishnan A,
Cohen C, Skinnider BF, Tickoo SK, Young AN and Amin MB:
Immunohistochemical analysis of mucinous tubular and spindle cell
carcinoma and papillary renal cell carcinoma of the kidney:
Significant immunophenotypic overlap warrants diagnostic caution.
Am J Surg Pathol. 30:13–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moll R, Divo M and Langbein L: The human
keratins: Biology and pathology. Histochem Cell Biol. 129:705–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferlicot S, Allory Y, Compérat E,
Mege-Lechevalier F, Dimet S, Sibony M, Couturier J and Vieillefond
A: Mucinous tubular and spindle cell carcinoma: A report of 15
cases and a review of the literature. Virchows Arch. 447:978–983.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szlasa W, Wilk K, Knecht-Gurwin K, Gurwin
A, Froń A, Sauer N, Krajewski W, Saczko J, Szydełko T, Kulbacka J
and Małkiewicz B: Prognostic and therapeutic role of CD15 and CD15s
in cancer. Cancers (Basel). 14:22032022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kenney PA, Vikram R, Prasad SR, Tamboli P,
Matin SF, Wood CG and Karam JA: Mucinous tubular and spindle cell
carcinoma (MTSCC) of the kidney: A detailed study of radiological,
pathological and clinical outcomes. BJU Int. 116:85–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu D, Yuan W, Zhu Q, Ye J, Zhu W and Chen
W: Comparative study of CT and MRI appearances in mucinous tubular
and spindle cell carcinoma and papillary renal cell carcinoma. Br J
Radiol. 94:202105482021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adamane SA, Menon S, Prakash G, Bakshi G,
Joshi A, Popat P and Desai SB: Mucinous tubular and spindle cell
carcinoma of the kidney: A case series with a brief review of the
literature. Indian J Cancer. 57:267–281. 2020.PubMed/NCBI
|
|
26
|
Zhu Q, Zhu W, Wang Z and Wu J: Clinical
and CT imaging features of mucinous tubular and spindle cell
carcinoma. Chin Med J (Engl). 127:1278–1283. 2014.PubMed/NCBI
|
|
27
|
Israel GM and Bosniak MA: How i do it:
Evaluating renal masses. Radiology. 236:441–450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oliva MR, Glickman JN, Zou KH, Teo SY,
Mortelé KJ, Rocha MS and Silverman SG: Renal cell carcinoma: t1 and
t2 signal intensity characteristics of papillary and clear cell
types correlated with pathology. AJR Am J Roentgenol.
192:1524–1530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Furubayashi N, Taguchi K, Negishi T, Miura
A, Sato Y, Miyoshi M and Nakamura M: Cytoreductive nephrectomy
after combination of nivolumab plus ipilimumab for mucinous tubular
and spindle cell carcinoma of the kidney with bone metastases: A
case report. In Vivo. 36:510–521. 2022. View Article : Google Scholar : PubMed/NCBI
|