Introduction
Marginal zone B-cell lymphoma (MZBL) represents
about 7% of all non-Hodgkin lymphomas and is the third most common
subtype after diffuse large B-cell lymphoma and follicular lymphoma
(1). Extranodal primary MZBL
originates from the mucosa-associated lymphoid tissue of several
organs, and the stomach is the most frequent site of extranodal
involvement (2). Rarely, primary
extranodal MZBL may develop in non-mucosal-associated tissue: in
this context, central nervous system (CNS) involvement is an
extremely rare event. According to the literature data, primary CNS
MZBL is usually localised within dura matter and manifests a better
prognosis compared to other primary CNS lymphoma subtypes: in fact,
most CNS lymphomas are high-grade diffuse large B-cell lymphomas
displaying aggressive behaviour, while primary CNS MZBL is
characterised by an indolent course and remains localised for a
long period of time (2). However,
the diagnosis of such an entity is tricky, given the disease
rarity, and most cases are initially misdiagnosed in the clinical
and on imaging. In fact, several pathologies share overlapping
radiological characteristics: meningioma represents the main
differential diagnosis, accounting for about 30% of all
intracranial tumours (3), but
aspergillosis, sarcoidosis, metastases, chloromas, gliomas and
schwannomas must also be considered (2). For this reason, histological
examination is essential to correctly and definitively diagnose
primary CNS MZBL. In fact, it is mainly characterised by the
presence of small B-cell lymphocytes admixed with plasma cells,
monocytoid cells, and scattered large immunoblasts (4). B cells are positive for CD20 and show
clonal rearrangements of IgH and k without expressing CD23,
Bcl-6, cyclin D1, CD5, and CD10 (5,6).
Tumour cells are also positive for Bcl-2, and 50% of cases are CD43
positive (7). The presence of a low
proliferation index confirms the indolent nature of such a
neoplasm, and this finding is typical of such pathology.
Multiple genetic abnormalities have been detected in
MZBL: chromosomal alterations, most often trisomies of chromosome
3, are occasionally detected (5,8),
inactivation of TNFAIP3 (common in cases with plasmacytic
differentiation) and activating NOTCH2 mutations accompanied
by inactivating TBL1XR1 mutations (common in cases with
monocytoid morphology) (9). Here,
the first case of a primary CNS extranodal MZBL is presented,
arising at the base of the choroid plexuses of the left lateral
ventricle.
Case report
In July 2021, a 48-year-old HIV-negative female
without a significant medical history (except for a head injury 1
year earlier) presented at the Hospital University Polyclinic ‘G.
Martino’ (Messina, Italy) after having worsening headaches, nausea,
vomiting, and dizziness. Neurological examination and routine
laboratory tests did not reveal any abnormalities.
The previous year, she underwent a computerised
tomography (CT) scan, which showed neither intracranial expansive
lesions nor haemorrhages (after a traumatic accident), while the
MRI performed after the worsening symptoms revealed an about 3-cm
extra-axial mass within the left lateral ventricle with contrast
enhancement (Fig. 1).
Clinically, the most likely diagnoses were
represented by an ependymoma, a choroid plexus papilloma, an
intraventricular meningioma or a metastasis, and less likely by an
inflammatory process or a lymphoma.
In November 2021, the patient was admitted to the
Division of Neurosurgery of the Hospital University Polyclinic ‘G.
Martino’ (Messina, Italy), where she underwent total resection of
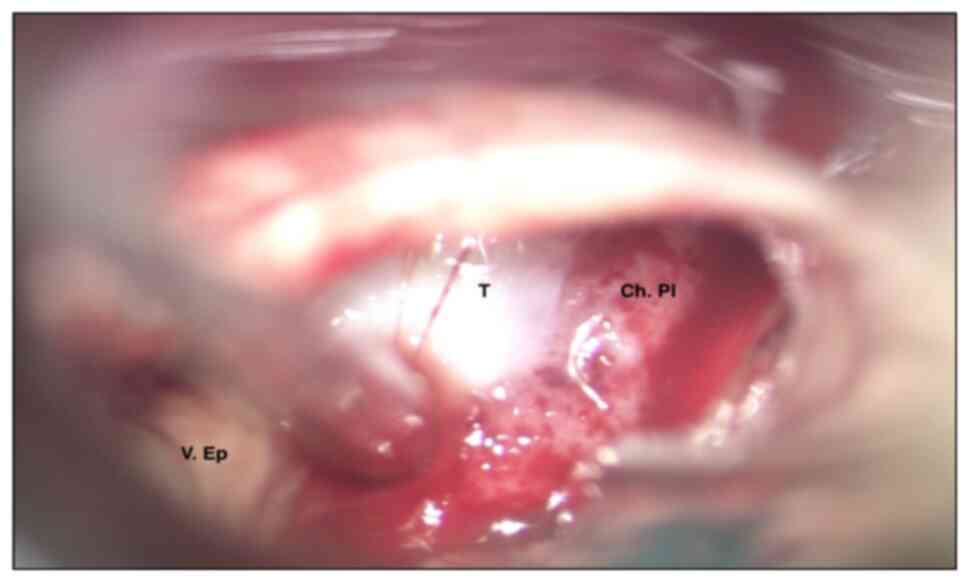
the lesion. At intraoperative macroscopic examination, it appeared
as a whitish mass with vascularised areas and a hard-friable
consistency (Fig. 2). After the
excision, the lesion shrank into multiple fragments that were fixed
in 10% neutral formalin for 24–36 h at room temperature, embedded
in paraffin at 56°C, and then cut into 5 µm thick serial sections
for routine haematoxylin/eosin histological staining and Congo red
stain. Moreover, parallel sections were cut and mounted on silane
coated glass for immunohistochemistry, then dewaxed in xylene and
rehydrated in graded ethanol. The immunohistochemical procedure was
performed using the automated Ventana BenchMark ULTRA platform with
Cell Conditioning 1 for 64 min, pre-peroxidase inhibition, and
primary antibody incubation for 16 min at 37°C. The OptiView DAB
IHC Detection Kit (Ventana Medical Systems, Inc.) was used to
detect protein expression of the primary antibodies shown in
Table I. Finally, all slides were
counterstained with Hematoxylin II (Ventana Medical Systems, Inc.)
and Bluing Reagent (Ventana Medical Systems, Inc.) for 4 min at
room temperature. To ensure the reliability of the results of the
immunohistochemical reactions, external positive and negative
controls were run according to the manufacturer's instructions. Of
note, immunohistochemical analyses were performed on parallel
sections from the same paraffin-embedded tissue.
 | Table I.List of antibodies used and their
immunoreactivity in our case. |
Table I.
List of antibodies used and their
immunoreactivity in our case.
| Antibody | Manufacturer | Catalogue number | Clone | Dilution | Immunoreactivity in
our case |
|---|
| Bcl2 | Ventana | 790-4604 | SP66 | Pre-diluted | Positive in
neoplastic small B lymphocytes |
| CD20 | Ventana | 760-2531 | L26 | Pre-diluted | Positive in
neoplastic small B lymphocytes |
| CD138 | Ventana | 760-4248 | B-A38 | Pre-diluted | Positive in clonal
plasma cells |
| Kappa | Ventana | 760-2514 | Polyclonal | Pre-diluted | Positive in clonal
plasma cells |
| Lambda | Ventana | 760-2515 | Polyclonal | Pre-diluted | Negative in clonal
plasma cells |
| IgG | Ventana | 760-2653 | Polyclonal | Pre-diluted | Non-specific
positivity of plasma cells |
| IgG4 | Ventana | 760-4614 | MRQ-44 | Pre-diluted | Non-specific
positivity of plasma cells |
| Bcl6 | Ventana | 760-4241 | GI191E/A8 | Pre-diluted | Negative in
neoplastic small B lymphocytes |
| CD23 | Ventana | 790-4408 | EP3093 | Pre-diluted | Negative in
neoplastic small B lymphocytes |
| Cyclin D1 | Ventana | 790-4508 | SP4-R | Pre-diluted | Negative in
neoplastic small B lymphocytes |
| SOX-11 | Ventana | 760-4868 | MRQ-58 | Pre-diluted | Negative in
neoplastic small B lymphocytes |
| CD5 | Ventana | 790-4451 | SP19 | Pre-diluted | Negative in
neoplastic small B lymphocytes |
| CD10 | Ventana | 790-4506 | SP67 | Pre-diluted | Negative in
neoplastic small B lymphocytes |
| Ki-67 | Ventana | 790-4286 | 30-9 | Pre-diluted | 5-10% of neoplastic
small B lymphocytes |
Microscopic examination showed a diffuse
proliferation of lymphoplasmacellular elements within the fragments
of a choroid plexus rich in fibrosclerotic tissue, also with the
presence of amorphous material deposits (Fig. 3A-D). Three main pathological
pictures represented the main diagnostic hypotheses: first of all,
a chronic/reactive post-traumatic inflammatory process, rich in
plasma cells; secondly, a hereditary transthyretin amyloidosis,
which usually has an adult-onset and is characterised by the
deposition of a misfolded transthyretin produced by the choroid
plexus and its consequent accumulation in the leptomeninges
(10); lastly, an IgG4-related
disease. However, the negative Congo red stain and non-specific
positivity of plasma cells for IgG and IgG4, together with normal
serum IgG4 levels, ruled out the latter two diagnostic hypotheses
(IgG and IgG4 immunohistochemical staining is shown in Fig. S1). A post-traumatic inflammatory
process represented the only plausible diagnostic hypothesis, but
the morphology and distribution pattern of the inflammatory
elements were not univocally attributable to such a process. In
fact, the lymphoplasmacellular infiltrate was mainly represented by
monomorphic small B lymphocytes with a marginal growth pattern and
marked plasma cell differentiation (Fig. 3A-D). Immunohistochemical examination
revealed positivity for CD20 and Bcl2 in small B lymphocytes
(Fig. 3E and F) and CD138
positivity (Fig. 3G) with k
clonal restriction in plasma cells (Fig. 3H).
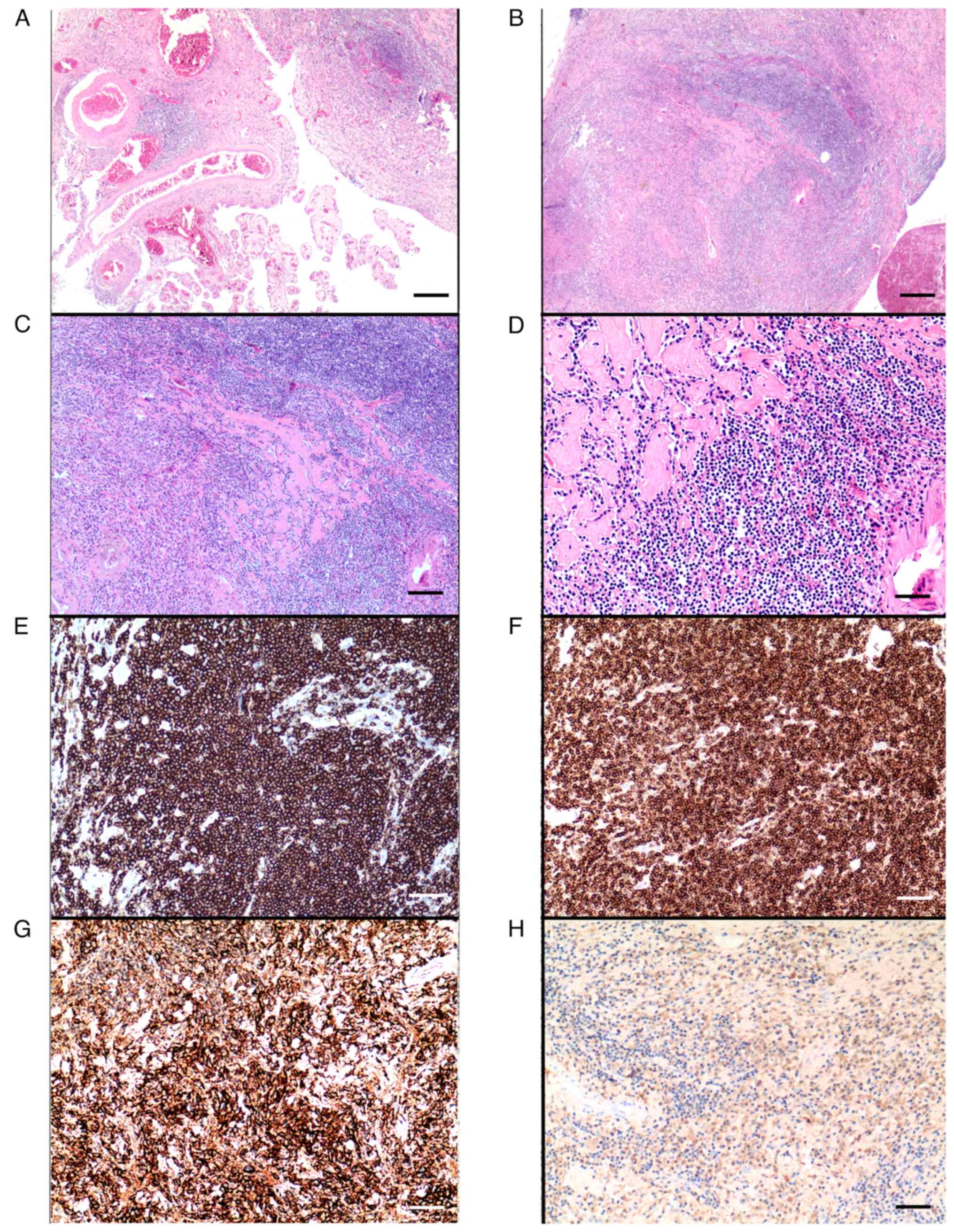 | Figure 3.(A-D) Haematoxylin and eosin staining
showing (A) fragments of a choroid plexus (×20; scale bar, 420 µm)
infiltrated (B) by a diffuse proliferation of lymphoplasmacellular
elements with a marginal growth pattern (×20; scale bar, 420 µm),
intermingled (C) with fibrosclerotic tissue (×40; scale bar, 210
µm; the same area is shown at different magnifications in B and C).
(D) Small monomorphic B lymphocytes, fibrosclerotic bands and
deposits of amorphous material are particularly evident at higher
magnification (×100; scale bar, 84 µm). (E-H) Immunohistochemical
staining showing a neoplastic B-lymphocyte population with diffuse
positivity for (E) CD20 (×100; scale bar, 84 µm) and (F) Bcl2
(×100; scale bar, 84 µm). The marked plasma cell differentiation is
evidenced by (G) CD138 positivity (×100; scale bar, 84 µm) with (H)
k clonal restriction (×100; scale bar, 84 µm). |
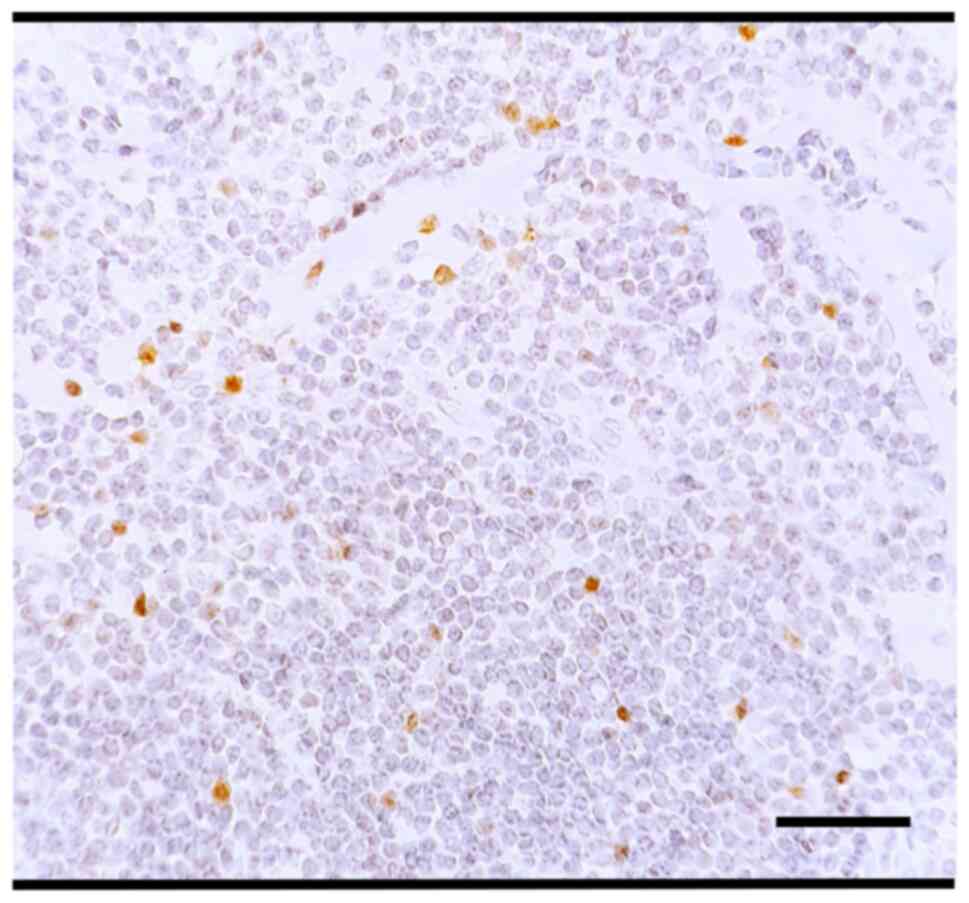
CD23, Bcl-6, cyclin D1, SOX-11, CD5 and CD10 were
all negative (Fig. S2;
immunoreactivity for each antibody is reported in Table I) and the ki-67 proliferation index
was very low (5–10%, Fig. 4). On
this basis, a lymphoproliferative process represented the only
possible diagnosis, both a CNS localisation of a systemic disease
or a primitive CNS lymphoma. Of note, the cytological analysis of
cerebrospinal fluid and bone marrow biopsy with flow cytometry and
cytogenetics showed no evidence of lymphomatous infiltration (data
not shown), and CT and positron emission tomography (PET) scans of
the thorax, abdomen and pelvis confirmed the absence of systemic
disease (Fig. S3 shows the
negative PET scan of the thorax, abdomen and pelvis). Therefore, it
was a primitive CNS lymphoma. Differential diagnoses included a
small lymphocytic lymphoma/chronic lymphocytic leukaemia (CLL/SLL),
lymphoplasmacytic lymphoma, follicular lymphoma, mantle cell
lymphoma and marginal zone lymphoma. CLL/SLL was unlikely due to
the absence of peripheral blood or bone marrow involvement and
negativity for CD5 and CD23. Similarly, in the absence of bone
marrow involvement, negative PET scan and no clinical history of
Waldenstrom's macroglobulinemia with the absence of both
MYD88 mutation and clonal circulating IgM by serum protein
electrophoresis and immunofixation electrophoresis (data not
shown), lymphoplasmacytic lymphoma involvement was unlikely.
Moreover, the lack of an overt morphological follicular appearance
and CD10 negativity made follicular lymphoma unlikely. Similarly,
negativity for cyclin D1, SOX-11 and CD5 ruled out mantle cell
lymphoma. Therefore, a primary CNS MZBL arising at the base of the
choroid plexuses of the left lateral ventricle represented the only
possible diagnosis. In this diagnostic challenge, considering the
unusual anatomical site for the localisation of such a
lymphoproliferative process, a polymerase chain reaction (PCR)
analysis of B lymphocyte clonality was performed. A monoclonal
population of B lymphocytes with a monoallelic rearrangement of the
IGH gene, FR2/JH, FR3/JH fragments and the k gene was
identified by testing for immunoglobulin heavy chain (IGH) and
kappa light chain gene (IGK) rearrangement using the PCR developed
by the collaborative European BIOMED-2 Concerted Action Study
Group. The monoallelic rearrangement was typically characterised by
the presence of a single peak (11), as in the case of the IGH gene,
FR2/JH fragment (Fig. S4), in
which the neoplastic elements showed a dominant fluorescent peak
indicative of a clonal population with identical PCR fragment sizes
at ~128 bp. Therefore, molecular studies corroborated the diagnosis
of a primary CNS MZBL with k plasma cell differentiation.
However, we currently do not have access to all PCR result graphs
since they are no longer visible in our digital archive, and this
issue can represent a limitation of our study. Nonetheless, all
missing PCR results can be retrieved by our hospital informatics
staff upon reasonable request. After the diagnosis, the patient
underwent standard clinical treatment for primary CNS extranodal
MZBL, receiving whole-brain external beam radiotherapy with a total
dose of 24 Gy, and follow-up CT (shown in Fig. S5) and MRI (not shown) showed the
disappearance of the gross disease (neither a mass effect nor an
abnormal enhancement was evident). She has also undergone a strict
follow-up to exclude treatment-related toxicities, particularly
regarding head and neck irradiated structures. Specifically,
thyroid function tests showed no signs of hypothyroidism (data not
shown). There were also no signs of treatment-related neurotoxicity
(like impaired cognitive function), xerostomia from parotid
irradiation and cataracts, or dry eye from orbital irradiation.
Currently, she has been alive and without symptoms for 12 months up
to the date of this report. The patient performed all follow-up
examinations privately, and therefore these were not available to
us at the moment of writing the manuscript. However, they have been
viewed by the neurosurgeons who managed her (MC and FA). The
corresponding author could request all the missing examinations
from the patient and provide them to anyone who asks upon
reasonable request.
Discussion
Extranodal MZBL arises from mucosa-associated
lymphoid tissue and has been described in association with specific
chronic inflammatory processes, both infectious and autoimmune,
such as within gastric mucosa colonised by H. pylori and
within lacrimal glands in Sjögren syndrome (3,4).
However, the CNS is devoid of mucosa-associated lymphoid tissue,
and the exact pathogenesis of primary CNS MZBL is poorly understood
(12). In most cases, primary CNS
MZBL is not directly linked to any infectious or autoimmune
condition and is, in fact, most frequently diagnosed in
immunocompetent middle-aged females, consistent with our patient's
presentation (12). The possible
mechanisms underlying the pathogenesis of CNS MZBL could be
ascribed to an implantation metastasis of undetected or vanishing
meningeal MZBL (13,14), a localisation of an undetected
primary extracranial MZBL, or chronic inflammation. This last
condition could derive from a direct chronic antigenic stimulation
of the dura, for example, from a long-standing history of untreated
HBV (15,16) or HCV (17). One patient with CNS MZBL had a long
history of white matter disease with some features of multiple
sclerosis (5), and another suffered
from a Chlamydia psittaci infection (18). Moreover, an association between
primary intracranial MZBLs and IgG4 expression was observed by
Venkataraman et al (8) in a
series of 32 dural-based MZBLs, a significant subset of which
showed IgG4 in light-chain-restricted clonal plasma cells.
In our case, the above-mentioned mechanism of
chronic inflammation, consequent to a previous head injury, could
have represented the pathogenetic substrate of MZBL onset: in fact,
chronic inflammation is associated with the genesis of organised
lymphoid tissue, and it has been demonstrated that the combination
of persistent BCR triggering, chronic T-cell help and TLR
stimulation elicited by chronic inflammation in these ectopically
formed lymphoid tissues can be overruled by genetic alterations
that guarantee constitutive NF-κB signalling. Thus, cell becomes
less dependent on the environmental stimuli, therefore predisposing
to MZBL development (19). It has
also been hypothesised that meningothelial cells of the arachnoid
membrane are comparable to epithelial cells in those organs in
which typically MALT lymphomas arise. These cells are ubiquitous in
the arachnoid membrane and are mainly concentrated not only in the
arachnoid granulations but also in the choroid plexus and
subarachnoid cisterns (20).
Therefore, this theory could well explain primary CNS MZBLs arising
extra-axially (21,22).
To our knowledge, this is the first
literature-reported case of a primary CNS MZBL arising at the base
of the choroid plexuses of a lateral ventricle; in fact, even the
most recent literature data do not report anything similar at this
anatomical site (14). Moreover,
our case is really peculiar because it is related to a history of
previous trauma and chronic inflammation, confirming what has
already been demonstrated about the role of chronic inflammation in
the pathogenesis of primary CNS MZBLs. Additionally, it could
represent a starting point for studies analysing the role of
meningothelial cells in the pathogenesis of primary CNS MZBL. Given
the singularity of our case, it would have been useful to exploit
whole slide imaging (WSI) systems to quickly share histological
slides with international experts in the field and also to preserve
high-quality images in a digital archive. However, we currently do
not have WSI scanners at our Institution, and the absence of images
in WSI format represents a limitation of our study. Nonetheless,
our experience underlines the therapeutic and prognostic importance
of a correct diagnosis of primary CNS MZBL since it is a
particularly radiosensitive low-grade disease with an indolent
clinical course, an excellent prognosis, and is effectively
treatable with localised radiotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
VF and MM confirm the authenticity of all the raw
data. VF, MM and LML conceptualised the manuscript. CP, ML and AI
made substantial contributions to the conception and design of the
manuscript. VF wrote the original draft. MC and FA managed the
patient. VF, MM, FP and LML participated in the pathological
evaluation. CP, ML, MC and FP retrieved data from the literature.
FA, GT and GF made substantial contributions to the acquisition of
clinicopathological data and their analysis and interpretation. AI,
FA, GT and GF reviewed and edited the manuscript. LML, GT and GF
supervised. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient's consent for publication
Written informed consent was obtained from the
patient for publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zucca E, Arcaini L, Buske C, Johnson PW,
Ponzoni M, Raderer M, Ricardi U, Salar A, Stamatopoulos K,
Thieblemont C, et al: Marginal zone lymphomas: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 31:17–29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sebastián C, Vela AC, Figueroa R, Marín MÁ
and Alfaro J: Primary intracranial mucosa-associated lymphoid
tissue lymphoma. A report of two cases and literature review.
Neuroradiol J. 27:425–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pavlou G, Pal D, Bucur S, Chakrabarty A
and van Hille PT: Intracranial non-Hodgkin's MALT lymphoma
mimicking a large convexity meningioma. Acta Neurochir (Wien).
148:791–793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goda JS, Gospodarowicz M, Pintilie M,
Wells W, Hodgson DC, Sun A, Crump M and Tsang RW: Long-term outcome
in localized extranodal mucosa-associated lymphoid tissue lymphomas
treated with radiotherapy. Cancer. 116:3815–3824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tu PH, Giannini C, Judkins AR, Schwalb JM,
Burack R, O'Neill BP, Yachnis AT, Burger PC, Scheithauer BW and
Perry A: Clinicopathologic and genetic profile of intracranial
marginal zone lymphoma: A primary low-grade CNS lymphoma that
mimics meningioma. J Clin Oncol. 23:5718–5727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lopetegui-Lia N, Delasos L, Asad SD, Kumar
M and Harrison JS: Primary central nervous system marginal zone
B-cell lymphoma arising from the dural meninges: A case report and
review of literature. Clin Case Rep. 8:491–497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sahm F, Reuss DE and Giannini C: WHO 2016
classification: Changes and advancements in the diagnosis of
miscellaneous primary CNS tumours. Neuropathol Appl Neurobiol.
44:163–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venkataraman G, Rizzo KA, Chavez JJ,
Streubel B, Raffeld M, Jaffe ES and Pittaluga S: Marginal zone
lymphomas involving meningeal dura: Possible link to IgG4-related
diseases. Mod Pathol. 24:355–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ganapathi KA, Jobanputra V, Iwamoto F,
Jain P, Chen J, Cascione L, Nahum O, Levy B, Xie Y, Khattar P, et
al: The genetic landscape of dural marginal zone lymphomas.
Oncotarget. 7:43052–43061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taipa R, Sousa L, Pinto M, Reis I,
Rodrigues A, Oliveira P, Melo-Pires M and Coelho T: Neuropathology
of central nervous system involvement in TTR amyloidosis. Acta
Neuropathol. 145:113–126. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langerak AW, Groenen PJ, Brüggemann M,
Beldjord K, Bellan C, Bonello L, Boone E, Carter GI, Catherwood M,
Davi F, et al: EuroClonality/BIOMED-2 guidelines for interpretation
and reporting of Ig/TCR clonality testing in suspected
lymphoproliferations. Leukemia. 26:2159–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bustoros M, Liechty B, Zagzag D, Liu C,
Shepherd T, Gruber D, Raphael B and Placantonakis DG: A rare case
of composite dural extranodal marginal zone lymphoma and chronic
lymphocytic leukemia/small lymphocytic lymphoma. Front Neurol.
9:2672018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saggioro FP, Colli BO, Paixão-Becker AN,
de Rezende GG, Santos AC and Neder L: Primary low-grade MALT
lymphoma of the dura. Histopathology. 49:323–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren J, Cai L, Ren J, Li S and Ding L:
Mucosa-associated lymphoid tissue in the central nervous system
presenting as meningioma: A case report. Oncol Lett. 26:2772023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ariani R and Ballas L: Primary CNS
extranodal marginal zone B-cell lymphoma: A case series of 2
patients treated with external beam radiation therapy. Case Rep
Oncol. 14:725–732. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ababou M, Mahtat EM, Jennane S, Elmaaroufi
H, Mikdame M and Doghmi K: Splenic marginal zone lymphoma
associated with hepatitis B virus infection, remission after viral
treatment, and splenectomy: A case report and review of the
literature. Hematol Oncol Stem Cell Ther. 14:153–155. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Villaume MT, Patel D, Lopez C, Patel V,
Diggs P, Harmsen H, Thompson MA and Morgan D: Dural marginal zone
lymphoma in a patient with a hepatitis C virus infection. World J
Oncol. 11:122–125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ponzoni M, Bonetti F, Poliani PL, Vermi W,
Bottelli C, Dolcetti R, Cangi MG, Ferreri AJ, Cin ED, Pasini E, et
al: Central nervous system marginal zone B-cell lymphoma associated
with Chlamydophila psittaci infection. Hum Pathol. 42:738–742.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bende RJ, van Maldegem F and van Noesel
CJ: Chronic inflammatory disease, lymphoid tissue neogenesis and
extranodal marginal zone B-cell lymphomas. Haematologica.
94:1109–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuller GN and Goodman JC: Practical review
of neuropathology. Lippincott, Williams & Wilkins;
Philadelphia: pp. 57–58. 2001, PubMed/NCBI
|
|
21
|
Kumar S, Kumar D, Kaldjian EP, Bauserman
S, Raffeld M and Jaffe ES: Primary low-grade B-cell lymphoma of the
dura: A mucosa associated lymphoid tissue-type lymphoma. Am J Surg
Pathol. 21:81–87. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aqil B, Rouah E and Verstovsek G: Primary
CNS marginal zone lymphoma: A case report and review of the
literature. Open J Pathol. 3:55–59. 2013. View Article : Google Scholar
|