Introduction
Cervical cancer is one of the most common types of
gynecological cancer, with an incidence of 7.7 per 100,000
population and mortality of 2.2 per 100,000 population worldwide
according to the Cancer Statistics (2023) (1,2).
Despite multi-therapy choice (such as surgery and chemoradiation)
for early and locally advanced-stage cervical cancer, treatment
options for patients with advanced cervical cancer patients are
limited and primarily include platinum-based chemotherapy regimens
(3–6). There are few therapeutic options
beyond first-line chemotherapy.
Immune checkpoint inhibitors (ICIs) provide another
therapeutic option for advanced cervical cancer (7–9). ICI
regimens have promising treatment outcomes [for example, 13.3–17.0%
of patients achieved objective response rate (ORR) in the
KEYNOTE-028 and KEYNOTE-158 trials (10,11)],
however ~80% of patients exhibit no response (10,11).
Hence, to minimize ineffective treatment, avoid the adverse
reactions of ICI regimens and reduce the consumption of medical
resources, it is urgent to find a potential biomarker to achieve
individualized ICI treatment in patients with advanced cervical
cancer.
Cell division cycle 42 (CDC42) is a small hydrolase
that regulates immune escape by several mechanisms, such as
regulating antigen-specific cytotoxic T lymphocyte-mediated
cytotoxicity and differentiation of macrophages into M2 phenotype
(12–14). For example, CDC42-deficient naïve T
cells preferentially differentiate to CD8+ effector
cells both in vitro and in vivo (15). Clinically, CDC42 could predict the
response of ICI treatment in several types of cancer, such as
hepatocellular carcinoma and colorectal cancer (16,17).
However, the effect of CDC42 on response of ICI treatment have not
been verified in patients with advanced cervical cancer. Hence, the
present study aimed to evaluate the potential of serum CDC42 in
predicting ICI treatment outcome in patients with advanced cervical
cancer.
Materials and methods
Patients
From July 2020 to December 2022, 46 patients (age,
36.0–70.0 years) with advanced cervical cancer who planned to
receive ICI treatment with or without antiangiogenic agents were
enrolled at Tongji Hospital (Wuhan, China). The inclusion criteria
were as follows: i) Histopathologically diagnosed as cervical
cancer; ii) confirmed as recurrent, persistent or metastatic
cervical cancer; iii) aged >18 years; iv) progression after at
least once standardized systemic chemotherapy; v) Eastern
Cooperative Oncology Group Performance Status (ECOG PS) (18) 0–1; vi) at least one measurable
lesion by the Response Evaluation Criteria in Solid Tumors (RECIST)
V.1.1 (19); vii) scheduled to
receive ICI treatment with or without antiangiogenic agents. The
exclusion criteria were as follows: i) Presence of other primary
malignant disease; ii) inadequate liver, kidney, heart, bone
marrow, and blood coagulation function; iii) history of ICI
treatment; iv) complicated with autoimmune disease; v) presence of
hematological diseases; vi) pregnancy or breastfeeding. The
patients provided written informed consent. The study was approved
by the Ethics Committee of Tongji Hospital, Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China;
approval no. ChiECRCT20200180).
Clinical characteristics
Clinical characteristics of patients were collected
from medical records, including age, International Federation of
Gynecology and Obstetrics (FIGO) (20) stage, ECOG PS, histology type, target
lesion size, metastasis, programmed death-ligand 1 combined
positive score [PD-L1 CPS; as previously described (21)] and treatment history.
Treatment
Patients received ICI treatment with or without
antiangiogenic agents according to their willingness, disease
condition and doctor's advice. The treatment was given in 3-week
cycles. ICI was given continually until intolerable toxicity
occurred or the disease progressed (up to 24 months) and included
camrelizumab (200 mg/cycle), sintilimab (200 mg/cycle),
pembrolizumab (200 mg/cycle) and atezolizumab (1,200 mg/cycle). For
patients who received ICI treatment combined with antiangiogenic
agents, antiangiogenic agents were given until the occurrence of
intolerable toxicity or disease progression and included
bevacizumab (15 mg/kg/cycle) and apatinib (250 mg/day). The dosing
adjustments were allowed based on conditions such as
intolerance.
Blood samples
Peripheral blood (5 ml) of patients was collected
before treatment (at baseline) and following two treatment cycles.
The serum was isolated (centrifugation at 1,800 × g for 10 min) and
serum CDC42 was detected by ELISA using commercial kits (cat. no.
JM-1116H1; Jiangsu Jingmei Biotechnology Co., Ltd.) according to
the manufacturer's instructions.
Assessment
Patients were routinely followed up until May 2023
and underwent imaging examinations (computed tomography or nuclear
magnetic resonance) after every two cycles of treatment. Based on
the imaging results after four cycles of treatment, the tumor
response was assessed via RECIST V.1.1 (19). Progression-free survival (PFS) and
overall survival (OS) were recorded for prognosis analysis.
CDC42 cutoff value
To explore the association of CDC42 with PFS and OS,
the CDC42 levels (baseline and following two treatment cycles) were
divided based on the cutoff value of 600 pg/ml (approximate median
value of CDC42 at baseline).
Statistical analysis
SPSS V.24.0 (IBM Corp.) was used for data
processing. Data are presented as median and interquartile range
(IQR). Comparisons were performed by the Wilcoxon or Kruskal-Wallis
H rank sum or Wilcoxon signed-rank test. Correlation analysis was
performed by Spearman's rank correlation test. PFS and OS were
determined by Kaplan-Meier curves and analyzed by log-rank test.
Factors associated with PFS and OS were determined by univariate
and backward-stepwise multivariate Cox's proportional hazard
regression models. A total of three independent experimental
repeats was performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics
The median (IQR) age of patients was 49.5
(45.8–52.0) years. A total of 15 (32.6%), 11 (23.9%), 8 (17.4%) and
12 (26.1%) patients had FIGO stage I, II, III and IV, respectively.
A total of 28 (60.9%) patients had squamous cell carcinoma; 15
(32.6%) patients had adenocarcinoma, while 3 (6.5%) patients had
adenosquamous carcinoma. The baseline median (IQR) CDC42 expression
was 599.0 (422.8–973.3) pg/ml. Baseline characteristics are shown
in Table I.
 | Table I.Clinical characteristics. |
Table I.
Clinical characteristics.
| Characteristic | Patients (n=46) |
|---|
| Age, years |
|
|
Median | 49.5 |
| IQR | 45.8–52.0 |
|
Range | 36.0–70.0 |
| FIGO stage at initial
diagnosis (%) |
|
| I | 15 (32.6) |
| II | 11 (23.9) |
| III | 8 (17.4) |
| IV | 12 (26.1) |
| ECOG PS (%) |
|
| 0 | 19 (41.3) |
| 1 | 27 (58.7) |
| Histology type
(%) |
|
|
Squamous cell carcinoma | 28 (60.9) |
|
Adenocarcinoma | 15 (32.6) |
|
Adenosquamous | 3 (6.5) |
| Target lesion size,
cm |
|
|
Median | 5.0 |
|
IQR | 4.0–7.5 |
|
Range | 2.0–14.0 |
| Pelvis metastasis
(%) | 22 (47.8) |
| Lung metastasis
(%) | 18 (39.1) |
| Liver metastasis
(%) | 13 (28.3) |
| Other distant
metastases (%) | 13 (28.3) |
| Previous platinum
(%) | 46 (100.0) |
| Previous paclitaxel
(%) | 46 (100.0) |
| Previous
bevacizumab (%) | 16 (34.8) |
| PD-L1 CPS (%) |
|
|
Positive | 33 (71.7) |
|
Negative | 10 (21.7) |
|
Unknown | 3 (6.5) |
| Treatment line
(%) |
|
|
1st | 0 (0.0) |
|
2nd | 19 (41.3) |
|
3rd | 19 (41.3) |
| 4th or
above | 8 (17.4) |
| Treatment (%) |
|
| ICI +
antiangiogenic therapy | 26 (56.5) |
|
ICI-alone | 20 (43.5) |
| CDC42 at baseline,
pg/ml |
|
|
Median | 599.0 |
|
IQR | 422.8–973.3 |
|
Range | 226.0–2161.0 |
High CDC42 at baseline is associated
with target lesion ≥5 cm and pelvis and lung metastases
CDC42 at baseline was increased in patients with
target lesion size ≥5 cm (P=0.020), the presence of pelvis
metastasis (P=0.031) and lung metastasis (P=0.043). However, CDC42
at baseline was not significantly different between patients with
other clinical characteristics including age (based on median
cutoff value of 50 years), ECOG PS and histology type (all
P>0.05; Table II). Furthermore,
CDC42 was not correlated with FIGO stage at initial diagnosis or
treatment line (both P>0.05) (Table
SI).
 | Table II.CDC42 in patients with different
clinical characteristics at baseline. |
Table II.
CDC42 in patients with different
clinical characteristics at baseline.
| Characteristic | Median CDC42 (IQR),
pg/ml | P-value |
|---|
| Age, years (%) |
| 0.531 |
|
<50 | 616.0 |
|
|
| (349.0–917.0) |
|
|
≥50 | 582.0 |
|
|
| (439.0–1296.0) |
|
| ECOG PS (%) |
| 0.251 |
| 0 | 554.0 |
|
|
| (425.0–735.0) |
|
| 1 | 616.0 |
|
|
| (416.0–1296.0) |
|
| Histology type
(%) |
| 0.905 |
|
Squamous cell carcinoma | 658.5 |
|
|
| (439.5–853.8) |
|
|
Adenocarcinoma | 503.0 |
|
|
| (301.0–1321.0) |
|
|
Adenosquamous | 554.0 |
|
|
| (462.0-NA) |
|
| Target lesion size
(%), cm |
| 0.020 |
|
<5 | 516.0 |
|
|
| (373.0–690.5) |
|
| ≥5 | 770.0 |
|
|
| (473.5–1388.0) |
|
| Pelvis metastasis
(%) |
| 0.031 |
| No | 511.0 |
|
|
| (329.8–674.3) |
|
|
Yes | 771.5 |
|
|
| (492.8–1127.3) |
|
| Lung metastasis
(%) |
| 0.043 |
| No | 511.0 |
|
|
| (361.0–798.5) |
|
|
Yes | 727.5 |
|
|
| (517.3–1354.5) |
|
| Liver metastasis
(%) |
| 0.414 |
| No | 582.0 |
|
|
| (411.0–893.0) |
|
|
Yes | 661.0 |
|
|
| (459.5–1315.0) |
|
| Other distant
metastases (%) |
| 0.134 |
| No | 576.0 |
|
|
| (352.0–915.5) |
|
|
Yes | 616.0 |
|
|
| (527.0–1183.5) |
|
| Previous
bevacizumab (%) |
| 0.106 |
| No | 532.0 |
|
|
| (353.5–903.5) |
|
|
Yes | 727.5 |
|
|
| (515.8–1212.5) |
|
| PD-L1 CPS (%) |
| 0.095 |
|
Positive | 516.0 |
|
|
| (406.5–838.5) |
|
|
Negative or unknown | 680.0 |
|
|
| (565.0–1308.5) |
|
| Treatment (%) |
| 0.278 |
| ICI +
antiangiogenic therapy | 532.0 |
|
|
| (337.0–945.5) |
|
|
ICI-alone | 668.0 |
|
|
| (472.3–995.8) |
|
CDC42 at baseline is associated with
worse treatment response
No patients with advanced cervical cancer achieved
the complete response (CR), 14 (30.4%) patients achieved the
partial response (PR), 22 (47.8%) patients exhibited stable disease
(SD), while 10 (21.7%) patients exhibited progressive disease (PD).
Therefore, ORR was 30.4%, and disease control rate (DCR) was 78.3%
(Table III). CDC42 at baseline
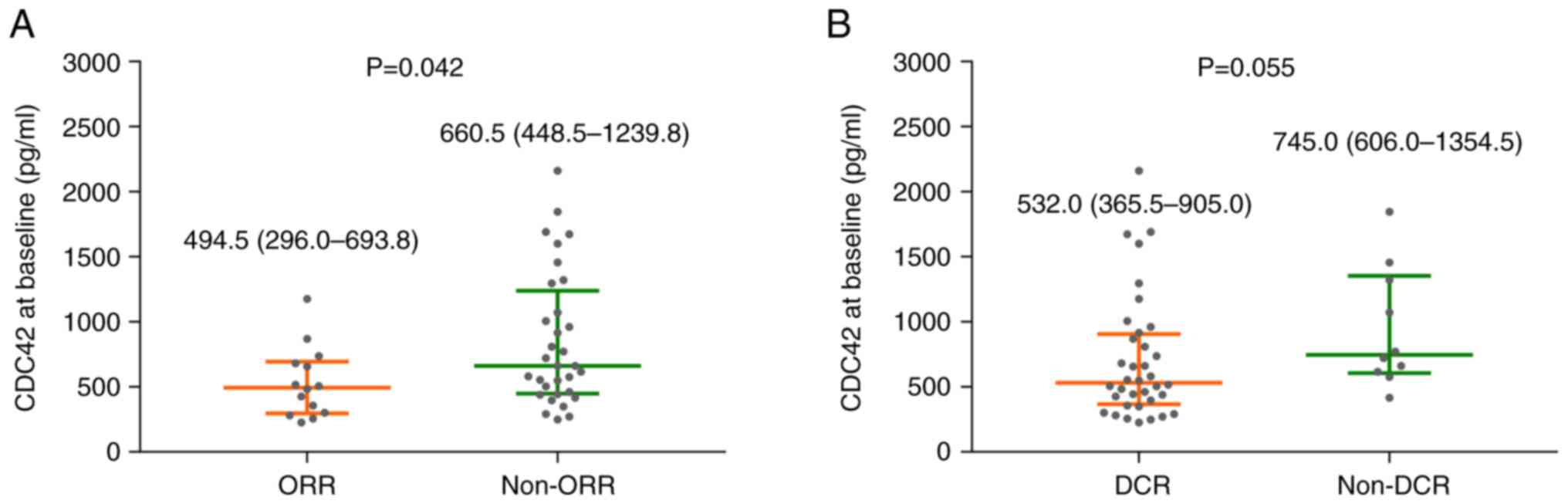
was decreased in patients who achieved the ORR compared with
non-ORR (P=0.042; Fig. 1A) but
remained unchanged between patients who achieved DCR and non-DCR
(P=0.055; Fig. 1B).
 | Table III.Tumor response. |
Table III.
Tumor response.
| Tumor response
(%) | Patients, n=46 |
|---|
| CR | 0 (0.0) |
| PR | 14 (30.4) |
| SD | 22 (47.8) |
| PD | 10 (21.7) |
| ORR | 14 (30.4) |
| DCR | 36 (78.3) |
CDC42 at baseline is associated with
worse survival profile
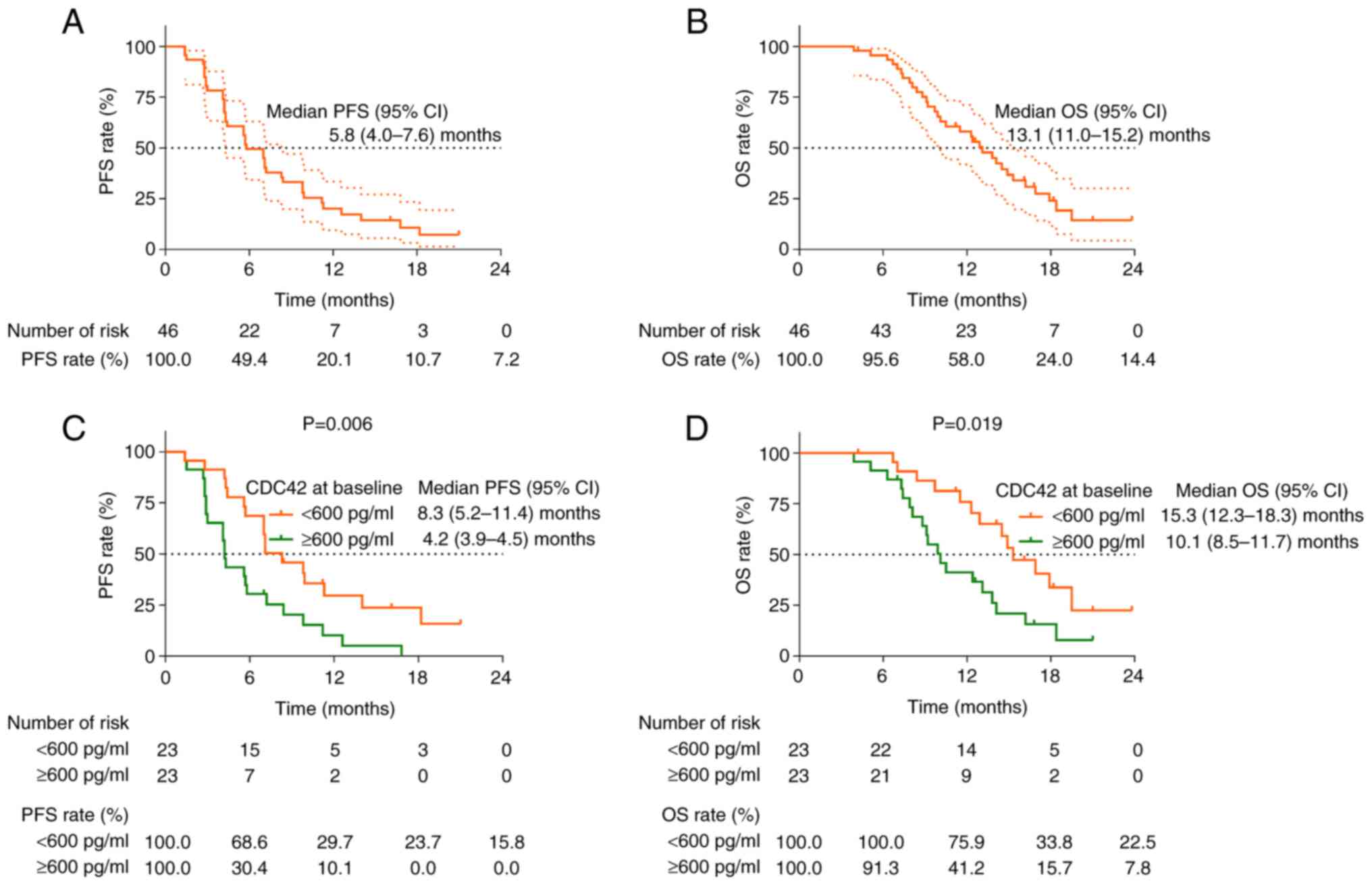
The median [95% confidential interval (CI)] PFS was
5.8 (4.0–7.6) months (Fig. 2A) and
the median OS was 13.1 (11.0–15.2) months (Fig. 2B) in all patients. PFS (P=0.006;
Fig. 2C) and OS (P=0.019; Fig. 2D) were reduced in patients with
baseline CDC42 ≥600 pg/ml compared with those with baseline CDC42
<600 pg/ml.
CDC42 following two treatment cycles
is associated with unfavorable treatment response
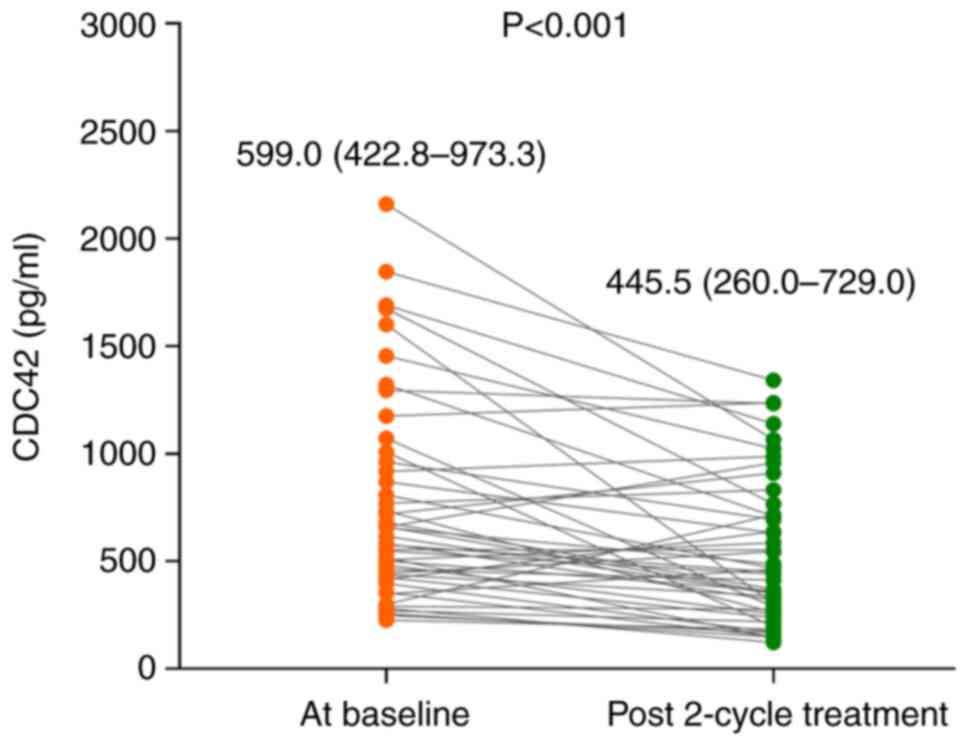
Following two treatment cycles, CDC42 expression
decreased (P<0.001; Fig. 3).
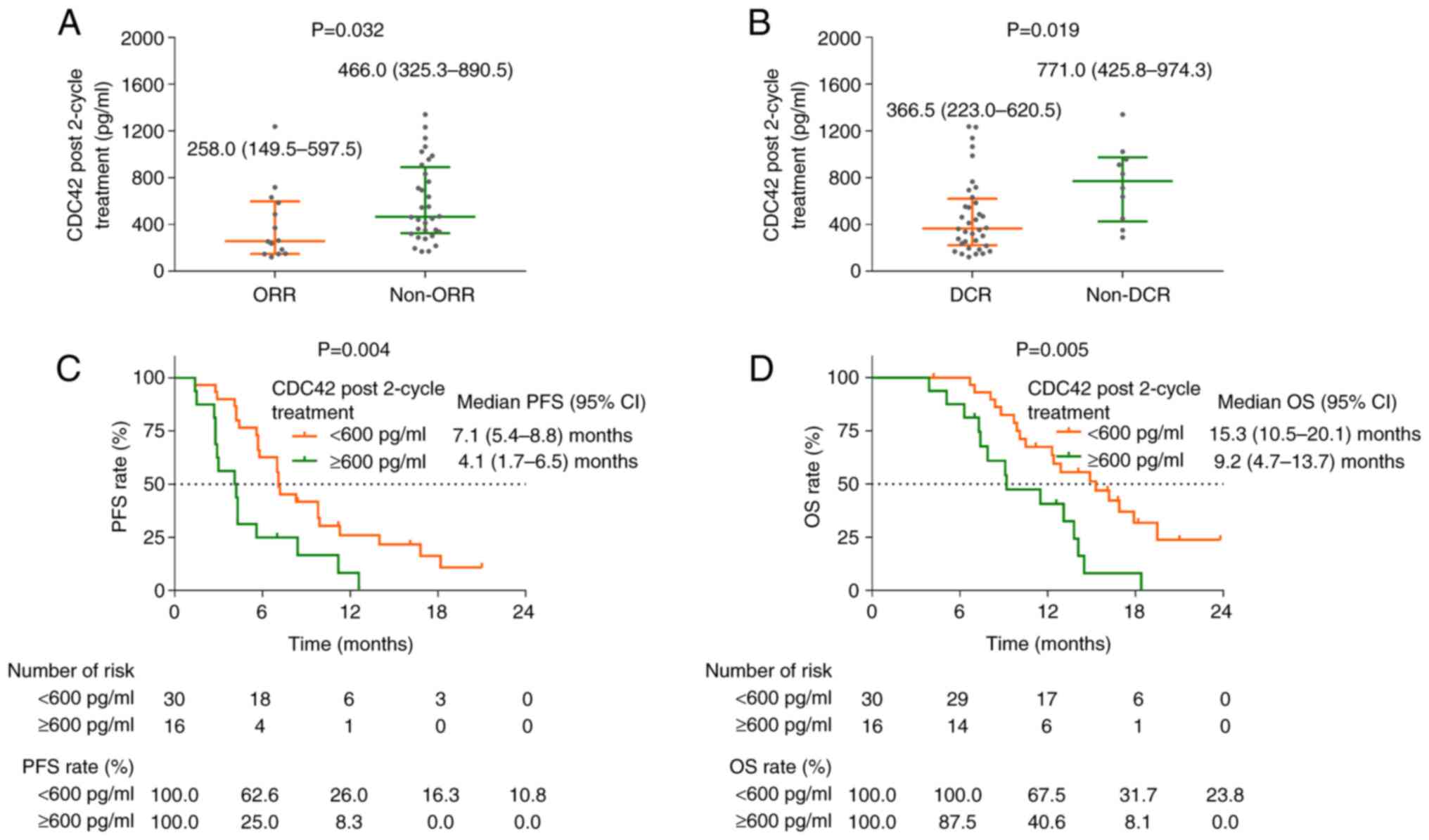
CDC42 following two treatment cycles showed a significant
difference between patients with ORR and non-ORR (P=0.032; Fig. 4A), as well as patients with DCR and
non-DCR (P=0.019; Fig. 4B).
CDC42 following two treatment cycles
is associated with unfavorable prognosis
PFS (P=0.004; Fig.
4C) and OS (P=0.005; Fig. 4D)
were also worse in patients with CDC42 ≥600 pg/ml compared with
CDC42 <600 pg/ml following two treatment cycles.
CDC42 following two treatment cycles
was an independent factors for shorter PFS and OS
After adjusting the confounders by the multivariate
Cox's regression analysis, CDC42 following two treatment cycles
(≥600 vs. <600 pg/ml) was independently associated with a
shorter PFS [P=0.022, hazard ratio (HR)=2.469]. Furthermore, ECOG
PS (1 vs. 0; P=0.007, HR=2.709) and lung metastasis (yes vs. no;
P=0.025, HR=2.517) independently predicted shorter PFS, while PD-L1
CPS (positive vs. negative or unknown; P=0.002, HR=0.284) and
treatment type (ICI + antiangiogenic therapy vs. ICI-alone;
P<0.001, HR=0.259) were associated with prolonged PFS (Table IV).
 | Table IV.Cox's proportional hazard regression
model for PFS. |
Table IV.
Cox's proportional hazard regression
model for PFS.
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Characteristic | P-value | HR | Lower | Upper |
|---|
| Univariate
model |
|
|
|
|
| CDC42
at baseline, ≥600 vs. <600 pg/ml | 0.009 | 2.402 | 1.249 | 4.618 |
| CDC42
following two treatment cycles, ≥600 vs. <600 pg/ml | 0.006 | 2.550 | 1.301 | 5.001 |
| Age,
≥50 vs. <50 years | 0.176 | 1.554 | 0.821 | 2.940 |
| Higher
FIGO stage at initial diagnosis | 0.164 | 1.220 | 0.922 | 1.615 |
| ECOG PS
(1 vs. 0) | 0.091 | 1.761 | 0.913 | 3.397 |
|
Histology type |
|
|
|
|
|
Squamous cell
carcinoma | Reference |
|
|
|
|
Adenocarcinoma | 0.354 | 1.402 | 0.686 | 2.866 |
|
Adenosquamous | 0.551 | 1.446 | 0.430 | 4.864 |
| Target
lesion size, ≥5 vs. <5 cm | 0.104 | 1.711 | 0.895 | 3.272 |
| Pelvis
metastasis, yes vs. no | 0.194 | 1.546 | 0.801 | 2.985 |
| Lung
metastasis, yes vs. no | 0.011 | 2.435 | 1.223 | 4.851 |
| Liver
metastasis, yes vs. no | 0.154 | 1.652 | 0.829 | 3.291 |
| Other
distant metastases, yes vs. no | 0.274 | 1.503 | 0.724 | 3.123 |
|
Previous bevacizumab, yes vs.
no | 0.142 | 1.618 | 0.852 | 3.073 |
| PD-L1
CPS, positive vs. negative or unknown | 0.017 | 0.441 | 0.224 | 0.866 |
| Higher
treatment line | 0.006 | 1.986 | 1.219 | 3.234 |
|
Treatment, ICI +
antiangiogenic therapy vs. ICI-alone | 0.007 | 0.396 | 0.202 | 0.778 |
| Backward-stepwise
multivariate model |
|
|
|
|
| CDC42
following two treatment cycles, ≥600 vs. <600 pg/ml | 0.022 | 2.469 | 1.139 | 5.352 |
| Age,
≥50 vs. <50 years | 0.101 | 1.918 | 0.881 | 4.176 |
| ECOG
PS, 1 vs. 0 | 0.007 | 2.709 | 1.310 | 5.603 |
| Lung
metastasis, yes vs. no | 0.025 | 2.517 | 1.124 | 5.636 |
| PD-L1
CPS, positive vs. negative or unknown | 0.002 | 0.284 | 0.130 | 0.618 |
|
Treatment, ICI +
antiangiogenic therapy vs. ICI-alone | <0.001 | 0.259 | 0.125 | 0.539 |
Multivariate cox's regression analysis for OS showed
that CDC42 following two treatment cycles (≥600 vs. <600 pg/ml)
was also associated with a shorter OS (P=0.013, HR=4.166). Age (≥50
vs. <50 years; P=0.003, HR=4.175), higher FIGO stage at initial
diagnosis (P=0.008, HR=1.621), ECOG PS (1 vs. 0; P=0.033,
HR=2.619), target lesion size (≥5 vs. <5 cm; P=0.001, HR=5.628)
and higher treatment line (P<0.001, HR=3.809) were linked with a
shorter OS, while PD-L1 CPS (positive vs. negative or unknown) was
associated with prolonged OS (P=0.001, HR=0.197; Table V).
 | Table V.Cox's proportional hazard regression
model for OS. |
Table V.
Cox's proportional hazard regression
model for OS.
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Characteristic | P-value | HR | Lower | Upper |
|---|
| Univariate
model |
|
|
|
|
| CDC42
at baseline, ≥600 vs. <600 pg/ml | 0.022 | 2.306 | 1.126 | 4.719 |
| CDC42
following two treatment cycles, ≥600 vs. <600 pg/ml | 0.007 | 2.737 | 1.321 | 5.667 |
| Age,
≥50 vs. <50 years | 0.066 | 1.982 | 0.956 | 4.109 |
| Higher
FIGO stage at initial diagnosis | 0.059 | 1.328 | 0.989 | 1.784 |
| ECOG
PS, 1 vs. 0 | 0.031 | 2.393 | 1.082 | 5.292 |
|
Histology type |
|
|
|
|
|
Squamous cell
carcinoma | Reference |
|
|
|
|
Adenocarcinoma | 0.176 | 1.723 | 0.784 | 3.786 |
|
Adenosquamous | 0.417 | 1.673 | 0.483 | 5.792 |
| Target
lesion size, ≥5 vs. <5 cm | 0.011 | 2.645 | 1.247 | 5.610 |
| Pelvis
metastasis, yes vs. no | 0.557 | 1.233 | 0.613 | 2.478 |
| Lung
metastasis, yes vs. no | 0.011 | 2.592 | 1.244 | 5.400 |
| Liver
metastasis, yes vs. no | 0.172 | 1.698 | 0.795 | 3.628 |
| Other
distant metastases, yes vs. no | 0.055 | 2.116 | 0.984 | 4.552 |
|
Previous bevacizumab, yes vs.
no | 0.430 | 1.332 | 0.654 | 2.714 |
| PD-L1
CPS, positive vs. negative or unknown | 0.032 | 0.447 | 0.214 | 0.932 |
| Higher
treatment line | 0.003 | 2.256 | 1.329 | 3.829 |
|
Treatment, ICI +
antiangiogenic therapy vs. ICI-alone | 0.012 | 0.401 | 0.197 | 0.816 |
| Backward-stepwise
multivariate model |
|
|
|
|
| CDC42
following two treatment cycles, ≥600 vs. <600 pg/ml | 0.013 | 4.166 | 1.349 | 12.860 |
| Age,
≥50 vs. <50 years | 0.003 | 4.175 | 1.613 | 10.811 |
| Higher
FIGO stage at initial diagnosis | 0.008 | 1.621 | 1.131 | 2.322 |
| ECOG
PS, 1 vs. 0 | 0.033 | 2.619 | 1.081 | 6.342 |
| Target
lesion size, ≥5 vs. <5 cm | 0.001 | 5.628 | 1.997 | 15.856 |
| PD-L1
CPS, positive vs. negative or unknown | 0.001 | 0.197 | 0.078 | 0.501 |
| Higher
treatment line | <0.001 | 3.809 | 2.061 | 7.040 |
Discussion
CDC42 is a key protein responsible for progression
of cervical cancer (22,23). Knockdown of CDC42 increases
C-terminal domain Ser phosphatases RNA polymerase II associated
protein 2 and F-cell production 1 (FCP1) in cervical cancer cells,
thus controlling cell proliferation (22). Another study reported that
activation of CDC42/p21-activated kinases 1 is associated with the
tumorigenic and invasive properties of tumorsphere cells enriched
from cervical cancer cell line HeLa (23). Nevertheless, the association between
CDC42 and the clinicopathological features of patients with
cervical cancer needs exploration. Here, serum CDC42 was elevated
in patients with advanced cervical cancer with larger tumor size,
pelvis metastasis and lung metastasis. These findings might be
explained by the proliferation- and invasion-enhancing property of
CDC42 in cervical cancer cells, (24–26).
However, CDC42 was not significantly different between patients
with PD-L1 CSP positive and those with PD-L1 CSP negative or known.
The potential reason is that the sample size was too small, which
resulted in low statistical power. CDC42 might participate in
regulation of immune escape via modulating CD8+ T cells,
rather than modulating the expression of PD-L1 (27).
In recent years, clinical investigations have
revealed that ICI is a potential treatment modality for patients
with advanced cervical cancer (28–30). A
recent phase II, single-arm study showed that in previously treated
patients with PD-L1-positive cervical cancer, serplulimab +
nab-paclitaxel achieves ORR of 51.7%, median PFS is 5.7 months and
the median OS is 15.5 months (28).
Balstilimab achieves ORR of 15% in patients with
recurrent/metastatic cervical cancer who receive prior
platinum-based treatment (31).
Another phase III trial revealed that in patients with cervical
cancer and disease progression after first-line platinum-based
chemotherapy, ORR (16.4 vs. 6.3%) and median OS (12.0 vs. 8.5
months) are both improved following treatment with cemiplimab
compared with chemotherapy (29).
In the present study, the ORR and DCR were 30.4 and 78.3%,
respectively, after ICI treatment in patients with advanced
cervical cancer. Median PFS and OS were 5.8 and 13.1 months,
respectively. These findings are in line with previous studies and
support application of ICI treatment in these patients (28,29,31).
CDC42 is reported to predict treatment response and
survival in patients with cancer who receive ICI treatment
(16,17). The present study revealed that serum
CDC42 at baseline was not significantly decreased in patients with
objective response achievement and disease control achievement; PFS
and OS after ICI treatment in patients with advanced cervical
cancer were decreased in patients with baseline CDC42 ≥600 pg/ml.
These findings might be because CDC42 negatively modulated the
differentiation of CD8+ T cells, which killed tumor
cells following suppression of immune escape by ICI treatment
(15,32). As aforementioned, CDC42 was
associated with larger tumor size, pelvis metastasis and lung
metastasis, which represent higher tumor burden. Patients with a
higher tumor burden might have worse survival. Patients with better
treatment responses tended to have better survival. Another key
finding of this study was that serum CDC42 was decreased following
two cycles of treatment and showed stronger prognostic value for
treatment response and survival in patients with advanced cervical
cancer compared with serum CDC42 at baseline. Moreover, the
association of serum CDC42 with survival was confirmed by
multivariate Cox's proportional hazard regression model. These
findings provide a potential prognostic tool for patients with
advanced cervical cancer, thus improving the management of these
patients.
Nevertheless, the present study had limitations.
First, the present study only enrolled patients with advanced
cervical cancer who received ICI treatment with or without
antiangiogenic agents. Therefore, the findings might not be
applicable in patients with cervical cancer who receive surgical
resection of the tumor. Second, the present study only detected
serum CDC42, however, the prognostic value of CDC42 from other
sources, such as tumors, should be explored in future. Third, the
sample size was not large enough to draw a definitive conclusion.
Further studies with larger sample sizes should be conducted to
verify the prognostic value of serum CDC42.
In conclusion, serum CDC42 was reduced after
treatment and its high expression reflected a lower possibility of
achieving treatment response and worse survival in patients with
advanced cervical cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by National Science Foundation
of China (grant no. 81802612).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LLG and CYW conceived the study. YS, XYL, WX and SLM
collected data and drafted the manuscript. LLG, YHL, WJW, XFL and
CYW analyzed data and revised the manuscript. LLG and CYW confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The patients provided written informed consent to
participate. Ethics Committee of Tongji Hospital, Tongji Medical
College, Huazhong University of Science and Technology (Wuhan,
China) provided ethics approval (approval no.
ChiECRCT20200180).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hillemanns P, Soergel P, Hertel H and
Jentschke M: Epidemiology and early detection of cervical cancer.
Oncol Res Treat. 39:501–506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cibula D, Raspollini MR, Planchamp F,
Centeno C, Chargari C, Felix A, Fischerova D, Jahnn-Kuch D, Joly F,
Kohler C, et al: ESGO/ESTRO/ESP guidelines for the management of
patients with cervical cancer-Update 2023. Virchows Arch.
482:935–966. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oncology NCPGi, . Cervical Cancer (version
1.2023). 2023.
|
|
5
|
Beckmann MW, Stubs FA, Koch MC, Mallmann
P, Dannecker C, Dietl A, Sevnina A, Mergel F, Lotz L, Hack CC, et
al: Diagnosis, therapy and follow-up of cervical cancer. Guideline
of the DGGG, DKG and DKH (S3-Level, AWMF Registry No. 032/033OL,
May 2021)-Part 1 with recommendations on epidemiology, screening,
diagnostics and therapy. Geburtshilfe Frauenheilkd. 82:139–180.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kusakabe M, Taguchi A, Sone K, Mori M and
Osuga Y: Carcinogenesis and management of human
papillomavirus-associated cervical cancer. Int J Clin Oncol.
28:965–974. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shieh KR, Huang A and Xu Y: Response to
immune checkpoint inhibitor treatment in advanced cervical cancer
and biomarker study. Front Med (Lausanne). 8:6695872021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Felice F, Giudice E, Bolomini G,
Distefano MG, Scambia G, Fagotti A and Marchetti C: Pembrolizumab
for advanced cervical cancer: Safety and efficacy. Expert Rev
Anticancer Ther. 21:221–228. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmidt MW, Battista MJ, Schmidt M, Garcia
M, Siepmann T, Hasenburg A and Anic K: Efficacy and safety of
immunotherapy for cervical cancer-a systematic review of clinical
trials. Cancers (Basel). 14:4412022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frenel JS, Le Tourneau C, O'Neil B, Ott
PA, Piha-Paul SA, Gomez-Roca C, van Brummelen EMJ, Rugo HS, Thomas
S, Saraf S, et al: Safety and efficacy of pembrolizumab in
advanced, programmed death ligand 1-positive cervical cancer:
Results from the phase Ib KEYNOTE-028 trial. J Clin Oncol.
35:4035–4041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oh DY, Algazi A, Capdevila J, Longo F,
Miller W Jr, Bing JT, Bonilla CE, Chung HC, Guren TK, Lin CC, et
al: Efficacy and safety of pembrolizumab monotherapy in patients
with advanced thyroid cancer in the phase 2 KEYNOTE-158 study.
Cancer. 129:1195–1204. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao J, Zhang C, Jiang GQ, Jin SJ, Wang Q,
Wang AQ and Bai DS: Identification of hepatocellular
carcinoma-related genes associated with macrophage differentiation
based on bioinformatics analyses. Bioengineered. 12:296–309. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia Y, Rao L, Yao H, Wang Z, Ning P and
Chen X: Engineering macrophages for cancer immunotherapy and drug
delivery. Adv Mater. 32:e20020542020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marques CA, Hahnel PS, Wolfel C, Thaler S,
Huber C, Theobald M and Schuler M: An immune escape screen reveals
Cdc42 as regulator of cancer susceptibility to lymphocyte-mediated
tumor suppression. Blood. 111:1413–1419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo F, Zhang S, Tripathi P, Mattner J,
Phelan J, Sproles A, Mo J, Wills-Karp M, Grimes HL, Hildeman D and
Zheng Y: Distinct roles of Cdc42 in thymopoiesis and effector and
memory T cell differentiation. PLoS One. 6:e180022011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Shao R, Zhang X, Yao D and Han S:
Serum cell division cycle 42 in advanced hepatocellular carcinoma
patients: Linkage with clinical characteristics and immune
checkpoint inhibitor-related treatment outcomes. Clin Res Hepatol
Gastroenterol. 47:1021492023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang L, Shen Y and Wang Y: Vertical level
of blood cell division cycle 42 predicts response and survival
benefits to PD-1 inhibitor-based regimen in metastatic colorectal
cancer patients. Scand J Clin Lab Invest. 83:103–110. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bergerot CD, Philip EJ, Bergerot PG, Hsu
J, Dizman N, Salgia M, Salgia N, Vaishampayan U, Battle D, Loscalzo
M, et al: Discrepancies between genitourinary cancer patients' and
clinicians' characterization of the Eastern Cooperative Oncology
Group performance status. Cancer. 127:354–358. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwartz LH, Litiere S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saleh M, Virarkar M, Bhosale P, El Sherif
S, Javadi S and Faria SC: Endometrial cancer, the current
international federation of gynecology and obstetrics staging
system, and the role of imaging. J Comput Assist Tomogr.
44:714–729. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robert ME, Ruschoff J, Jasani B, Graham
RP, Badve SS, Rodriguez-Justo M, Kodach LL, Srivastava A, Wang HL,
Tang LH, et al: High interobserver variability among pathologists
using combined positive score to evaluate PD-L1 expression in
gastric, gastroesophageal junction, and esophageal adenocarcinoma.
Mod Pathol. 36:1001542023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Zhong X, Sauane M, Zhao Y and
Zheng ZL: Modulation of the Pol II CTD phosphorylation code by Rac1
and Cdc42 small GTPases in cultured human cancer cells and its
implication for developing a synthetic-lethal cancer therapy.
Cells. 9:6212020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong Z, Yu C, Rezhiya K, Gulijiahan A and
Wang X: Downregulation of miR-146a promotes tumorigenesis of
cervical cancer stem cells via VEGF/CDC42/PAK1 signaling pathway.
Artif Cells Nanomed Biotechnol. 47:3711–3719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun X, Zhou L, Wang X, Li Y, Liu X, Chen
Y, Zhong Z and Chen J: FYCO1 regulates migration, invasion, and
invadopodia formation in HeLa cells through CDC42/N-WASP/Arp2/3
signaling pathway. Biochem Cell Biol. 100:458–472. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Chen B, Huang A, Ren C, Wang L, Zhu
T, Xiong J, Ding W and Wang H: LncRNA HCP5 enhances the
proliferation and migration of cervical cancer via
miR-216a-5p/CDC42 axis. J Cancer. 13:1882–1894. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen R and Zhang L: MiR-29a inhibits cell
proliferation and migration by targeting the CDC42/PAK1 signaling
pathway in cervical cancer. Anticancer Drugs. 30:579–587. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Serrano-Pertierra E, Cernuda-Morollon E
and Lopez-Larrea C: NKG2D- and CD28-mediated costimulation regulate
CD8+ T cell chemotaxis through different mechanisms: The role of
Cdc42/N-WASp. J Leukoc Biol. 95:487–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An J, Li X, Wang J, Zhu L, An R, Jiang K,
Huang Y, Wang K, Li G, Wang C, et al: Efficacy and safety of
serplulimab plus nab-paclitaxel in previously treated patients with
PD-L1-positive advanced cervical cancer: A phase II, single-arm
study. Front Immunol. 14:11422562023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tewari KS, Monk BJ, Vergote I, Miller A,
de Melo AC, Kim HS, Kim YM, Lisyanskaya A, Samouelian V, Lorusso D,
et al: Survival with cemiplimab in recurrent cervical cancer. N
Engl J Med. 386:544–555. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Monk BJ, Tewari KS, Dubot C, Caceres MV,
Hasegawa K, Shapira-Frommer R, Salman P, Yanez E, Gumus M, de
Mendoza MO, et al: Health-related quality of life with
pembrolizumab or placebo plus chemotherapy with or without
bevacizumab for persistent, recurrent, or metastatic cervical
cancer (KEYNOTE-826): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 24:392–402. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Malley DM, Oaknin A, Monk BJ, Selle F,
Rojas C, Gladieff L, Berton D, Leary A, Moore KN, Estevez-Diz MDP,
et al: Phase II study of the safety and efficacy of the anti-PD-1
antibody balstilimab in patients with recurrent and/or metastatic
cervical cancer. Gynecol Oncol. 163:274–280. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laletin V, Bernard PL, da Silva CS,
Guittard G and Nunes JA: Negative intracellular regulators of
T-cell receptor (TCR) signaling as potential antitumor
immunotherapy targets. J Immunother Cancer. 11:e0058452023.
View Article : Google Scholar : PubMed/NCBI
|